Abstract
Identifying the mechanisms behind neuronal fate specification are key to understanding normal neural development in addition to neurodevelopmental disorders such as autism and schizophrenia. In vivo cell fate specification is difficult to study in vertebrates. However, the nematode Caenorhabditis elegans, with its invariant cell lineage and simple nervous system of 302 neurons, is an ideal organism to explore the earliest stages of neural development. We used a comparative transcriptome approach to examine the role of cnd-1/NeuroD1 in C. elegans nervous system development and function. This basic helix-loop-helix transcription factor is deeply conserved across phyla and plays a crucial role in cell fate specification in both the vertebrate nervous system and pancreas. We find that cnd-1 controls expression of ceh-5, a Vax2-like homeobox class transcription factor, in the RME head motorneurons and PVQ tail interneurons. We also show that cnd-1 functions redundantly with the Hox gene ceh-13/labial in defining the fate of DD1 and DD2 embryonic ventral nerve cord motorneurons. These data highlight the utility of comparative transcriptomes for identifying transcription factor targets and understanding gene regulatory networks.
Keywords: NeuroD1, cell fate specification, Hox genes, homeobox genes, RNA-seq
Accurate control of gene expression is fundamental for the development and function of the central nervous system (CNS). Defects in CNS gene expression underlie many neurodevelopmental disorders, indicating a critical need for further study (Basu et al. 2009; Schizophrenia Working Group of the Psychiatric Genomics Consortium 2014). Gene expression is controlled by combinations of transcription factors that work in conjunction with chromatin remodeling complexes to promote or inhibit RNA polymerase access to the genome (Clapier and Cairns 2009). According to Waddington’s model of cellular differentiation, cell fate progressively refines over multiple rounds of cell division, first to tissue-type progenitors, then exiting the cell cycle to take on a tissue-specific terminal fate (Waddington 1957). The transcription factors and chromatin remodeling complexes required for these narrowing rounds of fate specification are not well understood.
The basic Helix-Loop-Helix (bHLH) super family of proneural transcription factors includes Atonal, NeuroD, Neurogenin, and Achaete/Scute, and has broad roles in nervous system development (Baker and Brown 2018). This family of bHLH transcription factors function as either homodimers or heterodimers and bind to E-box sequences of the motif CANNTG. In Drosophila, the bHLH family acts in neural cell fate specification and neurogenesis, with different family members having roles in external sensory organ formation, chordotonal organ development, and others (Lee 1997).
Vertebrate neurogenic differentiation 1 (NeuroD1) is a bHLH transcription factor that has a role in the transcriptional activation of proneural genes (Wang and Baker 2015). In addition, NeuroD1 is expressed abundantly in the brain after terminal fate specification, which suggests a secondary role in nervous system homeostasis and/or neural maturation and survival (Miyata et al. 1999). Ectopic expression of NeuroD1 in Xenopus embryos can convert non-neural ectodermal cells into fully differentiated neurons, indicating the potential role of NeuroD1 as a neural differentiator factor (Lee et al. 1995; Lee 1997). Humans bearing homozygous NeuroD1 mutations showed severe cerebral hypoplasia and developmental delay, in addition to defects in pancreatic β-cell maturation and islet formation, demonstrating the importance of this gene in nervous system and pancreatic development (Rubio-Cabezas et al. 2010). In the mouse, NeuroD1 is essential for the generation of granule cells in the hippocampus and the cerebellum (Miyata et al.1999; D’Amico et al. 2013). Despite extensive research on the role of NeuroD1 in cell fate specification and nervous system development (Seo et al. 2007; Pataskar et al. 2016), a comprehensive list of NeuroD1 targets has not been compiled and many questions on its role in neural development remain unanswered.
The nematode Caenorhabditis elegans, with its invariant cell linage and well-defined nervous system, is an excellent model to study cell lineage determination and terminal fate specification (Sulston and Horvitz 1977; Sulston et al. 1983). Once a neuroblast exits the cell cycle, it needs to extend growth cones and axons through the extra-cellular matrix, find its appropriate pre- and post-synaptic partners, assemble a synapse, gap junction, or neuromuscular junction, then package the various proteins required for synaptic transmission (Chisholm et al. 2016; Jin and Qi 2018). This leads to two major questions. First, what cascade of transcription factors is required to specify interim cell fates, prior to final specification of a neuron? Second, does a single transcription factor control the fate of a single neuron, or is terminal fate specified in a combinatorial manner, with multiple transcription factors controlling different aspects of the final cell fate? Extensive work has identified a battery of transcription factors known as “terminal selectors”, which are required for terminal fate specification in C. elegans neurons (Hobert 2016 and references therein). These transcription factors generally act in a combinatorial fashion to specify cell fates, although individual transcription factors may specify the fate of multiple cells that are unrelated by cell lineage, type, or circuit. Terminal selectors typically have autoregulatory properties, in that they positively regulate their own transcription to maintain neuronal identity throughout the life of an organism. In addition, they either directly or indirectly control the expression of “terminal effector” genes, which are required for that neuron’s post-mitotic function, for instance neurotransmitter biosynthesis, packaging, and release. Despite this depth of knowledge, the “proneural” transcription factors that act up-stream of terminal selector genes are not well described.
The C. elegans bHLH transcription factor cnd-1 is orthologous to the human NeuroD1 gene and is one of the earliest proneural genes to be activated during C. elegans embryonic development (Hallam et al. 2000). However, the only reported defects seen in cnd-1 loss-of-function mutants are a relatively mild back-coiler phenotype caused by misspecification of 2-3 dorsal D (DD) motorneurons required for inhibitory GABAergic neuromuscular innervation, in addition to axon guidance and synapse remodeling defects in the remaining D neurons (Hallam et al. 2000). To gain a better understanding of CND-1’s role during C. elegans neural development, we performed an RNA-seq assay comparing embryonic wild type and cnd-1(ju29) mutant transcriptomes. We find that CND-1 positively regulates the expression of homeobox transcription factor ceh-5/Vax2 in the head RME and tail PVQ neurons. We also confirm that CND-1 is required for the generation of cnd-1 expressing cells during ventral nerve cord fate specification. Finally, we show that cnd-1 functions in parallel with the Hox gene ceh-13/labial to specify a subset of embryonic DD class ventral nerve cord motorneuron fates.
Materials and Methods
Strains and maintenance
C. elegans strains were grown on nematode growth medium plates (NGM Lite) at 20° according to Brenner (1974). Bristol N2 strain was used as wild type and all analyses were conducted at 20°. The following alleles were used in this study: LGIII cnd-1(ju29), cnd-1(gk718) and ceh-13(sw1)/qC1 [dpy-19(e1259) glp-1(q339)]. Integrated transgenes used were juIs76 [unc-25p::GFP + lin-15(+)], kyIs39 [sra-6p::GFP + lin-15(+)], lhIs5 [unc-25p::mCherry], otIs356 [rab-3p(prom1)::2xNLS::TagRFP], pkIs586 [gpa-9p::GFP + dpy-20(+)], and stIs10055 [cnd-1p::his-24::mCherry + unc-119(ed3)]. Extra-chromosomal arrays used in this study were leEx2489 [ceh-5p::GFP + unc-119(+)] and dbEx724 [flp-6p::tax-2(cDNA)::SL2::GFP + lin-15(+)]. The cnd-1(gk718) allele was identified by the C. elegans deletion mutant consortium (2012). All mutants were outcrossed at least twice prior to analysis. Table S1 shows details of strains generated during the course of this study including strain numbers and sources.
A cnd-1(gk718) ceh-13(sw1)/qC1 line was built by crossing gk718/+ males into sw1/qC1, keeping lines that did not give rise to the qC1 dumpy/sterile phenotype (genotype gk718 +/ + sw1), selecting for gk718 homozygous animals (uncoordinated phenotype) then screening for the embryonic lethal sw1 phenotype (parent genotype gk718 sw1/ gk718 +). Two recombinants were identified from 200 gk718 animals screened, consistent with the 1 map unit distance between cnd-1 and ceh-13 on LG III. These lines were rebalanced over the qC1 chromosomal inversion prior to further characterization.
RNA extraction
Embryos were isolated from gravid worms grown in liquid culture as described previously (Hudson et al. 2006). Total RNA was extracted using RiboZol (AMRESCO) and followed the vendor’s protocol except that embryos were frozen in liquid nitrogen prior to grinding. Embryonic tissue was added to 1 mL of RiboZol and 500 μL aliquoted into two 5 PRIME Phase Lock Gel Tubes. At the isopropanol stage, 3μL of 20ng/ml Glycogen was added to improve RNA pellet visualization. RNA quality control was assayed by measuring the A260/A280 ratio using a Thermo Scientific NanoDrop and via an Agilent Bioanalyzer. All samples used for RNA-Seq had an RNA integrity number (RIN) above 9.2. Triplicate cnd-1(ju29) and N2 wild type samples were sent to the University of Kansas Genome Sequencing Core for RNA-seq library construction (Illumina TruSeq v2), barcoded, pooled, and sequenced in a single lane on an Illumina HiSeq 2500 system (high output, single read 100bp sequencing).
RNA-Seq expression analysis pipeline
Gene expression abundance were obtained using previous published RNA-seq analysis workflows. Briefly, read quality was determined using FastQC 0.11.5 (Andrews 2010). Reads were aligned back to the C. elegans reference genome (wbcel235) using Hisat2-2.1.0 (Kim et al. 2015). Samtools 1.5 (htslib 1.4.1) was used for file conversion and sorting (Li et al. 2009). Stringtie 1.3.3.b was used to assemble and quantify transcripts (Pertea et al. 2016). The script prepDE.py was used to generate gene and transcript count matrix files that were compatible with DESeq2 1.16.1 (R3.4.1), which was used to identify differentially expressed genes (Love et al. 2014). All programs were run locally.
Identification of differential isoform usage
This was performed using DEXSeq (Anders et al. 2017). Raw RNA-seq reads were mapped with HiSat2 to the C. elegans annotated reference genome obtained from Ensembl. A python script provided by the DEXSeq package was used to combine all isoforms of a gene into one global schematic representation with marked intron/exon boundaries. The analysis was performed following the developers’ recommendations with the following inclusion “- s” for no strand specific reads.
Characterization of the ken2 deletion allele
2.0X Taq RED Master Mix Kit (Apex Bioresearch Products) was used to amplify the 3′ end of the ptrn-1 gene. Reverse primers at approximately 1kb intervals were used in combination with a
single forward primer within the ptrn-1 coding region to amplify the ptrn-1 3′ untranslated region (UTR). Primer R9, located 10kb downstream of the F1 forward primer were the only pair which amplified cnd-1(ju29) genomic DNA, and gave a 3kb amplicon. Sanger sequencing was used to confirm the ken2 breakpoints, which corresponds to a 6908bp deletion/80bp insertion. Coordinates of the ken2 breakpoints along with insertion and flanking sequences are; LG X
AGAGATACACATGTTTTTGGTGCTTTGTAGAAACCAGTACACGCGCATTTTCACTTACTTTTTTTATTTTTTTCCGTTTCTTTCTGTTTCTAATTTTGCAGATT (17,022,165)/
CTAATTTTGCAGATTCGGTGTTCTCCGAGGTTTTTTAAATCGGTGGGCAGGTGGAAATATTTTGTCATAGTTTTTCGAAG / (17,015,257)
TATCAGGTTGTCCCATAAGTTTTTGTACTATTTTTTTTTTTGAAAAAAATTTATTTCTCTCAAGCGACAAGTAGTACTATTCACACAAGTATTCACCATTAGTGT. Note that the 3′ breakpoint lies in a transposon sequence and is difficult to identify via BLAST search. The ken2 deletion removes all of F35B3.1, F35B3.10, F35B3.4, and around 250bp of the ptrn-1 3′ untranslated region. Primer sequences used for PCR characterization of the ken2 allele were as follows and show expected wild type amplicon size:
ptrn-1F1 (gtgaccaaatccaaccgtg); ptrn-1R1 (ttgcctcagtgcattttgg, 593bp); ptrn-1R2 (ctttaaggaaggcatgggatg, 1164bp); ptrn-1R4 (gtcaaggcatcaagtggttag, 2245bp); ptrn-1R5 (tttgaataatgcctctttaaagtgaatg, 3670bp); ptrn-1R6 (aggtaactttctgagcccac, 4717bp); ptrn-1R7 (gtggaacctgaagtgaataatgg, 6116bp); ptrn-1R8 (caaaaccgtctgccacg, 7119bp); ptrn-1R9 (gaagtagtagatcagccatatgc, 9896bp).
Plasmid constructs
Plasmids were constructed using Gateway Technology (Invitrogen). Target gene promoters were PCR amplified from genomic DNA using Phusion polymerase (Thermo Scientific), gel purified, A-overhangs added by incubating with Taq RED Master Mix Kit (Apex Bioresearch Products), cloned into the pCR8/GW/TOPO vector (Thermo Scientific) following the manufacturer’s specifications then transformed into NEB 5-alpha Competent E. coli (New England BioLabs). Plasmid digestion was used to confirm the forward orientation of the amplicon. Promoters were recombined into the promoterless Gateway GFP expression vector pCZGY32 vector (a kind gift from Yishi Jin, UC San Diego) using Gateway LR Clonase II Enzyme Mix (Invitrogen) following the vendor’s instructions and transformed into NEB 5-alpha Competent E. coli. Plasmid digestion was used to confirm identity. Constructs were microinjected into the germlines of either wild type or stIs10055[ cnd-1p::his-24::mCherry unc-119(+)] worms at 20ng/μL in conjunction with either unc-122::GFP (coelomocyte::GFP) or ttx-3p::RFP co-injection plasmids and pBlueScript II to a final concentration of 50ng/μL. F1 hermaphrodites that expressed the co-injection marker were single plated and screened to obtain stable lines.
cDNA synthesis and RT-qPCR
Total RNA from mixed staged embryos was treated with DNAase (New England Biolabs) and cleaned using the RNA Clean & Concentrator kit (Zymo Research). First-strand synthesis was done using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). cdc-42 transcript levels were used to normalize for differences in input cDNA. Three or four biological samples, in triplicate, were run on a LightCycler 480 real-time PCR system and relative expression rations were calculated according to Pfaffl (2001).
Dye-filling assay
Animals were washed off plates, washed three times with M9 buffer then incubated for one hour in 100 ng/μl Vybrant DiD cell-labeling solution (Invitrogen) as described previously (Schultz and Gumienny 2012). After destaining for one hour on NGMLite plates seeded with OP50 E. coli, animals were imaged by confocal microscopy at 40x magnification for DiD uptake (647nm excitation) in the amphid neurons and GFP expression (488nm excitation).
Microscopy
Well-fed worms grown under standard conditions were used for expression pattern characterization. Images were captured on either a Zeiss LSM 700 confocal microscope, Zeiss Axiovision compound microscope, or Olympus BX61 compound microscope. Expression patterns in L1 larvae were imaged within 1 hr of hatching. For quantitative imaging of cnd-1p::his-24::mCherry expression in wild type and cnd-1 mutant backgrounds, 2-4 cell embryos were isolated from gravid hermaphrodites and mounted on a bead pad (Murray et al. 2008). After 6-7 hr, when the embryos reached comma stage, a single image stack (to eliminate possible photobleaching) was captured for each embryo using a Zeiss LSM 700 confocal microscope at 40x magnification under identical settings, ensuring no detector saturation. Images were processed using Fiji (Schindelin et al. 2012) using a Z-project - Sum Slices workflow which rendered the summated stacks as 32-bit images. Pixel values and counts for the whole image (1024 × 1024 pixels) were obtained using the Analyze - Histogram function (using the pixel value range) then processed in Microsoft Excel (sum [pixel value x pixel count]).
Individual DD neuron identities was inferred by imaging unc-25p::GFP in L1 larvae then measuring the nose-to-neuron, neuron-to-neuron, or neuron-to-tail distances along the anterior-posterior body axis using the segmented line function in Fiji. DD neuron identity in cnd-1(gk718) and ceh-13(sw1) mutant combinations were mapped to the closest relative location in wild type L1 larvae. Total ventral nerve cord cells were determined by double-labeling with juIs76 [unc-25p::GFP] and otIs356 [rab-3p(prom1)::2xNLS::TagRFP] then counting from DD1 or the posterior end of the terminal pharyngeal bulb (if DD1 was absent) to the anus. In wild type, this included most of the 22 DA, DB and DD class motorneurons, plus some cells in the retrovesicular ganglion and tail region.
Neuroanatomy
Cell identity was confirmed by crossing transgenic arrays into previously characterized strains then imaging as above. Table S1 shows the strains used for cell identification.
Statistical analysis
Student t-tests were performed in Microsoft Excel, Mann-Whitney tests were performed in R, and two-tailed Fisher Exact tests were performed using GraphPad QuickCalcs. Graphs were generated in Excel or SAS. RNA-seq significance values reported in the text are not adjusted for false discovery. Benjamini-Hochberg corrections for multiple comparisons are available in the supplemental data. Bonferroni corrections were applied to other data where appropriate.
Data availability
All reagents are available on request. Table S1 shows strains generated in this study. Tables S2 and S3 show lists of significantly down-regulated and up-regulated genes in the cnd-1(ju29) comparative transcriptome. Figure S1 shows a volcano plot summarizing the cnd-1(ju29) comparative transcriptome data. Figure S2 shows DEXseq hits identified in this work along with any expression validations. Figure S3 compares predicted CND-1 wild type and ju29 mutant protein sequences. Figure S4 shows cnd-1p::his-24::mCherry and unc-25p::GFP co-localization. Raw and processed transcriptome files generated in this study are publicly available via the Gene Expression Omnibus (Edgar et al. 2002), accession number GSE125051 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE125051). Supplemental material available at figshare: https://doi.org/10.25387/g3.12567827.
Results
cnd-1 controls the expression of multiple genes During embryogenesis
Previous studies demonstrated that the proneural transcription factor cnd-1 is active early in embryogenesis, with expression first being seen at the 14-cell stage and persisting until just prior to hatching (Hallam et al. 2000; Murray et al. 2012). cnd-1 reporter gene expression decreases rapidly beyond the L1 stage but persists at low levels in adult head and ventral neurons (Kratsios et al. 2011). To gain a better understanding of how CND-1 controls gene expression during early nervous system development, we performed RNA-seq on RNA isolated from three samples each of N2 wild type and cnd-1(ju29) mutant mixed stage embryos and analyzed the data using the DSeq2 package (Love et al. 2014). cnd-1(ju29) is a G-to-A transition in the splice acceptor of intron 2 and behaves as a strong loss-of-function recessive allele (Hallam et al. 2000). cnd-1(ju29) mutant embryos show 105 genes with significantly lower transcript levels (P < 0.05) when compared to wild type (Table S2) and 46 genes with significant higher transcript levels (Table S3, Figure S1). Table 1 shows the top 40 most significant hits sorted by up- vs. down-regulation and p-value. Surprisingly, only a single transcription factor gene, ceh-5, was identified in the down-regulated dataset, whereas three transcription factors genes (nhr-68, nhr-77, and cnd-1 itself) were found in the up-regulated dataset. This suggests that cnd-1 functions close to the end of a transcriptional regulatory cascade during C. elegans embryogenesis. This is in contrast to ngn-1/neurogenin, which controls expression of at least eight downstream transcription factors (Christensen et al. 2020).
Table 1. Top 40 differentially expressed genes in the cnd-1(ju29) RNA-seq dataset based on P-value. (A) Down-regulated genes; (B) up-regulated genes. Gene ID, gene identity; gene name, commonly used gene name/cosmid name; base mean, mean of normalized counts for that gene; log2 fold-change, log2 change in gene expression level when compared to wild type; P-value, significance; P-adj, significance with Benjamini-Hochberg adjustment for false discovery rate.
| A. cnd-1(ju29) transcriptome down-regulated genes (most significant p - value) | |||||
|---|---|---|---|---|---|
| Gene_ID | Gene Name | Base mean | log2 fold change | P-value | P-adj |
| WBGene00018031 | F35B3.4 | 402 | −1.7 | 6.5E-22 | 1.76E-17 |
| WBGene00005832 | srw-85 | 428 | −1.2 | 3.7E-20 | 4.96E-16 |
| WBGene00014955 | Y102A5C.6 | 158 | −1.0 | 2.6E-10 | 1.19E-06 |
| WBGene00010212 | fbxa-192 | 262 | −0.9 | 2.6E-08 | 7.00E-05 |
| WBGene00044213 | Y102A5C.36 | 131 | −0.9 | 1.2E-07 | 2.96E-04 |
| WBGene00014954 | Y102A5C.5 | 66 | −0.9 | 7.1E-09 | 2.40E-05 |
| WBGene00007454 | C08F11.7 | 54 | −0.9 | 3.6E-10 | 1.38E-06 |
| WBGene00015990 | C18H2.3 | 120 | −0.9 | 1.3E-16 | 1.15E-12 |
| WBGene00014454 | MTCE.7 | 1917 | −0.7 | 8.3E-06 | 0.016 |
| WBGene00010958 | ndfl-4 | 70 | −0.7 | 8.4E-06 | 0.016 |
| WBGene00010209 | fbxa-191 | 31 | −0.7 | 1.1E-08 | 3.38E-05 |
| WBGene00007201 | exos-4.1 | 947 | −0.7 | 3.1E-05 | 0.056 |
| WBGene00014472 | MTCE.33 | 2190 | −0.6 | 6.5E-04 | 0.93 |
| WBGene00016953 | C55C3.3 | 65 | −0.5 | 1.1E-03 | 1.00 |
| WBGene00015044 | cyp-34A9 | 82 | −0.5 | 1.0E-03 | 1.00 |
| WBGene00016506 | abhd-5.1 | 95 | −0.5 | 1.9E-03 | 1.00 |
| WBGene00006650 | tts-1 | 1948 | −0.5 | 2.8E-04 | 0.42 |
| WBGene00000754 | col-181 | 39 | −0.5 | 3.1E-03 | 1.00 |
| WBGene00000430 | ceh-5 | 332 | −0.5 | 6.2E-03 | 1.00 |
| WBGene00014672 | C08F11.6 | 27 | −0.4 | 7.8E-04 | 1.00 |
| WBGene00077585 | T01G5.8 | 7 | −0.3 | 5.7E-05 | 0.096 |
| WBGene00202498 | Y60C6A.2 | 35 | −0.3 | 2.0E-03 | 1.00 |
| WBGene00022013 | Y60C6A.1 | 59 | −0.3 | 2.1E-03 | 1.00 |
| WBGene00002013 | hsp-12.6 | 164 | −0.2 | 4.4E-03 | 1.00 |
| WBGene00012790 | Y43D4A.4 | 6 | −0.2 | 1.4E-04 | 0.22 |
| WBGene00015549 | C06G3.3 | 24 | −0.2 | 2.9E-03 | 1.00 |
| WBGene00008396 | D1086.9 | 27 | −0.2 | 7.2E-03 | 1.00 |
| WBGene00017371 | sre-39 | 4 | −0.2 | 2.2E-03 | 1.00 |
| WBGene00020178 | T02H6.8 | 4 | −0.1 | 3.5E-03 | 1.00 |
| WBGene00045311 | Y57G11C.57 | 3 | −0.1 | 5.7E-03 | 1.00 |
| WBGene00044293 | K08D12.7 | 3 | −0.1 | 6.2E-03 | 1.00 |
| WBGene00044390 | ZK177.11 | 3 | −0.1 | 7.0E-03 | 1.00 |
| WBGene00011429 | T04C12.7 | 7 | −0.1 | 7.8E-03 | 1.00 |
| B. cnd-1(ju29) transcriptome up-regulated genes (most significant p - value) | |||||
|---|---|---|---|---|---|
| gene_ID | name | base mean | log2 fold change | P-value | P-adj |
| WBGene00018031 | lgc-34 | 1214 | 1.3 | 4.30E-16 | 2.91E-12 |
| WBGene00005832 | F35B3.3 | 41 | 0.9 | 2.37E-11 | 1.28E-07 |
| WBGene00014955 | ctc-3 | 66934 | 0.7 | 6.72E-06 | 0.015 |
| WBGene00010212 | ZC21.10 | 882 | 0.5 | 0.003 | 1.00 |
| WBGene00044213 | cnd-1 | 958 | 0.5 | 0.004 | 1.00 |
| WBGene00008677 | col-171 | 8 | 0.1 | 0.007 | 1.00 |
| WBGene00004567 | C32E8.4 | 3 | 0.1 | 0.007 | 1.00 |
We also analyzed our data using DEXseq, a variant of the DSeq2 workflow (Anders et al. 2017). This analysis compares data sets by aligning individual sequence blocks (exons, alternative transcriptional start sites, and alternative splice sites) and is a sensitive way to identify splice or transcriptional variants between two datasets. Using this approach, aak-2, srw-85, and ptrn-1 were found to have at least one significantly different transcript block in cnd-1(ju29) mutants when compared to wild type (Figure S2). aak-2 belongs to the AMP-activated protein kinase (AMPK) family and has roles in DAF-2-mediated insulin signaling, lifespan, and temperature-dependent dauer larva formation (Apfeld et al. 2004; Hardie 2014). In cnd-1 mutants, the first exon of an internally transcribed aak-2c variant is expressed at a significantly higher level (P < 0.05), suggesting that CND-1 may repress this internally transcribed variant in wild type animals (Figure S2A).
DEXseq identification of ptrn-1 transcript differences were resolved by visual inspection of the ptrn-1 genomic locus using Integrated Genome Viewer (Robinson et al. 2011; Thorvaldsdottir et al. 2013). This revealed loss of all gene transcription in the 7-8kb region immediately downstream of ptrn-1. Genomic PCR coupled with Sanger sequencing confirmed this to be a novel 6906bp deletion/ 80bp insertion allele that removes most of the ptrn-1 3′ UTR including the poly-adenylation signal, along with three downstream genes (F35B3.1, F35B3.4 and F35B3.10), of which F35B3.1 and F35B3.4 were also differentially expressed in our transcriptome (Figure S2I-L and Table S2). F35B3.10 codes for a predicted snRNA whose transcript was not represented in either wild type or cnd-1(ju29) datasets. ptrn-1 codes for a known neuronal microtubule stabilizing protein (Chuang et al. 2014; Marcette et al. 2014; Richardson et al. 2014), so it is possible that this ptrn-1(ken2) deletion may enhance the cnd-1(ju29) uncoordinated phenotype and be selected for during out-cross. To control for this possibility, we performed validation assays using the cnd-1(gk718) mutation, which is a large deletion allele, predicted to be a null mutant, and was shown by genomic PCR not to contain the ken2 deletion (Figure S2K).
cnd-1 controls ceh-5 expression in a subset of neurons
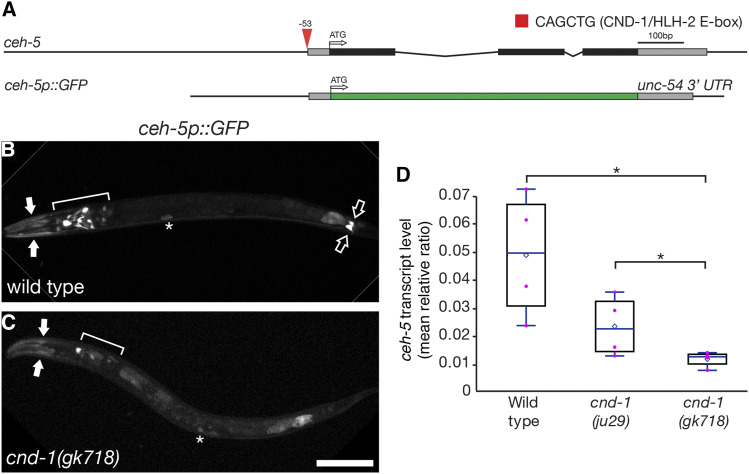
Our comparative transcriptome showed that ceh-5 was significantly down-regulated in cnd-1(ju29) mutants when compared to wild type (Table 1). ceh-5 is the C. elegans ortholog of the mammalian transcription factor ventral anterior homeobox 2 gene (Vax2), which is required for correct dorsoventral patterning of the eye (Take-uchi et al. 2003; Liu et al. 2008; Alfano et al. 2011). A sequence search across the ceh-5 locus found a single candidate cnd-1 CATATG E-box binding site about 50bp 5′ to the ceh-5 translational start site (Figure 1A). To better understand the role of cnd-1 in controlling ceh-5 expression, we used a ceh-5p::GFP reporter gene to compare expression patterns in wild type and cnd-1 mutants (Figure 1, Table 2) (Reece-Hoyes et al. 2007). In wild type L1 larvae, ceh-5p::GFP showed robust expression in head muscles, a subset of head neurons (including the RME neurons), and five or six cells in the tail including the PVQL/R neurons (Figure 1B). Weak ceh-5p::GFP expression was also seen in the coelomocytes (asterisks, Figure 1B) and the pharyngeal terminal bulb. In cnd-1(gk718) mutants, ceh-5p::GFP expression was lost from many head neurons including the RMEs, and also the tail neurons (Figure 1C), but was retained in some head muscles and also the coelomocytes. We used quantitative PCR to further validate ceh-5 transcript levels; these were significantly lower in both cnd-1(ju29) and cnd-1(gk718) RNA samples, with gk718 showing lower ceh-5 transcript levels when compared to ju29, suggesting that CND-1(ju29) may retain some function (Figure 1D). Together, these data indicate that CND-1 is necessary for ceh-5p::GFP expression in a subset of cells including the RME head and PVQ tail neurons, although it is not known if these cells are lost, changing fate, or are merely losing ceh-5 reporter gene expression.
Figure 1.
cnd-1 controls ceh-5 expression in a subset of neurons. (A) Schematic of the ceh-5 genomic region showing predicted CND-1/HLH-2 binding site (Grove et al. 2009), and structure of the ceh-5 reporter gene used in this study. (B, C) ceh-5p::GFP reporter gene expression in wild type (B) and cnd-1(gk718) mutants (C). Filled arrows, head muscles; open arrows, PVQL/R neurons; asterisks, coelomocytes. Bracketed regions show RME plus other head neurons. Scale bar = 25μm. (D) Box and whisker plot showing average quantitative RT-PCR levels of ceh-5 mRNA transcript in wild type, cnd-1(ju29) and cnd-1(gk718) mutants. Open diamond, average; box shows median, first, and third quartiles. Whiskers show data extremes in 1.5 × interquartile range. Data are relative to cdc-42 mRNA. * P < 0.025, Student’s t-test with Bonferroni correction for multiple comparisons.
Table 2. Summary of ceh-5p::GFP expression in L1 larvae. (A) Average number of head neurons, head muscle bundles, and tail neurons observed in wild type and cnd-1(gk718) mutants respectively (+/− standard error of the mean). ** P < 0.01, Mann-Whitney U-test with continuity correction. (B) Percentage of animals showing expression in other tissues. Note that the GFP reporter stain used in this assay was an extra-chromosomal array and showed some expression variability between animals. ** P < 0.01, Fisher Exact test.
| A. ceh-5p::GFP expression in neurons and head muscles | |||
|---|---|---|---|
| Strain | # head neurons | # head muscles | # tail neurons |
| wild type (n = 11) | 12.5 (1.6) | 4.0 (0) | 2.2 (0.3) |
| cnd-1(gk718) (n = 5) | 4.8 (0.8)** | 2.2 (0.7)** | 0.0 (0)** |
| B. ceh-5p::GFP expression in other tissues | |||
|---|---|---|---|
| Strain | pharynx | coelomocytes | gut |
| wild type (n = 11) | 100% | 73% | 100% |
| cnd-1(gk718) (n = 5) | 20%** | 60% | 100% |
CND-1 controls the fate of some cnd-1-expressing cells during embryonic nervous system development
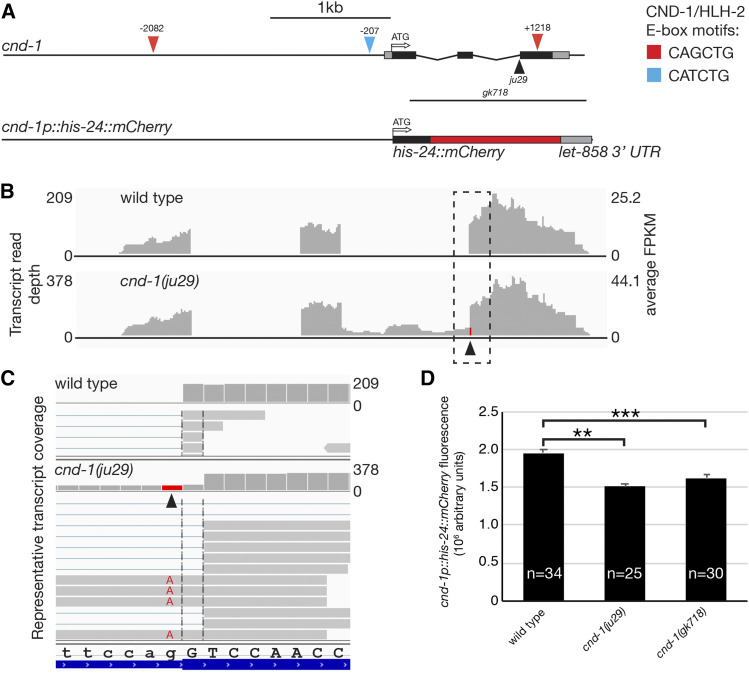
The cnd-1 locus is comprised of three exons spanning a 1.5kb region of chromosome III (Figure 2A). As mentioned previously, the cnd-1(ju29) allele used in our transcriptome analysis is a G-to-A transition in the splice acceptor of intron 2 and was predicted to force a splice onto a non-canonical splice acceptor leading to a frame shift (Hallam et al. 2000). When cnd-1 RNA-seq data were viewed using Integrated Genome Viewer, we confirmed that cnd-1(ju29) transcripts showed the G-to-A transition at the ju29 base change and also a 1bp shift in the splice acceptor (Figure 2B, red column at the start of exon 3; Figure 2C; Figure S3). In addition, around 20% of transcripts show inclusion of intron 2, presumably because the ju29 mutation creates a weak splice acceptor. Figure 2B shows representative read depth across the cnd-1 locus. In terms of raw reads and when normalized to Fragments per Kilobase Million (FPKM), cnd-1 transcript levels are almost twice as high in cnd-1(ju29) compared to wild type, suggesting that CND-1 may be partially responsible for regulating its own transcript levels via transcriptional repression.
Figure 2.
cnd-1(ju29) transcript levels are significantly up-regulated when compared to wild type, although embryonic cnd-1p::mCherry fluorescence is reduced in cnd-1 mutants. (A) Schematic of the cnd-1 genomic region showing location of predicted CND-1/HLH-2 binding sites, and the stIs10055 [cnd-1p::his-24::mCherry] reporter gene used extensively in this study. (B) Integrated Genome Viewer output of wild type and cnd-1(ju29) RNA-seq reads showing raw transcript depth and average Fold Per Kilobase Million coverage. Arrowhead shows location of the ju29 mutation. (C) Inset of boxed region in (B), showing representative reads, the ju29 G-to-A mutation, and the non-canonical 3′ splice acceptor used in the ju29 mutant. (D) Quantitative fluorescence of cnd-1p::his-24::mCherry expression in comma-stage embryos in wild type, cnd-1(ju29) and cnd-1(gk718) mutant embryos. Error bars show standard error of the mean. ** P < 0.005, *** P < 0.0005, Student’s t-test with Bonferroni correction for multiple comparisons.
To further explore these data, we performed quantitative confocal microscopy on wild type and cnd-1 mutant comma stage embryos carrying an integrated cnd-1p::his-24::mCherry transcriptional reporter gene (Murray et al. 2012). The comma stage is easily identified during embryonic development and provides a defined time point to quantitatively compare mCherry expression levels. Contrary to what RNA-seq revealed, we found that cnd-1p::mCherry reporter gene expression was significantly lower in cnd-1 mutants when compared to wild type (Figure 2D, P < 0.005, n = 34, 25, and 30 embryos analyzed for wild type, cnd-1(ju29) and cnd-1(gk718) mutants respectively). The reason for this discrepancy was unclear but may be assay dependent. For instance, inclusion of intron 2 within the cnd-1(ju29) mRNA transcript may increase its representation within the RNA-seq dataset, causing it to appear up-regulated. Alternatively, it may increase the stability of cnd-1(ju29) transcripts, causing an apparent up-regulation of RNA levels.
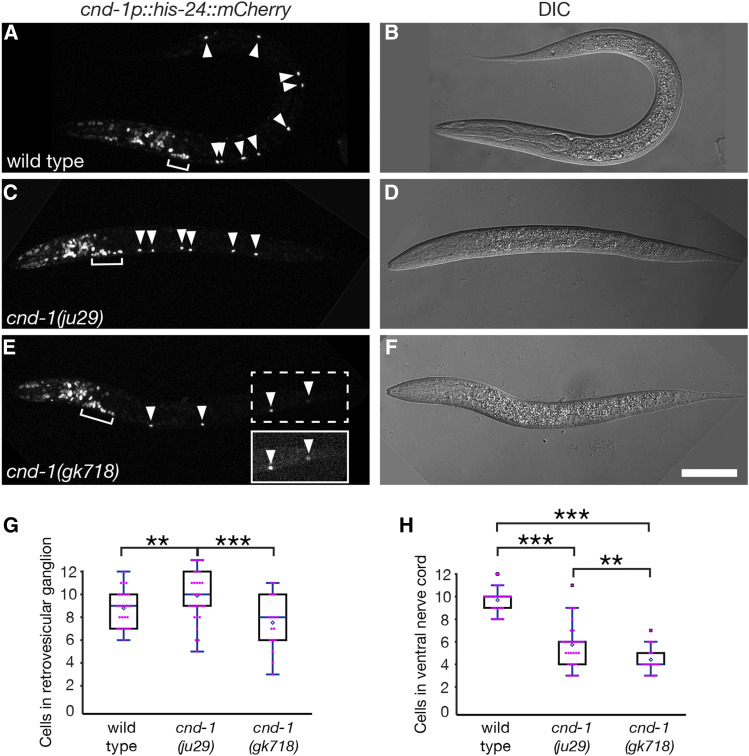
To clarify the role of cnd-1 during nervous system development, we also analyzed cnd-1 reporter gene expression in L1 larvae. Only three types of ventral cord motorneuron (cholinergic DA and DB, and GABAergic DD class) are born during embryogenesis and can be easily assayed in young L1 larvae using genetically encoded reporter genes. Previous data showed that cnd-1(ju29) mutants exhibit variable loss of all three embryonic motor neuron types (Hallam et al. 2000). We examined cnd-1p::mCherry expression in early L1 larvae, within an hour of hatching, counting nuclei in the retrovesicular ganglion and also the ventral nerve cord (Figure 3). The retrovesicular ganglion is a linear cluster of cells located on the ventral midline of the worm immediately posterior to the pharynx, and contains the anterior most DA, DB and DD motorneurons (DA1, DB1, DB2 and DD1), along with eight additional cells. Wild type animals showed an average of nine cnd-1p::mCherry nuclei in the retrovesicular ganglion and 10 in the ventral nerve cord (n = 26). As there are 22 motorneurons in early L1 larvae, this suggests that only a subset express the cnd-1 reporter gene. Based on their location along the ventral nerve cord, co-labeling with unc-25p::GFP (a known DD neuron marker), and by corroborating against single cell RNA-seq expression data (Packer et al. 2019), we tentatively conclude that cnd-1p::mCherry is expressed in DA1-5, DB1, DB3, and DD1-6 (Table 3 and Figure S4). In cnd-1(ju29) mutants, the average number of cnd-1-positive cells in the retrovesicular ganglion increased to 10 (P < 0.005), although the number of cells in the ventral nerve cord dropped dramatically to around six (n = 19, P < 0.001). In cnd-1(gk718) mutants, the number of cnd-1-positive cells dropped to an average of seven in the retrovesicular ganglion and four in the ventral nerve cord (P < 0.0005). When comparing cnd-1p::mCherry expression between the cnd-1(ju29) and cnd-1(gk718) backgrounds, both retrovesicular ganglion and ventral nerve cord cell counts are significantly different (P < 0.0005 for retrovesicular ganglion cells, and P < 0.005 for ventral nerve cord cells). Based on these reporter gene studies, we conclude that CND-1 is required for the fate specification of a subset of embryonic ventral nerve cord neurons, confirming data reported by Hallam et al. (2000). In addition, the above data suggests that the CND-1(ju29) protein retains some activity, or in some contexts behaves in a neomorphic manner to affect the developmental outcome of CND-1-dependent cell fates (Figure S4). For this reason, all remaining analyses were performed using the cnd-1(gk718) allele.
Figure 3.
cnd-1 controls the fate of some cnd-1-expressing cells in the retrovesicular ganglion and ventral nerve cord. (A-F) Confocal and DIC micrographs of cnd-1p::his-24::mCherry expression in (A) wild type, (C) cnd-1(ju29), and (E) cnd-1(gk718) mutants. (B, D, F) DIC images of the above. Arrowheads show cnd-1-positive nuclei in the ventral nerve cord. Bracket shows retrovesicular ganglion. Inset in (E) is contrast-stretched to highlight the two most posterior cnd-1-positive neurons. Scale bar in (F) = 25μm. (G, H) Box and whisker plots showing average number of cnd-1-positive cells in the retrovesicular ganglion and ventral nerve cord respectively. n = 26, 19, and 17 animals for wild type, cnd-1(ju29), and cnd-1(gk718) respectively. Open diamond shows the average; box shows median, first, and third quartiles; whiskers show data extremes in 1.5 × interquartile range with outliers shown beyond. ** P < 0.005; *** P < 0.0005, Mann-Whitney U-test with continuity correction and Bonferroni correction for multiple comparisons.
Table 3. cnd-1 and unc-25 markers co-express in sub-sets of ventral cord motorneurons. N = 21 wild type animals scored. Cells are listed from left-to-right in the anterior-to-posterior order they appear in L1 larvae. *In two animals, we saw an additional cell body (tentatively identified as RIGL) between DB3 and DA2. #Three animals had cnd-1-positive cells at the DB5 location. However, we suspect these are animals where DA4 and DB5 switched position during development.
| Ventral cord neuron | DB2 | DD1 | DB1 | DA1 | DB3 | RIGL* | DA2 | DD2 | DA3 | DB4 | DA4 | DD3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| unc-25p::GFP | 0 | 21 | 0 | 0 | 0 | 0 | 0 | 21 | 0 | 0 | 0 | 21 |
| cnd-1p::mCherry | 0 | 21 | 20 | 21 | 18 | 2 | 21 | 21 | 21 | 0 | 18 | 21 |
| Ventral cord neuron | DB5 | DA5 | DD4 | DB6 | DA6 | DD5 | DB7 | DA7 | DD6 | DA8 | DA9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| unc-25p::GFP | 0 | 0 | 21 | 0 | 0 | 21 | 0 | 0 | 21 | 0 | 0 | |
| cnd-1p::mCherry | 3# | 21 | 21 | 0 | 0 | 21 | 0 | 0 | 21 | 0 | 0 |
cnd-1 and ceh-13 are co-expressed in a subset of ventral cord motorneurons
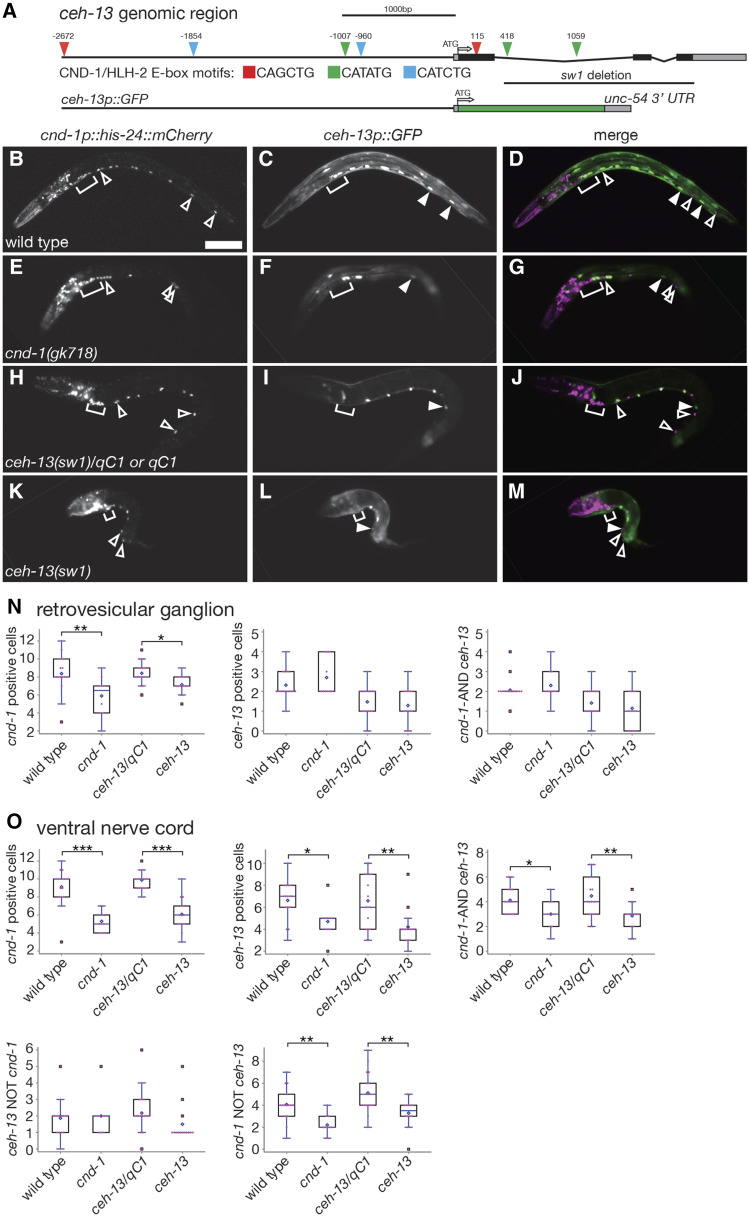
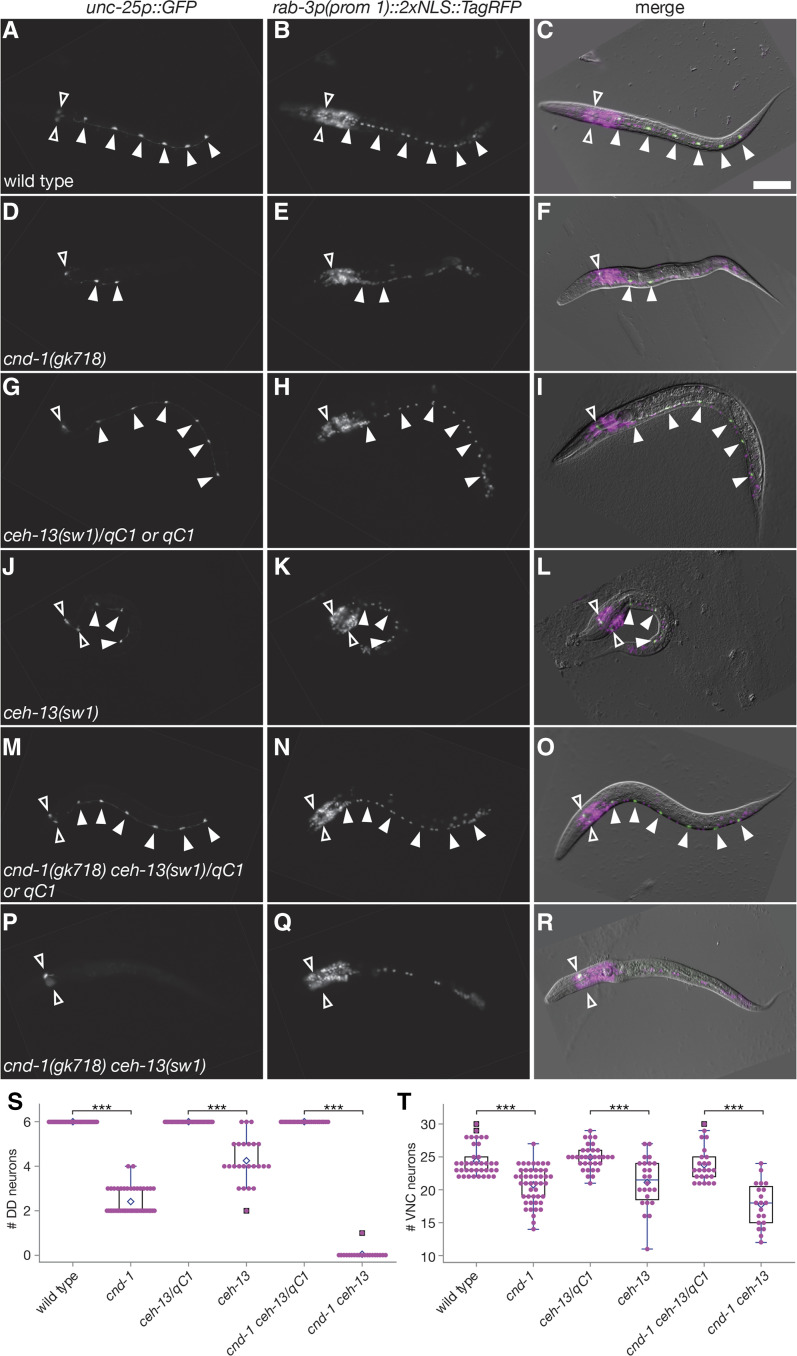
Previous work using RNA-seq analysis of FACS-isolated cnd-1p::mCherry-positive cells showed that Hox gene ceh-13/labial transcripts were enriched in cnd-1-expressing cells when compared to negative controls (Burdick et al. 2016). The ceh-13 locus has multiple consensus CND-1 E-box binding sites (Figure 4A), raising the possibility that cnd-1 may control aspects of ceh-13 transcription. In addition, ceh-13 mutants exhibit loss of ventral cord motorneurons similar to that seen in cnd-1 mutants (Stefanakis et al. 2015). This led us to investigate the relationship between cnd-1 and ceh-13 in controlling embryonic motorneuron cell fate specification. We examined ceh-13p::GFP and cnd-1p::mCherry expression in the retrovesicular ganglion and ventral nerve cords of wild type, cnd-1(gk718), ceh-13(sw1)/qC1 heterozygotes, and ceh-13(sw1) homozygous mutant L1 larvae (Figure 4B-G). ceh-13(sw1) is a 1.5kb deletion of that removes most of intron 1, and all of exons 2 and 3 (Figure 4A) and behaves as a recessive null allele (Brunschwig et al. 1999). ceh-13(sw1) homozygous animals are 97% embryonic lethal, with the remaining 3% of surviving larvae showing strong body morphology defects. We used this phenotype to identify L1 stage sw1 homozygotes to analyze for ceh-13p::GFP and cnd-1p::mCherry expression. In wild type and ceh-13(sw1)/qC1 heterozygotes, cnd-1p::mCherry and ceh-13p::GFP showed a complex and partially overlapping expression pattern in the retrovesicular ganglion and ventral nerve cord, with around two cells co-expressing cnd-1 and ceh-13 in the ganglion and an average of 4.1 (wild type) and 4.7 cells (ceh-13/qC1) co-expressing in the ventral nerve cord (Figure 4B-O, Tables 4 and 5). In cnd-1(gk718) and ceh-13(sw1) homozygotes, the average number of cells co-expressing each marker in the ventral nerve cord dropped significantly to 3.0 and 2.8 for cnd-1 and ceh-13 respectively. Both cnd-1 and ceh-13 homozygous mutants also showed a significant reduction in cells that expressed cnd-1p::mCherry only, but not ceh-13p::GFP. Based on the similarity in phenotypes shown, we conclude that both cnd-1 and ceh-13 have roles in controlling a subset of ventral nerve cord cell fates during embryogenesis. We note that the ganglion and ventral cord cell counts in this double reporter gene assay were slightly different from the data reported in Figure 3. However, the data in Figure 3 was captured on a confocal microscope whereas the data in Figure 4 was captured using epifluorescence, which gives slightly lower resolution and may have led to an undercount of cells that were directly adjacent to or behind each other.
Figure 4.
ceh-13 and cnd-1 show partially overlapping expression in the ventral nerve cord. (A) Schematic of the ceh-13 genomic region showing predicted CND-1/HLH-2 binding sites, location of the ceh-13(sw1) mutation, and structure of the ceh-13 reporter gene used in this study. (B-M) Expression patterns of cnd-1p::his-24::mCherry and ceh-13p::GFP in wild type, cnd-1(gk718), ceh-13(sw1)/qC1 (or qC1), and ceh-13(sw1) mutants. while most VNC cells express both cnd-1 and ceh-13, a distinct subset does not, and multiple cells are absent in both cnd-1 and ceh-13 mutants. Open arrowheads, cnd-1 expressing cells only; filled arrowheads, ceh-13 expressing cells only; bracket, retrovesicular ganglion. Scale bar in (B) = 25μm. (N, O) Box and whisker plots of cnd-1 and ceh-13 reporter genes showing average cell counts in the retrovesicular ganglia and ventral nerve cord respectively. Open diamond shows the average; box shows median, first, and third quartiles; whiskers show data extremes in 1.5 × interquartile range with outliers shown beyond. * P < 0.05; ** P < 0.01; *** P < 0.001, Mann-Whitney U-test with continuity correction.
Table 4. cnd-1 and ceh-13 markers co-express in mid-body DA and DD ventral cord motorneurons. Percentage of cnd-1p::mCherry and ceh-13p::GFP-positive neurons scored each for ventral nerve cord cell assayed. N = 10 wild type animals scored. Cells are listed from left-to-right in the anterior-to-posterior order they appear in L1 larvae. Strains that were highly mosaic for the ceh-13p::GFP reporter gene were omitted from this analysis.
| DA2 | DD2 | DA3 | DA4 | DD3 | DA5 | DD4 | DA6 | DD5 | DA7 | DD6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cnd-1p::mCherry | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 0 | 100 |
| ceh-13p::GFP | 50 | 50 | 80 | 90 | 60 | 100 | 40 | 90 | 0 | 80 | 0 |
Table 5. Summary of cnd-1p::mCherry and ceh-13p::GFP expression in L1 larvae. (A, B) Average number of cells showing reporter gene expression in the retrovesicular ganglion and ventral nerve cord respectively (+/− standard error of the mean). * P < 0.05; ** P < 0.01, *** P < 0.001, Mann-Whitney U-test with continuity correction (cnd-1 compared against wild type and ceh-13 compared against ceh-13/qC1). The ceh-13p::GFP reporter stain used in this assay was an extra-chromosomal array and showed some expression variability between animals.
| A. Cells in retrovesicular gangion | ||||||
|---|---|---|---|---|---|---|
| N | cnd-1p:: mCherry | ceh-13p:: GFP | cnd-1 AND ceh-13 | ceh-13 NOT cnd-1 | cnd-1 NOT ceh-13 | |
| wild type | 16 | 8.4(0.5) | 2.3(0.2) | 2.1(0.2) | — | — |
| cnd-1(gk718) | 10 | 5.9(0.7) ** | 2.7(0.3) | 2.3(0.3) | — | — |
| ceh-13(sw1)/qC1 or qC1 | 17 | 8.5(0.4) | 1.3(0.2) | 1.3(0.2) | — | — |
| ceh-13(sw1) | 14 | 6.9(0.3) * | 1.2(0.3) | 1.0(0.3) | — | — |
| B. Cells in ventral nerve cord | ||||||
| wild type | 16 | 9.1(0.5) | 6.6(0.4) | 4.1(0.3) | 1.9(0.3) | 4.1(0.4) |
| cnd-1(gk718) | 10 | 5.3(0.4) *** | 4.7(0.5) * | 3.0(0.4) * | 2.0(0.4) | 2.2(0.3) ** |
| ceh-13(sw1)/qC1 or qC1 | 17 | 10.0(0.2) | 6.5(0.6) | 4.7(0.4) | 1.4(0.4) | 4.8(0.5) |
| ceh-13(sw1) | 14 | 5.8(0.4) *** | 4.2(0.5) ** | 2.8(0.3) ** | 1.6(0.3) | 3.3(0.4) ** |
cnd-1 and ceh-13 function redundantly to induce DD1 and DD2 motorneuron fate
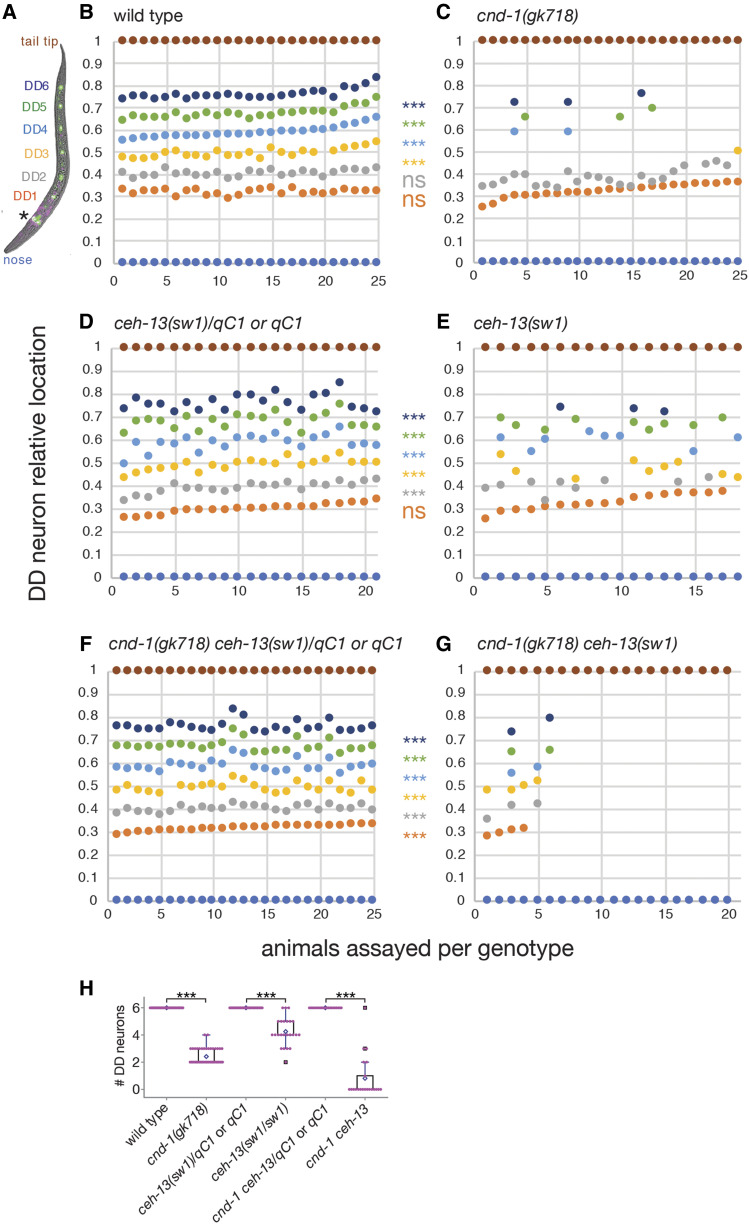
The similarity in cnd-1 and ceh-13 loss-of-function phenotypes and their effect on each other’s reporter gene expression suggest that they may function together, either to cross-regulate each other or to specify ventral nerve cord motor neuron fate. To clarify this, we used an unc-25p::GFP reporter gene (Jin et al. 1999) to examine DD motorneuron fate in cnd-1(gk718) and ceh-13(sw1) single mutants, and cnd-1(gk718) ceh-13(sw1) double mutant L1 larvae (Figure 5). unc-25p::GFP is expressed in the six DD neurons (annotated in color, Figure 5A), in addition to the four RME head neurons (asterisk, Figure 5A). We also plotted the cell body location of each DD neuron relative to the nose and tail tip, to establish which cells were more sensitive to loss of cnd-1 or ceh-13. Wild type animals showed GFP expression in all six DD neurons (Figure 5B, H), in agreement with previous studies (Jin et al. 1999). However, cnd-1(gk718) mutants showed an average of 2.5 DD neurons (Figure 5C, H, n = 25, P < 0.001), with DD1 and DD2 being retained and DD3-6 being lost. L1 larvae of ceh-13(sw1)/qC1 or qC1 genotype (i.e., those with wild type morphology) showed GFP expression in all 6 DD neurons (n = 21 larvae scored). In contrast, ceh-13(sw1) homozygous animals (identified by body morphology defects) showed on average four DD neurons, with DD1 generally being present but with variable loss of DD2-6 (Figure 5E, H, n = 18, P < 0.001). cnd-1 ceh-13/qC1 balanced double mutants again showed a wild type DD neuron induction pattern consistent with both cnd-1 and ceh-13 displaying recessive phenotypes (Figure 5F, H). However, 12/20 cnd-1(gk718) ceh-13(sw1) homozygous double mutants showed no DD neuron fate induction, with the remaining animals showing variable induction of one or more DD neurons (Figure 5G, H, n = 20, P < 0.001). One animal with body morphology defects had six DD neurons. We speculate that this may have been a cnd-1 ceh-13/qC1 heterozygous or qC1 homozygous animal that happened to have a body morphology defect. Overall, this suggests that cnd-1 is primarily required for fate induction of DD3 through DD6, with ceh-13 playing only a minor role in this process. In contrast, cnd-1 and ceh-13 have redundant roles in DD1 and probably DD2 fate induction, with loss of either transcription factor still allowing robust fate specification. Note that distinguishing between DD2 and DD3 neurons was difficult in some animals, so DD2 fate is likely to be under-counted in ceh-13 mutants.
Figure 5.
cnd-1 and ceh-13 function redundantly to control a subset of DD motorneuron cell fates. (A) Representative image of unc-25p::GFP and cnd-1p::his-24::mCherry expression in an L1 larva showing DD neuron color key. Asterisk shows RME head neurons. (B-G) Plots of DD neuron relative location in (B) wild type, (C) cnd-1(gk718), (D) ceh-13(sw1)/qC1, (E) ceh-13(sw1), (F) cnd-1(gk718) ceh-13(sw1)/qC1, and (G) cnd-1(gk718) ceh-13(sw1) L1 larvae. Body morphology defects were used to identify sw1 or gk718 sw1 homozygous animals. *** P < 0.001, Fisher exact test, presence vs. absence of each DD neuron type between control and experimental group (ns = not significant). (H) Box and whisker plots showing average number of DD neurons in the above strains. Open diamond shows the average; box shows median, first, and third quartiles; whiskers show data extremes in 1.5 × interquartile range with outliers shown beyond. *** P < 0.001, Mann-Whitney U-test with continuity correction.
cnd-1 and ceh-13 are redundantly required for setting ventral nerve cord cell number
Our data above indicate that both cnd-1 and ceh-13 have roles in regulating expression of unc-25p::GFP, a reporter gene for GABAergic terminal fate specification of DD motorneurons. However, loss of unc-25p::GFP expression does not necessarily mean loss of neural fate induction. For instance, DA2-DA5 cholinergic motorneurons share the same great-grandmother cell that gives rise to the DD neurons, raising the possibility that DD cells might switch fates in cnd-1 or ceh-13 mutants (Sulston et al. 1983). To address this possibility, we examined DD fate induction using unc-25p::GFP in the presence of pan-neuronal terminal fate marker rab-3p(prom1)::2xNLS::TagRFP (Stefanakis et al. 2015). Wild type animals (n = 37) showed an average of 24 ventral nerve cord cells, of which six were unc-25p::GFP-positive consistent with our previous data (Figure 6A - C). However, in cnd-1(gk718) mutants (n = 46), only 20 ventral cord cells were observed (Figure 6D - F, P < 0.001 compared to wild type). In addition, they showed a parallel loss of around four DD cells similar to Figure 5C and 5H. ceh-13(sw1) homozygous mutants (n = 24) showed similar phenotypes, with 21 ventral cord cells counted compared to 25 in sw1/qC1 balanced heterozygotes (n = 33) (Figure 6G - L, P < 0.001). Finally, cnd-1(gk718) ceh-13(sw1) double homozygous mutants (n = 20) showed around 18 cells in the ventral cord when compared to an average of 24 cells in gk718 sw1/qC1 balanced heterozygotes (n = 24) (Figure 6M - R). Figure 6S and 6T summarize average DD neuron and ventral cord motorneuron counts for the above assays. Overall, the difference in ventral cord neuron count parallels the loss of DD neurons observed in cnd-1 and ceh-13 mutant backgrounds and argues against a change of DD neuron fate to another neuronal cell type.
Figure 6.
cnd-1 and ceh-13 control the birth of DD motorneurons but have no obvious role in DA or DB motorneuron birth. (A-R) Representative images of DD neurons (identified by unc-25p::GFP expression) and all ventral cord motorneurons (identified by rab-3p(prom 1)::2xNLS::TagRFP expression) in (A-C) wild type, (D-F) cnd-1(gk718), (G-I) ceh-13(sw1)/qC1, (J-L) ceh-13(sw1), (M-O) cnd-1(gk718) ceh-13(sw1)/qC1, and (P-R) cnd-1(gk718) ceh-13(sw1) L1 larvae. Body morphology defects were used to identify sw1 or gk718 sw1 homozygous animals. Filled arrowheads show DD motor neurons; open arrowheads show RME head neurons, which also express the unc-25p::GFP reporter. Scale bar in (C) = 25μm. (S, T) Box and whisker plots showing average number of (S) DD neurons and (T) all ventral cord motorneurons in the above strains. Open diamond shows the average; box shows median, first, and third quartiles; whiskers show data extremes in 1.5 × interquartile range with outliers shown beyond. *** P < 0.001, Mann-Whitney U-test with continuity correction.
Discussion
The role of cnd-1 as a proneural transcription factor
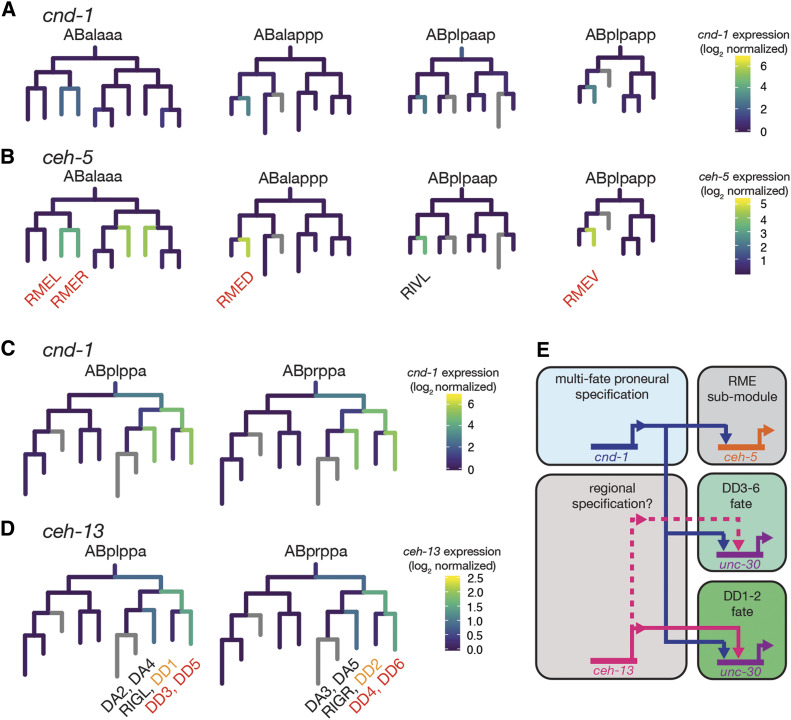
Our comparative transcriptome data expand on cnd-1’s role as a proneural transcription factor, identifying the homeobox gene ceh-5 as a novel downstream target of cnd-1 (Figure 1). We find that cnd-1 controls ceh-5 gene expression in the RME neurons, suggesting that ceh-5 may function as a terminal selector transcription factor in this cell type. Analysis of publicly available single-cell RNA-seq data allows us to contextualize the relationship between cnd-1 and ceh-5 (Packer et al. 2019). Figure 7 shows sub-lineages of single-cell RNA-seq data visualized using the Viscello package. Onset of cnd-1 expression in the RME parent cell lineages (Figure 7A) occurs at the same time as onset of ceh-5 expression (Figure 7B). However, our ceh-5p::GFP reporter gene data suggests that cnd-1 controls ceh-5 expression in the RME neurons. It may be that the single-cell RNA-seq data lacks the temporal resolution to define when one transcription factor is transcribed relative to another. Alternatively, cnd-1 and ceh-5 may function collaboratively to maintain ceh-5 expression, for instance in other head neuron and muscle cell types, where we see ceh-5p::GFP expression drop but is not eliminated in cnd-1 mutants. It should be noted that some aspects of RME neuron fate appear to be preserved in cnd-1 mutants as they continue to express unc-25p::GFP (a known RME marker gene), even when unc-25p::GFP is lost in posterior DD neurons (Figure 5 and 6). This suggests that ceh-5 may control a sub-module of RME terminal fate but not the actual fate of the neuron itself. The co-expression of cnd-1 and ceh-13 in terminal fate cells such as the RMEs may be predictive of cnd-1’s ability to control ceh-5 expression in other neurons. Viscello data shows that these two transcription factors are co-expressed in RIVL/R, FLPL/R and PVQL/R neurons (Packer et al. 2019). Our data shows that cnd-1(gk718) mutants lose ceh-5p::GFP expression in PVQ neurons, supporting this hypothesis. Overall, our data adds to previously published work, placing cnd-1 as a proneural transcription factor upstream of ceh-5, unc-3, unc-4, and unc-47, to control aspects of RME, PVQ, DA, DB, and DD neuron fate respectively (Miller et al. 1992; Jin et al. 1994; Prasad et al. 1998; Kratsios et al. 2011).
Figure 7.
Summary of cnd-1’s role in controlling a ceh-5-dependent RME sub-module and DD motorneuron fate. (A - D) Single-cell RNA-seq expression lineages of (A, C) cnd-1, (B) ceh-5, and (D) ceh-13 expression. Data derived from Packer et al. (2019) and visualized using the Viscello data display tool (https://cello.shinyapps.io/celegans/). Co-expression of cnd-1 and ceh-5 transcripts occurs in all RME-class neurons. We find that ceh-5p::GFP expression in RME neurons is lost in cnd-1(gk718) mutants suggesting that cnd-1 is responsible for driving ceh-5 expression in these cells. Based on the above expression overlap, we predict that cnd-1 also controls ceh-5p::GFP expression in RIVL/R. Note that ceh-5 does not control RME neuron fate, because those cells can still be visualized using an unc-25p::GFP reporter gene. Similarly, cnd-1 and ceh-13 are co-expressed in DD3-6 motorneurons but not in DD1 and DD2. unc-25p::GFP expression in DD3-6 is primarily controlled by cnd-1, with a weak contribution from ceh-13. However, unc-25p::GFP expression in DD1 and DD2 is redundantly controlled by both cnd-1 and ceh-13. An alternative interpretation is that both cnd-1 and ceh-13 are required for successful induction of ABpl/rppapp fates. Loss of these genes may mean that this cell division is lost, leading to a default anterior fate that permits aspects of DA2-6 to be specified normally but leads to loss of all DD neurons. (E) model summarizing cnd-1, ceh-5, and ceh-13 function in the control of RME sub-module transcription and DD neuron fate specification.
CND-1 regulation of cnd-1-expressing cells
Our RNA-seq data shows that cnd-1 transcription appears to be up-regulated in cnd-1(ju29) animals (Table 1B, Figure 2B and C). While there is evidence of intron inclusion, it does not appear to be sufficient to explain the almost twofold increase in cnd-1(ju29) transcript levels. We postulate that the intron inclusion may positively affect transcript stability, leading to higher levels of transcript for longer. We used two separate reporter gene assays to further explore the role of cnd-1 in nervous system development. First, quantitative imaging of cnd-1p::his24::mCherry in comma-stage embryos shows reduced cnd-1 expression in the two cnd-1 mutant alleles examined. Second, cnd-1p::his24::mCherry and unc-25p::GFP assays in ventral cord motorneurons reveals significantly lower cell counts in cnd-1 mutants, with cnd-1(ju29) mutants displaying strong DD neuron induction defects, although not as strong as those seen in cnd-1(gk718) mutants. This indicates that cnd-1 is required for the fate specification of cells that normally express cnd-1. Whether this is via a self-regulatory mechanism is not known.
CND-1 functions redundantly with CEH-13 to specify DD1 and DD2 cell fate
Our data corroborate previous work showing that loss-of-function in the Hox gene ceh-13/labial leads to loss of ventral cord motorneurons in a manner similar to that seen in cnd-1 mutants (Hallam et al. 2000; Stefanakis et al. 2015). While ceh-13 is not significantly different in our whole embryo RNA-seq dataset, a previous RNA-seq study, using Fluorescence-Activated Cell Sorting to enrich for cnd-1-labeled embryonic cells, revealed that ceh-13 transcripts are up-regulated in that tissue relative to background (Burdick et al. 2016). This led us to investigate the genetic interaction between these two highly conserved transcription factors with regard to DD neuron fate specification (Figures 5 and 6). While both genes show similar loss-of-function DD neuron phenotypes, there are subtle differences. In cnd-1(gk718) mutants, cell fate specification of DD1 and DD2 ventral motorneurons appears normal, whereas DD3-6 are generally eliminated (Figure 5B and C). However, ceh-13(sw1) mutants show a weaker, variable loss of DD3-6, but with robust induction of DD1 and perhaps DD2. Analysis of cnd-1(gk718) ceh-13(sw1) double mutants reveals a striking synergy with almost complete loss of all DD neurons (Figure 5F and G). This is not due to a change of cell fate, as analysis of all ventral nerve cord cells shows a corresponding cell count difference that mirrors the loss of DD neurons (Figure 6). Based on total ventral cord cell counts, we tentatively conclude that DA and DB motorneurons are not obviously affected by loss of cnd-1 and/or ceh-13. This is in contrast to previously published work showing less cholinergic DA and DB ventral cord neurons when labeled by acr-2p::YFP (Hallam et al. 2000). One possible explanation for this discrepancy may be the transgenes used to label the cells. Hallam et al. (2000) used acr-2p::YFP and unc-25p::GFP (which are driven from a cholinergic receptor and glutamate decarboxylase promoter sequences respectively) to label all embryonic motorneurons and reported that some cnd-1(ju29) ventral cord cells lacked expression of both reporters. Despite this, those cells were apparently present, based on the stereotyped location of cell bodies and nuclei identified using Differential Interference Contrast microscopy. The reporter gene used in our assay was driven from a rab-3 promoter element that was previously shown to label all neurons apart from the CAN associated neurons (Stefanakis et al. 2015). It may be that all DA and DB type neurons are born in cnd-1 and/or ceh-13 mutants but lack a complete battery of terminal selectors leading to apparent loss of identity, depending on the reporter gene used to label that cell.
Figure 7C and D shows the relationship between cnd-1 and ceh-13 transcript expression as identified via single-cell RNA-seq (Packer et al. 2019). Similar to the relationship between cnd-1 and ceh-5, there does not appear to be any temporal sequence in their expression. Intriguingly, this visualization shows loss of cnd-1 and ceh-13 expression in the DD1 and DD2 mother cells (ABplppappa and ABprppappa respectively). We speculate that this renders DD1 and DD2 resistant to changes in either cnd-1 or ceh-13 expression, such that both cells are correctly specified in either single mutant background. Perhaps loss of both cnd-1 and ceh-13 promotes premature cell cycle exit in DD mother cells (as postulated by Hallam et al. 2000), preventing any DD neurons (and presumably RIGL and RIGR) from being born. While the DA2-5 mother cells express cnd-1 and ceh-13, their grandparents (ABplppapa and ABprppapa) only express cnd-1 (and at a lower level than the posterior daughter). This raises the possibility that this anterior branch of the lineage is less sensitive to these transcription factors and may give rise to a default set of cell fates, which means that DA2-5 are born whereas DD1-6 are lost. Figure 7E summarizes our analysis on the genetic interactions between cnd-1 and ceh-13 and the sub-fate terminal selector transcription factors ceh-5 in RME and unc-30 in DD neurons respectively. unc-30 was previously reported to control the GABAergic neurotransmission module of DD and RME neurons (Eastman et al. 1999). While our RNA-seq assay does not reveal significant changes in unc-30 expression, it may have been below the threshold for statistical significance.
Acknowledgments
This work is dedicated to the memory of John Salerno, a great colleague and mentor, who passed away on December 25th, 2015. We thank Brian Ackley for providing feedback on this work prior to publication. We thank Ian Hope for the ceh-5p::GFP reporter strain and Parmida Jamshidi for building the ceh-13(sw1)/qC1; cnd-1p::mCherry strain. We also thank Yishi Jin for the pCZGY32 promoterless Gateway-GFP plasmid. We are grateful to Erik Lundquist and Jenny Hackett at the University of Kansas Genome Sequencing Core (GSC) for sequencing library construction, Illumina sequencing, and advice on RNA handling. KU GSC is supported by the NIH National Institute of General Medical Sciences under award number P20GM103638. The cnd-1(gk718) mutant was provided by the C. elegans Reverse Genetics Core Facility at the University of British Columbia, which is part of the international C. elegans Gene Knockout Consortium. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). MLH is funded by NIH grant 1R15NS100632-01, and from grants from Kennesaw State University’s Office of the Vice President for Research. WAN was funded by NIH grant R25GM111565 (PI Jonathan McMurry). Instrumentation used in this work was funded by NSF Major Research Instrument grants DBI-1624654, DBI-1337791, and DBI-1229237.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25387/g3.12567827.
Communicating editor: J. Kim
These authors contributed equally to this work.
Present Addresses: Molecular Biosciences Graduate Program, University of Kansas, Lawrence, KS 66045
Graduate Program in Genetics and Genomics, Duke University Medical Center, Durham, NC 27710
Biomedical Sciences Graduate Program, University of Connecticut Health Sciences Center, Farmington, CT 06030
Development, Disease Models & Therapeutics Graduate Program, Baylor College of Medicine, Houston, TX 77030
Medical Program, Trinity School of Medicine, VC0100, St. Vincent
Literature Cited
- Alfano G., Conte I., Caramico T., Avellino R., Arnò B. et al. , 2011. Vax2 regulates retinoic acid distribution and cone opsin expression in the vertebrate eye. Development 138: 261–271. 10.1242/dev.051037 [DOI] [PubMed] [Google Scholar]
- Anders S., Reyes A., and Huber W., 2017. Detecting differential usage of exons from RNA-seq data. Genome Res. 22: 2008–2017. 10.1101/gr.133744.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S., 2010. FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Apfeld J., Connor G. O., Mcdonagh T., Distefano P. S., and Curtis R., 2004. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 18: 3004–3009. 10.1101/gad.1255404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. E., and Brown N. L., 2018. All in the family: proneural bHLH genes and neuronal diversity. Development 145: 1–9. 10.1242/dev.159426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. N., Kollu R., and Banerjee-Basu S., 2009. AutDB: a gene reference resource for autism research. Nucleic Acids Res. 37: D832–D836. 10.1093/nar/gkn835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunschwig K, Wittmann C., Schnabel R., Bürglin T. R., Tobler H., et al. , 1999. Anterior organization of the Caenorhabditis elegans embryo by the labial-like Hox gene ceh-13. Development 126: 1537–1546. [DOI] [PubMed] [Google Scholar]
- Burdick J., Walton T., Preston E., Zacharias A., Raj A. et al. , 2016. Overlapping cell population expression profiling and regulatory inference in C. elegans. BMC Genomics 17: 159 10.1186/s12864-016-2482-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. elegans Deletion Mutant Consortium 2012. Large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 (Bethesda) 2: 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm A. D., Hutter H., Jin Y., and Wadsworth W. G., 2016. The Genetics of Axon Guidance and Axon Regeneration in Caenorhabditis elegans. WormBook 204: 849–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E. L., Beasley A., Radchuk J., Mielko Z. E., Preston E. et al. , 2020. ngn-1/neurogenin activates transcription of multiple terminal selector transcription factors in the Caenorhabditis elegans nervous system. 2020. G3 (Bethesda) 10: 1949–1962. 10.1534/g3.120.401126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang M., Goncharov A., Wang S., Oegema K., Jin Y. et al. , 2014. The microtubule minus-end-binding protein patronin/ PTRN-1 is required for axon regeneration in C. elegans. Cell Rep. 9: 874–883. 10.1016/j.celrep.2014.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapier C. R., and Cairns B. R., 2009. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78: 273–304. 10.1146/annurev.biochem.77.062706.153223 [DOI] [PubMed] [Google Scholar]
- D’Amico L. A., Boujard D., and Coumailleau P., 2013. The neurogenic factor NeuroD1 is expressed in post-mitotic cells during juvenile and adult xenopus neurogenesis and not in progenitor or radial glial cells. PLoS One 8: e66487 10.1371/journal.pone.0066487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman C., Horvitz H. R., and Jin Y., 1999. Coordinated Transcriptional Regulation of the unc-25 Glutamic Acid Decarboxylase and the unc-47 GABA Vesicular Transporter by the Caenorhabditis elegans UNC-30 Homeodomain Protein. J. Neurosci. 19: 6225–6234. 10.1523/JNEUROSCI.19-15-06225.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., and Lash A. E., 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30: 207–210. 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove C. A., De Masi F., Barrasa M. I., Newburger D. E., Alkema M. J. et al. , 2009. A multiparameter network reveals extensive divergence between C. elegans bHLH transcription factors. Cell 138: 314–327. 10.1016/j.cell.2009.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam S., Singer E., Waring D., and Jin Y., 2000. The C. elegans NeuroD homolog cnd-1 functions in multiple aspects of motor neuron fate specification. Development 127: 4239–4252. [DOI] [PubMed] [Google Scholar]
- Hardie D. G., 2014. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J. Intern. Med. 276: 543–559. 10.1111/joim.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., 2016. Terminal Selectors of Neuronal Identity. Curr. Top. Dev. Biol. 116: 455–475. 10.1016/bs.ctdb.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Hudson M. L., Kinnunen T., Cinar H. N., and Chisholm A. D., 2006. C. elegans Kallmann syndrome protein KAL-1 interacts with syndecan and glypican to regulate neuronal cell migrations. Dev. Biol. 294: 352–365. 10.1016/j.ydbio.2006.02.036 [DOI] [PubMed] [Google Scholar]
- Jin Y., Hoskins R., and Horvitz H. R., 1994. Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature 372: 780–783. 10.1038/372780a0 [DOI] [PubMed] [Google Scholar]
- Jin Y., Jorgensen E., Hartwieg E., and Horvitz H. R., 1999. The C. elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19: 539–548. 10.1523/JNEUROSCI.19-02-00539.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., and Qi Y. B., 2018. Building stereotypic connectivity: mechanistic insights into structural plasticity from C. elegans. Curr. Opin. Neurobiol. 48: 97–105. 10.1016/j.conb.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Langmead B., and Salzberg S. L., 2015. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12: 357–360. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratsios P., Stolfi A., Levine M., and Hobert O., 2012. Coordinated regulation of cholinergic motor neuron traits through a conserved terminal selector gene. Nat. Neurosci. 15: 205–214. 10.1038/nn.2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E., 1997. Basic helix-loop-helix genes in neural development. Curr. Opin. Neurobiol. 7: 13–20. 10.1016/S0959-4388(97)80115-8 [DOI] [PubMed] [Google Scholar]
- Lee, J. E., S. M. Hollenberg, L. Snider, D. L. Turner, N. Lipnick et al, 1995. Conversion of xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 268: 836–844. 10.1126/science.7754368 [DOI] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J. et al. , 2009. The Sequence Alignment / Map format and SAMtools. Bioinformatics 25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Liu Y., Liu Y., Lupo G., Lan L. et al. , 2008. A Role for Xvax2 in Controlling Proliferation of Xenopus Ventral Eye and Brain Progenitors. Dev. Dyn. 237: 3387–3393. 10.1002/dvdy.21763 [DOI] [PubMed] [Google Scholar]
- Love M. I., Huber W., and Anders S., 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15: 1–21. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcette J. D., Chen J. J., and Nonet M. L., 2014. The Caenorhabditis elegans microtubule minus-end binding homolog PTRN-1 stabilizes synapses and neurites. eLife 2014: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M. I., Shen M. M., Shamu C. E., Bürglin T. R., Ruvkun G. et al. , 1992. C. elegans unc-4 Gene Encodes a Homeodomain Protein That Determines the Pattern of Synaptic Input to Specific Motor Neurons. Nature 355: 841–845. 10.1038/355841a0 [DOI] [PubMed] [Google Scholar]
- Miyata T., Maeda T., and Lee J. E., 1999. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 13: 1647–1652. 10.1101/gad.13.13.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. I., Bao Z., Boyle T. J., Boeck M. E., Barbara L. et al. , 2008. Automated analysis of embryonic gene expression with cellular resolution in C. elegans. Nat. Methods 5: 703–709. 10.1038/nmeth.1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. I., Boyle T. J., Preston E., Vafeados D., Mericle B. et al. , 2012. Multidimensional regulation of gene expression in the C. elegans embryo. Genome Res. 22: 1282–1294. 10.1101/gr.131920.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer J. S., Zhu Q., Huynh C., Sivaramakrishnan P., Preston E. et al. , 2019. A lineage-resolved molecular atlas of C. elegans embryogenesis at single-cell resolution. Science 365 10.1126/science.aax1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataskar A., Jung J., Smialowski P., Noack F., Calegari F. et al. , 2016. NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. EMBO J. 35: 24–45. 10.15252/embj.201591206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M., Kim D., Pertea G. M., Leek J. T., and Salzberg S. L., 2016. Transcript-level expression analysis of RNA- seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 11: 1650–1667. 10.1038/nprot.2016.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. C., Ye B., Zackhary R., Schrader K., Seydoux G. et al. , 1998. unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O / E family of transcription factors. Development 125: 1561–1568. [DOI] [PubMed] [Google Scholar]
- Reece-Hoyes J. S., Shingles J., Dupuy D., Grove C. A., Walhout A. J. M. et al. , 2007. Insight into transcription factor gene duplication from Caenorhabditis elegans Promoterome-driven expression patterns. BMC Genomics 8: 27 10.1186/1471-2164-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. E., Spilker K. A., Cueva J. G., Perrino J., Goodman M. B. et al. , 2014. PTRN-1, a microtubule minus end-binding CAMSAP homolog, promotes microtubule function in Caenorhabditis elegans neurons. eLife 2014: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421–427. 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. T., Thorvaldsdottir H., Winckler W., Guttman M., Lander E. S. et al. , 2011. Integrative Genomics Viewer. Nat. Biotechnol. 29: 24–26. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Cabezas O., Minton J. A., Kantor I., Williams D., Ellard S. et al. , 2010. Homozygous mutations in NEUROD1 are responsible for a novel syndrome of permanent neonatal diabetes and neurological abnormalities. Diabetes 59: 2326–2331. 10.2337/db10-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Pietzsch T. et al. , 2012. Fiji - an Open Source platform for biological image analysis. Nat. Methods 9: 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. D., and Gumienny T. L., 2012. Visualization of Caenorhabditis elegans Cuticular Structures Using the Lipophilic Vital Dye DiI. J. Vis. Exp. 59: e3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S., Lim J., Yellajoshyula D., Chang L., and Kroll K. L., 2007. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 26: 5093–5108. 10.1038/sj.emboj.7601923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanakis N., Carrera I., and Hobert O., 2015. Regulatory Logic of Pan-Neuronal Gene Expression in C. elegans. Neuron 87: 733–750. 10.1016/j.neuron.2015.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., and Horvitz H. R., 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. 10.1016/0012-1606(77)90158-0 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., and Thomson J. N., 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119. 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- Take-uchi M., Clarke J. D. W., and Wilson S. W., 2003. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development 130: 955–968. 10.1242/dev.00305 [DOI] [PubMed] [Google Scholar]
- Thorvaldsdottir H., Robinson J., and Mesirov J. P., 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14: 178–192. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington C. H., 1957. The Strategy of the Genes. A Discussion of Some Aspects of Theoretical Biology, Allen & Unwin, London. [Google Scholar]
- Wang L., and Baker N. E., 2015. E proteins and ID proteins: Helix-loop-helix partners in development and disease. Dev. Cell 35: 269–280. 10.1016/j.devcel.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All reagents are available on request. Table S1 shows strains generated in this study. Tables S2 and S3 show lists of significantly down-regulated and up-regulated genes in the cnd-1(ju29) comparative transcriptome. Figure S1 shows a volcano plot summarizing the cnd-1(ju29) comparative transcriptome data. Figure S2 shows DEXseq hits identified in this work along with any expression validations. Figure S3 compares predicted CND-1 wild type and ju29 mutant protein sequences. Figure S4 shows cnd-1p::his-24::mCherry and unc-25p::GFP co-localization. Raw and processed transcriptome files generated in this study are publicly available via the Gene Expression Omnibus (Edgar et al. 2002), accession number GSE125051 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE125051). Supplemental material available at figshare: https://doi.org/10.25387/g3.12567827.