Abstract
Neuropathic pain can be generated by chronic compression of dorsal root ganglion (CCD). Stimulation of primary motor cortex can disrupt the nociceptive sensory signal at dorsal root ganglion level and reduce pain behaviors. But the mechanism behind it is still implicit. Protein kinase C gamma is known as an essential enzyme for the development of neuropathic pain, and specific inhibitor of protein kinase C gamma can disrupt the sensory signal and reduce pain behaviors. Optogenetic stimulation has been emerged as a new and promising conducive method for refractory neuropathic pain. The aim of this study was to provide evidence whether optical stimulation of primary motor cortex can modulate chronic neuropathic pain in CCD rat model. Animals were randomly divided into CCD group, sham group, and control group. Dorsal root ganglion-compressed neuropathic pain model was established in animals, and knocking down of protein kinase C gamma was also accomplished. Pain behavioral scores were significantly improved in the short hairpin Protein Kinase C gamma knockdown CCD animals during optic stimulation. Ventral posterolateral thalamic firing inhibition was also observed during light stimulation on motor cortex in CCD animal. We assessed alteration of pain behaviors in pre-light off, stimulation-light on, and post-light off state. In vivo extracellular recording of the ventral posterolateral thalamus, viral expression in the primary motor cortex, and protein kinase C gamma expression in dorsal root ganglion were investigated. So, optical cortico-thalamic inhibition by motor cortex stimulation can improve neuropathic pain behaviors in CCD animal, and knocking down of protein kinase C gamma plays a conducive role in the process. This study provides feasibility for in vivo optogenetic stimulation on primary motor cortex of dorsal root ganglion-initiated neuropathic pain.
Keywords: Optogenetics, neuropathic pain, dorsal root ganglia, protein kinase C gamma, thalamus, motor cortex
Introduction
Chronic lower back pain with sciatica, hyperalgesia, and other tormenting conditions are common symptoms associated with many diseases of the lumbosacral spine and can result in functional disability along with chronic neuropathic pain.1–4 The pharmacological intervention has been unsuccessful in improving neuropathic pain cases; moreover, sometimes it is accompanied by adverse effects, whereupon understanding the implicit neuronal mechanisms have become a burning question.
The primary motor cortex (M1) plays a key role in the modulation of pain in various chronic pain syndromes. The use of chronic motor cortex stimulation (MCS) for treating medically intractable chronic pain disorders, including trigeminal neuropathic pain, central deafferentation pain, phantom limb pain, and pain caused by multiple sclerosis, spinal cord injury, and peripheral nerve injury, has been increased over the past couple of decades.5 M1 stimulation is thought to beget alterations in other systems by the activation of cortico-striatal-thalamo-cortical loops, as well as influences both descending and ascending pain processing pathway.6,7 However, it remains argumentative because few published articles exhibited negative results on modulation of cortico-thalamic pathway by MCS.8–11
Previous studies revealed that activation of protein kinase C gamma (PKCγ) in dorsal horn neurons occurs in a number of pain models, and the administration of PKCγ inhibitors alleviates neuropathic pain which makes it a striking therapeutic target for many human ailments.12–16 In the present study, we used short hairpin Protein Kinase C gamma (shPKCγ), which is a selective inhibitor of PKCγ, to observe whether M1 stimulation in PKCγ knockdown condition provides momentous pain reduction or not rather than PKCγ active condition.
The advancement of optogenetic science strategies enables the precise temporal and spatial control of specific neural populations via the delivery of specific wavelengths of light following the introduction of genes encoding for light-sensitive transmembrane channels.17–19 Multiple brain areas of different animal models have been studied using optogenetics to understand underlying interactions with chronic pain and its therapeutic options. Different studies have already proved that the optogenetic stimulation of brain structures impacts both physical and emotional aspects of pain which ensures the optogenetic approach in the control of pain perception as it provides higher temporal specificity compared to pharmacologic and electrical intervention.20–22
Therefore, we schemed our study to determine the effects of the M1 optogenetic stimulation in chronic compression of dorsal root ganglion (CCD) neuropathic pain rat model with PKCγ knockdown and active state. We conducted behavior tests and electrophysiological methods to observe any alterations in the cortico-thalamic pathway resulted by optical stimulation of channelrhodopsin in motor cortex. Here, we hypothesized that MCS by optogenetic technique could exhibit better antinociceptive effect with PKCγ knockdown state.
Materials and methods
Experimental animal and ethical review
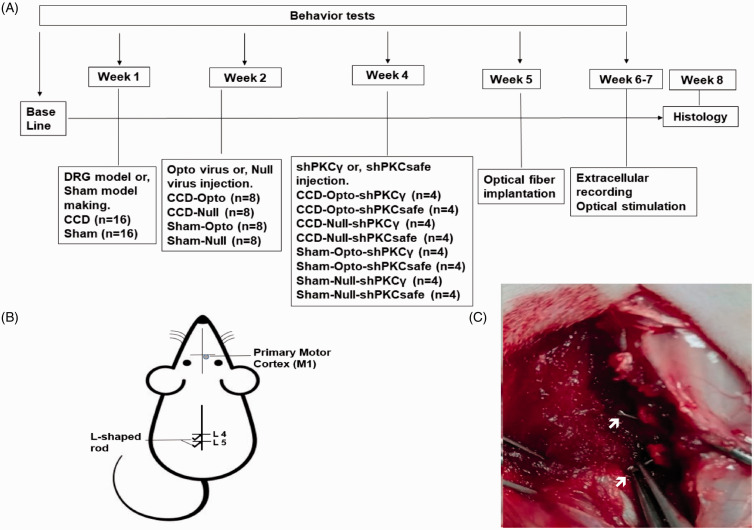
A total of 36 adult female Sprague-Dawley rats (aged 8 weeks; Koatech, Pyeongtaek, Korea) weighing 200 to 250 g on arrival were housed having free access to ad libitum fresh food and water and maintained in a room with 12 hr of light and 12 hr of darkness per day, 50% to 60% humidity. We used female rats because among diverse traits of neuroscience, female rats are not more variable than male rats in neuroscience research. Some types of neuroscience tests may yield more precise, or less variable, data values, but this does not differ by sex. Females exhibited higher variability in the category of nonbrain measures. In addition, male rats often show dominating behavior to other cage mates and results in fighting.23 Animal tests were conducted by randomized, double-blind, controlled animal trial. The animals were randomly divided into animal with CCD group (n = 16), sham group (n = 16), and control group (n = 4). The timeline of experimental protocol is shown in Figure 1(A).
Figure 1.
Experimental animal model and timeline: (A) experimental timeline, (B) schematic diagram of experimental animal model and optogenetic virus injection site, and (C) stainless steel rod insertion at L4 and L5 intervertebral foramen to induce DRG compression.
DRG: dorsal root ganglion; CCD: chronic compression of DRG; PKCγ: protein kinase C gamma.
We conducted all experiments in accordance with ethical review permitted by the Institutional Animal Care Committee and Animal Use of Chungbuk National University, Republic of Korea. We performed all animal experiments at Laboratory Animal Research Center, Chungbuk National University during light period.
Behavior confirmation of hypersensitivity
Mechanical threshold and latency
The rats were accustomed to the behavior test room for a minimum of 30-min period. Baseline data test was performed one day before surgery, and then comparing behavior change data tests were done on every three days interval after surgery. The behavior test was performed based on a prior study related to mechanical and thermal hypersensitivity. Mechanical hypersensitivity was assessed by measuring paw withdrawal threshold and latency to an increasing pressure stimulus placed onto the plantar area of the hind paw using a Dynamic Plantar Aesthesiometer (Ugo Basile, Varese, Italy). For mechanical threshold and latency measurement, the rats were placed individually in a transparent plexiglass chamber (20 × 20 × 14 cm) with a metal mesh floor. Calibration was performed by applying a metal filament (0.5 mm diameter) to the hind paw with increasing force from 0 to 50 g. When rats responded to the stimulation, they withdrew their hind paw from the mesh floor. The force intensity and latency appeared on the device’s monitor.24
Thermal latency
The thermal latency (TL) was measured by conventional hot plate test. Rats were placed on a temperature-controlled Peltier plate (Hot/Cold Plate, Ugo Basile, Varese, Italy) set at 50°C after 10 min of adaptation at 25°C, and the time taken to observe a nociceptive response (hind paw lick, flinch or jump) was recorded.24
All analyses were surveyed three times for each rat, and the mean value was taken for evaluation.
Chronic compression of dorsal root ganglions
The CCD model making was performed by following previously instructed methods which has been used generally for neuropathic pain treatment and drug development studies.25–27 Under general anesthesia induced by intraperitoneal (i.p.) injection of a mixture of 15 mg/kg Zoletil and 9 mg/kg Rompun in saline, the rats were mounted onto the surgical field in a prone position. The back skin was incised, and paraspinal muscles were separated from the mamillary process at the area of left lumber 4 to lumber 6 vertebrae to expose the L4 and L5 intervertebral foramens. To dispense chronic compression on the L4 and L5 dorsal root ganglions (DRGs) and nerve root, at first, an L-shaped needle was thrusted into the foramen and observed to detect a slight, transient twitch of the ipsilateral hind leg which is the proof of reaching needle tip to DRG. After witnessing the twitch, needle was revoked from the foramen, and a sterilized stainless steel rod (0.7 mm diameter and 4 mm length) was inserted along the path of needle in the space of the fourth and fifth intervertebral foramen (Figure 1(C)). After completion of implanting rod, which was meant to generate a resolute compression, the musculature and skin were sutured with silk (3–0). A sham operation was also done in the same way just the stainless steel rods were not inserted. After surgery, the rats were given fresh pelleted food and water ad libitum and checked for the survival every day for at least one week.
Optogenetic viral vector injection
Animals of the CCD group were randomly divided into two groups. Eight rats were subjected to optogenetic viral vector AAV-CaMKII-hChR2-EYFP (titer 1 × 1013 GC/ml, Korea Institute of Science and Technology, Seoul, South Korea), while the remaining eight rats were subjected to null virus AAV-CaMKII-EYFP (titer 1 × 1013 GC/ml, Korea Institute of Science and Technology, Seoul, South Korea) into M1 layer 5 region (anteroposterior (AP): 1 mm, mediolateral (ML): 1.5 mm, dorsoventral (DV): 1.5 mm),28–30 by intracranial injection under general anesthesia. Animals of the sham group were also divided into two groups for injection of either the optogenetic virus or null virus. We injected adeno-associated virus carrying the ChR2-EYFP fusion protein under the control of an excitatory neuron-specific calcium/calmodulin-dependent protein kinase II (CaMKII) promoter in our study. Before injecting virus, the animals were anesthesized by an i.p. injection of a mixture of 15 mg/kg Zoletil (Zoletil50®, Virbac Laboratories, Carros, France) and 9 mg/kg Rompun (Rompun®, Bayer, Seoul, South Korea). Then, 2 µl of virus was injected at a rate of 0.3 µl/min using a Hamilton syringe and an automatic microsyringe pump. After injection, the needle was kept in the same place for 5 min to have the virus absorbed and then retracted in a very slow manner.
shPKCγ injection in L4 and L5 DRG
Three weeks after generation of the CCD model and virus injection, animals in each group (CCD-Opto, CCD-Null, Sham-Opto, and Sham-Null) were again divided into two sub-groups of four animals each. Animals in one group received an injection of shPKCγ (1 µg/µl, pFBAAVmu6-shPKCg1CMVeGFP, VVC, University of Iowa, USA) as a PKCγ antagonist directly into ipsilateral L4 and L5 DRG by performing laminectomy procedure as described previously,31 while animals in the other group were injected with shPKCsafe where PKCγ remained in active state. Animals were laid down in ventral recumbency under general anesthesia induced by i.p. injection of a mixture of 15 mg/kg Zoletil and 9 mg/kg Rompun in saline. Then, after exposing the L4 and L5 intervertebral foramens by cleaning the lateral aspect of the vertebrae with the help of blunt dissection, the transverse process of L4 and L5 along with the marginal laminar rim caudal to the L4 and L5 ganglion were removed using a small rongeur to expose DRG. Then, 1 µl of shPKCγ was injected in each DRG taking 3 min for each site. After giving the injection, the syringe should not be withdrawn for additional 3 min to allow pressure within the ganglion to equalize and minimize backflow. The musculature and skin were sutured after that. A sham operation was executed by following the same procedures but without injecting anything in DRGs.
Optic fiber implantation
Two weeks after optogenetic virus inoculation, each rat was positioned in a stereotactic frame after anesthesized. An optic fiber was implanted into the skull at AP: 1 mm, ML: 1.5 mm to send a laser pulse to the hindlimb motor cortex contralateral to lesioned side. Optic fibers (200 µm core, 230 µm outer diameter, numerical aperture of 0.48, hard polymer cladding type, Doric Lenses; Québec City, Québec, Canada) were cut to a length of 1.4 mm to optimize M1 depth. Dental cement was used to fix the fiber firmly in place (Ortho-Jet Pound Package, Lang Dental, USA).
Optical stimulation
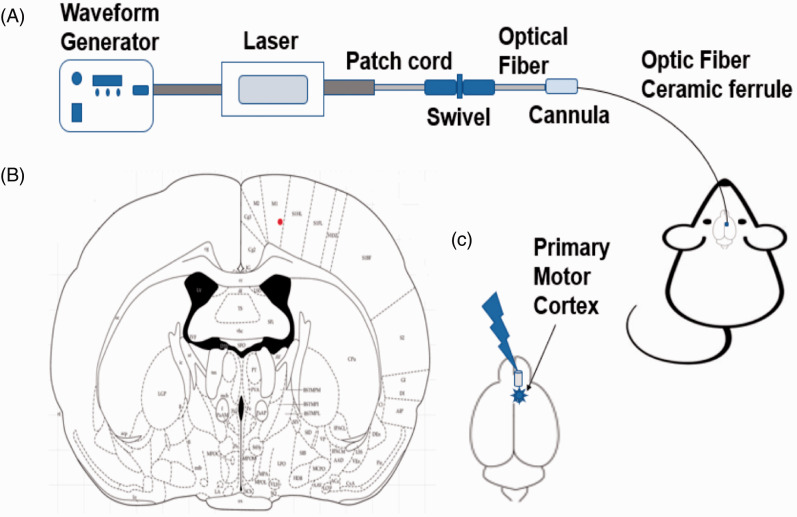
We used a laser power supply with a wavelength of 473 nm (ADR-700D, Shanghai, China) and a waveform generator (Keysight 33511 b-CFG001, Keysight, Santa Rosa, CA, USA) to regulate the waveform and pulse width of the laser (Figure 2). The laser’s intensity was set at 10 mW, the pulse width was set at 4 ms, the pulses were set at 20 Hz, and the duration of stimulation was 5 min. Mechanical and thermal test differences were observed on optogenetic virus-inoculated animals under optic stimulation (pre, stim, post) to determine the optical neuromodulation effects.
Figure 2.
Optogenetic stimulation procedure: (A) complete setup of optic stimulation, (B) optic fiber implantation position, and (C) stimulation giving by blue laser on primary motor cortex area.
In vivo extracellular recording
After 4 weeks of DRG compression, rats were anesthetized with 15 mg/kg tiletamine/zolazepam and 9 mg/kg xylazine to prepare for extracellular recordings. Extracellular recordings were obtained from the ventral posterolateral (VPL) (AP: 2.5 mm, ML: 3 mm, DV: 6.5 mm) using a single electrode. We recorded thalamic neuronal spikes and firing rate during the pre-stimulation, optical stimulation (blue-473 nm), and post-stimulation states after a 20-min resting state in vivo. A glass-insulated carbon fiber microelectrode (Cat. No.: E1011-20, Carbostar-1, Kation Scientific, LLC, MN 55414 USA) was used for recording in the thalamus. Duration of each stage was 5 min, and between two stages, there were 5 min of gap. We chose well-isolated clusters and recorded neuronal signals using a Digital Lynx SX (Neuralynx, Bozeman, USA) data-acquisition system along with Cheetah software. We digitized and bandpass filtered at 40 kHz and 1 Hz to 5 kHz, respectively. We sorted offline using Spike Sorter 3 D (Neuralynx Inc., MT, USA). We observed neuronal discharge in the VPL thalamus of lesioned and sham animals. We analyzed the rate histograms (spikes/s) of the lesioned models under different optical condition with NeuroExplorer (Neuralynx Inc.).
Histological examinations
The rats were deeply anesthetized and transcardially perfused with phosphate buffered saline (PBS), followed by 4% paraformaldehyde. The brains and L4-L5 DRGs were extracted and fixed overnight in the same post-fixed solution, followed by dehydration in 30% sucrose solution.
We embedded brains and DRGs in optimal cutting temperature compound (Tissue Tek® - Sakura, USA) and cryopreserved with liquid nitrogen and isopentane at –79°C. Coronal sections of the brains and DRGs were cut by cryostat (Thermo Scientific, Waltham, MA, USA) with 20 µm and 10 µm thickness, respectively, and mounted on slides. The brain sections were incubated with 4',6-diamidino-2-phenylindole (DAPI) and mounted with coverslips to examine under fluorescence microscope. Immunostaining of the DRG sections were done following procedure described before. The DRGs sections were incubated serum block solution for 1 hr and with anti-PKC gamma antibody (1:200, ab4145, Abcam) overnight. The corresponding secondary antibody was applied before the sections were stained with DAB (Vector Laboratory, CA, USA). Nuclei were counterstained with hematoxylin. Finally, the sections were dehydrated and mounted with coverslips and examined under microscope.
Analysis of the bursting and firing rates
The activity in thalamic neurons was divided into bursts rates (bursts/s) and overall firing rates (spikes/s), according to the type of optical stimulation, using NeuroExplorer software (Neuralynx Inc.). Activity was assessed for 5 min in each state: pre, laser-on, and post states. We defined burst rates as a group of spikes with a maximum 4-ms interval between spikes and a minimum of three spikes, with a 100-ms interval between bursts. We selected similar interspike interval histograms and compared in all groups.
Statistical analysis
Data were analyzed using GraphPad Prism 7 (GraphPad Software, Inc., San Diego, CA, USA), and data from the behavior test and the in vivo recordings were shown as mean ± standard deviation (SD). We performed either an unpaired t-test, two-way analysis of variance (ANOVA) with Tukey’s post hoc test, or a repeated measures test, depending on the conditions of the experiment. Behavioral tests were assessed based on the mean values for each of the three optical states. Unpaired t-tests were used to compare firing rate between trigeminal neuralgia model animals and sham-operated animals.
Results
Pain behavior tests
Behavioral responses after CCD of the rat
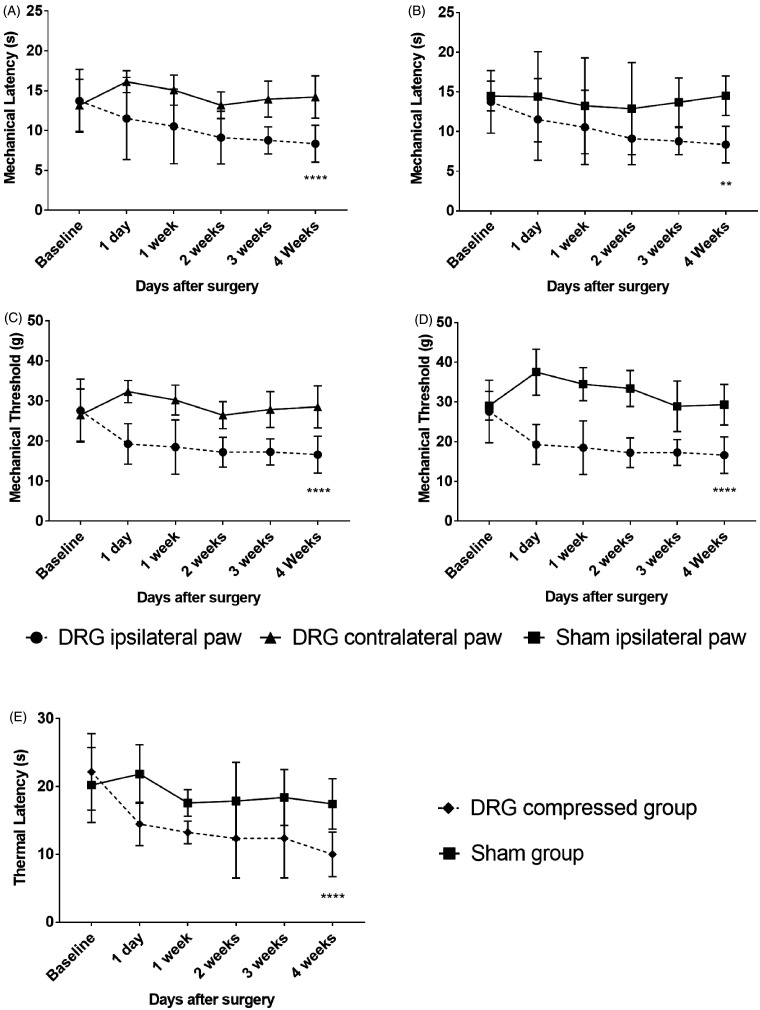
Mechanical withdrawal threshold, latency, and TL were used to confirm changing of responses due to CCD by stainless steel rod. In all tests after surgery, a gradual reduction in threshold and latency indicator scores was exhibited by the CCD group over time in comparison with the sham group. Mechanical latency of ipsilateral hind paw of CCD group decreased from 13.73 ± 3.94 to 8.36 ± 2.32, with a two-way ANOVA showing a significant difference after surgery, F(1, 180) = 78.58, p < 0.0001. Mechanical threshold of ipsilateral hind paw of CCD group decreased from 27.61 ± 7.86 to 16.62 ± 4.59, with a two-way ANOVA showing a significant difference after surgery, F(1, 180) = 163.2, p < 0.0001. TL of DRG-compressed group also abated from 22.15 ± 5.65 to 10 ± 3.29, with a two-way ANOVA also showing a significant difference after surgery, F(1, 180) = 55.06, p < 0.0001. There were significant successive differences between the ipsilateral and contralateral hind paw of CCD-grouped animals as well. An ANOVA with Tukey’s post hoc test was used to determine whether there were differences between different groups and days (Figure 3(A) to (E)).
Figure 3.
Behavioral pain responses to mechanical and thermal tests of CCD group and sham group. (A) Paw withdrawal latency in response to mechanical pain with a plantar aesthesiometer of ipsilateral and contralateral hind paw in CCD group. (B) Ipsilateral hind paw withdrawal latency between CCD group and sham group. (C) Paw withdrawal threshold of ipsilateral and contralateral hind paw in CCD group. (D) Ipsilateral hind paw withdrawal threshold between CCD group and sham group. (E) Hind paw thermal latency in hot plate test between DRG-compressed group and sham group. **p < 0.01, ****p < 0.0001 significant difference between the indicated values (ANOVA).
DRG: dorsal root ganglion.
Alleviation of hyperalgesia by optic stimulation in PKCγ knockdown animal
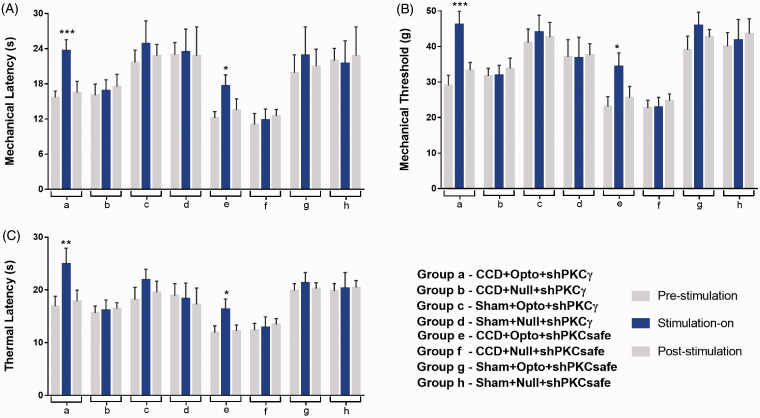
The optogenetic neuromodulation on motor cortex of PKCγ knockdown and active animals was observed in mechanical and thermal test in three optical states (pre-stimulation, during stimulation, and post-stimulation) (Figure 4). CCD-Opto-shPKCγ-grouped animals with blue light stimulation on M1 resulted in significant alterations of behavior test scores. Mechanical latency of ipsilateral hind paw was increased during optic stimulation (15.67 ± 1.1 at pre state, 23.67 ± 1.89 at blue laser “on” state, and 16.51 ± 1.93 at post state (mean ± SD)). A two-way ANOVA showed significant effects of optic stimulation, F(2, 24) = 91.91, p < 0.001 (Figure 4A(a)). Mechanical threshold of ipsilateral hind paw was also increased during optic stimulation (29.067 ± 2.867 at pre state, 46.15 ± 3.77 at blue laser-on state, and 33.317 ± 2.183 at post state, F(2, 24) = 104.7, p < 0.001, Figure 4B(a)). TL of CCD group showed increased score during stimulation (16.89 ± 1.92 at pre state, 24.93 ± 2.98 at blue laser “on” state, and 17.85 ± 2.12 at post state, F(2, 24) = 50.75, p < 0.01, Figure 4C(a)).
Figure 4.
Alterations of hyperalgesia in animal groups with blue laser stimulation. (A) Paw withdrawal latency scores, (B) paw withdrawal threshold scores, and (C) thermal latency scores of all eight animal groups. Only CCD-Opto-shPKCγ group (a) and CCD-Opto-shPKCsafe group (e) exhibited significant changes of behavioral scores with blue light “ON” state. In other animal groups, blue laser stimulation “ON” state did not have any significant alterations in behavioral response. *p < 0.05; **p < 0.01, ***p < 0.001 significant difference between the indicated values (ANOVA).
CCD: chronic compression of dorsal root ganglion; PKCγ: protein kinase C gamma.
Less significant behavioral test score changes were also seen in animals of CCD-Opto-shPKCsafe group. Mechanical test score was found 12.17 ± 1.1 at pre state, 17.67 ± 1.89 at blue laser “on” state, and 13.51 ± 1.93 at post state. A two-way ANOVA showed significant effects of optic stimulation, F(2, 24) = 31.89, p < 0.05 (Figure 4A(e)). Mechanical threshold was found 23.067 ± 2.75 at pre state, 33.45 ± 3.29 at blue laser “on” state, and 25.617 ± 2.183 at post state, F(2, 24) = 35.43, p < 0.05 (Figure 4B(e)). TL of CCD group also showed test scores of 11.88 ± 1.37 at pre state, 16.33 ± 1.9 at blue laser-on state, and 12.25 ± 1.16 at post state, F(2, 24) = 32.69, p < 0.05 (Figure 4C(e)).
All the other animal groups showed very less or no changes in behavior test results.
Our results here support that primary MCS abates mechanical and thermal hyperalgesia in chronic neuropathic pain condition.
In vivo extracellular recording data
Changes of neural activity in the VPL of CCD animals during MCS by optogenetic virus
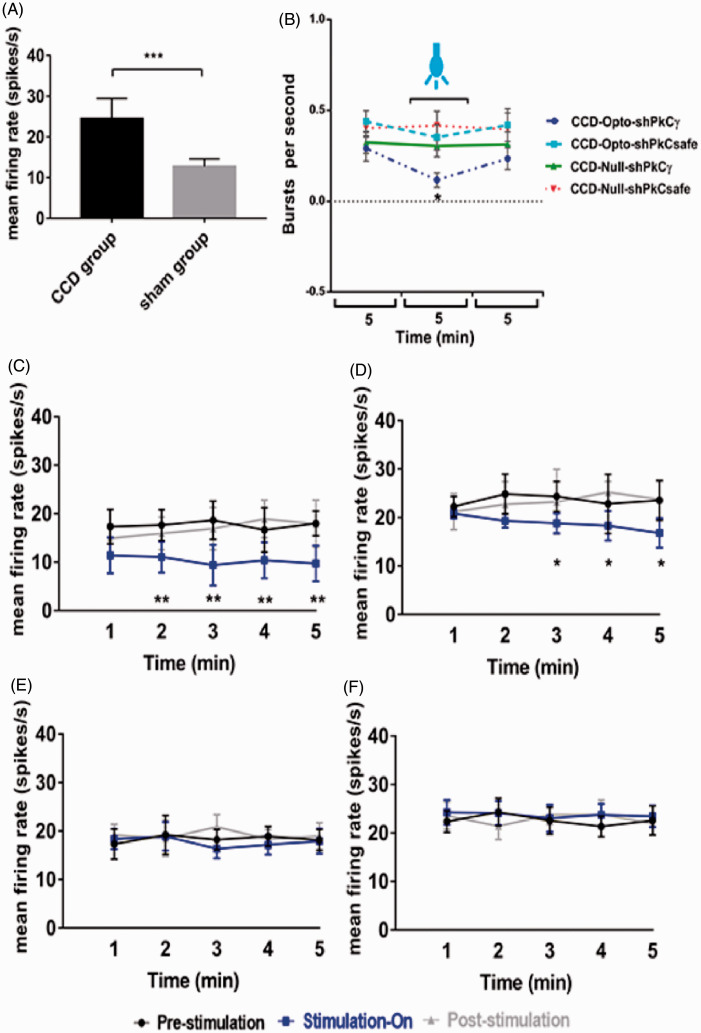
To confirm the neuronal activity, we monitored the firing rates of posterolateral thalamic neurons among CCD, sham, and control animals. Significantly higher firing rates were found in CCD-lesioned rats (24.47 ± 5.24 spikes/s) compared with those in sham animals (12.73 ± 1.93 spikes/s, unpaired t-test, p < 0.001; Figure 5(A)). After three weeks of optogenetic virus injection, we assessed the effects of optical stimulation on M1 in the CCD-Opto and CCD-Null group by in vivo extracellular recording. Burst rates appeared to decrease during optic stimulation in CCD-Opto group. The thalamic burst rates were 0.29 ± 0.08/s at the pre state, 0.11 ± 0.04/s at blue laser “on” state, and 0.23 ± 0.10/s at post state of CCD-Opto-shPKCγ-grouped animals. A two-way ANOVA showed significant effects of optic stimulation, F(2, 3588) = 173.2, p < 0.05 (Figure 5(B)). Optical stimulation of CCD-Opto-shPKCsafe-grouped animals also showed slight reduction of burst rate, but it was not significant.
Figure 5.
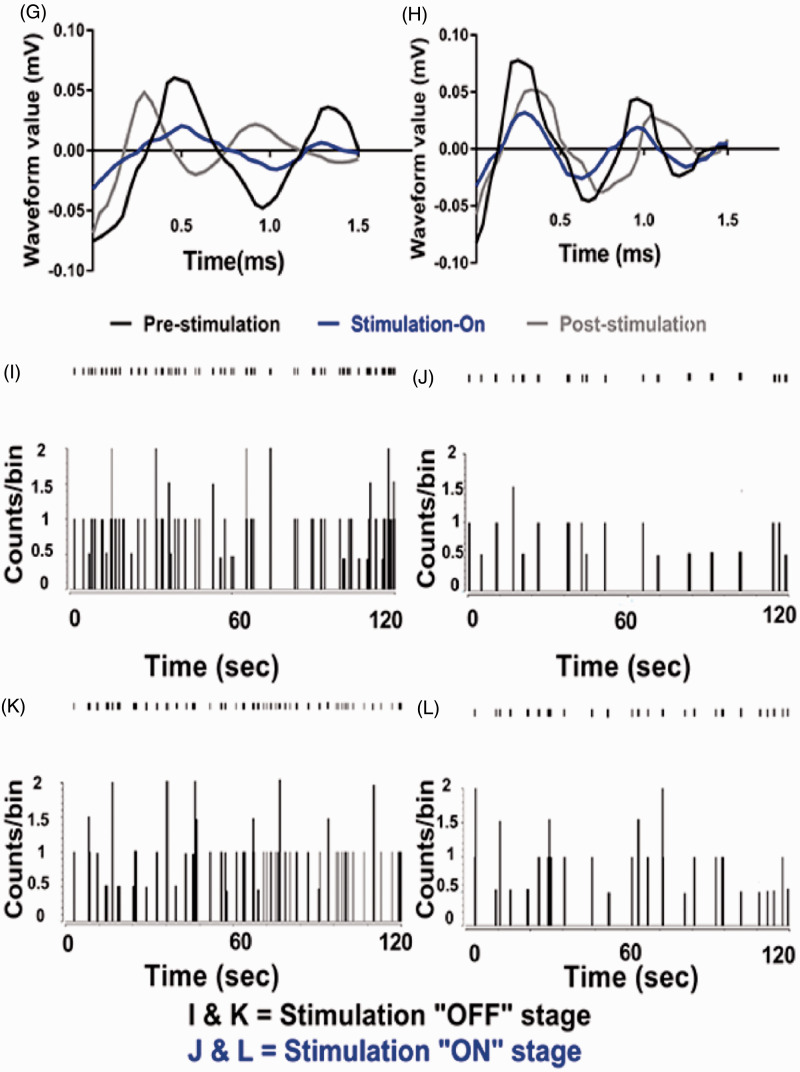
Electrophysiology results of thalamic output by optic stimulation in motor cortex. (A) Evoked firing rates in the neurons of VPL thalamus of CCD animals compared to sham-operated animals. Unpaired t-test was used to compare between sham group and CCD group. (B) Burst firing rates of CCD-grouped animals following optical stimulation. Significant change was seen in only CCD-Opto-shPKCγ group. Burst rates decreased in CCD-Opto-shPKCsafe group also, but it was not significant. (C, D) In vivo recording of CCD-Opto-shPKCγ-grouped animals (C) and CCD-Opto-shPKCsafe-grouped animals (D) from the VPL thalamus. Firing output (spikes/s) declines under blue laser stimulation, which is higher in the pre- and post-light states. (E, F) No changes of firing rates in CCD-Null-shPKCγ and CCD-Null-shPKCsafe group, respectively. Two-way ANOVA test was used to compare neuronal activity according to the optical stimulation. *p < 0.05; **p < 0.01; ***p<0.001 significant difference between the indicated values (ANOVA). (G) Average action potential waveform of VPL neuron of CCD-Opto-shPKCγ-grouped animal. (H) Average action potential waveform of CCD-Opto-shPKCsafe-grouped animal. (G, H) In both cases, amplitude got decreased during optical stimulation on motor cortex. (I, J) Perievent raster histogram of responses of CCD-Opto-shPKCγ-grouped animal’s VPL neurons. (K, L) Raster plot responses of CCD-Opto-shPKCsafe-grouped animal’s VPL neurons. (I, K) Increased firing response when there is no optical stimulation present. (J, L) During optical stimulation, VPL firing response got decreased. Bin size = 50 ms.
CCD: chronic compression of dorsal root ganglion; PKCγ: protein kinase C gamma.
We found significant alterations in the neural action of VPL thalamus also depending on optogenetic stimulation in CCD-Opto group. We recorded the data in three stages: pre-stimulation stage, blue laser-on stage, and post-stimulation stage. Each stage was conducted for 5 min. In pre-stimulation, stimulation-on, and post-stimulation condition of CCD-Opto-shPKCγ-grouped animals, the spikes/s data were 17.66 ± 3.58, 10.38 ± 3.71, and 16.92 ± 3.87, respectively. A two-way ANOVA showed significant effects of optic stimulation, F(2, 2691) = 491.1, p < 0.01 (Figure 5(C)). In case of CCD-Opto-shPKCsafe-grouped animals, spikes/s data were 23.56 ± 6.12 at pre-stimulation state, 18.81 ± 3.04 at stimulation-on state, and 23.23 ± 6.71 at post-stimulation state. A two-way ANOVA showed significant effects of optic stimulation, F(2, 2691) = 313.4, p < 0.05 (Figure 5(D)). In CCD-Null-shPKCγ and CCD-Null-shPKCsafe group, optical stimulation did not show any effect on thalamic firing rate (Figure 5(E) and (F)). In both CCD-Opto-shPKCγ and CCD-Opto-shPKCsafe group, amplitude of average waveform got decreased during optical stimulation “ON” stage (Figure 5(G) and (H)), and responses of thalamic neurons showed inhibition of thalamic output in perievent raster histogram in optic stimulation “ON” state than stimulation “OFF” state.
These results depict that MCS attenuates thalamic discharge of CCD animals with ChR2 group, and as a result, the repetitive firing rate got decreased during stimulation with blue laser, and once the stimulation was withdrawn, thalamic firing rate get increased again. In other two CCD groups, there were no effect of blue laser on motor cortex area, and no distinctive changes in the thalamic firing pattern were found among the three stages.
Confirmation of viral expression in the motor cortex and PKCγ expression in DRG
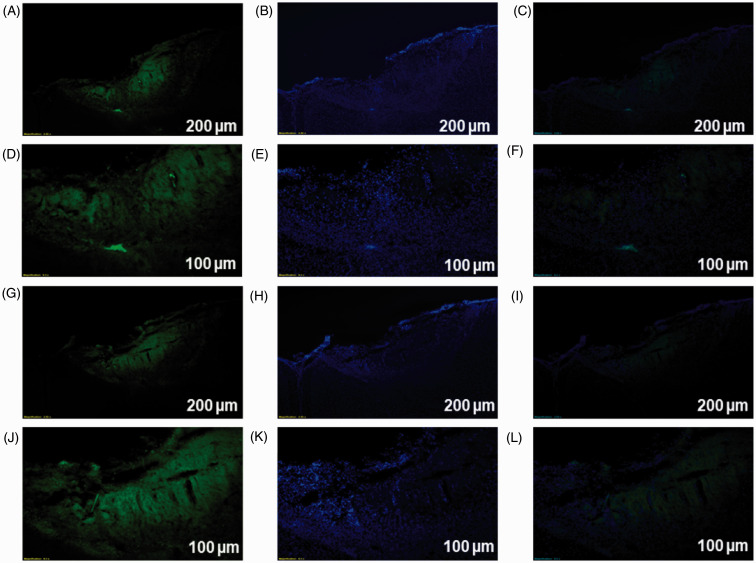
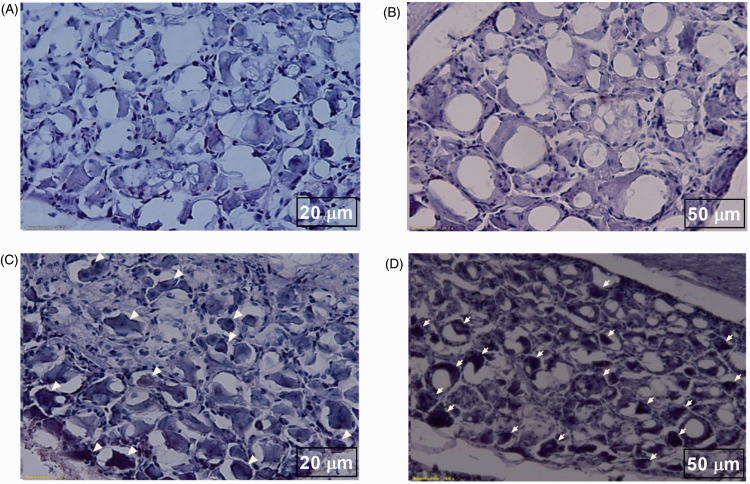
The optogenetic target in this study was the layer 5 region of motor cortex, which was stereotaxically located using a rat atlas. We observed expression of opto virus and null virus in the contralateral motor cortex neurons of animals using immunofluorescence. Viral expression in DAPI and enhanced yellow fluorescent protein (EYFP) was obtained using a fluorescence microscope and ImageJ software (Figure 6). In shPKCγ- and shPKCsafe-injected animals, absence of anti-PKCγ antibody binding and presence of anti-PKCγ antibody binding with PKCγ within the DRG cells were seen respectively (Figure 7).
Figure 6.
Immunofluorescence results confirmed viral expression in primary motor cortex area (A–L). The low-magnification figures (A to C) and higher magnification figures (D to F) showed the optogenetic virus-infected and DAPI-stained neurons in the motor cortex region. (G to I) and (J to L), respectively, showed the low-magnification and higher magnification of histological section of null virus-infected and DAPI-stained neurons in the motor cortex region. (A, D, G, J) EYFP, (B, E, H, K) DAPI, and (C, F, I, L) Merge. (A, B, C, G, H, I) Scale bar = 200 µm. (D, E, F, J, K, L) Scale bar = 100 µm.
Figure 7.
Immunohistochemistry results of DRG cells of CCD animals. (A, B) DRG cells of shPKCγ-injected animals showing no bindings with anti-PKCγ antibody as shPKCγ inhibits the activation of PKCγ within DRG cells. (C, D) DRG cells of shPKCsafe-injected animals showing bindings (arrow bars) anti-PKCγ antibody with activated PKCγ within DRG cells. (A, C) Scale bar = 20 µm. (B, D) Scale bar = 50 µm.
Discussion
We showed that optical stimulation of layer 5 M1 with PKCγ inhibition modulates VPL thalamic discharge aptly and has significant role in neuropathic pain behaviors alteration in CCD animal. As PKCγ is an important element of CCD-induced pain, we used shPKCγ and shPKCsafe along with MCS to inquire whether MCS alone can provide effective analgesia or with another effective treatment manner it provides better improvement. We found that CCD-Opto-shPKCγ group had shown more significant improvement than CCD-Opto-shPKCsafe group.
DRG is a known clinical target for the delivery of anti-inflammatory steroids, surgery, radio-frequency ablation, pulse-radio frequency, and electrical neuromodulation therapies.32–36 DRG functions as a portal for spinothalamic projections of peripheral signals with or without adding an impulsive context to nociception.12,37 Many experiments showed similar results that CCD induces chronic hypersensitivity because macrophages along with different small and large molecules can cross the satellite glial cell (SGC) overlay of DRG neuron that makes Schwann cells and SGCs to release proinflammatory mediators which influence nociceptors to lead to neuropathic pain.25,26,38–44
For having an ideal anatomical localization to transmit nociceptive stimuli, PKCγ is a necessary secondary messenger and plays a key role in providing linkage between peripheral stimuli and ascending transmission neurons and thus induces neuropathic pain by various cellular processes.12,15,45–47 Findings in PKCγ inhibitor and knockout animal studies delineate the importance for spinal PKCγ in developing central sensitization, especially in inflammatory and neuropathic pain after nerve injury.16,46,48,49 These results point out that with selective inhibitor of PKCγ, it may be possible to attenuate neuropathic pain conditions that resulted from nerve injury, avoiding the vivid side effects that are imminent with nonselective inhibitors of PKCγ which had been used in several preceding studies.48,50 In our study, we injected shPKCγ in the DRG to inhibit PKCγ expression, and behavioral test scores were less in the shPKCγ-injected animals than the results found in shPKCsafe group.
MCS is comparatively a promising neurosurgical technique because of the low perpetration of complication, the lower propensity to cause seizures, and ability to apply it noninvasively as the stimulation electrodes or fibers for MCS are placed in epidural space.51–56 Stimulating other structures including the internal capsule, the periaqueductal gray (PAG)–periventricular gray complex, the thalamus, etc. were tried for altering chronic neuropathic nociceptive signals, but MCS has been found simpler and easier to implement than other methods such as direct nerve stimulation, neurectomy, etc. and more effective since its first introduction.28,57 For several cortical areas including motor cortex, layer-specific patterns of local circuit connectivity are well established. By understanding neural circuitry involving in the modulation of distant neural structures by M1 and its connection with brain behaviors, motor cortex can be used as a potent marker to lead neuromodulatory therapeutic options.58,59 Motor cortex consists of six layers, and each layer has distinct role in cerebral function. Among them, layer 2/3 axons usually send excitatory inputs to the layer 5 and layer 5 pyramidal neurons of motor cortex project into different regions. Among them, two projection classes which are important for motor control are the cortico-spinal and cortico-striatal neurons, which means cortico-striatal fibers originate from layer 5 cells of motor cortex, and these pyramidal neurons project into striatum.60,61 Hence, after being excited, layer 5 MCS appears to trigger the striatum which in turn causes rapid and phasic alteration in the lateral thalamus by gamma-Aminobutyric acid producing (GABAergic) neurons, which is then followed by a cascade of events of longer time-course in medial thalamus, PAG matter, and anterior cingulate/orbitofrontal cortices.62,63 This focus on M1 neuromodulation will be helpful for focusing on other fundamental studies in different types of pain.64–68
We used optogenetics technology to stimulate M1 for producing circuit-specific neuromodulation to regulate neuronal activities by overexpressing light-sensitive proteins (opsins) in M1 layer 5 cells.60 Optogenetic stimulation was accomplished by using viral vectors that infect only definite neuron types through cell type-specific promoter CaMKIIα, which will localize optogenetic proteins to excitatory neurons.22,69 To rein cellular activity with high spatiotemporal resolution, optogenetics is consisted of heterologous expression of photosensitive actuators such as channelrhodopsin2 (ChR2).70 ChR2 allows proper quantitative coupling between optical excitation and neuronal activation.71,72 Since ChR2 is genetically targetable, its expression is used as a powerful tool to increase cytoplasmic Ca2+ concentration or to depolarize the cell membrane.73,74 These channels open when activated by blue light (∼473 nm) and are used to induce neuronal excitation.4,75 CaMKII is a glutamatergic, neuron-specific promoter to drive ChR2 expression. In this manner, ChR2 expression is specific to the excitatory CaMKIIα-expressing cortical neuron population. As a result, the optical neural interface selectively actuates excitatory cortical neurons.
M1 area is the center for planning and executing movement activity with other motor areas, and through the cortico-spinal tract, it projects to internal capsule and spinal cord.57,76 Present hypotheses point out that MCS may function through two mechanisms: descending pathway and ascending pathway.28,77 In descending pathway, MCS simultaneously affects the PAG by increasing the activity of zona incerta to cause the PAG to release high amount of serotonin, which is a known descending pain modulator such as opioids.2,78–82 In ascending pathway, glutamate secretion from PAG excites GABAergic interneurons in lamina II of the dorsal horn. It increases the release of GABA on the second-order neurons to hyperpolarize them, thereby inhibiting them from sending nociceptive stimuli.12 Behavior test results of our study also showed improvement from hypersensitivity after MCS which remain in accordance with our expectation.
Electrophysiology results showed that CCD-grouped animals produce long periods of repetitive firing in thalamus due to painful responses of CCD similar to that found in peripheral injury models.83–85 After the dorsal root entrance of nociceptors, central nociceptive terminals amalgam to second-order neurons mainly placed in lamina II (pure nociceptive) and lamina V (mixed nociceptive and mechanosensory). The main neurotransmitters involved in these first relays are glutamate, but also substance p, acting as a co-transmitter in peptidergic nociceptors, is valuable to experience mild to intense pain.86 The thermal and nociceptive information reach thalamus via spinothalamic tract from the second-order neuron. Therefore, thalamus gets somatosensory input, and as a result, neuronal activity in the VPL drastically increased by painful stimulation.1,87
Optical stimulation of ChR2-transfected layer 5 pyramidal neurons in M1 inhibits the sensory inputs in spinothalamic tract and modulates cortico-striatal neural circuitry, hence reduces abnormal thalamic firing. The overall effect of MCS in VPL neurons is inhibitory. Our data corroborate with these findings, and as anatomic connections between motor cortex and ipsilateral thalamus are very robust, thalamic burst rate also get decreased while ChR2 excites cortical neurons of motor cortex on blue light infliction. Several studies found similar result of VPL thalamic firing rate alteration after MCS.76,88
In our study, we did not examine the effects of other cortical structure’s stimulation. The anesthetic condition could have influenced on spontaneous thalamic discharge in our study. However, we avoided the effect of stimulation with von Frey filaments. We performed electrophysiological study in the brain to monitor VPL thalamic activity in response to pain but not in the DRG. Future studies could utilize the novel technique to determine different dopaminergic neuron’s distinct roles in MCS-induced pain modulation. Those studies will provide experimental evidence to demonstrate the role of endogenous dopamine system in MCS-produced analgesia.
Our study suggests optical stimulation of M1 layer 5 cortico-spinal and cortico-striatal neural circuitries can improve neuropathic pain behavior in CCD rat model. Here, behavioral tests and electrophysiological studies were combined to define the role of the PKCγ, motor cortex, VPL thalamus, and DRG in pain that goes way beyond motor functioning. Also, we need to elaborate our knowledge about this specific area and its cortico–cortico and cortico–subcortico interactions, and how it can modulate different bottom-up (such as median nerve stimulation) or top-down (such as TMS or tDCS) interventions or vice versa.
Author Contributions
JI and YSP were responsible for the study concept and design. JI was responsible for the animal experiment and surgery. JI and EK were responsible for the electrophysiology, data analysis, and immunostaining. JI and YSP wrote the manuscript. BHO, HCM, and YSP were responsible for data interpretation and manuscript review. All authors approve the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was funded by the National Research Foundation of Korea (NRF, 2020R1F1A1052716). This work was financially supported by the Research Year of Chungbuk National University in 2018.
ORCID iD
Young Seok Park https://orcid.org/0000-0001-7685-6292
References
- 1.Kim J, Ryu SB, Lee SE, Shin J, Jung HH, Kim SJ, Kim KH, Chang JW. Motor cortex stimulation and neuropathic pain: how does motor cortex stimulation affect pain-signaling pathways? J Neurosurg 2016; 124: 866–876. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage 2007; 37: S71–S79. [DOI] [PubMed] [Google Scholar]
- 3.Lefaucheur J-P, Drouot X, Cunin P, Bruckert R, Lepetit H, Créange A, Wolkenstein P, Maison P, Keravel Y, Nguyen J-P. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain 2009; 132: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Tao F. Application of optogenetics-mediated motor cortex stimulation in the treatment of chronic neuropathic pain. J Transl Sci 2016; 2: 286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JA, Barbaro NM. Motor cortex stimulation for central and neuropathic pain: current status. Pain 2003; 104: 431–435. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, Brandao R, Coutinho L, Truong D, Datta A, Shani-Hershkovich R, Weiss M, Laufer I, Reches A, Peremen Z, Geva A, Parra LC, Fregni F. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain 2016; 17: 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fregni F, Gimenes R, Valle AC, Ferreira MJL, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS. A randomized, sham‐controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum 2006; 54: 3988–3998. [DOI] [PubMed] [Google Scholar]
- 8.Cioni B, Meglio M. Motor cortex stimulation for chronic non-malignant pain: current state and future prospects In: Sakas DE, Simpson BA. (eds) Operative neuromodulation. Vienna: Springer, 2007, pp.45–49. [DOI] [PubMed] [Google Scholar]
- 9.Fujii M, Ohmoto Y, Kitahara T, Sugiyama S, Uesugi S, Yamashita T, Shiroyama Y, Ito H. Motor cortex stimulation therapy in patients with thalamic pain. No Shinkei Geka Neurological Geka 1997; 25: 315–319. [PubMed] [Google Scholar]
- 10.Radic JA, Beauprie I, Chiasson P, Kiss ZH, Brownstone RM. Motor cortex stimulation for neuropathic pain: a randomized cross-over trial. Can J Neurol Sci 2015; 42: 401–409. [DOI] [PubMed] [Google Scholar]
- 11.Sachs AJ, Babu H, Su YF, Miller KJ, Henderson JM. Lack of efficacy of motor cortex stimulation for the treatment of neuropathic pain in 14 patients. Neuromodulation 2014; 17: 303–311. [DOI] [PubMed] [Google Scholar]
- 12.Velázquez KT, Mohammad H, Sweitzer SM. Protein kinase C in pain: involvement of multiple isoforms. Pharmacol Res 2007; 55: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Huang L-Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature 1992; 356: 521–523. [DOI] [PubMed] [Google Scholar]
- 14.Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J 1993; 291: 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igwe O, Chronwall B. Hyperalgesia induced by peripheral inflammation is mediated by protein kinase C βII isozyme in the rat spinal cord. Neuroscience 2001; 104: 875–890. [DOI] [PubMed] [Google Scholar]
- 16.Martin WJ, Malmberg AB, Basbaum AI. PKCγ contributes to a subset of the NMDA-dependent spinal circuits that underlie injury-induced persistent pain. J Neurosci 2001; 21: 5321–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci 2011; 34: 389–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalanithi PS, Henderson JM. Optogenetic neuromodulation. Int Rev Neurobiol 2012; 107: 185–205. [DOI] [PubMed] [Google Scholar]
- 19.DosSantos MF, Moura BS, DaSilva AF. Reward circuitry plasticity in pain perception and modulation. Front Pharmacol 2017; 8: 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang SJ, Kwak C, Lee J, Sim S-E, Shim J, Choi T, Collingridge GL, Zhuo M, Kaang B-K. Bidirectional modulation of hyperalgesia via the specific control of excitatory and inhibitory neuronal activity in the ACC. Mol Brain 2015; 8: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M, Manders TR, Eberle SE, Su C, D'amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci 2015; 35: 5247–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aravanis AM, Wang L-P, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 2007; 4: S143–S156. [DOI] [PubMed] [Google Scholar]
- 23.Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ 2016; 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kc E, Moon HC, Kim S, Kim HK, Won SY, Hyun SH, Park YS. Optical modulation on the nucleus accumbens core in the alleviation of neuropathic pain in chronic dorsal root ganglion compression rat model. Neuromodulation 2020; 23: 167–176. [DOI] [PubMed] [Google Scholar]
- 25.Song X-J, Hu S-J, Greenquist KW, Zhang J-M, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol 1999; 82: 3347–3358. [DOI] [PubMed] [Google Scholar]
- 26.Hu S-J, Xing J-L. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain 1998; 77: 15–23. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y-J, Wang Y-H, Wang C-Z, Ho M-L, Kuo P-L, Huang M-H, Chen C-H. Effect of low level laser therapy on chronic compression of the dorsal root ganglion. PLoS One 2014; 9: e89894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonoff ET, Dale CS, Pagano RL, Paccola CC, Ballester G, Teixeira MJ, Giorgi R. Antinociception induced by epidural motor cortex stimulation in naive conscious rats is mediated by the opioid system. Behav Brain Res 2009; 196: 63–70. [DOI] [PubMed] [Google Scholar]
- 29.Veinante P, Deschênes M. Single‐cell study of motor cortex projections to the barrel field in rats. J Comp Neurol 2003; 464: 98–103. [DOI] [PubMed] [Google Scholar]
- 30.Zilles K. The cerebral cortex of the rat. A stereotaxic atlas. New York: Springer-Verlag, 1985. [Google Scholar]
- 31.Puljak L, Kojundzic SL, Hogan QH, Sapunar D. Targeted delivery of pharmacological agents into rat dorsal root ganglion. J Neurosci Methods 2009; 177: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manchikanti L. Transforaminal lumbar epidural steroid injections. Pain Physician 2000; 3: 374–398. [PubMed] [Google Scholar]
- 33.Vad VB, Bhat AL, Lutz GE, Cammisa F. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine 2002; 27: 11–15. [DOI] [PubMed] [Google Scholar]
- 34.Acar F, Miller J, Golshani KJ, Israel ZH, McCartney S, Burchiel KJ. Pain relief after cervical ganglionectomy (C2 and C3) for the treatment of medically intractable occipital neuralgia. Stereotact Funct Neurosurg 2008; 86: 106–112. [DOI] [PubMed] [Google Scholar]
- 35.de Louw AJ, Vles HS, Freling G, Herpers MJ, Arends JW, Kleef M. The morphological effects of a radio frequency lesion adjacent to the dorsal root ganglion (RF‐DRG)—an experimental study in the goat. Eur J Pain 2001; 5: 169–174. [DOI] [PubMed] [Google Scholar]
- 36.Van Zundert J, Patijn J, Kessels A, Lamé I, van Suijlekom H, van Kleef M. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain 2007; 127: 173–182. [DOI] [PubMed] [Google Scholar]
- 37.Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med 2014; 15: 1669–1685. [DOI] [PubMed] [Google Scholar]
- 38.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CBA, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005; 114: 386–396. [DOI] [PubMed] [Google Scholar]
- 39.Sweitzer S, Martin D, DeLeo J. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience 2001; 103: 529–539. [DOI] [PubMed] [Google Scholar]
- 40.Zelenka M, Schäfers M, Sommer C. Intraneural injection of interleukin-1β and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 2005; 116: 257–263. [DOI] [PubMed] [Google Scholar]
- 41.Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain 2010; 149: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamae T, Ochi M, Olmarker K. Pharmacological inhibition of tumor necrosis factor may reduce pain behavior changes induced by experimental disc puncture in the rat: an experimental study in rats. Spine 2011; 36: E232–E236. [DOI] [PubMed] [Google Scholar]
- 43.Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol 2012; 12: 55–61. [DOI] [PubMed] [Google Scholar]
- 44.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci USA 2007; 104: 20151–20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain 2010; 151: 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou W, Song Z, Guo Q, Liu C, Zhang Z, Zhang Y. Intrathecal lentiviral-mediated RNA interference targeting PKCγ attenuates chronic constriction injury–induced neuropathic pain in rats. Hum Gene Ther 2011; 22: 465–475. [DOI] [PubMed] [Google Scholar]
- 47.Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan L-Y, Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep 2015; 13: 1246–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science 1997; 278: 279–283. [DOI] [PubMed] [Google Scholar]
- 49.Labombarda F, Coronel MF, Villar MJ, De Nicola AF, González SL. Neuropathic pain and temporal expression of preprodynorphin, protein kinase C and N-methyl-d-aspartate receptor subunits after spinal cord injury. Neurosci Lett 2008; 447: 115–119. [DOI] [PubMed] [Google Scholar]
- 50.Malmberg AB, Brandon EP, Idzerda RL, Liu H, McKnight GS, Basbaum AI. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J Neurosci 1997; 17: 7462–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arle JE, Shils JL. Motor cortex stimulation for pain and movement disorders. Neurotherapeutics 2008; 5: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canavero S, Bonicalzi V. Extradural cortical stimulation for central pain. In: Sakas D.E. and Simpson B.A. (eds.) Operative neuromodulation Springer, 2007, pp. 27–36. [DOI] [PubMed]
- 53.Fagundes-Pereyra WJ, Teixeira MJ, Reyns N, Touzet G, Dantas S, Laureau E, Blond S. Motor cortex electric stimulation for the treatment of neuropathic pain. Arq Neuropsiquiatr 2010; 68: 923–929. [DOI] [PubMed] [Google Scholar]
- 54.Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg 2009; 110: 251–256. [DOI] [PubMed] [Google Scholar]
- 55.Mo J-J, Hu W-H, Zhang C, Wang X, Liu C, Zhao B-T, Zhou J-J, Zhang K. Motor cortex stimulation: a systematic literature-based analysis of effectiveness and case series experience. BMC Neurol 2019; 19: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahimpour S, Lad SP. Surgical options for atypical facial pain syndromes. Neurosurg Clin N Am 2016; 27: 365–370. [DOI] [PubMed] [Google Scholar]
- 57.Lucas JM, Ji Y, Masri R. Motor cortex stimulation reduces hyperalgesia in an animal model of central pain. Pain 2011; 152: 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carvalho S, Gonçalves ÓF, Soares JM, Sampaio A, Macedo F, Fregni F, Leite J. Sustained effects of a neural-based intervention in a refractory case of Tourette syndrome. Brain Stimul 2015; 8: 657–659. [DOI] [PubMed] [Google Scholar]
- 59.DaSilva AF, Mendonca ME, Zaghi S, Lopes M, DosSantos MF, Spierings EL, Bajwa Z, Datta A, Bikson M, Fregni F. tDCS‐induced analgesia and electrical fields in pain‐related neural networks in chronic migraine. Headache 2012; 52: 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson CT, Sheets PL, Kiritani T, Shepherd GM. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci 2010; 13: 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiler N, Wood L, Yu J, Solla SA, Shepherd GM. Top-down laminar organization of the excitatory network in motor cortex. Nat Neurosci 2008; 11: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003; 6: 750–757. [DOI] [PubMed] [Google Scholar]
- 63.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci 2000; 23: 393–415. [DOI] [PubMed] [Google Scholar]
- 64.Botelho LM, Morales-Quezada L, Rozisky JR, Brietzke AP, Torres IL, Deitos A, Fregni F, Caumo W. A framework for understanding the relationship between descending pain modulation, motor corticospinal, and neuroplasticity regulation systems in chronic myofascial pain. Front Hum Neurosci 2016; 10: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caumo W, Deitos A, Carvalho S, Leite J, Carvalho F, Dussán-Sarria JA, Lopes Tarragó MG, Souza A, Torres I, Fregni F. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Front Hum Neurosci 2016; 10: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu X-S, Fisher CA, Munz SM, Toback RL, Nascimento TD, Bellile EL, Rozek L, Eisbruch A, Worden FP, Danciu TE, DaSilva AF. Feasibility of non-invasive brain modulation for management of pain related to chemoradiotherapy in patients with advanced head and neck cancer. Front Hum Neurosci 2016; 10: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci 2016; 10: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Brien AT, Amorim R, Rushmore RJ, Eden U, Afifi L, Dipietro L, Wagner T, Valero-Cabré A. Motor cortex neurostimulation technologies for chronic post-stroke pain: implications of tissue damage on stimulation currents. Front Hum Neurosci 2016; 10: 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C, Bamberg E. Ultra light-sensitive and fast neuronal activation with the Ca 2+-permeable channelrhodopsin CatCh. Nat Neurosci 2011; 14: 513–518. [DOI] [PubMed] [Google Scholar]
- 70.Beaudry H, Daou I, Ase AR, Ribeiro-da-Silva A, Séguéla P. Distinct behavioral responses evoked by selective optogenetic stimulation of the major TRPV1+ and MrgD+ subsets of C-fibers. Pain 2017; 158: 2329–2339. [DOI] [PubMed] [Google Scholar]
- 71.Zhang F, Wang L-P, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 2006; 3: 785–792. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y-P, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nat Methods 2007; 4: 139–141. [DOI] [PubMed] [Google Scholar]
- 73.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 2003; 100: 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci 2010; 13: 387–392. [DOI] [PubMed] [Google Scholar]
- 75.Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci 2007; 27: 14231–14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain 2012; 153: 2359–2369. [DOI] [PubMed] [Google Scholar]
- 77.DosSantos MF, Ferreira N, Toback RL, Carvalho AC, DaSilva AF. Potential mechanisms supporting the value of motor cortex stimulation to treat chronic pain syndromes. Front Neurosci 2016; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silva GD, Lopes PS, Fonoff ET, Pagano RL. The spinal anti-inflammatory mechanism of motor cortex stimulation: cause of success and refractoriness in neuropathic pain? J Neuroinflammation 2015; 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holsheimer J, Nguyen J-P, Lefaucheur J-P, Manola L. Cathodal, anodal or bifocal stimulation of the motor cortex in the management of chronic pain? In: Sakas D.E. and Simpson B.A. (eds.) Operative neuromodulation Springer, 2007, pp. 57–66. [DOI] [PubMed]
- 80.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010; 3: 95–118. [DOI] [PubMed] [Google Scholar]
- 81.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 2007; 69: 827–834. [DOI] [PubMed] [Google Scholar]
- 82.Pagano RL, Assis DV, Clara JA, Alves AS, Dale CS, Teixeira MJ, Fonoff ET, Britto LR. Transdural motor cortex stimulation reverses neuropathic pain in rats: a profile of neuronal activation. Eur J Pain 2011; 15: 268–e1–e14.. [DOI] [PubMed] [Google Scholar]
- 83.Watanabe K, Yabuki S, Sekiguchi M, Kikuchi S-I, Konno S-I. Etanercept attenuates pain-related behavior following compression of the dorsal root ganglion in the rat. Eur Spine J 2011; 20: 1877–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ab Aziz CB, Ahmad AH. The role of the thalamus in modulating pain. Malays J Med Sci 2006; 13: 11–18. [PMC free article] [PubMed] [Google Scholar]
- 85.Moon HC, Heo WI, Kim YJ, Lee D, Won SY, Kim HR, Ha SM, Lee YJ, Park YS. Optical inactivation of the anterior cingulate cortex modulate descending pain pathway in a rat model of trigeminal neuropathic pain created via chronic constriction injury of the infraorbital nerve. J Pain Res 2017; 10: 2355–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol 2001; 429: 23–37. [DOI] [PubMed] [Google Scholar]
- 87.Jung HH, Shin J, Kim J, Ahn S-H, Lee SE, Koh CS, Cho JS, Kong C, Shin H-C, Kim SJ, Chang JW. Rostral agranular insular cortex lesion with motor cortex stimulation enhances pain modulation effect on neuropathic pain model. Neural Plast 2016; 2016: 3898924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci 2009; 29: 10264–10271. [DOI] [PMC free article] [PubMed] [Google Scholar]