Abstract
As of now, therapeutic strategies for the novel coronavirus (SARS-CoV-2) are limited and much focus has been placed on social distancing techniques to “flatten the curve”. Initial treatment efforts including ventilation and hydroxychloroquine garnered significant controversy and today, SARS-CoV-2 outbreaks are still occurring throughout the world. Needless to say, new therapeutic strategies are needed to combat this unprecedented pandemic. Nature Reviews Immunology recently published an article hypothesizing the pathogenesis of TAM (Tyro3, Axl, and Mer) receptor signaling in COVID-19. In it they expressed that hypercoagulation and immune hyper-reaction could occur secondary to decreased Protein S (PROS1). And hypoxia has been recently discovered to significantly decrease expression of PROS1. Regarding the cause of hypoxia in COVID-19; NIH funded research utilizing state-of-the-art topologies has recently demonstrated significant metabolomic, proteomic, and lipidomic structural aberrations in hemoglobin (Hb) secondary to infection with SARS-CoV-2. In this setting, Hb may be incapacitated and unable to respond to environmental variations, compromising RBCs and oxygen delivery to tissues. The use of red blood cell exchange would target hypoxia at its source; representing a Gemini of therapeutic opportunities.
Keywords: RBC exchange, COVID-19, Gemini, Therapeutic opportunity
Introduction
It has been 102 years since the outbreak of the 1918 Spanish flu; today, we face the ever-evolving lexicon of the coronavirus pandemic [1]. The outbreak of novel coronavirus (SARS-CoV-2) began in Wuhan, China and has spread rapidly throughout the world [2]. Leaving health, economic, environmental and social consequences for the entire world [3]. Currently, treatment strategies are lacking and the use of ventilation therapy in COVID-19 also faces criticism [4]. For hydroxychloroquine, studies showing therapeutic efficacy are also lacking, while side effects from usage still occur [5]. Today, social distancing tactics as well as wide spread mask usage have been crucial for “flattening the curve” [6]. However, outbreaks are still occurring throughout the world and novel therapeutic strategies are needed to combat this unprecedented virus.
Hypothesis
I hypothesize that Red Blood Cell (RBC) exchange could be used for the successful treatment of COVID-19. This hypothesis was sparked by a recent publication illustrating the effects of SARS-CoV-2 on the protenomic and lipid structure of hemoglobin (Hb). If such is the case for Hb, these patients would be unable to bind oxygen and ventilation may not be the answer to treating hypoxia in this setting. And perhaps this is why some COVID-19 patients are responding to High-flow oxygen [7]. This may also be the reason behind the COVID-19 clinical picture, reminiscent of methemoglobinemia, with very low oxygen saturations (SpO2). This is seen in methemoglobinemia, where methodologic problems consistently hinder oximetry and elevated methemoglobin results in the underestimation of SpO2. For methemoglobinemia, RBC exchange has actually been demonstrated to be superior to methylene blue for therapeutic treatment [8], [9].
In addition, hypoxia has been most recently discovered to affect the production of Protein S (PROS1) [10]. And academics are beginning to believe that hypercoagulability and immune hyper-reaction in COVID-19 is mechanistically linked to Protein S [11].
Hemoglobin structural protein and membrane lipid induced hypoxia:
COVID-19 disease is most commonly associated with shortness of breath, dry cough and fever [12]. Some experience symptoms most reminiscent of hypoxia and now, red blood cells (RBCs) have been found altered in patients infected with SARS-CoV-2. One of the most significant studies utilized state-of-the-art topologies to investigate the metabolomic, proteomic, and lipidomic effects of COVID-19 on RBCs [13].
In this COVID-19 study, RBCs were collected from 23 healthy patients and 29 molecularly-diagnosed COVID-19 patients [13]. Differences in RBCs from COVID-19 patients were observed; increased levels of glycolytic intermediates resulted in oxidation and fragmentation of ankyrin, spectrin beta, in addition to the N-terminal cytosolic domain of band 3 (AE1) [13]. Aberrant lipid metabolism was also observed in the short and medium chain saturated fatty acids, acyl-carnitines and sphingolipids [13].
Similar to other studies, they did not notice as many significant aberrations in clinical hematological parameters including: RBC count, hematocrit, and mean corpuscular hemoglobin concentration [13].
It has been long understood that enhancing the release of membrane-bound glycolytic enzymes to the cytosol, results in the induction of glycolysis which shifts to the production of 2,3-bisphosphoglycerate (2,3-BPG) facilitating O2 release [14]. Although this would improve the capacity of hemoglobin to off-load oxygen, it would result in difficulty for Hb to bind oxygen in the lungs. At the same time, as the N-terminus of AE1 is also affected and is responsible for stabilizing deoxyhemoglobin for finely tunes oxygen off-loading [13]. In this setting, RBCs affected by SARS-CoV-2 may be incapacitated and unable to respond to environmental variations in Hb oxygen saturation, resulting in an inability to transport and deliver oxygen to tissues. This undoubtably would result in severe hypoxia despite normal hematological parameters, such as those seen in SARS-CoV-2 [15].
Hypoxia, thrombosis and the role of protein S:
It has been long understood that hypoxia can cause thrombosis [16], [17]. Hypoxia is known to be caused by many factors including: high altitude, smoking, lung failure and sickle cell anemia [10], [18], [19], Interesting, per se however, is that hypoxia has more recently been found to decrease the body’s natural anticoagulant - Protein S, with an increased risk for blood clotting from thrombosis [10]. This is why deficiencies of protein S occur in diseases such as sickle cell anemia and at a high altitude. This suggests that hypoxia and PROS1 deficiency, in turn, elevate one’s thrombotic risk.
More specifically, hypoxia drives the dimeric transcription factor hypoxia inducible factor 1 (HIF1), something expressed constitutively in many tissues. O2 deficiency inhibits HIF1α destruction; thereby stabilizing HIF1α [20]. An analysis in one study revealed that hypoxia accelerated HIF1α transcription - from 100% to 340% [10]. At the same time, decreasing O2 from 20% to 1%, in turn, decreased PROS1 transcription from 100% to 20% [10].
This decrease in PROS1 in the setting of hypoxia could be responsible for significant disease related thrombosis now termed, Coronavirus-associated coagulopathy (CAC), by the International Society on Thrombosis and Haemostasis [21], [22]. Most interestingly, is that in a Spanish series of non-ICU hospitalized COVID-19 patients, pulmonary emboli were found in the absence of deep–vein thrombosis [23]. A finding which suggests the presence of small-vessel thrombosis in the setting of hyperinflammatory immune responses.
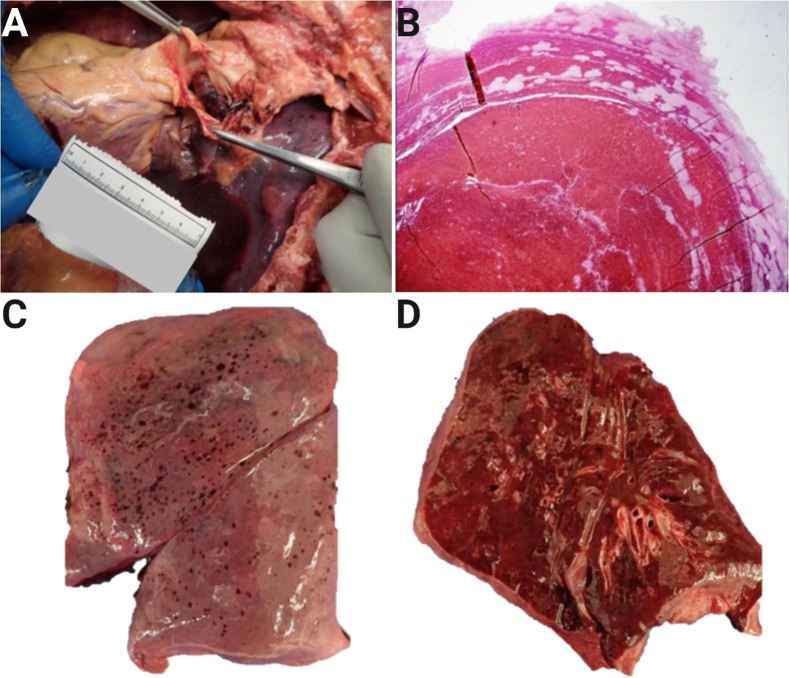
This has also been a common finding in our regional autopsy service based in the New York Metropolitan Area. An example being this 48-year-old patient, who developed a saddle pulmonary embolus secondary to COVID-19 disease (Fig. 1 ).
Fig. 1.
Autopsy findings in coronavirus-associated coagulopathy. A-B, Saddle pulmonary embolism from a 48-year-old patient; C-D, Pleural petechiae and homogeneous, slightly firm cut surfaces with abundant expressed fluid from the same patient. Courtesy of Alex K. Williamson, MD; Northwell Regional Autopsy Service.
Protein S and immunomodulation:
TAM (Tyro3, Axl, and Mer) receptors are part of the larger receptor tyrosine kinases (RTK) family [24]. Today, some feel that excessive coagulation and immune modulation may be intrinsically linked to the TAM receptors and Protein S. And Nature Reviews Immunology recently published an article hypothesizing the pathogenesis of TAM receptor signaling in SARS-CoV-2 [11]. This hypothesis is based on the premise that PROS1 also functions as an activating ligand for TAM RTKs, in addition to functioning as an anticoagulant [24].
In the human immune system, TAM RTKs are almost exclusively expressed by MER receptors on the surface of phagocytic tissue macrophages [11]. Its kinase signaling pathway is activated by Protein S, which subsequently binds to the extracellular domain of MER8 [11]. The activation of MER in immune cells yields a immunosuppressive function, which hinders immune response mechanisms including: TNF, cytokines and interferons released in response to SARS-CoV-2 in COVID-19 disease [25].
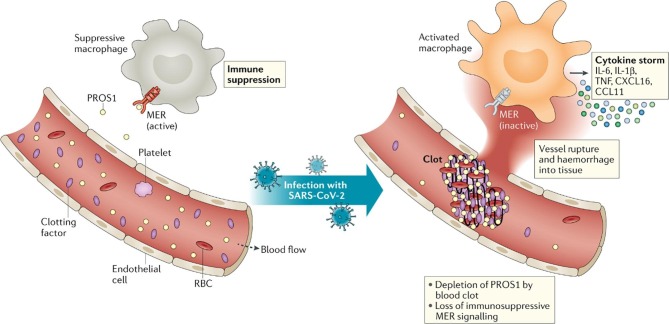
We have also known that SARS-CoV-2 causes significant hypercoagulation. Hypercoagulation is known to weaken vessels and result in tissue hemorrhage [11]. However, expanding clot work to consume many important clotting factors; this includes the anticoagulant PROS1, a crucial ligand responsible for the immunosuppressive through MER, a previously mentioned TAM RTK expressed on immune cells [11] (Fig. 2 ). PROS1 depletion also decreased MER signaling, which is probably responsible for immune cells secreting inflammatory cytokines, commonly referred to as a ‘cytokine storm’ [11], [25].
Fig. 2.
Does protein S link blood clotting and cytokine storms in COVID-19? PROS1, Protein S; RBC, red blood cell; TNF, tumor necrosis factor. Reprinted by permission from Springer Nature, Nature Reviews Immunology[11](Blood clots and TAM receptor signalling in COVID-19 pathogenesis, Lemke G and Silverman GJ), (2020).
As mentioned previously, hypoxia is also an important contributor to deficiencies in Protein S and could be the initial event triggering Coronavirus-associated coagulopathy. However, the role of hypoxia and fulminant blood clotting in SARS-CoV-2 is not currently well understood. In future, treatments targeting RBC based oxygenation could be the key to clinical heterogeneity in COVID-19. And patients expected to have poor outcomes could experience dramatic changes in clinical course [26].
Role of Red blood cell exchange and addressing hypoxia:
Replenishing the oxygen carrying capacity of blood could be the key to addressing COVID-19 induced hypoxia and its downstream consequences. This is supported by a case report demonstrating a patient with cardiac arrest and multiple comorbidities including: chronic obstructive pulmonary disease, congestive heart failure, and anemia secondary gastrointestinal bleeding [26]. The patient subsequently tested positive for SARS- CoV-2 and this ultimately progressed to pulmonary disease with bilateral interstitial infiltrates on his chest X-ray. The patient was anemic and treatment with packed red blood cells was undertaken. Initially, the patient was intubated for ventilation of acute respiratory failure. To the surprise of attending clinicians, oxygen stats improved and the patient was later extubated.
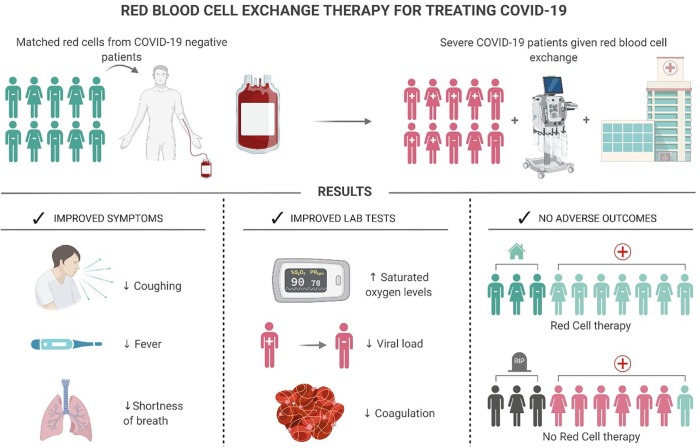
Compared to regular transfusion and SARS-CoV-2, the use of RBC exchange could actually be a much more effective approach for improving oxygenation. And RBC exchange is commonly performed for many hemoglobin disorders; the most notable being sickle cell disease [27]. RBC exchange also offers a lower risk of iron accumulation, while also replacing pathologically aberrant erythrocyte populations [27]. Disadvantages include higher operating costs, along with the requirement for apheresis devices and trained hospital staff [27]. In theory, RBC exchange could improve both symptomatology and laboratory abnormalities, without real significant adverse outcomes (Fig. 3 ).
Fig. 3.
Theoretical efficacy of red blood cell exchange therapy for treating COVID-19.
RBC exchange has also been used in ‘Blackwater fever’, or severe falciparum malaria, clinically characterized by intravascular hemolysis [28], [29]. Where RBC exchange results in the clearance of peripheral parasitemia and prevents hemolysis of RBCs [28]. It is entirely possible that RBC exchange could also lower viral load through this same mechanism; although definitive laboratory data will be needed to confirm this.
Finally, simple transfusion is traditionally recommended for the treatment of symptomatic anemia with hemoglobin (Hb) levels less than 9 g/dL [27], [30]. On the other hand, red cell exchange is indicated to prevent or treat complications arising from the presence of abnormal hemoglobin proteins, such as hemoglobin S (HbS) in sickle cell disease [30]. And the goal of exchange is to lower HbS level to less than 30% [30]. In this setting, with such significant Hb protein and lipid structural damages, the use of RBC exchange would likely be more beneficial than that of simple transfusion.
Discussion
SARS-CoV-2 has likely been causing prolonged and progressive hypoxia secondary to downstream alterations in both the Hb protein and lipid membrane structures [13]. This likely has been leading to a failure of blood to carry oxygen, along with the multi-organ failure and mortality seen in COVID-19 disease.
Interestingly, many COVID-19 patients come to the hospital with low peripheral oxygen concentrations (SpO2) at presentation [31]. This is because initially, COVID-19 pneumonia causes oxygen deprivation without significant symptoms. Something termed “silent” hypoxia; a phenomena commonly seen in COVID-19 disease [32]. You wouldn’t know this from talking to these patients though, as they don’t seem starved of oxygen.
This is something also seen with methemoglobinemia and many patients with methemoglobin levels of 30% or higher do not have symptoms of hypoxia - despite very low SpO2 [33], [34], [35]. Methodologic problems consistently hinder oximetry in methemoglobinemia and elevated methemoglobin results in the underestimation of SpO2 [36]. If this SARS-CoV-2 has the ability to adversely affect the structure of hemoglobin, this could also bias oximetry estimates of SpO2.
For the discussion of this hypothesis, it is important to understand that hypoxia can have downstream consequences: possibly result in increased HIF1α, decreased protein S with resultant hypercoagulation, along with impaired immune modulation through the TAM RTKs. The inverse relationship between HIF1α and PROS1 could represent the bodies normal adaptive compensation to O2 concentrations. And functionally, HIF1 may work to regulate PROS1. However, in the setting of COVID-19, pathological stabilization of HIF1α is likely responsible for significant complications and patient demise.
Pertaining to Protein S, deficiencies are probably also exacerbated by dysregulated blood coagulation and are not solely a consequence of hypoxia [11]. Future studies examining the effects of RBC exchange on hypoxia, admission status and overall patient outcomes, will be paramount for determining the efficacy of this therapeutic.
It is important to acknowledge that blood shortages could become a problem through the remaining duration of the COVID-19 pandemic. And RBC exchange does carry a somewhat high blood requirement, as some erythrocytes transfused ultimately become removed throughout the process of apheresis [37]. Currently, it seems that reductions in donor blood products have largely been matched by a significant reduction in the demand for transfusion during this pandemic [38].
In summary, from pathological findings and the silent hypoxia seen in COVID-19 clinical disease; along with extensive review of the literature. I hypothesize that hypoxia secondary to altered structural Hb, may well be responsible for mortality in COVID-19 disease. The use of red blood cell exchange would target hypoxia at the source; representing a Gemini of therapeutic opportunities.
Declaration of Competing Interest
The author declares that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Fig. 1, Fig. 3 were created with BioRender.com. Fig. 2 was reprinted by permission from Springer Nature, Nature Reviews Immunology [11] (Blood clots and TAM receptor signalling in COVID-19 pathogenesis, Lemke G and Silverman GJ), (2020). Fig. 1 is courtesy of Alex K. Williamson, MD; Northwell Regional Autopsy Service.
References
- 1.Potter P: Edvard Munch (1863-1944). Self-portrait after the Spanish Flu (1919-20). Emerg Infect Dis 2003, 9:407.
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty I., Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel B.K., Kress J.P., Hall J.B. Alternatives to invasive ventilation in the COVID-19 pandemic. JAMA. 2020;324:43–44. doi: 10.1001/jama.2020.9611. [DOI] [PubMed] [Google Scholar]
- 5.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian M., Jiang J. COVID-19 and social distancing. Z Gesundh Wiss. 2020:1–3. doi: 10.1007/s10389-020-01321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J., Fink J.B., Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020;55:2000892. doi: 10.1183/13993003.00892-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taleb M., Ashraf Z., Valavoor S., Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs. 2013;13:325–330. doi: 10.1007/s40256-013-0027-2. [DOI] [PubMed] [Google Scholar]
- 9.Khetarpal A., Kotwal U. Role of Automated therapeutic red cell exchange in the setting of acute methemoglobinemia: our experience. Indian J Hematol Blood Transfus. 2018;34:143–145. doi: 10.1007/s12288-017-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilli V.S., Datta A., Afreen S., Catalano D., Szabo G., Majumder R. Hypoxia downregulates protein S expression. Blood. 2018;132:452–455. doi: 10.1182/blood-2018-04-841585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemke G., Silverman G.J. Blood clots and TAM receptor signalling in COVID-19 pathogenesis. Nat Rev Immunol. 2020;20:395–396. doi: 10.1038/s41577-020-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas T., Stefanoni D., Dzieciatkowska M., Issaian A., Nemkov T., Hill R.C. Evidence for structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. medRxiv. 2020;2020(06) doi: 10.1021/acs.jproteome.0c00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun K., Zhang Y., D'Alessandro A., Nemkov T., Song A., Wu H., Liu H. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun. 2016;7:12086. doi: 10.1038/ncomms12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X., Zhang R., He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;99:1421–1428. doi: 10.1007/s00277-020-04103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta N., Zhao Y.-Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Ninivaggi M., de Laat M., Lancé M.M.D., Kicken C.H., Pelkmans L., Bloemen S. Hypoxia induces a prothrombotic state independently of the physical activity. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0141797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stream J.O., Luks A.M., Grissom C.K. Lung disease at high altitude. Expert Rev Respir Med. 2009;3:635–650. doi: 10.1586/ers.09.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fricker M., Goggins B.J., Mateer S., Jones B., Kim R.Y., Gellatly S.L. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight. 2018;3 doi: 10.1172/jci.insight.94040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginouvès A., Ilc K., Macías N., Pouysségur J., Berra E. PHDs overactivation during chronic hypoxia “desensitizes” HIFalpha and protects cells from necrosis. Proc Natl Acad Sci USA. 2008;105:4745–4750. doi: 10.1073/pnas.0705680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landi A., De Servi S. The burden of thrombotic complications in critically ill patients with COVID-19: charting the uncharted. Intern Emerg Med. 2020;15:893–895. doi: 10.1007/s11739-020-02393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois-Silva Á., Barbagelata-López C., Mena Á., Piñeiro-Parga P., Llinares-García D., Freire-Castro S. Pulmonary embolism and screening for concomitant proximal deep vein thrombosis in noncritically ill hospitalized patients with coronavirus disease 2019. Intern Emerg Med. 2020;15:865–870. doi: 10.1007/s11739-020-02416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothlin C.V., Ghosh S., Zuniga E.I., Oldstone M.B., Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Ejigu T., Patel N., Sharma A., Vanjarapu J.M.R., Nookala V. Packed red blood cell transfusion as a potential treatment option in COVID-19 patients with hypoxemic respiratory failure: a case report. Cureus. 2020;12 doi: 10.7759/cureus.8398. e8398-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stussi G., Buser A., Holbro A. Red blood cells: exchange, transfuse, or deplete. Transfus Med Hemother. 2019;46:407–416. doi: 10.1159/000504144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auer-Hackenberg L., Staudinger T., Bojic A., Locker G., Leitner G.C., Graninger W. Automated red blood cell exchange as an adjunctive treatment for severe Plasmodium falciparum malaria at the Vienna General Hospital in Austria: a retrospective cohort study. Malar J. 2012;11:158. doi: 10.1186/1475-2875-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huggan P.J., Ng C.H., Ho J., Lin R., Chavatte J.M. A case of blackwater fever with persistent Plasmodium falciparum parasitaemia detected by PCR after artemether-lumefantrine treatment. Malar J. 2018;17:35. doi: 10.1186/s12936-018-2180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biller E., Zhao Y., Berg M., Boggio L., Capocelli K.E., Fang D.C. Red blood cell exchange in patients with sickle cell disease-indications and management: a review and consensus report by the therapeutic apheresis subsection of the AABB. Transfusion. 2018;58:1965–1972. doi: 10.1111/trf.14806. [DOI] [PubMed] [Google Scholar]
- 31.Sun R., Liu H., Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21:541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teo J. Early detection of silent hypoxia in Covid-19 Pneumonia using smartphone pulse oximetry. J Med Syst. 2020;44:134. doi: 10.1007/s10916-020-01587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Husseini A., Azarov N. Is threshold for treatment of methemoglobinemia the same for all? a case report and literature review. Am J Emerg Med. 2010;28 doi: 10.1016/j.ajem.2009.10.014. 748.e5-.e10. [DOI] [PubMed] [Google Scholar]
- 34.Khanal R., Karmacharya P., Pathak R., Poudel D.R., Ghimire S., Alweis R. Do all patients with acquired methemoglobinemia need treatment? a lesson learnt. J Community Hosp Intern Med Perspect. 2015;5:29079. doi: 10.3402/jchimp.v5.29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright R.O., Lewander W.J., Woolf A.D. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–656. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 36.Feiner J.R., Bickler P.E., Mannheimer P.D. Accuracy of methemoglobin detection by pulse CO-oximetry during hypoxia. Anesth Analg. 2010;111:143–148. doi: 10.1213/ANE.0b013e3181c91bb6. [DOI] [PubMed] [Google Scholar]
- 37.Kelly S., Quirolo K., Marsh A., Neumayr L., Garcia A., Custer B. Erythrocytapheresis for chronic transfusion therapy in sickle cell disease: survey of current practices and review of the literature. Transfusion. 2016;56:2877–2888. doi: 10.1111/trf.13800. [DOI] [PubMed] [Google Scholar]
- 38.Stanworth S.J., New H.V., Apelseth T.O., Brunskill S., Cardigan R., Doree C., Germain M. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]