Abstract
For basic research, rodents are often housed in individual cages prior to behavioral testing. However, aspects of the experimental design, such as duration of isolation and timing of animal manipulation, may unintentionally introduce variance into collected data. Thus, we examined temporal correlates of acclimation of C57Bl/6J mice to single housing in a novel environment following two commonly used experimental time periods (7 or 14 days, SH7 or SH14). We measured circulating stress hormones (adrenocorticotropic hormone and corticosterone), basally or after injection stress, hippocampal gene expression of transcripts implicated in stress and affect regulation: the glucocorticoid receptor (GR), the mineralocorticoid receptor (MR), including the MR/GR ratio, and fibroblast growth factor 2 (FGF2). We also measured signaling in the mammalian target of rapamycin (mTOR) pathway. The basal elevation of stress hormones in the SH14 group is accompanied by a blunting in the circadian rhythms of GR and FGF2 hippocampal gene expression, and the MR/GR ratio, that is observed in SH7 mice. Following mild stress, the endocrine response and hippocampal mTOR pathway signaling are decreased in the SH14 mice. These neural and endocrine changes at 14 days of single housing likely underlie increased anxiety-like behavior measured in an elevated plus maze test. We conclude that multiple measures of stress responsiveness change dynamically between one and two weeks of single housing. The ramifications of these alterations should be considered when designing animal experiments since such hidden sources of variance might cause lack of replicability and misinterpretation of data.
Keywords: HPA axis, Glucocorticoid receptor, Mineralocorticoid receptor, Fibroblast growth factor 2, Ribosomal protein S6, Anxiety
1. Introduction
Animals, like humans, are constantly interacting with the environment, including both its physical and social aspects, while simultaneously defining their own territory. A disruption of the balance between territoriality and social interactions, such as crowding or social isolation, triggers stress responses with neurobiological consequences. These responses, which can be adaptive or maladaptive depending on the time frame, manifest as changes in the hypothalamic-pituitary-adrenal (HPA) axis as well as in the neural circuitry that regulates stress responsivity and affect regulation. Replicability and consistency of experimental results are essential for rigorous scientific research. However, hidden sources of variability may exist in an experimental design. Rodents are usually acclimated to a new environment prior to experimental testing, often with changes in how they are housed. Yet, the temporal aspects of animals’ responses to this adaptation are often not considered.
Approximately seven days of acclimation has been typically considered to be sufficient time for stress hormones such as corticosterone (CORT) to return to basal levels in rodents, based on reports that concentrations of plasma CORT of single-housed animals are similar to those of group-housed animals at 7 days (Bartolomucci et al., 2003; Haller et al., 2000; Reis et al., 2012; Tuli et al., 1995), 14 days and up to 42 days (Arndt et al., 2009; Bartolomucci et al., 2003; Hunt and Hambly, 2006; Tuli et al., 1995). One study has shown resting CORT levels in isolated rodents to be even lower than group-housed animals with no differences in circadian rhythm at 18 days of housing (Nichols and Chevins, 1981).
Single housing is the standard condition for experiments in many laboratories. For example, researchers employing surgical procedures often single house animals at least following surgery so that there is no interference among animals while they heal. Other researchers house animals individually to eliminate order effects during behavioral testing (Arndt et al., 2009; Chesler et al., 2002; Lyte et al., 2005). When animals are group-housed, the experimenter needs to remove one animal at a time for testing. Once a cage is disturbed by removing an animal, the other animals exhibit stress responses that would likely confound their performance in a variety of behavioral tests (Arndt et al., 2009). For example, when testing anxiety-like behavior in the elevated plus maze (EPM) or the light/dark box, the goal is to measure spontaneous behavior in a novel environment that is not influenced by prior handling or cage disturbance. Thus, it is easier to test behavior in animals, with and without prior surgery, when they are single-housed.
Another rationale for single housing animals comes into play when determining the effects of genotype on phenotype. Genotype may have the predominant effect on phenotype in single-housed animals, as Nagy et al. (2002) report when examining body composition. In contrast, increased variance in group-housed animals, as compared to those single-housed, may be due to the effects of behavioral and social interactions on the phenotype. Thus, single housing animals may enhance the probability of detecting significant differences because of lower variances in the dependent variables among experimental groups, especially if the findings of Nagy et al. (2002) can be extrapolated to behavioral variables.
Group housing can modify behavior and hormonal levels in variable ways since co-housed mice form social hierarchies (Wang et al., 2014). For example, subordinate CD-1 male mice have higher plasma corticosterone when group-housed in the presence of an alpha male. Conversely, alpha males have higher levels of this stress hormone when mice are pair-housed (Williamson et al., 2017). When social isolation is used, the length can range from 24 h of single housing prior to a specific behavioral test (Parmigiani et al., 1999) to behavioral testing performed after extended periods of time such as 3–8 weeks (Simler et al., 1982; Kempf et al., 1984; van der Veen et al., 2007). Since the goal of our study was to determine how a set of inter-related variables changes dynamically in C57Bl/6J mice when they are single-housed for 7 or 14 days immediately after delivery, conditions often used by researchers, group housing was not included as an experimental variable.
Thus, we examined temporal aspects of rodents’ acclimation to single housing in a new environment by assessing stress hormone levels and expression of stress- and affect-related genes in the brain. We determined how the response to single housing, in and of itself, might be a significant variable affecting stress hormone levels over this time period, and following an acute stress (PBS injection). We also measured gene expression of the glucocorticoid receptor (GR), the mineralocorticoid receptor (MR), and fibroblast growth factor 2 (FGF2), all key players in regulating stress and affect. We focused on the hippocampus (HPC) which is involved in controlling the basal tone and rhythmicity of the HPA axis (Akil et al., 1991), terminating the stress response (Herman et al., 2005; Cullinan et al., 1993), and regulating emotional responsiveness (Admon et al., 2009; Chaudhury et al., 2014; Eren-Kocak et al., 2011). We measured phosphorylation of ribosomal protein S6 (S6) in the HPC as a marker of mTOR pathway activation since mTOR signaling is linked in the brain to translational control, synaptic plasticity, developmental disorders, and psychiatric illness (Bockaert and Marin, 2015; Hoeffer and Klann, 2010; Huber et al., 2015). Finally, we tested PBS-injected mice in the EPM for anxiety-like behavior as an end point known to be sensitive to changes in the level of stress. This multivariate study reveals that acclimation to a novel environment, from gene expression to behavior, does not reach a stable endpoint at one week, but rather is dynamic. Therefore, this acclimation feature should be taken into consideration when designing animal research studies.
2. Materials and methods
2.1. Animals and experimental design
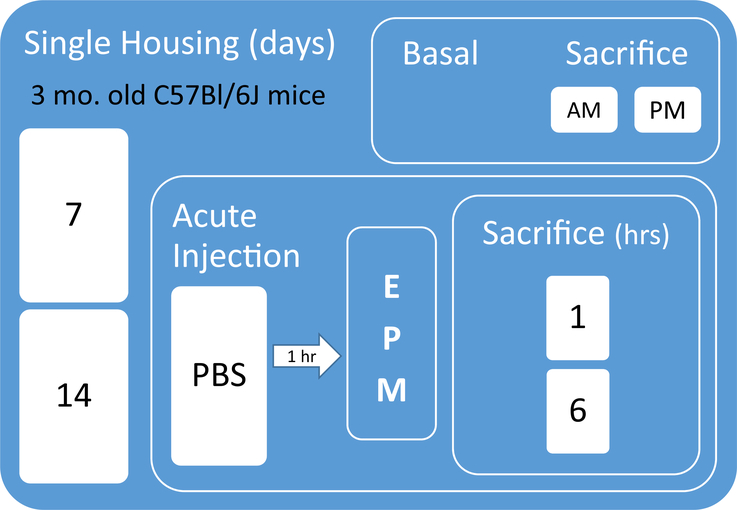
Twelve-week-old male C57Bl/6J mice (N = 81) were obtained from Jackson Laboratory (Bar Harbor, Maine) and single housed in standard mouse cages (18.6 cm × 29.8 cm × 12.8 cm; 484 sq. cm) for either 7 (SH7) or 14 (SH14) days prior to sacrifice for basal measurements or acute mild injection stress, behavioral testing, and sacrifice. Fig. 1 shows the experimental design and timeline. Mice were housed on a 14:10 light/dark cycle (lights on at 0600 h) with ad libitum access to food and water. Basal mice (N = 32) were kept unhandled in their home cages until removal for sacrifice on either day 7 or 14 in the AM (between 0900 and 1100 h) or the PM (between 1300 and 1500 h). Given that numerous molecules have circadian rhythms, especially stress hormones, we chose to collect blood and brains from the mice at these two time points during the day at which researchers often test rodents. To assess reactivity to an acute stress, other mice were given one injection of phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (5 μl/g; IP) between 0700 and 0900 h and returned to their home cages for 1 h before behavioral testing in the EPM. Two separate cohorts of mice were tested in the EPM 1 h after this acute injection stress (N = 24 SH7, 25 SH14) and, since the behavior was similar between the two cohorts, the EPM data are reported together. For the second cohort only, half of the SH7 (N = 8) and SH14 (N = 8) mice were sacrificed immediately following the five-minute EPM test in the AM (between 0900 and 1100 h; 1 h post-PBS injection), and the other half (N = 8 SH7, 8 SH14) were returned to their home cages and sacrificed in the PM (between 1300 and 1500 h; 6 h post-PBS injection). Both basal mice and those that received acute injection stress plus the EPM test were sacrificed at similar AM and PM time points. Brains from separate groups of SH7 and SH14 mice from each time point were used for in situ hybridization (ISH) and immunoblotting as described in the relevant subsections of the methods. All procedures were conducted in accordance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the University Committee for the Use and Care of Animals at the University of Michigan.
Fig. 1.
Experimental design and timeline. PBS = phosphate-buffered saline, EPM = elevated plus maze.
2.2. Adrenocorticotropic hormone and corticosterone radioimmunoassays
To determine basal stress hormone levels, trunk blood was collected from mice within 30 s after removal from their cages from 0900 to 1100 h or 1300 to 1500 h. For stress-induced hormone levels, trunk blood was collected from mice 1 h (immediately following the EPM) or 6 h following acute injection stress (and five hours following the EPM). Blood was centrifuged within 1 h of collection and plasma stored at −80 °C until assayed. Plasma adrenocorticotropic hormone (ACTH) and CORT were measured using commercially available radioimmunoassay kits (MP Biomedicals, Orangeburg, NY) according to package instructions. The sensitivity of the ACTH and CORT radioimmunoassays were 5.7 pg/ml and 0.77 μg/dl, respectively. The ACTH and CORT intra- and inter-assay coefficients of variation were<11%.
2.3. GR, MR, and FGF2 in situ hybridization
Gene expression of stress-related molecules was examined in brains from basal mice via ISH. Mice were sacrificed by rapid decapitation, and their brains removed, snap frozen in precooled isopentane, and stored at −80 °C. Brains were cryostat sectioned at 10 μm, and sections were mounted on Fisherbrand Superfrost/Plus Microscope Slides. Sections were processed for ISH as previously described (Hebda-Bauer et al., 2013). The GR probe is a 597-bp fragment directed against the mouse GR mRNA. The MR probe is a 281-bp fragment directed against the mouse MR mRNA. The mouse FGF2 probe is a 316-bp fragment directed against the mouse FGF2 mRNA. All cRNA probes were directed against the coding sequence of each mRNA and synthesized in our laboratory. Slides were placed in autoradiography cassettes with Kodak XAR film (Eastman Kodak, Rochester, NY) for 6 (MR), 17.5 (GR), or 56 (FGF2) days. Autoradiograms were digitized using a ScanMaker 1000XL Pro (Microtek, Carson, CA) with LaserSoft Imaging software (AG, Kiel, Germany). Digitized images were analyzed using Image J (NIH) at the rate of 63 pixels/mm. Optical density measurements were taken from the left and right sides of the brain at 70 μm intervals (1 in 7 series) throughout the rostro-caudal extent of each region of interest. Thus, for each animal, an average of 16–18 sections were analyzed from four subregions of the dorsal HPC (Cornu Ammonis fields CA1–CA3 and the dentate gyrus) for GR, MR, and FGF2, and 5 sections from the paraventricular nucleus of the hypothalamus (PVN) for GR. In addition, given the prominent FGF2 gene expression in the fasciola cinereum (FC) and a more medial area of the CA2 (i.e., medial CA2 in this paper) that is rostral to and continuous with the FC (Lein et al., 2005; Williams et al., 1996), we analyzed these regions separately. Adjacent sections were stained with cresyl violet for anatomical localization. Optical density measurements were corrected for background (i.e., tissue area without signal) on each brain slice. Signal pixels of a region of interest were defined as being 3.5 standard deviations above the mean of the background. The mean optical density was determined for each region of each animal and then group averages were calculated and compared statistically. Data are reported as the mean optical density ± the standard error of the mean (SEM) or percent change in mean optical density ± standard error of the percent change for each region or subregion in each experimental group.
2.4. Immunoblotting for mTOR pathway activation
The mTOR/p70S6K/S6 pathway determines synaptic plasticity in neurons through modulation of protein synthesis and is responsive to acute and chronic stress (Chandran et al., 2013; Hoeffer and Klann, 2010; Polman et al., 2012; Yang et al., 2008). Moreover, activation of the mTOR pathway through neuron-specific deletion of an endogenous protein repressor of the pathway, phosphatase and tensin homolog (PTEN), increases phosphorylation of the downstream pathway components, Akt and S6, in conjunction with decreased anxiety in the EPM (Lugo et al., 2014). Therefore, in this study, S6 phosphorylation is used as a marker of mTOR pathway activation in the HPC. Dorsal HPC punches were obtained from frozen brains of SH7 and SH14 mice under basal conditions or after acute stress and the tissue was homogenized in a buffer containing 100 mM NaCl, 10 mM NaPO4 (pH 7.4), 5 mM EDTA (pH 8.0), 5 mM EGTA (pH 7.0), 10 mM Na2P2O7, 50 mM NaF, 2 mM Na3VO4 and 1% Triton X-100 with the addition of one Complete Mini protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN) per 10 ml of solution. The amount of total protein was determined via a BCA Protein Assay kit (Pierce, Rockford, IL). Samples from the dorsal HPC containing equal amounts of protein were resolved with SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblotting was performed for phosphorylated S6 with a phospho-specific antibody that recognizes Ser235/236. Phosphorylation was normalized to the total amount of S6 by immunoblotting with an antibody that recognizes both the phospho- and dephospho-forms of the protein that are resolved from one another with the SDS-PAGE conditions that were used. Both primary antibodies were obtained from Cell Signaling Technology, Inc., Danvers, MA. Immunoreactive bands were visualized on Amersham Hyperfilm™ ECL following incubation with an anti-rabbit-peroxidase conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO). Immunoreactivity was quantified by densitometric analysis using UNSCAN-IT™ gel digitizing software (Version 6.1, Silk Scientific Corporation Inc., Orem, UT). Data are expressed as the mean ± SEM of results from at least triplicate experiments.
2.5. Behavioral testing
Mice were tested in the EPM 1 h after acute injection stress, between 0800 and 1000 h. A videotracking system (Ethovision, Noldus Technology) was used to collect behavioral data during this test. The EPM consists of four arms (27 cm × 6 cm) arranged in a plus form and elevated 51 cm from the floor. Two opposing arms are surrounded with 14-cm-high clear Plexiglas walls (closed arms), whereas the other arms are devoid of walls (open arms). The light intensity in the open arms was 275 lx. At the intersection of the four arms is a central 8 cm × 8 cm square platform giving access to all arms. Mice were gently placed in the center area and their behavior monitored for 5 min. An entry is defined as a mouse having all four paws in an arm. Dependent measures included the time spent and number of entries into the open and closed arms and the center area and the latency to enter the open arms.
2.6. Statistical analyses
Data were analyzed using linear mixed models (proc mixed) in SAS statistical software. The mixed models for ISH data contain three fixed effects (single housing duration, time of sacrifice, and HPC subregion) for the HPC and two fixed effects for the PVN (single housing duration and time of sacrifice), as well as a random effect parameter due to the subjects (i.e., mice). Standard errors for percent change in mean optical density for ISH data were calculated using linearized ratio estimates based on the estimates and standard errors from the mixed models. Data from the ACTH and CORT radioimmunoassays were analyzed with two-way ANOVAs (single housing duration x time of sacrifice), S6 immunoblotting with two-way ANOVAs (single housing duration x treatment condition [i.e., basal and sacrifice 1 or 6 h after PBS injection]), and the EPM with Student’s t-tests. Post hoc least-squared means tests with slices were performed with the linear mixed models and ANOVAs to determine effects of single housing duration, time of sacrifice, and treatment condition in specific groups. The effect sizes for all reported data are included in the text. Cohen’s d is reported for two-sample comparisons, semipartial eta squared (ηp2) is reported for ANOVAs, and marginal R2 is reported for linear mixed models. The marginal R2 for linear mixed models was computed with the function rsquared in the R package piecewiseSEM (Lefcheck, 2016). The significance value for all tests was α ≤ 0.05.
3. Results
3.1. Prolonged single housing modifies basal stress hormone levels and blunts the peripheral stress response
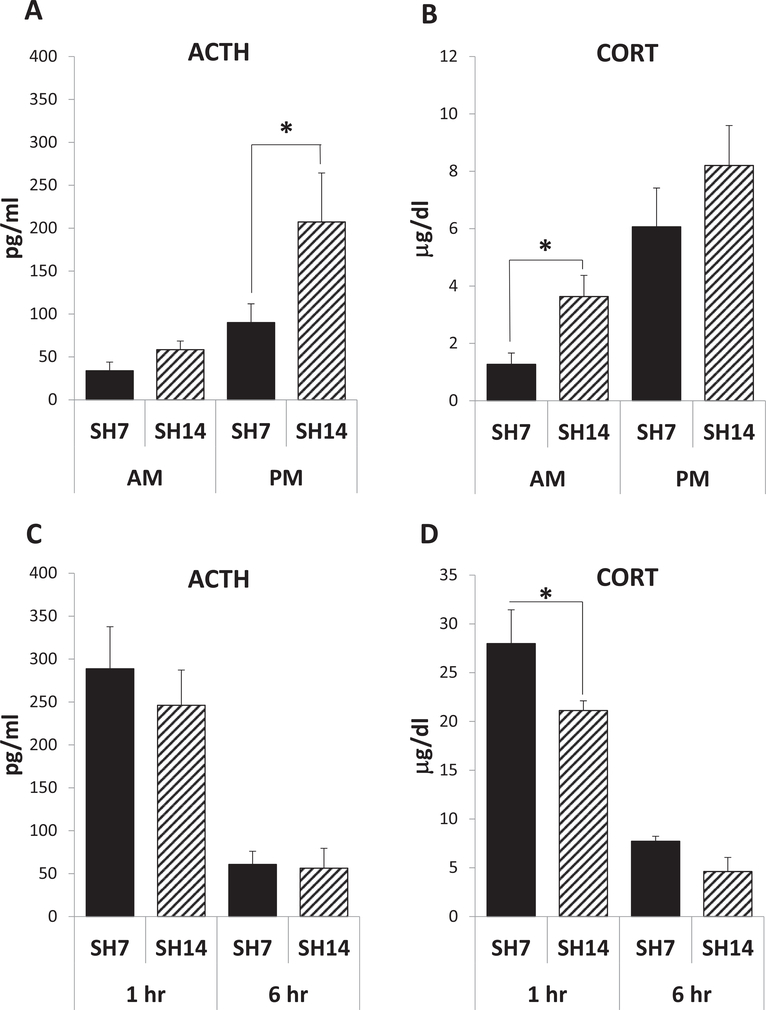
As a first measure of how the duration of single housing can affect the HPA axis, basal hormone levels in the blood were measured in the AM and the PM (Fig. 2A and B). All mice, at 7 and 14 days of single housing (SH7 and SH14), show a rise in ACTH and CORT levels from morning to afternoon, as expected for the normal daily rhythm of these circulating hormones (ACTH: F(1,25) = 9.00, p = 0.0060, ηp2 = 0.2192; CORT: F(1,25) = 17.71, p = 0.0003, ηp2 = 0.3826). However, extended isolation increases indices of HPA axis activation, with the SH14 mice exhibiting significantly higher basal ACTH and CORT levels than SH7 mice in the PM and AM, respectively (ACTH: F(1,25) = 4.31, p = 0.0484, ηp2 = 0.1049; CORT: F(1,25) = 4.10, p = 0.0538, ηp2 = 0.0885). Hormone levels in the blood were also measured 1 and 6 h following an acute stress challenge (i.e., a single injection) after 7 and 14 days of single housing (Fig. 2C and D). Large ACTH and CORT increases were noted 1 h post-injection when compared to basal levels of these hormones (compare Fig. 2C and D to Fig. 2A and B). In addition to higher circulating basal AM CORT levels when compared to SH7 mice (Fig. 2B), SH14 mice exhibit a lower magnitude in the CORT response to acute stress (single housing duration main effect: F(1,26) = 5.71, p = 0.0244, ηp2 = 0.0503; posthoc test – 1 h sacrifice time: F(1,26) = 5.42, p < 0.0280; Fig. 2D). However, all SH7 and SH14 mice show a decrease in ACTH and CORT levels from one to 6 h post-injection (ACTH: F(1,26) = 36.00, p < 0.001, ηp2 = 0.5763; CORT: F(1,26) = 77.81, p < 0.0001, ηp2 = 0.6854). Thus, extended isolation leads to altered basal hormone levels and reactivity to acute stress at the peripheral level, indicating altered HPA regulation and a state of chronic stress in the SH14 mice. The data also indicate that measurement of ACTH or CORT at single time points may not adequately capture changes in hormonal levels during different intervals of single housing.
Fig. 2.
Prolonged single housing modifies basal stress hormones and blunts the peripheral stress response. All mice show a rise in ACTH (A) and CORT (B) from AM to PM, as expected for the normal daily rhythms of these circulating hormones (p < 0.01). However, SH14 mice exhibit significantly higher basal ACTH and CORT levels than SH7 mice in the PM (A) and AM (B), respectively. All mice show a decrease in ACTH (C) and CORT (D) levels from 1 to 6 h post injection and EPM testing (p < 0.001). However, SH14 mice exhibit a significantly smaller rise in CORT than SH7 mice 1 h following injection stress and EPM testing (D). Data represent mean ± SEM. *p < 0.05.
3.2. Prolonged single housing blunts diurnal GR gene regulation
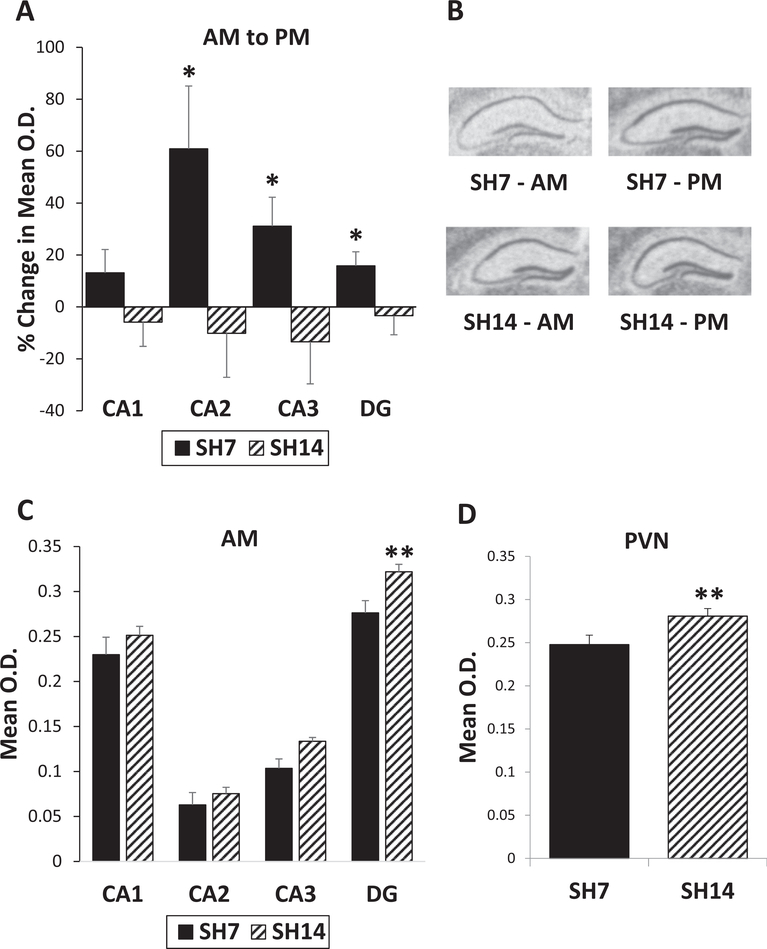
Since extended isolation alters peripheral indices of the HPA system, basal gene expression in the brain of the stress- and affect-related molecules, GR, MR, and FGF2, was examined in subregions of the dorsal HPC via ISH after 7 and 14 days of single housing. In the SH7 mice, basal GR gene expression increases in the PM relative to AM levels in all of the HPC fields (Fig. 2A and B). In contrast, the SH14 mice exhibit no change or a small decrease in GR mRNA levels from AM to PM. A mixed model analysis (marginal R2 = 0.8749) shows significant interactions between single housing duration and sacrifice time (F(1, 1756) = 3.96, p = 0.0469) and region (F(3,1756) = 14.81, p < 0.0001), as well as a significant main effect for region (F(3,1756) = 3666.61, p < 0.0001). Posthoc tests show significant upregulation from AM to PM in GR mRNA expression of SH7 mice in the CA2 (F(1,1756) = 3.83, p = 0.0505), CA3 (F(1,1756) = 3.81, p = 0.0512), and dentate gyrus (F(1,1756) = 6.70, p = 0.0097) subregions of the HPC (Fig. 3A). Further, SH14 mice show higher AM GR mRNA levels in all areas of the dorsal HPC compared to SH7 mice, and these higher levels reach significance in the dentate gyrus (F(1,1756) = 7.53, p = 0.0061; Fig. 3C). GR gene expression is also higher in the PVN of SH14 mice compared to SH7 mice (F(1,136) = 6.82, p = 0.0100, marginal R2 = 0.3588; Fig. 3D). Since there was no AM to PM diurnal regulation of GR gene expression in the PVN, the AM and PM time points were combined for this region. These GR gene expression data in the dorsal HPC and PVN show a loss of regulation and overall higher AM GR levels in SH14 mice, indicating that extended isolation impacts the regulation of stress-related genes.
Fig. 3.
Prolonged single housing blunts diurnal GR gene regulation. (A) SH7 mice show basal upregulation of GR mRNA expression in all areas of the dorsal HPC from AM to PM, while SH14 mice do not. (B) Representative GR in situ hybridization autoradiographs of the dorsal HPC from SH7 and SH14 mice sacrificed in the AM and PM. (C) SH14 mice show higher AM GR mRNA levels in all areas of the dorsal HPC, compared to SH7 mice. (D) GR mRNA levels are also significantly higher in the PVN of SH14 mice compared to SH7 mice. Data in (A) represent percent change from AM to PM ± the standard error of the percent change. Data in (C) and (D) represent mean ± SEM. *p < 0.05; **p < 0.01.
3.3. Prolonged single housing alters MR gene regulation and the MR/GR ratio
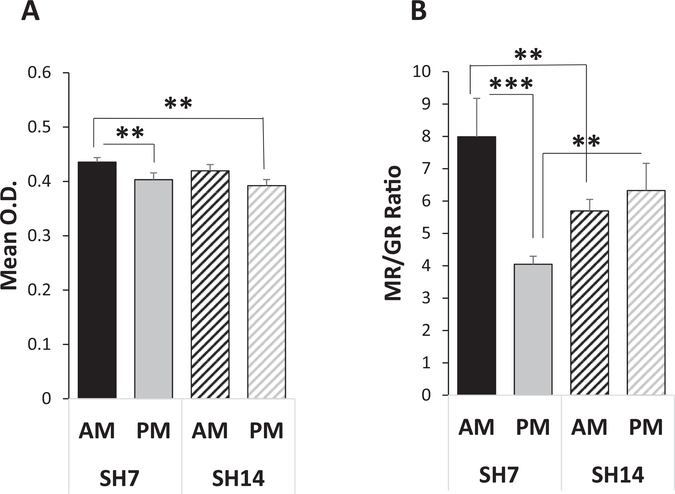
Similar analysis of MR gene expression reveals a significant main effect for time of day (F(1,1757) = 5.36, p = 0.0208), but not for single housing duration (F(1,1757) = 1.13, p = 0.2888). Both SH7 and SH14 mice show a downregulation of MR gene expression in all areas of the dorsal HPC from AM to PM, with a significant decrease only in the CA2 subregion of SH7 mice (posthoc test: F(1,1757) = 6.14, p = 0.0133; Fig. 4A). In addition to a significant main effect for region (F(3,1757) = 889.35, p < 0.0001), a mixed model analysis (marginal R2 = 0.9083) reveals a significant single housing duration by sacrifice time by region interaction (F(3,1757) = 2.85, p = 0.0363). For example, in the CA2 region, posthoc tests show that SH14 mice exhibit significantly lower MR mRNA expression in the PM than SH7 mice in the AM (CA2: t(1757) = 3.09, p = 0.0020). Thus, the changes in gene expression for MR, in comparison to GR, are more limited, occur in the opposite direction from AM to PM time points, and are mainly in the CA2 region.
Fig. 4.
Prolonged single housing alters MR gene regulation and the MR/GR ratio in the CA2 subregion of the HPC. (A) Both SH7 and SH14 mice show a downregulation in MR gene expression in the dorsal HPC from AM to PM; the CA2 region is shown. However, SH14 mice sacrificed in the PM exhibit lower MR mRNA levels than SH7 mice sacrificed in the AM. (B) SH7 mice exhibit a significant decrease from AM to PM in the MR/GR mRNA ratio in the CA2 subregion of the dorsal HPC, in contrast to the SH14 mice that show little change from AM to PM. SH14 mice exhibit significantly lower and higher MR/GR ratios in the AM and PM, respectively, compared to SH7 mice. Data represent mean ± SEM. **p < 0.01; ***p < 0.001.
An optimal MR/GR balance is necessary for promoting homeostasis and helping the stress system adapt to change (Oitzl et al., 2010). Although MR gene expression in the dorsal HPC is not affected by the duration of single housing as extensively as that of GR gene expression, the MR/GR mRNA ratio in the CA2 subregion is dramatically affected. A mixed model analysis (marginal R2 = 0.1171) of the MR/GR ratio in the dorsal HPC reveals a significant main effect for region (F(3,36) = 75.45, p < 0.0001) and two significant interactions: single housing duration by sacrifice time (F(1,36) = 4.40, p = 0.0429) and single housing duration by sacrifice time by region (F(3,36) = 4.15, p = 0.0127). Posthoc tests show that SH7 mice exhibit a significant decrease in the MR/GR ratio in the CA2 subregion from AM to PM (F(1,36) = 22.69, p < 0.0001), while the SH14 mice show little time of day change in the ratio (F(1,36) = 0.58, p = 0.4510; Fig. 4B). Also, SH7 mice exhibit significantly higher and lower MR/GR ratios in the CA2 subregion than SH14 mice in the AM and PM, respectively (posthoc tests - AM: F(1,36) = 7.67, p = 0.0088; PM: F(1,36) = 7.60, p = 0.0091). These MR/GR ratio alterations with extended isolation may reflect an imbalance that increases vulnerability to sub-optimal stress responses and recovery.
3.4. Prolonged single housing blunts FGF2 gene regulation
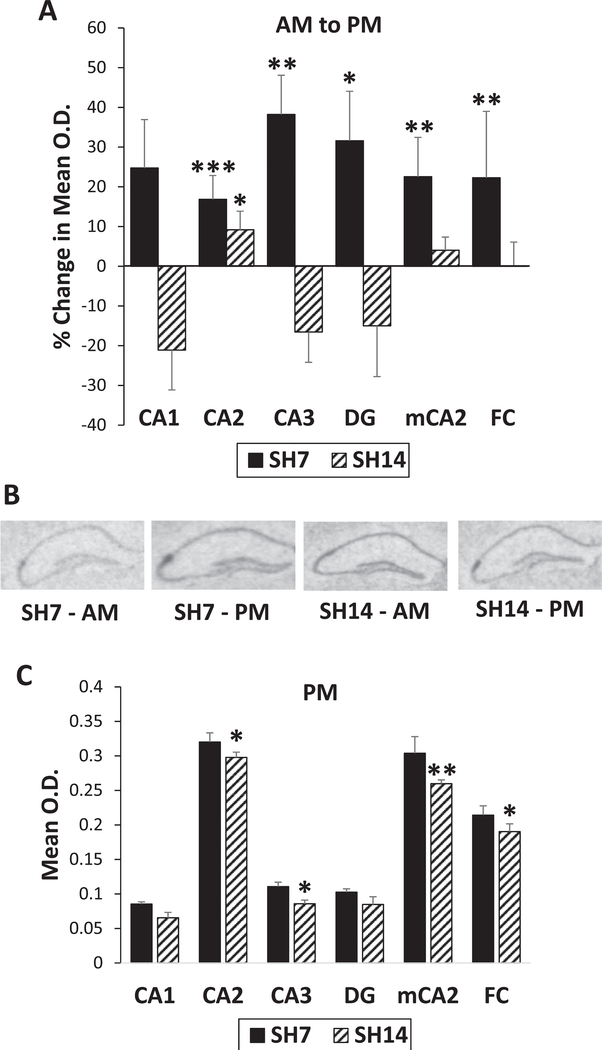
Due to the dramatic alterations in the CA2 MR/GR ratio with prolonged single housing and the knowledge that FGF2 levels are sensitive to glucocorticoids (Molteni et al., 2001), we examined FGF2 gene expression in subregions of the dorsal HPC as a function of the duration of single housing. Similar to GR gene expression, the affect-related FGF2 gene exhibits an upregulation in mRNA from AM to PM in SH7 mice, and this upregulation is lost with extended isolation in SH14 mice, except in the CA2 subregion (Fig. 5A and B). A mixed model analysis (marginal R2 = 0.8299) of FGF2 gene expression reveals significant main effects for sacrifice time (F(1,1987) = 4.69, p = 0.0304) and region (F(5,1987) = 1515.56, p < 0.0001), but not single housing duration (F(1,1987) = 0.80, p = 0.3725). However, single housing duration by sacrifice time (F(1,1987) = 6.05, p = 0.0140) produces a significant interaction, as well as sacrifice time by region (F(5,1987) = 8.68, p < 0.0001). Posthoc tests show that FGF2 mRNA levels significantly increase from AM to PM in SH7 mice in all areas of the dorsal HPC, except for the CA1 subregion (CA1: F(1,1987) = 2.54, p = 0.1110; CA2: F(1,1987) = 13.55, p = 0.0002; CA3: F(1,1987) = 6.81, p = 0.0091; DG: F(1,1987) = 4.05, p = 0.0443; mCA2: F(1,1987) = 9.55, p = 0.0020; FC: F(1,1987) = 9.39, p = 0.0022; Fig. 5A and B). In contrast, FGF2 mRNA levels do not increase from AM to PM with extended isolation, except in the CA2 subregion (F(1,1987) = 5.38, p = 0.0204). As expected (Williams et al., 1996), there is a strong mRNA expression of the FGF2 gene in the CA2 subregion (Fig. 5B), as well as in its component areas, the medial CA2 and the FC. Moreover, SH14 mice exhibit lower PM FGF2 levels compared to that of SH7 mice in four of the HPC subregions (CA2: F(1,1987) = 3.85, p = 0.0499; CA3: F(1,1987) = 4.62, p = 0.0316; mCA2: F(1,1987) = 6.28, p = 0.0123; FC: F(1,1987) = 4.43, p = 0.0355), indicating a blunted regulation of FGF2 expression in these mice (Fig. 5C).
Fig. 5.
Prolonged single housing blunts FGF2 gene regulation in the HPC. (A) SH7 mice exhibit an increase in FGF2 gene expression from AM to PM in all areas of the dorsal HPC, while SH14 mice do not, except for the CA2 subregion. The FGF2 gene is highly expressed in the CA2, including its associated areas that are more medial: mCA2 that is more rostral to and continuous with the FC. (B) Representative FGF2 in situ hybridization autoradiographs of the dorsal HPC from SH7 and SH14 mice sacrificed in the AM and PM. (C) SH14 mice also show lower PM FGF2 levels compared to that of SH7 mice in several of the HPC subregions. Data in (A) represent percent change from AM to PM ± the standard error in the percent change. Data in (C) represent mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
3.5. Prolonged single housing abrogates mTOR pathway activation in the HPC following acute stress
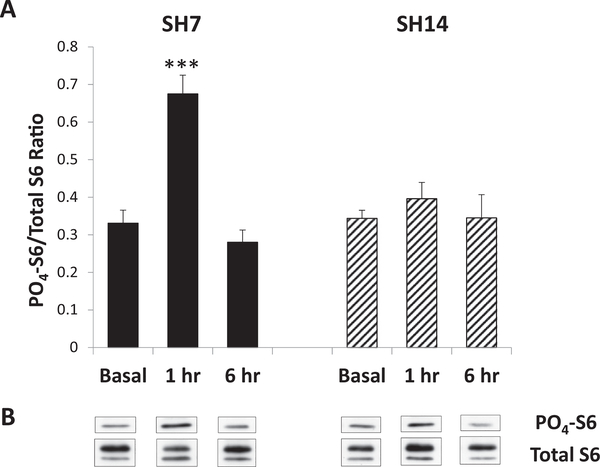
Since the duration of single housing impacts gene expression and peripheral basal and stress-induced circulating hormone levels, the existence of functional changes in HPC signaling was examined via measurement of S6 phosphorylation, a marker of mTOR pathway activation that has been correlated with the behavioral state of mice (Lugo et al., 2014). Phosphorylation of S6 was determined by immunoblotting and expressed as a ratio of PO4-S6 to total S6 in each dorsal HPC sample. Since it was found that time of day did not affect basal PO4-S6/total S6 ratios (data not shown), AM and PM values were combined. The data reveal that the duration of single housing also did not affect basal levels of S6 phosphorylation, as SH7 and SH14 basal mice exhibit similar PO4-S6/total S6 ratios (posthoc test - F(1,27) = 0.08, p = 0.7780; Fig. 6A and B). SH7 mice, however, exhibit increased phosphorylation of S6 in the dorsal HPC 1 h following acute stress, while SH14 mice do not. A mixed model analysis reveals significant main effects for duration of single housing (F(1,27) = 4.42, p = 0.0450, ηp2 = 0.0538) and treatment condition (i.e., basal and sacrifice one or 6 h after PBS injection; F(2,27) = 18.02, p < 0.0001, ηp2 = 0.4387) and a significant duration of single housing by treatment condition interaction (F(2,27) = 10.27, p = 0.0005, ηp2 = 0.2499). Posthoc tests reveal that 1 h following injection stress, and immediately after the five-minute EPM test, SH7 mice exhibit significantly higher S6 phosphorylation compared to basal mice (SH7: t(27) = 6.21, p < 0.0001; SH14: t(27) = 6.11, p < 0.0001) and SH14 mice (F(1,27) = 22.10, p < 0.0001; Fig. 6A and B). This rise in S6 phosphorylation in the dorsal HPC of SH7 mice returns to basal levels within 6 h (t(27) = 6.66, p < 0.0001). In contrast, SH14 mice show PO4-S6/total S6 ratios one and 6 h following acute mild stress that are similar to basal levels. Thus, prolonged single housing abrogates mTOR activation in the dorsal HPC following acute mild stress.
Fig. 6.
Prolonged single housing abrogates mTOR pathway activation in the HPC following acute stress. (A) Basal levels of S6 phosphorylation in the dorsal HPC are similar in SH7 and SH14 mice, but 1 h following acute injection stress and EPM testing, SH7 mice exhibit a significantly higher PO4-S6/total S6 ratio than SH14 mice. This increased S6 phosphorylation in SH7 mice returns to basal levels by 6 h post-injection and behavior. (B) Representative immunoblots for PO4- and total S6 corresponding to collated data for each experimental condition as shown in (A). Data represent mean ± SEM. ***p < 0.001.
3.6. Prolonged single housing promotes anxiety-like behavior
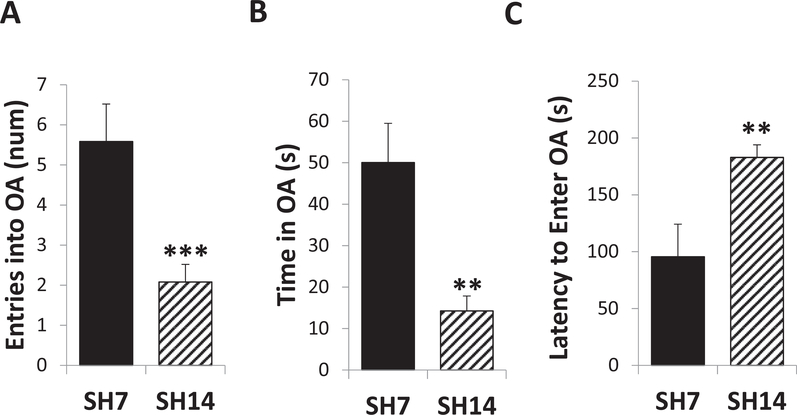
We have previously determined that higher GR levels in concert with lower FGF2 levels in the rat HPC are associated with increased anxiety-like behavior (Chaudhury et al., 2014). Since similar changes in GR (AM) and FGF2 (PM) expression were found at SH14 in this study with mice in combination with other markers of altered stress reactivity, anxiety-like behavior was tested with the EPM in SH7 and SH14 mice 1 h following a single PBS injection. After one week of single housing plus an acute mild stress, SH7 mice show relatively high exploration and low anxiety-like behavior in the EPM (compared to wild-type mice of other strains bred in our lab; unpublished data). In contrast, two weeks of single housing plus an acute stress generates very different behavior. SH14 mice exhibit high anxiety-like behavior by making fewer entries (t(32.727) = 3.39, p = 0.0019, d = 0.9686), spending less time (t(29.533) = 3.53, p = 0.0014, d = 1.0086), and taking longer to enter (t(47) = −2.69, p < 0.0099, d = 0.7686) the open arms of the EPM than SH7 mice (Fig. 7A, B, and C, respectively). Thus, the duration of single housing, without any other environmental changes, makes a dramatic difference in spontaneous exploratory behavior in a novel environment following an acute mild stress. When challenged with an acute mild stress, one week of single housing fosters exploratory behavior, while two weeks of single housing attenuates this exploration.
Fig. 7.
Prolonged single-housing promotes anxiety-like behavior in the EPM. One hour following acute injection stress, SH14 mice made fewer entries (A), spent less time (B), and took longer to first enter (C) the open arms (OA) of the EPM than SH7 mice. Data represent mean ± SEM. **p < 0.01; ***p < 0.001.
4. Discussion
The results of the present study demonstrate that acclimation to a single-housing environment is dynamic and evolves even over a short time. We have used three types of markers that have been implicated in coping with the environment - classic elements of the HPA axis, a growth factor linked to anxiety and mood disorders, and a signaling pathway involved in neuroplasticity. The temporal differences observed with extended single housing are revealed in altered basal and stress-induced circulating hormone levels and stress- and affect-related gene expression and mTOR pathway signaling in the brain. These effects are indicative of a stressed state and likely underlie the anxiety-like behavior that is observed when the duration of single housing of C57Bl/6J mice is increased from 7 to 14 days.
Single housing, in and of itself, produces hormonal and molecular differences basally in the absence of an acute stressor. Although all single-housed mice in the current study exhibited a rise in both ACTH and CORT from morning to afternoon, as expected for the normal diurnal rhythm for these stress hormones (Akil and Morano, 1996), mice single housed for 14 days showed higher basal hormone levels and a blunted rise in CORT following acute injection stress. The majority of researchers have not tested animals exposed to different durations of single housing in the same experiment as was performed in the current study, with one exception. Bartolomucci et al. (2003) report similar CORT levels among single-housed Swiss CD-1 mice tested at 1, 7, 14, 21, or 42 days. It was not until single-housed mice (for 21 days) were exposed to an acute mild stress that they showed higher basal CORT levels the morning following acute stress exposure. A strength of the current study is that, in addition to more than one duration of single housing, hormone levels were measured not with a single daily measurement but at two time points during the day, both basally and following an acute mild stressor, based on the observation that an acute stress might be needed to elicit hormonal changes. Although not attempting to report on 24-h hormonal rhythms, the current data do provide an indication of circulating hormonal differences during times of day in which behavior of animals is often assessed and reveal changes in the circulating stress hormone patterns with extended isolation that indicate a chronically-activated stress system (Dallman et al., 2004). It should be noted that the peripheral blood levels of CORT and ACTH show significant changes at certain time points and not others and, if taken as the primary measure, a single determination may lead to the erroneous conclusion that the system is stable. The brain measures and stress challenge are also essential to a more complete understanding of dynamic changes caused by housing conditions.
The HPC serves as an integrator of the molecular elements responsible for activation or inhibition of the stress- and affect-systems (Akil et al., 1991). Interplay between neuronal circuitry and hormonal mechanisms is evident in the basal tone of the stress system that fluctuates with the rest-activity cycle and the activated state in response to stressors. The HPC shows changes in MR and GR mRNA levels at two points in the diurnal cycle, consistent with the hypothesis that the HPC acts as an integrator of HPA signals across a 24-h period (Herman et al., 1993). MR and GR in the HPC are specifically receptive to information concerning time of day and glucocorticoid (i.e., CORT) levels. In the current study, C57Bl/6J mice single housed for 7 days clearly show an upregulation of GR mRNA in the HPC from the morning to the afternoon. However, this GR regulation in the HPC is lost when isolation is extended to 14 days. Further, GR gene expression in the PVN, the primary controller of HPA-mediated CORT release (Herman et al., 2002) is higher with extended isolation. The lack of diurnal rhythm of GR mRNA in the PVN of mice exposed to either duration of single housing in the current study is consistent with previous reports (Herman et al., 1993). A loss of GR mRNA regulation in the HPC and higher GR mRNA levels in the HPC (in the AM) and the PVN with 14 days of single housing suggest continued activation of the stress system. Since GR is considered a sensor of stress and a key player in the negative feedback that turns off the stress response, expression levels of GR need to be optimized by the organism to ensure appropriate, but not excessive, detection of stress and provide efficient termination of the ensuing stress response (Akil and Morano, 1996).
Although only minimal changes in MR gene expression in the HPC are observed from 7 to 14 days of single housing in most subregions, an exception is the CA2. Moreover, the MR/GR mRNA ratio in the CA2 subregion of the HPC shows a dramatic time of day effect at 7 days and a loss of this AM to PM difference with more extended isolation. These results are consistent with a potential role of MR in determining plasticity through regulation of gene expression in the CA2 subregion (McCann et al., 2018). The MR/GR balance hypothesis predicts that MR and GR operate in combination, and that balanced activation of MR and GR is crucial for neuronal excitability, stress responsivity, and behavioral adaptability (De Kloet et al., 1998; Oitzl et al., 2010). Upon imbalance of the MR/GR ratio, threats to homeostasis are less well processed and, over a certain threshold, may lead to neuroendocrine dysregulation and impaired behavioral adaptation. Human postmortem studies show gene expression alterations within brain regions that regulate the stress- and affect-systems, including altered ratios of MR and GR mRNAs in the HPC of depressed patients (Lopez et al., 1998). Animal studies using the MR/GR ratio as an indicator of HPA function report either an increased or decreased ratio concomitant with chronic stress or long-term adaptation to stress (Brureau et al., 2013; Garcia-Fuster et al., 2012; Ladd et al., 2004; Liberzon et al., 1999; Topic et al., 2008; Zhang et al., 2011). Our laboratory has previously shown a time of day effect in the MR/GR mRNA ratio in the HPC, with a higher ratio in the morning compared to the afternoon (Kerman et al., 2012), consistent with the current findings in C57Bl/6J mice single housed for 7 days. Loss of the MR/GR mRNA ratio difference relative to time of day in the HPC further indicates adverse effects from 7 to 14 days of single housing since altered expression of MR and GR have long-lasting consequences for stress responsiveness and emotional regulation.
Interestingly, the key single-housing duration changes in the MR/GR ratio occur in the CA2 region of the hippocampus where the strongest FGF2 mRNA expression is also found. This hippocampal area has recently received attention as a distinct region incorporating many unique anatomical, synaptic, and molecular features that differentiate it from the other hippocampal areas (see reviews by Benoy et al., 2018 and Carstens and Dudek, 2019). Recent evidence also suggests a role for this area in social behavior and memory (Dudek et al., 2016). The CA2 region has place cells similar to that of the CA1 and CA3 areas, but CA2 place cells are unique in that they link social and novel contextual information with representations of space. These cells have the capacity to update their spatial representations in response to social or contextual changes in the environment (Alexander et al., 2016). This place cell feature has ramifications in light of the temporal dynamics of single housing observed in the current study, namely in the MR/GR mRNA ratio and FGF2 mRNA levels.
FGF2 in the HPC plays a role in emotional regulation, an essential component of the stress- and affect systems that guide responses and adaptation to the physical and social environment, including anticipating, responding, and coping with stress (Turner et al., 2012). The FGF system is also reactive to stress and glucocorticoids (Molteni et al., 2001). Similar to that of stress-related hormones and steroid receptors, FGF2 gene expression shows a daily rhythm as we have previously reported (Turner et al., 2015). In the current study, C57Bl/6J mice single housed for 7 days show an upregulation of FGF2 mRNA in most areas of the HPC from the morning to the afternoon, consistent with the data obtained from group-housed rats. This regulation, however, is lost and the overall PM FGF2 gene expression is decreased with extended isolation, consistent with increased anxiety-like behavior in the 14-day single-housed mice. Our laboratory has shown that in humans and Sprague-Dawley rats, FGF2 gene expression is decreased in conjunction with anxiety and depression (Evans et al., 2004; Perez et al., 2009; Turner et al., 2008a, 2008b). The current data extend these findings by showing that lower endogenous FGF2 gene expression in the HPC is correlated with high anxiety-like behavior of the SH14 mice in conjunction with higher AM GR and lower PM FGF2 mRNA levels in the HPC, consistent with our current working model of the relationship between the molecular organizers, GR and FGF2, with anxiety behavior (Chaudhury et al., 2014).
The mouse data also suggest how FGF2 gene expression changes in conjunction with altered hormone levels and stress-gene expression signifying an activated stress system. FGF2 mRNA levels initially increase when exposed to stress, but then, with continued or repeated stress, they decrease (Bland et al., 2006; Turner et al., 2008a). The initial rise in FGF2 in response to stress serves a neuroprotective function (Anderson et al., 1988; Mark et al., 1997; Otto and Unsicker, 1990), but the decline with continued stress may reflect a reduction and long-term impairment in brain function (Riva, 1995). A decline in FGF2 gene expression in the HPC from 7 to 14 days of single housing in the current study suggests impaired cellular and behavioral (i.e., altered emotional regulation) resilience with extended isolation. Further research is needed to document a possible causal relationship between alterations in CORT/GR and FGF2 levels and to verify the impact of changes in FGF2 gene expression on resilience to stress in this model.
Compromised resilience with extended single housing is also evidenced by changes in the mTOR/p70S6k/S6 signaling pathway, known to be responsive to stress (Chandran et al., 2013; Hoeffer and Klann, 2010; Polman et al., 2012; Yang et al., 2008). In the current study, increased mTOR signaling was observed in the HPC of C57Bl/6J mice after 7 days of single housing followed by a mild stress and exposure to a novel environment (i.e., PBS injection and EPM). Since no changes were observed in basal S6 phosphorylation, this enhanced mTOR pathway activity appears to be a response to acute stress. Increased mTOR signaling in mice single housed for 7 days is also consistent with other studies correlating decreased anxiety-like behavior in the EPM with phosphorylation of S6. Suo et al. (2013) found such a relationship in the prefrontal cortex, but not the HPC, when adolescent Sprague-Dawley rats were exposed to twenty-eight days of predictable chronic mild stress (PCMS) followed by testing in the EPM as adults. Such PCMS, especially during adolescence, is purported to confer resilience and, thereby, promote low anxiety-like and depressive-like behavior. A neuron-specific deletion of PTEN creates a hyperactive mTOR pathway in mice on a FVB-based mixed background with an increase in phosphorylated Akt and S6 concomitant with decreased anxiety in the EPM and increased activity in the open field (Lugo et al., 2014). Conversely, increased anxiety-like behavior follows mTOR signaling blockade with rapamycin (Hadamitzky et al., 2014; Tsai et al., 2013). We find that enhanced mTOR signaling is lost in the HPC of mice exposed to 14 days of single housing and acute stress in association with anxiety-like behavior in the EPM, another indication that the normal response to stress is altered when the period of single housing is extended. Such correlations between the state of activation of the mTOR pathway and behavior may be relevant within a broader context to conditions which are characterized by an anxious phenotype. A link between mTOR signaling and emotional regulation exists, as components of the mTOR pathway are altered in brains of people with major depressive disorder, schizophrenia, and autism (Bockaert and Marin, 2015; Huber et al., 2015; Jernigan et al., 2011).
The results of this study must be considered within the context of the behavioral traits and genetic background of C57Bl/6J mice. Substrains of C57 mice are relatively nonemotional, more active, and less aggressive than other inbred strains (BALB/c, DBA/2, C3H/He) with corresponding neurotransmitter phenotypes and differences in their stress responses (Cabib et al., 2002; Kempf et al., 1984; Kopp et al., 1999; Lalonde and Strazielle, 2008; Oliverio et al., 1973; Peeler and Nowakowski, 1987; Simler et al., 1982; van der Veen et al., 2007). In contrast to outbred (Swiss-Webster, Swiss CD-1) or wild mice, they show less basal, anxiety-related, or exploratory activities, less aggression, and more risk assessment (Hsieh et al., 2017; Parmigiani et al., 1999; Rodgers et al., 2002). In some studies, C57Bl/6 mice display a high level of anxiety-like behavior in the EPM and adopt a hyper-immobile response in the forced swim task (Avgustinovich et al., 2000; Cabib et al., 2002; Griebel et al., 2000). In contrast, other investigators report that C57 substrains are more exploratory, even in the open arms of the EPM, or less anxious than other strains (Carola et al., 2002; Lepicard et al., 2000; Lalonde and Strazielle, 2008; Rodgers et al., 2002). These differing results have been attributed to experimental factors, such as variable lighting conditions during the EPM test (Lalonde and Strazielle, 2008). The C57BL/6 mouse strain shows only intermediate reactivity in the light-dark box test and is unable to learn an active avoidance task (Bovet et al., 1969; Cabib et al., 2002; Griebel et al., 2000). Given these observations, the EPM was chosen for the behavioral part of this study with C57Bl/6J mice as a protocol appropriate for measurement of anxiety-like behavior.
The duration of single housing dramatically impacts anxiety-like behavior in the EPM following an acute stress challenge, as shown in the current study. Exploration marked the behavior of the C57Bl/6J mice single housed for 7 days. These mice explored the whole EPM, entered the open arms many times, and spent a considerable amount of time in them.
Although single housing is thought to be a type of social isolation stress, it may initially have some positive features for male mice, as adequate space appears to be an extremely important characteristic of a positive environment for them (Latham and Mason, 2004; Sherwin and Nicol, 1997). Males often display territorial behavior, and single housing provides the individual mouse with its own, unchallenged territory. In the current study, however, there was a dramatic shift in behavior from 7 to 14 days of single housing when the C57Bl/6J mice displayed high anxiety-like behavior by spending little time and making few entries into the open arms of the EPM. This aversive behavior in combination with underlying alterations observed over a range of stress-related variables suggests that, between 7 and 14 days, single housing switches from a positive to a negative experience. As stated above, determination of whether C57 substrains are anxious in the EPM has yielded discrepant experimental results. This study suggests that one source of such variability may be the nature and duration of housing conditions under which mice are kept prior to testing in addition to the lighting conditions during the test.
When facing challenges or stressors, the HPA system becomes activated in response to the organism’s innate need for homeostasis, enabling adaptation to environmental changes. Although achieving homeostasis implies that behavioral and physiological indices have returned to baseline levels, it may, in fact, reflect stability that is achieved only through continual change in relation to environmental conditions, or allostasis (McEwen and Wingfield, 2010). Such indices may never return to the original baseline values, but may now reflect a new “baseline” by which comparisons can be made. It is evident that isolation for 14 days leads to a different state than the one observed at 7 days. The question remains as to whether the mice in this study have reached stability by day 14 of single housing or are still responding to this environmental change. Continued activation of the HPA system, beyond the behavior of mice single housed for 14 days, could eventually become deleterious and lead to increased vulnerability to illness.
While the ability to acclimate to novel environments has been well documented in many species, researchers still know very little about the neuronal and neuroendocrine mechanisms that are responsible. The results from the current study demonstrate that acclimation is a process involving the stress- and affect-systems and does not necessarily reach a clear end-point. Further, temporal aspects of acclimation reveal that such adaptation does not always elicit beneficial outcomes. The ramifications of these stress- and affect-system alterations during the acclimation process should be considered when designing animal experiments. Identifying sources of variance, such as housing conditions and timing of data collection, is essential to standardize experimental parameters. This study shows how duration of isolation elicited by single housing and a few hours difference in the time of day, can significantly alter numerous, inter-related variables, from gene expression to behavior. Stability in measured variables is key for obtaining valid, reliable data. Determining how to achieve this stability and reproducibility is a continuing challenge for researchers using animal models.
Acknowledgements
This work was supported by the Hope for Depression Research Foundation and the Pritzker Neuropsychiatric Research Consortium (H. Akil and S.J. Watson). The authors would like to thank James Stewart, Angela Koelsch, and Yu Tang for their technical assistance and Zachary Freeman, DVM, PhD, DACLAM for helpful discussions on this manuscript.
Footnotes
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
References
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T, 2009. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences of the United States of America 106, 14,120–14,125. 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Morano I, 1996. The biology of stress: from periphery to brain In: Watson SJ (Ed.), Biology of Schizophrenia and Affective Disease. Raven Press, New York, pp. 15–48. [Google Scholar]
- Akil H, Kwak S, Morano I, Herman JP, Taylor L, Watson S, 1991. Interplay between glucocorticoid receptors and neuronal pathways in controlling circadian and stress response patterns In: Costa E, Paul SM. (Eds.), Neurosteroids and Brain Function Thieme Medical Publishers. Inc, New York, pp. 31–35. [Google Scholar]
- Alexander GM, Farris S, Pirone JR, Zheng C, Colgin LL, Dudek SM, 2016. Social and novel contexts modify hippocampal CA2 representations of space. Nat Commun 7, 10,300 10.1038/ncomms10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KJ, Dam D, Lee S, Cotman CW, 1988. Basic fibroblast growth factor prevents death of lesioned cholinergic neurons in vivo. Nature 332, 360–361. 10.1038/332360a0. [DOI] [PubMed] [Google Scholar]
- Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van’t Klooster J, Ohl F, 2009. Individual housing of mice–impact on behaviour and stress responses. Physiology & behavior 97, 385–393. 10.1016/j.physbeh.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Lipina TV, Bondar NP, Alekseyenko OV, Kudryavtseva NN, 2000. Features of the genetically defined anxiety in mice. Behav Genet 30, 101–109. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE, Parmigiani S, 2003. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology 28, 540–558. [DOI] [PubMed] [Google Scholar]
- Benoy A, Dasgupta A, Sajikumar S, 2018. Hippocampal area CA2: an emerging modulatory gateway in the hippocampal circuit. Exp Brain Res 236, 919–931. 10.1007/s00221-018-5187-5. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF, 2006. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport 17, 593–597. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Marin P, 2015. mTOR in brain physiology and pathologies. Physiol Rev. 95, 1157–1187. 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- Bovet D, Bovet-Nitti F, Oliverio A, 1969. Genetic aspects of learning and memory in mice. Science 163, 139–149. [DOI] [PubMed] [Google Scholar]
- Brureau A, Zussy C, Delair B, Ogier C, Ixart G, Maurice T, Givalois L, 2013. Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer’s disease rat model. Neurobiology of aging 34, 1426–1439. 10.1016/j.neurobiolaging.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S, Ventura R, 2002. The contribution of comparative studies in inbred strains of mice to the understanding of the hyperactive phenotype. Behav Brain Res 130, 103–109. [DOI] [PubMed] [Google Scholar]
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P, 2002. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134, 49–57. [DOI] [PubMed] [Google Scholar]
- Carstens KE, Dudek SM, 2019. Regulation of synaptic plasticity in hippocampal area CA2. Curr Opin Neurobiol 54, 194–199. 10.1016/j.conb.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran A, Iyo AH, Jernigan CS, Legutko B, Austin MC, Karolewicz B, 2013. Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog Neuropsychopharmacol Biol Psychiatry 40, 240–245. 10.1016/j.pnpbp.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury S, Aurbach EL, Sharma V, Blandino P Jr., Turner CA, Watson SJ, Akil H, 2014. FGF2 is a target and a trigger of epigenetic mechanisms associated with differences in emotionality: partnership with H3K9me3. Proceedings of the National Academy of Sciences of the United States of America 111, 11,834–11,839. 10.1073/pnas.1411618111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS, 2002. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 26, 907–923. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ, 1993. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. The Journal of comparative neurology 332, 1–20. 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F, 2004. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann N Y Acad Sci 1018, 141–150. 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M, 1998. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 19, 269–301. 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Alexander GM, Farris S, 2016. Rediscovering area CA2: unique properties and functions. Nat Rev. Neurosci 17, 89–102. 10.1038/nrn.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren-Kocak E, Turner CA, Watson SJ, Akil H, 2011. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biological psychiatry 69, 534–540. 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H, 2004. Dysregulation of the fibroblast growth factor system in major depression. Proceedings of the National Academy of Sciences of the United States of America 101, 15,506–15,511. 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Flagel SB, Mahmood ST, Watson SJ, Akil H, 2012. Cocaine withdrawal causes delayed dysregulation of stress genes in the hippocampus. PLoS One 7, e42092 10.1371/journal.pone.0042092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ, 2000. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 148, 164–170. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Herring A, Keyvani K, Doenlen R, Krugel U, Bosche K, Orlowski K, Engler H, Schedlowski M, 2014. Acute systemic rapamycin induces neurobehavioral alterations in rats. Behavioural brain research 273, 16–22. 10.1016/j.bbr.2014.06.056. [DOI] [PubMed] [Google Scholar]
- Haller J, Halasz J, Makara GB, 2000. Housing conditions and the anxiolytic efficacy of buspirone: the relationship between main and side effects. Behavioural pharmacology 11, 403–412. [DOI] [PubMed] [Google Scholar]
- Hebda-Bauer EK, Simmons TA, Sugg A, Ural E, Stewart JA, Beals JL, Wei Q, Watson SJ, Akil H, 2013. 3xTg-AD mice exhibit an activated central stress axis during early-stage pathology. J Alzheimers Dis 33, 407–422, doi: 10.3233/JAD-2012-121,438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Watson SJ, Chao HM, Coirini H, McEwen BS, 1993. Diurnal regulation of glucocorticoid receptor and mineralocorticoid receptor mRNAs in rat hippocampus. Mol Cell Neurosci 4, 181–190. 10.1006/mcne.1993.1022. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG, 2002. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci 16, 381–385. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H, 2005. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29, 1201–1213. 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E, 2010. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33, 67–75. 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LS, Wen JH, Miyares L, Lombroso PJ, Bordey A, 2017. Outbred CD1 mice are as suitable as inbred C57BL/6J mice in performing social tasks. Neurosci Lett 637, 142–147. 10.1016/j.neulet.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Klann E, Costa-Mattioli M, Zukin RS, 2015. Dysregulation of mammalian target of rapamycin signaling in mouse models of autism. J Neurosci 35, 13,836–13,842. 10.1523/JNEUROSCI.2656-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C, Hambly C, 2006. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group housed males. Physiology & behavior 87, 519–526. 10.1016/j.physbeh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B, 2011. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35, 1774–1779. 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf E, Puglisi-Allegra S, Cabib S, Schleef C, Mandel P, 1984. Serotonin levels and turnover in different brain areas of isolated aggressive or non-aggressive strains of mice. Prog Neuropsychopharmacol Biol Psychiatry 8, 365–371. [PubMed] [Google Scholar]
- Kerman IA, Clinton SM, Simpson DN, Bedrosian TA, Bernard R, Akil H, Watson SJ, 2012. Inborn differences in environmental reactivity predict divergent diurnal behavioral, endocrine, and gene expression rhythms. Psychoneuroendocrinology 37, 256–269. 10.1016/j.psyneuen.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Vogel E, Misslin R, 1999. Comparative study of emotional behaviour in three inbred strains of mice. Behav Processes 47, 161–174. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM, 2004. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological psychiatry 55, 367–375. 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C, 2008. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J Neurosci Methods 171, 48–52. 10.1016/j.jneumeth.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Latham N, Mason G, 2004. From house mouse to mouse house: the behavioural biology of free-living Mus musculus and its implications in the laboratory. Applied Animal Behaviour Science 86, 261–289. 10.1016/j.applanim.2004.02.006. [DOI] [Google Scholar]
- Lefcheck Jonathan S., 2016. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods in Ecology and Evolution. 7 (5), 573–579. 10.1111/2041-210X.12512. [DOI] [Google Scholar]
- Lein ES, Callaway EM, Albright TD, Gage FH, 2005. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. The Journal of comparative neurology 485, 1–10. 10.1002/cne.20426. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G, 2000. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol Biochem Behav 67, 739–748. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Lopez JF, Flagel SB, Vazquez DM, Young EA, 1999. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol 11, 11–17. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ, 1998. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biological psychiatry 43, 547–573. [DOI] [PubMed] [Google Scholar]
- Lugo JN, Smith GD, Arbuckle EP, White J, Holley AJ, Floruta CM, Ahmed N, Gomez MC, Okonkwo O, 2014. Deletion of PTEN produces autism-like behavioral deficits and alterations in synaptic proteins. Front Mol Neurosci 7, 27 10.3389/fnmol.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, Opitz N, Goehler LE, Gaykema RP, Overmier JB, 2005. Recommended housing conditions and test procedures can interact to obscure a significant experimental effect. Behav Res Methods 37, 651–656. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Keller JN, Kruman I, Mattson MP, 1997. Basic FGF attenuates amyloid beta-peptide-induced oxidative stress, mitochondrial dysfunction, and impairment of Na+/K+-ATPase activity in hippocampal neurons. Brain research 756, 205–214. [DOI] [PubMed] [Google Scholar]
- McCann KE, Lustberg DJ, Carstens KE, Farris S, Shaughnessy EK, Zhoa M, Alexander GM, Dudek SM, 2018. Novel role for mineralocorticoid receptors in the development and maintenance of hippocampal area CA2 pyramidal cell phenotype Abstract No. 643.01, 2018 Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience. [Google Scholar]
- McEwen BS, Wingfield JC, 2010. What is in a name? Integrating homeostasis, allostasis and stress. Hormones and behavior 57, 105–111. 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, Melcangi RC, Riva MA, 2001. Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res Brain Res Rev. 37, 249–258. [DOI] [PubMed] [Google Scholar]
- Nagy TR, Krzywanski D, Li J, Meleth S, Desmond R, 2002. Effect of group vs. single housing on phenotypic variance in C57BL/6J mice. Obes Res 10, 412–415. 10.1038/oby.2002.57. [DOI] [PubMed] [Google Scholar]
- Nichols DJ, Chevins PF, 1981. Effects of housing on corticosterone rhythm and stress responses in female mice. Physiology & behavior 27, 1–5. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER, 2010. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 34, 853–866. 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Oliverio A, Eleftheriou BE, Bailey DW, 1973. Exploratory activity: genetic analysis of its modification by scopolamine and amphetamine. Physiol Behav 10, 893–899. [DOI] [PubMed] [Google Scholar]
- Otto D, Unsicker K, 1990. Basic FGF reverses chemical and morphological deficits in the nigrostriatal system of MPTP-treated mice. The Journal of neuroscience: the official journal of the Society for Neuroscience 10, 1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani S, Palanza P, Rogers J, Ferrari PF, 1999. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci Biobehav Rev. 23, 957–969. [DOI] [PubMed] [Google Scholar]
- Peeler DF, Nowakowski RS, 1987. Genetic factors and the measurement of exploratory activity. Behav Neural Biol 48, 90–103. [DOI] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H, 2009. A new role for FGF2 as an endogenous inhibitor of anxiety. The Journal of neuroscience: the official journal of the Society for Neuroscience 29, 6379–6387. 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman JA, Hunter RG, Speksnijder N, van den Oever JM, Korobko OB, McEwen BS, de Kloet ER, Datson NA, 2012. Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology 153, 4317–4327. 10.1210/en.2012-1255. [DOI] [PubMed] [Google Scholar]
- Reis FM, Albrechet-Souza L, Franci CR, Brandao ML, 2012. Risk assessment behaviors associated with corticosterone trigger the defense reaction to social isolation in rats: role of the anterior cingulate cortex. Stress 15, 318–328. 10.3109/10253890.2011.623740. [DOI] [PubMed] [Google Scholar]
- Riva MA, 1995. The role of neurotrophic factors in the stress response In: Steckler T, Kalin NH, Reul JMHM(Eds.), Handbook of Stress and the Brain. Elsevier B. V, Amsterdam. [Google Scholar]
- Rodgers RJ, Davies B, Shore R, 2002. Absence of anxiolytic response to chlordiazepoxide in two common background strains exposed to the elevated plus-maze: importance and implications of behavioural baseline. Genes Brain Behav 1, 242–251. [DOI] [PubMed] [Google Scholar]
- Sherwin CM, Nicol CJ, 1997. Behavioral demand functions of caged laboratory mice for additional space. Anim Behav 53, 67–74. [Google Scholar]
- Simler S, Puglisi-Allegra S, Mandel P, 1982. gamma-Aminobutyric acid in brain areas of isolated aggressive or non-aggressive inbred strains of mice. Pharmacol Biochem Behav 16, 57–61. [DOI] [PubMed] [Google Scholar]
- Suo L, Zhao L, Si J, Liu J, Zhu W, Chai B, Zhang Y, Feng J, Ding Z, Luo Y, Shi H, Shi J, Lu L, 2013. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 38, 1387–1400. 10.1038/npp.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topic B, Oitzl MS, Meijer OC, Huston JP, de Souza Silva MA, 2008. Differential susceptibility to extinction-induced despair and age-dependent alterations in the hypothalamic-pituitary-adrenal axis and neurochemical parameters. Neuropsychobiology 58, 138–153. 10.1159/000182890. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Greene-Colozzi E, Goto J, Anderl S, Kwiatkowski DJ, Sahin M, 2013. Prenatal rapamycin results in early and late behavioral abnormalities in wildtype C57BL/6 mice. Behav Genet 43, 51–59. 10.1007/s10519-012-9571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli JS, Smith JA, Morton DB, 1995. Stress measurements in mice after transportation. Lab Anim 29, 132–138. [DOI] [PubMed] [Google Scholar]
- Turner CA, Calvo N, Frost DO, Akil H, Watson SJ, 2008a. The fibroblast growth factor system is downregulated following social defeat. Neuroscience letters 430, 147–150. 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H, 2008b. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain research 1224, 63–68. 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Watson SJ, Akil H, 2012. The fibroblast growth factor family: neuromodulation of affective behavior. Neuron 76, 160–174. 10.1016/j.neuron.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Eren-Kocak E, Inui EG, Watson SJ, Akil H, 2015. Dysregulated fibroblast growth factor (FGF) signaling in neurological and psychiatric disorders. Semin Cell Dev Biol. 10.1016/j.semcdb.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R, Piazza PV, Deroche-Gamonet V, 2007. Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology (Berl) 193, 179–186. 10.1007/s00213-007-0777-0. [DOI] [PubMed] [Google Scholar]
- Wang F, Kessels HW, Hu H, 2014. The mouse that roared: neural mechanisms of social hierarchy. Trends in neurosciences 37, 674–682. 10.1016/j.tins.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Williams TE, Meshul CK, Cherry NJ, Tiffany NM, Eckenstein FP, Woodward WR, 1996. Characterization and distribution of basic fibroblast growth factor-containing cells in the rat hippocampus. The Journal of comparative neurology 370, 147–158. . [DOI] [PubMed] [Google Scholar]
- Williamson CM, Lee W, Romeo RD, Curley JP, 2017. Social context-dependent relationships between mouse dominance rank and plasma hormone levels. Physiol Behav 171, 110–119. 10.1016/j.physbeh.2016.12.038. [DOI] [PubMed] [Google Scholar]
- Yang PC, Yang CH, Huang CC, Hsu KS, 2008. Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. The Journal of biological chemistry 283, 2631–2643. 10.1074/jbc.M706954200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang J, Sun H, Liu H, Yang Y, Yao Z, 2011. Exposure to enriched environment restores the mRNA expression of mineralocorticoid and glucocorticoid receptors in the hippocampus and ameliorates depressive-like symptoms in chronically stressed rats. Curr Neurovasc Res 8, 286–293. [DOI] [PubMed] [Google Scholar]