Abstract
Although treating COVID-19 is shown to be challenging, NANOtechnology is around the corner to overcome potential drawbacks. The use of NANOtechnologies will definitely shape the worldwide approaches and tools to treat COVID-19. Here we highlight the importance of going NANO on the COVID-19 pandemic.
Main Text
On the January 30, 2020, the WHO director-general declared the novel coronavirus (2019-nCoV or COVID-19) outbreak a pandemic and a public health emergency of international concern. On this same day the world changed unimaginably. This new and natural virus—SARS-CoV-2—is the seventh coronavirus known to infect humans and cause severe acute respiratory syndrome. When this coronavirus pandemic broke out it transformed the modes in which we live and perceive the world, changing the way we interact, with social distancing encouraged to stop the spread of the 2019-nCoV. From blank canals in Venice to empty streets in Paris, this new coronavirus changed life, every day, for all of us around the world.
Although preventing and treating COVID-19 has a lot of puts and takes, NANOtechnology is around the corner to do so. Being a highly multi-disciplinary and translational-focused field, NANOtechnology is able to quickly pivot and refocus unique engineering approaches on much-needed solutions for many of the problems this pandemic poses, as well as to alleviate the already overstrained medical treatment centers. The idea of using NANOtechnology is that viruses like COVID-19 work on a similar scale as NANOmaterials. At this scale, structures are just ten times bigger than individual atoms. Therefore, the interaction of NANOstructures with microorganisms like viruses is in fact gaining momentum in the biomedical field by offering a huge potential in both diagnostic and therapeutic approaches.
This idea started more than 60 years ago, when in December of 1959, Richard Feynman (an American theoretical physicist) gave a lecture called “There's Plenty of Room at the Bottom” at an annual meeting of the American Physical Society at Caltech. In this well-known talk, Feynman placed the theoretical foundations for the field now called NANOtechnology, when he could perceive the world where things could be miniaturized, or when huge volumes of intel could be programmed onto smaller and even smaller devices, and when machines could be made significantly reduced and compact. Despite the fact that 1959 was the time when computers were the size of entire rooms, Feynman asked his audience: “I don't know how to do this on a small scale in a practical way, but I do know that computing machines are very large; they fill rooms. Why can't we make them very small, make them of little wires, little elements, and by little, I mean little?”1
In fact, the concept of miniature medical minions isn’t new. Richard Feynman at that time already suggested the possibility of “swallowing the doctor.” Feynman told us “it would be interesting in surgery if you could swallow the surgeon (small machine)… It goes into the heart and looks around. It finds out which valve is the faulty and repairs it.”1 That inspired others to consider the possibility of manipulating individual atoms as a powerful tool for synthetic chemistry and synthetic biology.
And that’s how NANOtechnology was born. Since then, NANOtechnology is everywhere and is everyday practice. From the initial vision of using NANOrobots, NANOmedicine has emerged as a powerful platform that has led to the development of novel materials and devices with a wide range of applications, especially in imaging, diagnostics, and therapy, which contributed to the early detection and treatment of diseases.2
The promise of NANOtechnology is lying in the potential of manipulating matter on the small scale used either by nature or by humans. This opens new vistas to work at the scale of the cells and microbe-like viruses. These NANOmaterials that have exceptional features and cues, otherwise absent at a macroscopic level, could have the potential to present efficient functions and benefits that can be applied to medical science and clinics. This is no exception for the detection and treatment of COVID-19. From these applications, we can only envision that the use of NANOtechnologies will shape the worldwide epidemiologic trace of COVID-19.
And we have lots of tools available to create numerous materials to probe, sense, and treat COVID-19. A range of stimuli responsive properties such as genetic, molecular, enzymatic, targeting, optical, or chemical could be incorporated into NANOmaterials, and NANOmaterials can be engineered to have some of or all these properties in one single device. Those smart materials have the potential to provide highly active and specific therapies with minimal side effects and maximum therapeutic outcome. So, we can design materials to overcome the most common delivery obstacles, which are often associated with circulation time and stability as well as tissue extravasation and cell internalization. This will be essential to find viral particles in an efficient way and target them for destruction by developing NANOvaccines involved in host cell protection and immune and immunity response and/or anti-viral NANOagents, involved in inhibiting viral attachment, cell entry, and systemic infection (Figure 1 ).
Figure 1.
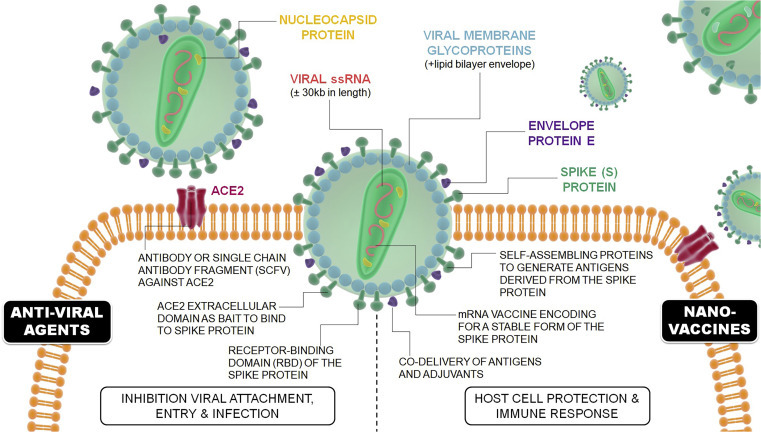
Potential Therapeutic Approaches by which NANOtechnology Can Contribute against COVID-19
NANOvaccines, involved in host cell protection and immune and immunity response, and anti-viral agents, involved in inhibiting viral attachment, cell entry, and systemic infection.
Where Are We at with NANOvaccines?
A series of different drug candidates have already been patented for severe acute respiratory syndrome (SARS) virus (SARS-CoV), including Middle East respiratory syndrome (MERS) virus (MERS-CoV) and COVID-19.3 In a general manner, a vaccine contains one or more key components of the virus such as the envelope, spike, or a membrane protein. Injection of the vaccine in the body gives the immune system a preview of the virus without causing the disease, leading to awareness of the immune system to seek out and attack the virus containing those specific proteins if the real virus ever shows up. Typically, the use of NANOparticles in vaccine formulations could fulfill three different purposes: (1) enhanced antigen stability by protecting them from premature degradation by proteolytic enzymes, (2) enhanced immunogenicity as immuno-stimulant adjuvant to provoke an immune response, and (3) targeted delivery where NANOparticles are used as delivery systems to facilitate antigen uptake and processing by antigen-presenting cells.4 However, only recently inspired by the idea of virus-like NANOparticles, scientists were capable of decorating self-assembling protein NANOparticles (1c-SApNPs) with SARS-CoV-2 protein spikes, as stated by StatNano.com. This system, reportedly, can induce the immune system to rapidly generate antibodies to neutralize (or deactivate) the coronavirus, ultimately offering a recipient protection against the real SARS-CoV-2 virus. This is rather a unique approach to use the extraordinary surface properties of NANOparticles (associated with their high surface-area-to-volume ratio) in combination with virus surface proteins to draw an immune response. Of course, large-scale production of viral proteins, associated with these vaccines, brings with it several challenges including the need for a manufacturing process that is scalable and cost effective.5 To address these shortcomings, the new vaccines based on messenger RNA (mRNA) has been suggested. These vaccines provide a synthetic mRNA of the virus, which the host body then uses to produce the viral proteins itself. In fact, Moderna, a clinical stage biotechnology company pioneering messenger RNA (mRNA) therapeutics, is already testing a mRNA-based vaccine candidate (mRNA-1273) against COVID-19. mRNA-1273 is an mRNA vaccine against SARS-CoV-2 encoding for a prefusion stabilized form of the spike protein, which was selected by Moderna in collaboration with the Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID). According to the National Institutes of Health (NIH), during this Phase 1 study, under the Investigational New Drug (IND) the synthesized mRNA that is required for preparing the vaccine is embedded in lipid NANOparticles (in StatNano.com). Due to instability of mRNA molecule, this group is using a novel lipid NANOparticle (LNP) to encapsulate the mRNA vaccine.
Furthermore on the development of NANOvaccines, Novavax, a late-stage biotechnology company developing next-generation vaccines for serious infectious diseases, announced that it has developed a coronavirus vaccine candidate, NVX-CoV2373, a stable and highly immunogenic prefusion protein made embedded on a Matrix-M adjuvant in order to enhance immune responses and stimulate high levels of neutralizing antibodies.
These NANOvaccines are examples as to how NANOtechnology can further enhance the therapeutic effect of COVID-19 vaccines. For instance, we know by now that angiotensin-converting enzyme 2 (ACE2) receptor, a common receptor in human airway epithelia as well as lung parenchyma, is the major receptor for both the SARS-CoV and the related human respiratory coronavirus NL63.6 Hence, one could imagine the realization of an oral multi-modal NANOvaccine for targeted delivery of a synthetic mRNA of the virus to the respiratory tract, with the purpose of enhancing the immunostimulatory activity of the vaccine, by simply including antibodies or small molecules that could target the interaction sites between ACE2 and SARS-CoV.
NANO-based Anti-virals as Potential Alternatives?
Unlike vaccines that are typically designed for boosting a certain aspect of the immune system (either directly or indirectly), anti-viral agents are simply interfering with certain stages of the virus replication cycle (viral entry, viral replication, or viral shedding) to inhibit their actions. Along these lines, in the context of coronavirus, NANOmaterials have already entered the fray as NANOvehicles capable of carrying therapeutic molecules to manipulate the virus mechanism of action. One avenue that researchers have explored was design of NANOparticle-bound ligands that can act as protective barriers on cell surface to efficiently prevent virus attachment, entry, and budding. Another interesting approach is design of NANOparticles loaded with compounds with intrinsic anti-viral properties (such as polyphenols) not only to inhibit the attachment of virus to the cell membrane, but also to promote production of interferon-stimulating genes (ISGs) and pro-inflammatory cytokines by the host cell that can suppress the synthesis of negative-strand RNA of the virus.7 , 8 One other proposed approach is to use NANOparticulate RNA interference (RNAi) formulations to downregulate genes that facilitate the viral progression.9 Although such studies produced promising results in vitro against coronavirus, their efficacy in vivo (as well as in clinic) is yet to be explored. Considering that viruses could be phylogenetically unrelated and structurally different, and given that most vaccines are virus specific, a promising approach would be that of developing broad-spectrum anti-viral NANOparticles to fight COVID-19 and future pandemics.10 Overall, anti-viral agents can potentially be employed as the first line of treatment against most viral diseases.
Currently, there are no specific vaccine or anti-viral treatments available for COVID-19, but a wide range of fields of science and technology, such as NANOtechnology, has great potential to help in the prevention, diagnosis, and treatment of COVID-19. Here we highlighted the latest developments in how the NANO world is taking root against this unprecedent fight and could help us get closer to a treatment for COVID-19.
Acknowledgments
J.C. is funded by the European Research Council Starting Grant (ERC- StG-2019- 848325).
References
- 1.Feynman R.P. There’s plenty of room at the bottom. Eng. Sci. 1960;23:22–36. [Google Scholar]
- 2.Wagner V., Dullaart A., Bock A.-K., Zweck A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 3.Thanh Le T., Andreadakis Z., Kumar A., Gómez Román R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L., Seth A., Wibowo N., Zhao C.X., Mitter N., Yu C., Middelberg A.P. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 5.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B., Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting D., Dong N., Fang L., Lu J., Bi J., Xiao S., Han H. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Applied Nano Mater. 2018;1:5451–5459. doi: 10.1021/acsanm.0c00970. [DOI] [PubMed] [Google Scholar]
- 8.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L., Xiao S., Han H. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl. Mater. Interfaces. 2018;10:4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- 9.Ufaz S., Balter A., Tzror C., Einbender S., Koshet O., Shainsky-Roitman J., Yaari Z., Schroeder A. Anti-viral RNAi nanoparticles protect shrimp against white spot disease. Mol. Syst. Des. Eng. 2018;3:38–48. [Google Scholar]
- 10.Cagno V., Andreozzi P., D’Alicarnasso M., Jacob Silva P., Mueller M., Galloux M., Le Goffic R., Jones S.T., Vallino M., Hodek J. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018;17:195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]


