Highlights
-
•
COVID-19 can cause a hyperinflammatory state akin to cytokine release syndrome (CRS).
-
•
Our report shows the efficacy and safety of using anti-IL-6/IL-6-R therapy in 31 patients.
-
•
Treatment at the onset of severe CRS may be the ideal window for intervention.
-
•
Long term effects of these agents are currently lacking.
Keywords: Infectious disease, SARS-CoV-2, IL-6, C-reactive protein
Abbreviations: (ALC), Absolute Lymphocyte Count; (ARDS), Acute respiratory distress syndrome; (anti-IL-6/IL-6-R), Anti-IL-6/IL-6-Receptor; (PaO2/FiO2), Arterial oxygen partial pressure/fraction of inspired oxygen; (BMI), Body mass index; (CRP), C-reactive protein; (CRS), Cytokine release syndrome; (DNR/DNI), Do not resuscitate/do not intubate; (ECMO), Extracorporeal membrane oxygenation; (ESR), Erythrocyte sedimentation rate; (IRB), Institutional review board; (LDH), Lactate dehydrogenase; (NIV), Noninvasive ventilation; (SpO2), Peripheral capillary oxygen saturation; (RT-PCR), Reverse-transcriptase polymerase-chain-reaction; (RWMC), Roger Williams Medical Center; (SITC), Society for Immunotherapy of Cancer
Abstract
The SARS-CoV-2 virus responsible for the COVID-19 pandemic can result in severe or fatal disease in a subset of infected patients. While the pathogenesis of severe COVID-19 disease has yet to be fully elucidated, an overexuberant and harmful immune response to the SARS-CoV-2 virus may be a pivotal aspect of critical illness in this patient population. The inflammatory cytokine, IL-6, has been found to be consistently elevated in severely ill COVID-19 patients, prompting speculation that IL-6 is an important driver of the pathologic process. The inappropriately elevated levels of inflammatory cytokines in COVID-19 patients is similar to cytokine release syndrome (CRS) observed in cell therapy patients. We sought to describe outcomes in a series of severely ill patients with COVID-19 CRS following treatment with anti-IL-6/IL-6-Receptor (anti-IL-6/IL-6-R) therapy, including tocilizumab or siltuximab. At our academic community medical center, we formed a multi-disciplinary committee for selecting severely ill COVID-19 patients for therapy with anti-IL-6 or IL-6-R agents. Key selection criteria included evidence of hyperinflammation, most notably elevated levels of C-reactive protein (CRP) and ferritin, and an increasing oxygen requirement. By the data cutoff point, we treated 31 patients with anti-IL-6/IL-6-R agents including 12 who had already been intubated. Overall, 27 (87%) patients are alive and 24 (77%) have been discharged from the hospital. Clinical responses to anti-IL-6/IL-6-R therapy were accompanied by significant decreases in temperature, oxygen requirement, CRP, IL-6, and IL-10 levels. Based on these data, we believe anti-IL-6/IL-6-R therapy can be effective in managing early CRS related to COVID-19 disease. Further study of anti-IL-6/IL-6-R therapy alone and in combination with other classes of therapeutics is warranted and trials are underway.
1. Introduction
It is increasingly evident that the SARS-CoV-2 virus infection responsible for the COVID-19 pandemic is associated with profound immune dysfunction [1], [2], [3], [4], [5]. Emerging evidence also indicates that severely ill patients have markedly elevated C-reactive protein (CRP), ferritin, and IL-6 levels [1], [5], [6], [7], [8], [9]. The pathophysiology of severe COVID-19 disease may relate to dysfunctional immune and inflammatory responses to the SARS-CoV-2 infection. In such cases, patients develop a hyperinflammatory state akin to cytokine release syndrome (CRS) as seen in cell therapy patients [10], [11], where IL-6 is a critical mediator of the pathologic process. Patients with CRS physiology are at risk for rapid progression to multiorgan failure resulting from inappropriately elevated cytokine levels and immune dysregulation [3], [5], [9], [12]. This has encouraged the medical community to use existing IL-6 or IL-6 receptor (IL-6-R) blocking monoclonal antibodies in such scenarios [13], [14], [15], [16]. Two IL-6-R blocking antibodies (tocilizumab and sarilumab) and one IL-6 blocking agent (siltuximab) are FDA approved for rheumatological and other lymphoproliferative disorders. Moreover, tocilizumab is additionally FDA approved to manage CRS in patients who receive CAR T-cell therapy [17], [18], with which our center has experience. Tocilizumab was approved in China in March 2020 for the treatment of patients with COVID-19 and severe pulmonary disease associated with elevated serum IL-6 levels [5]. Emerging data suggest that anti-IL-6/IL-6-R therapy may offer meaningful clinical benefit for COVID-19 patients with CRS and related manifestations, including severe pulmonary hypoxemia and acute respiratory distress syndrome (ARDS) [12], [19], [20], [21], [22], [23]. Small series and case reports indicate significant improvement with the use of these agents in both intubated and non-intubated patients [13], [14], [15], [16].
Based on the totality of these data, several centers in the US and across the world have moved quickly to formally study the safety and efficacy of anti-IL-6/IL-6-R antibodies in patients with COVID-19 [NCT04310228, NCT04317092, NCT04324073, NCT04306705, NCT04320615, NCT04315298]. The Society for Immunotherapy of Cancer (SITC) has issued a statement supporting the use of tocilizumab for the treatment of COVID-related CRS [24]. It is becoming increasingly clear that the management of patients with severe COVID-19 related disease requires a nimble and agile multi-disciplinary approach. The fatal manifestations of COVID-19 disease involves not only the viral infection itself, but an over-exuberant and dangerous host immune response that can be fatal if left unchecked.
The aim of our report is to describe the clinical outcomes and correlative cytokine profiles among patients with COVID-19 who received tocilizumab or siltuximab in our institution based on our protocol for off-label use. While this single-arm series is relatively small, we demonstrate consistent responses and encouraging survival rates following anti-IL-6/IL-6-R blockade in patients with COVID-related CRS, along with correlative data indicating reversal of CRS physiology. These data support ongoing studies that seek to determine if anti-IL-6/IL-6-R therapy should be broadly adopted for management for COVID-related CRS and severe acute pulmonary disease.
2. Methods
2.1. Study population, setting, data collection, statistical analysis
The current report includes patients with laboratory-confirmed severe COVID-19 infection who were admitted to Roger Williams Medical Center (RWMC) between March 18 and May 3, 2020. A confirmed case of COVID-19 was defined by a positive result on a reverse-transcriptase polymerase-chain-reaction (RT-PCR) assay of a nasopharyngeal swab specimen. Only laboratory-confirmed cases were included. COVID-19 was considered severe for this analysis if they had evidence of acute respiratory decompensation defined as tachypnea (respiratory rate ≥ 30/minute) and/or hypoxemia, despite being on standard supportive care treatment. Hypoxemia was defined as arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio ≤ 300 mmHg and/or peripheral capillary oxygen saturation (SpO2) ≤ 93% on room air regardless of the oxygenation method, including nasal cannula or mask with oxygen flow rate of greater than 12 L per minute, high-flow oxygen through nasal cannula, noninvasive ventilation (NIV), and invasive ventilation through tracheal intubation. Pregnant women, prisoners, and children (those younger than 18 years of age) were not among the patients who received the intervention. The institutional review board (IRB) approved the use of biospecimens and data from COVID-19 patients, including those treated with tocilizumab or siltuximab. Informed consent was obtained as part of an existing IRB approved biospecimen protocol, in addition to a specific informed consent for the treatment. We obtained demographic data, information on clinical symptoms or signs at presentation, their comorbid conditions and laboratory and radiologic results during admission as well as through the rest of the hospitalization. All laboratory tests and radiologic assessments were performed at the discretion of the treating physician(s).
2.2. Treatment protocol
Patients were deemed to be candidates for tocilizumab or siltuximab if they met all the following criteria per Table 1. This criteria was modified from the existing consensus grading for CRS associated with immune effector cells [10]. There should be established presence of hyperinflammation as evidenced by serial monitoring of ferritin, CRP, D-dimers, and other inflammatory markers like erythrocyte sedimentation rate (ESR) and fibrinogen. Empiric levels of CRP ≥ 80 mg/L and/or ferritin ≥ 600 ng/mL were used as evidence of hyperinflammation. No specific levels were set for other markers. Acute severe infection from sources other than SARS-CoV-2 were excluded. In patients who were consented per tissue bank protocol, cytokine levels were drawn as part of the protocol. The tissue bank protocol allows for the storage of biospecimens from patients with benign or malignant tumors, or from patients with infectious disease. All subjects provided consent for the collection of their blood to study the effect of COVID-19 and its associated treatments on cytokines levels.
Table 1.
Clinical criteria for tocilizumab or siltuximab administration.
| Confirmed SARS-CoV-2 infection with documentation of the following | ||
|---|---|---|
| Fever (≥38 °C or ≥ 100.4°F) | ||
| With one or more of the following: | ||
| Hypotension | Inflammatory markers | Hypoxia |
| SBP < 90 mm Hg or < 20% of baseline | CRP ≥ 80 mg/L or rapid doubling | Increasing O2 requirements (≥5 L/min) nasal cannula, facemask, non-rebreather mask, high flow nasal cannula, CPAP, or BiPAP. |
| Requiring vasopressor support | Serum ferritin ≥ 600 ng/mL or rapid doubling | Rapid decompensation with intubation and mechanical ventilation. |
All patients who met the above criteria were reviewed in a daily multidisciplinary team meeting that included critical care medicine, immunotherapy, hematology, bone marrow transplant, infectious disease and consultant of record. Treatment was initiated after discussion with and approval from all the members. Tocilizumab was dosed at 4 mg/kg or 400 mg as a single intravenous infusion. The dose was rounded to the nearest whole vial available (80 mg, 200 mg or 400 mg). One additional dose was considered ≥ 12 h later if clinical symptoms worsened or there was no improvement; this was subject to approval from the multidisciplinary team. A maximum of 2 doses per patient per course of COVID-19 was allowed. Siltuximab was dosed at 11 mg/kg as a single intravenous infusion with no additional doses within a 3 week period.
2.3. Cytokine assays
Quantification of serum cytokines was performed using a Luminex Magpix (Luminex Co., Austin, TX) and High Sensitivity Cytokine Panel A kits (R&D Systems Inc., Minneapolis, MN). Day 0 time point specimens were drawn 12 h post-anti-IL-6/IL-6-R treatment to assess cytokine activity early in the post-treatment period. Cytokine data was generated for specimens collected from 16 patients treated with anti-IL-6/IL-6-R therapy. These were patients who consented to the tissue bank and had no missing data points.
2.4. Data analysis and statistics
Descriptive statistics were used to summarize the clinical data and results are reported as medians and interquartile ranges or means and standard deviations, as appropriate for continuous variables. Categorical variables were summarized as counts and percentages. Wilcoxon signed-rank test were used for comparison of paired medians over time and two-tailed t-tests were used for comparison of means. No imputation was made for missing data.
3. Results
3.1. Demographics and clinical characteristics of the patients
We identified 31 patients from March 18, 2020 to May 3, 2020 who were admitted to RWMC for severe COVID-19 infection. The basic demographics and the clinical history of these patients are as documented in Table 2. Median age was 61 years, ranging from 32 years to 89 years with majority (64.5%) men. Body mass index (BMI) ≥ 30 kg/m2 was noted in eleven (35%) cases. Majority of them (77%) were admitted from home and 4 of the 31 patients were healthcare workers. Fourteen (75%) had history of contact with another COVID-19 positive person and in three cases the contact was a family member who was a healthcare worker. Most common presenting symptom was shortness of breath (84%) and cough (77%). Documented fever was noted in 75% of cases. Hypertension was the most common comorbidity in 61% of the cases.
Table 2.
Clinical characteristics of patients at baseline.
| Characteristic | N = 31 (%) |
|---|---|
| Median age (range) | 61 years (32 – 89) |
| Sex (M:F) | 20:11 |
| Median weight (range) | 82 kg (45–159) |
| BMI (kg/m2) | |
|
20 (65) |
|
11(35) |
| Admission source | |
|
24 (77) |
|
7 (23) |
| Healthcare worker | 4 (13) |
| Symptoms at presentation | |
|
24 (77) |
|
20 (65) |
|
26 (84) |
|
12 (39) |
|
10 (32) |
|
7 (23) |
|
5 (16) |
| Comorbid conditions | |
|
8 (26) |
|
9 (29) |
|
19 (61) |
|
3 (10) |
|
6 (19) |
|
0 |
|
1 (CKD) |
| History of sick contact | 18 (58) |
| Vitals on admission | |
|
20 (65) |
|
18 (58) |
|
23 (74) |
On admission, the majority of patients had normal total leukocyte counts (77%) with lymphocytopenia (Absolute Lymphocyte Count/ALC) ≤ 1500 cells/mm3) noted in 97% of the cases (Table 3). Mean ALC was 993 cells/mm3. Platelet counts were normal in 87% of the cases while 4 patients had presenting platelets less than normal with the lowest being 98 x103/µL. Elevated serum creatinine levels were noted in 26% of patients, AST elevation in 48%, and ALT elevation in 10%. Median lactate dehydrogenase (LDH) was slightly elevated at 342 U/L. Median serum lactate levels were normal. Median D-dimer levels were mildly elevated at 0.82 mg/L FEU. Chest radiographs were obtained in all patients on admission, majority (77%) of which showed bilateral or multifocal infiltrates (Fig. 1A-C). Four had normal chest radiographs at presentation which quickly progressed to bilateral pneumonia within 24 h of admission. Three patients had computed tomography of the chest on admission which showed bilateral ground glass opacities.
Table 3.
Lab parameters and imaging findings on admission.
| Lab data | N = 31 (%) |
|---|---|
| WBC | |
| - Median | 7.6 × 103/µL |
|
24 (77) |
|
6 (19) |
|
30/31 (97%) |
|
993 cells/mm3 |
| Platelet count | |
|
211 × 103/µL |
|
27 (87) |
|
4 (13) |
| ESR greater than 40 mm/h | 12/24 (50) |
| Ferritin (N 23.9 – 336.2 ng/mL) | |
| - Median | 455.3 ng/mL |
|
10 (32) |
|
11 (35) |
|
3 (10) |
|
7 (23) |
| CRP (N 0.00 – 7.30 mg/L) | |
|
130-0.44 mg/L |
|
0 |
|
10 (32) |
|
14 (45) |
|
7 (23) |
| LFT | |
|
15 (48) |
|
3 (10) |
|
3 (10) |
| Serum creatinine | |
|
8 (26) |
| Lactate median (N 0.5–2.0 mmol/L) | 1.7 (1.0–2.8) |
| LDH median (N 140–271 U/L) | 343 (129 – 1017) |
| D-dimer (N 0.19 – 0.52 mg/L FEU) | 0.82 (0.27 – 17.83) |
| Chest X ray findings | |
|
4 (13) |
|
24 (77) |
|
3 (10) |
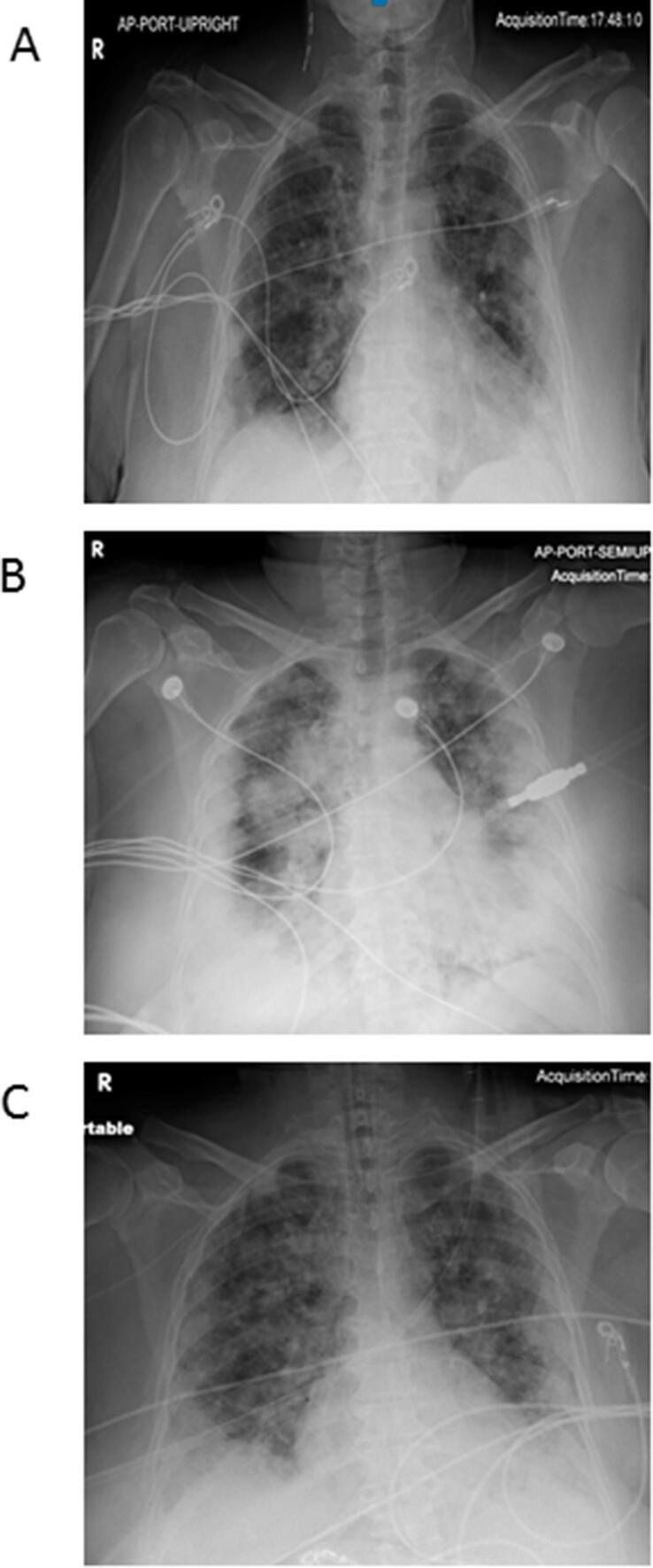
Fig. 1.
Radiological changes noted in chest X-ray (CXR) in response to tocilizumab in one of the patients. (A) Admission CXR showing moderate multifocal airspace disease with diffuse bilateral infiltrates. (B) CXR within 24 h prior to tocilizumab showing marked worsening of bilateral airspace infiltrates. (C) CXR 36 h after tocilizumab infusion showing improvement in the infiltrates.
3.2. Clinical and radiographic responses to IL-6/IL-6-R blocking therapy
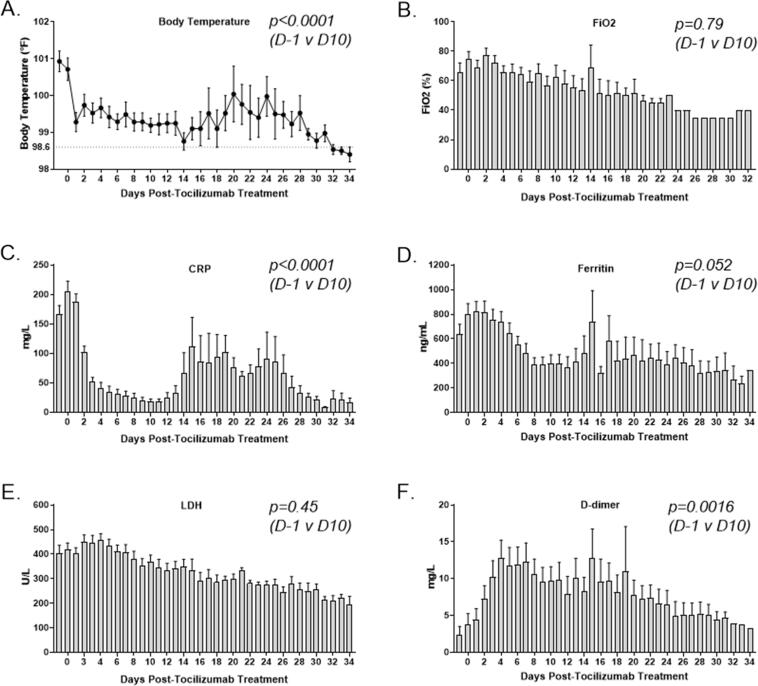
Following treatment with anti-IL-6/IL-6-R agents, patient temperature elevations decreased significantly (p = 0.0001, Fig. 2A), with a mean of 100.9°F prior to treatment and a mean of 99.2°F 10 days following treatment. In addition, the oxygen requirements as measured by the FiO2 decreased over time, with a mean of 65% prior to tocilizumab treatment to 40% at day 25 (p = 0.32, Fig. 2B). Three patients had marked improvement of their intractable diarrhea within seventy-two hours of treatment. Admission levels of serum ferritin were normal in 32% of the cases while none of the patients had normal CRP. Ferritin level ≥ 1000 ng/mL and CRP ≥ 200 mg/L were noted in 23% of patients. Prior to infusion, the mean CRP level was 166.2 mg/mL and decreased to 18.62 mg/mL 10 days following treatment (p < 0.0001, Fig. 2C), but began to increase around day 12 possibly as the drug was eliminated by the body. Similarly, the pre-treatment mean serum ferritin level was 638 ng/mL with a decrease to 399.7 ng/mL on day 10 (p = 0.052, Fig. 2D). LDH levels trended down (p = 0.45), as well (Fig. 2E). Consistent with the known hypercoagulable state in severely ill COVID-19 patients [25], the mean D-dimer increased from 2.3 to 9.7 mg/mL (p < 0.0016, Fig. 2F).
Fig. 2.
Clinical response to anti-IL-6/IL-6-R therapy. (A) Body temperature elevations decreased quickly and significantly and remained significantly lowered through day 10 (p < 0.001). (B) FiO2 levels following treatment trended downward. Comparisons for day 10 (p = 0.78) and day 25 (p = 0.32) were not significant. (C) CRP levels decreased dramatically, beginning within two days of therapy and were significantly lower at day 10 (p < 0.0001). (D) Ferritin decreased slowly, reaching a significantly reduced level by day 10 (p = 0.052). (E) LDH decreased slowly, but not significantly within ten days of anti-IL-6/IL-6-R therapy (p = 0.45). (F) D-dimer increased gradually and was significantly elevated on day 10 (p = 0.0016).
3.3. Cytokine levels following IL-6/IL-6-R blocking therapy
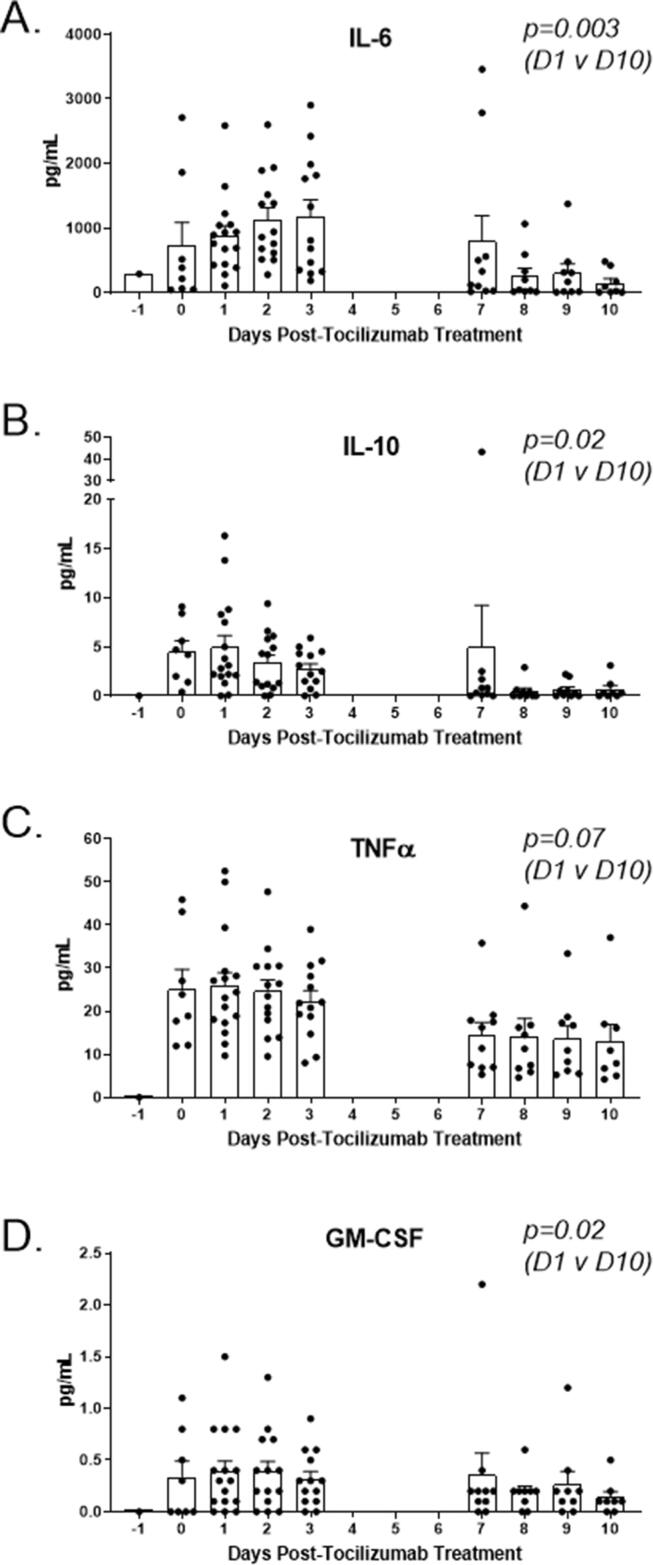
IL-6 may be a critical driver of severe COVID-19 disease and CRS, which along with lymphopenia, is reflective of a dysregulated immune response. COVID-19 also has a distinct inflammatory cytokine profile when compared to SARS and MERS [26]. In our patients, the IL-6 levels decreased from a mean of 881 pg/mL to 152.6 pg/mL following anti-IL-6/IL-6-R infusion (p = 0.003, Fig. 3A). IL-10, an immunosuppressive cytokine, decreased significantly by day 10, as well (p = 0.02, Fig. 3B). Mean TNFα levels decreased nearly two-fold following treatment but the difference was not significant (p = 0.07) while GM-CSF did decrease significantly (p = 0.02, Fig. 3C-D).
Fig. 3.
Serum cytokine response to anti-IL-6/IL-6-R therapy. (A) Serum IL-6 gradually increased over several days but decreased significantly by day 10 (p = 0.003). (B) Serum IL-10 decreased slowly, reaching a significantly reduced level by day 10 (p = 0.002). (C) Serum TNFα remained elevated for several days but decreased slowly, although not significantly (p = 0.07). (D) Serum GM-CSF decreased significantly ten days following anti-IL-6/IL-6-R therapy (p = 0.02).
3.4. Clinical outcomes
Median time of follow up for the entire cohort is 21 days with a minimum follow up of 14 days or discharge from the last dose of tocilizumab administration. Median time from admission to tocilizumab administration was 3 days among mechanically ventilated patients and patients who did not require intubation. Among the patients in this series, 15 required mechanical ventilation (Table 4 and Fig. 4). Median PaO2/FiO2 ratio and PEEP after intubation were 86 mmHg and 18 cm H20 respectively. Eleven of the 15 patients had PaO2/FiO2 ratio ≤ 100 mmHg. Twelve patients received tocilizumab after intubation and mechanical ventilation. Six of these patients received the drug on the day of intubation itself, while the other 6 received it within 48 h of intubation. Three patients required intubation after receiving tocilizumab (median 3 days), 2 of whom were extubated, the third patient remains intubated. Fourteen patients never required mechanical ventilation after receiving tocilizumab. Twenty-five patients received a dose of 400 mg, 5 patients received 320 mg (4 mg/kg) and one patient received siltuximab (11 mg/kg). A second dose of tocilizumab was administered in 4 patients. Three of the 4 patients have been extubated and discharged. One of the patients continues to be intubated.
Table 4.
Clinical outcomes including ICU level therapies.
| IL-6/IL-6-R Therapy | N (=31) |
|---|---|
| Mechanical ventilation - Median PaO2/FiO2 ratio at intubation (IQR) - PaO2/FiO2 ratio ≤ 100 mm Hg - Median PEEP at intubation (IQR) Did not require mechanical ventilation Neuromuscular blockade Vasopressors Inhaled pulmonary vasodilators ECMO Prone positioning Tracheostomy |
15 86 mm Hg (62–140) 11/15 18 cm H20 (8–20) 16/31 6/15 7/15 0 0 2/15 1/15 |
| Extubated patients as of last follow up | 11/15 |
| Median duration of mechanical ventilation [IQR] | 11 days [9–33] |
| Median length of ICU stay (extubated) [IQR] | 13 days [8–34] |
| Median time from tocilizumab to extubation [IQR] | 10.5 days [5–31] |
| Terminal extubation | 2/15a |
Sustained end organ damage after ICU stay
|
6/11 extubated 0/9 0/9 |
| Number of patients who died after Tocilizumab | 4/31b |
| Patients discharged from the hospital | 24/31 (11 intubated,13 not intubated) |
Median length of hospital stays
|
27 days (13–43 days) 15 days (3–24 days) 24 days |
both terminally extubated at request of family after change in goals of care
Two patients with advanced directives of DNR/DNI prior to need for intubation and the 2 patients referred to above, where family changed goals of care to comfort measures only while on ventilator.
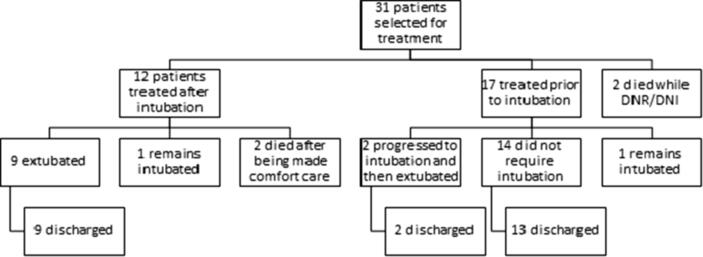
Fig. 4.
Clinical outcomes of all treated patients. The diagram depicts the clinical outcomes for all patients in our series.
Among the 31 patients who received anti-IL-6/IL-6-R therapy, only 2 patients currently remain intubated (Fig. 4). Twelve of these 31 patients received tocilizumab after intubation, of which 9 have been extubated, 2 patients died, and 1 currently remains intubated. The other 19 of the 31 patients were also given tocilizumab, of which 14 never required intubation, 3 progressed to intubation while 2 patients with advanced directives of do not resuscitate/do not intubate (DNR/DNI) status died. Among the 3 patients who progressed to intubation after tocilizumab, 2 have been extubated, and 1 currently remains intubated. Among all the 15 intubated patients, 40% had concurrent hypotension requiring vasopressors and 48% required neuromuscular blockade. No patients were given inhaled pulmonary vasodilators or required to be transferred for extracorporeal membrane oxygenation (ECMO). One patient required tracheostomy.
Eleven of the 15 intubated patients have been extubated at the time of last follow up. Two patients died, while 2 currently remain intubated. Among extubated patients, median duration of mechanical ventilation was 11 days and median ICU stay was 13 days. Twenty-five of the 31 patients (81%) currently remain ventilator free. Twenty-four patients (77%) have been discharged, 11 of whom required mechanical ventilation during their hospital stay. Four of the 31 patients (13%) expired at the time of last follow up, 1 patient was an octogenarian with advanced directives of DNR/DNI and died after 3 days of admission. The second person was in his 70′s also with DNR/DNI directives and died on the sixth day of his admission. The third person was a nursing home resident with significant psychosocial issues and was terminally extubated on the sixth day of admission per family’s wishes. The fourth patient was terminally extubated after 22 days as family opted to change the goals of care to comfort measures only. The remaining 27 patients are alive at the time of last follow up, resulting in a survival rate of 87% including 8 of the 10 who were 70 years or older.
4. Discussion
This single center series describes the clinical outcomes of 31 patients who presented with severe COVID-19 related acute respiratory failure, who received anti-IL-6/IL-6-R therapy. To our knowledge, this is the first reported case series in the U.S. describing the use of anti-IL-6/IL-6-R agents for COVID-19 patients. The outcomes were favorable in this series following multi-disciplinary patient assessment and treatment with anti-IL-6/IL-6-R therapy. Inflammatory markers and cytokine levels decreased significantly following treatment, which supports the hypothesis that interruption of IL-6 signaling in COVID-19 CRS has the potential to halt progression toward more severe and fatal outcomes.
The patients were predominantly men in their 60′s, including 10 patients ≥ 70 years, with the majority of them presenting from home. Typical respiratory and systemic symptoms including shortness of breath, cough, fever, and flu-like symptoms were noted as consistent with the reported literature of SARS-CoV-2 [1], [2], [27]. Enteric symptoms including diarrhea was noted only in 32% of the cases. A little more than sixty percent had chronic hypertension while close to a third had diabetes. Lymphocytopenia with an ALC ≤ 1500 cells/mm3 was seen in 97% of cases suggesting severe disease and immune dysregulation [1], [2]. Only 32% of the patients had normal ferritin on admission and none had a normal CRP, suggestive of severe disease and the presence of significant systemic inflammation.
Our case fatality rate (13%) is low compared to 50% or more among critically ill COVID-19 patients [1], [27], [28], [29] and our outcomes compare favorably to the tocilizumab experience from two other single center series [13], [16]. Importantly, the majority of the patients in our series, unlike others, did not receive any concomitant COVID-19 specific treatments, suggesting that interruption of IL-6 activity was an important therapeutic intervention. In our series, 81% (25/31) of patients have remained ventilator free at the time of this publication. Of the patients who were extubated, age range was 40 to 80 years which suggests that age may not be the sole indicator for successful extubation.
We believe that the timing of tocilizumab administration and multi-disciplinary input were important determinants of outcome in this study. We used the rapid upward trend in inflammatory markers (CRP ≥ 80 mg/L, Ferritin ≥ 600 ng/mL) along with clinical deterioration with increasing oxygen requirements (≥5 L) as a harbinger of CRS to trigger anti-IL-6/IL-6-R therapy. Treatment at the onset of severe CRS physiology, but prior to progression to severe multi-organ failure syndrome may be the ideal window for intervention with anti-IL-6/IL-6-R agents. We speculate that IL-6 is a critical driver of the initial phases of COVID-related CRS, but multiple cytokines and humoral mediators assume prominent roles as the disease progresses.
During the time of this study, the majority of our patients had no access to other therapies including antivirals such as remdesivir, or convalescent plasma (CP). Although some patients (11 of 31) received hydroxychloroquine prior to anti-IL-6/IL-6-R therapy, their clinical status deteriorated, necessitating escalation of care. Five of the patients did receive CP, three of which were late in the course of the disease, two of whom remain intubated, and the third non-intubated at the time of this analysis. The treatment decision consensus cut off of ferritin (≥600 ng/mL) and CRP (≥80 mg/L) were based on the retrospective data from Wuhan exhibiting mean ferritin and CRP levels (1297.6 ng/mL and 126.6 mg/L respectively) among non-survivors vs survivors (ferritin 614.0 ng/ml and CRP 34.1 mg/L) p < 0.001 [5], [9]. Furthermore, the median CRP levels in the other published case series of tocilizumab use in severe COVID-19 at the time of our study were 75.06 ± 66.80 mg/L [10]. The doses of tocilizumab (4–8 mg/kg) and siltuximab (11 mg/kg) were derived from CAR-T cell CRS management literature [30], [31] and other published evidence of use of these agents [13], as the dose(s) and frequency for optimal efficacy in COVID-19 CRS remains part of active clinical trials. Treatment with tocilizumab was associated with prompt reductions in serum CRP as well as other inflammatory markers as has been shown in other studies [13], [16]. Our observation from this series shows that CRP might be a better predictor of virally driven hyperinflammation and a better surrogate marker of CRS.
Among mechanically ventilated patients, the oxygen requirements paralleled those of patients with severe ARDS with median PaO2/FiO2 ratio of 86 mmHg and median PEEP of 18 cm H20. These were not immediately reversed after anti-IL-6/IL-6-R therapy administration as the median time from anti-IL-6/IL-6-R therapy initiation to extubation was 11 days. While these therapies may halt the cytokine storm process itself if timed appropriately, microvascular and epithelial damage in the airways will likely take additional time to resolve. For such reasons, as well as with our observation that close to half of the patients (14/31 patients) who received anti-IL-6 therapy did not deteriorate to the point of requiring mechanical ventilation, we believe that the most appropriate time to intervene with anti-IL-6/IL-6-R therapy is at the onset or early stages of COVID-19 CRS. It is important to note, however, the D-dimer levels in our cohort significantly increased within a week of treatment, especially in mechanically ventilated patients. This raises the question as to whether microvascular thrombotic complications may have led to impaired oxygenation and greater dependency on ventilatory support. While we do not yet have established parameters to predict this progression, rapidly increasing inflammatory markers along with rapid increases in oxygenation requirements seems to herald the onset of severe hyperinflammatory syndromes.
Serum cytokine changes in response to treatment resonated well with what we observed with respect to routine inflammatory markers and clinical outcomes. While an initial spike in IL-6 was noted following treatment with anti-IL-6/IL-6-R therapy, over time a significant decrease was documented. We speculate that the initial rise in IL-6 following treatment was due to receptor occupancy and increases in free serum levels. As the therapeutic effect took hold and the inflammatory response subsided, serum IL-6 levels decreased. Accordingly, we also noted significant decreases in IL-10 and GM-CSF, along with a favorable trend in TNFα levels. The decrease in IL-10, an immunosuppressive cytokine, may have facilitated development of anti-viral immune responses. Other cytokines, including IL-1β, IL-2, IL-8, IFN-γ, IL-17, MCP-1, and IL-4 were not assessed in this series but may be physiologically important, as well. We also did not assess changes in peripheral T cell phenotype or function, which may also be informative with respect to COVID-19 immune dysfunction and response to therapy [21].
Our case series has several limitations. Firstly, it is a single center retrospective small case series which is hypothesis generating, and not definitive. While each subject did not have complete data at all time points, statistical analyses confirmed the significance of several comparisons. Our follow up is short, including 2 patients who are currently intubated at the time of censoring and the outcomes of these patients are not known. Moreover, we do not yet have an understanding of what the long-term effects may be following recovery from COVID-19 CRS and severe acute pulmonary disease. One theoretical concern of IL-6 blockade would be the potential hampering of anti-COVID IgG-mediated humoral immune response prolonging viral clearance [32], although other reports claim this may not be necessarily relevant [21]. None of our patients sustained any serious adverse events from the treatment in terms of disseminated or invasive fungal infections, irreversible hepatotoxicity or gastrointestinal perforation. One patient with prolonged intensive care stay had a transient candidemia associated with a peripherally inserted central catheter which resolved quickly with treatment.
Use of anti-IL-6/IL-6-R therapy resulted in highly favorable outcomes in severely ill COVID-19 patients treated in an academic community medical center. Positive clinical outcomes were associated with significant improvements in IL-6 levels, among other inflammatory markers and cytokines. Our encouraging experience supports ongoing controlled studies designed to assess the impact of anti-IL-6/IL-6-R therapy in severely ill COVID-19 patients. While antivirals, CP, and other investigational agents may be important in managing severe COVID-19 disease, our study along with rapidly emerging data from other centers, suggests that anti-IL-6/IL-6-R agents deserve strong consideration as part of the treatment algorithms given the positive outcomes and mechanistic rationale. Timing of anti-IL-6/IL-6-R treatment may be a critical determinant of therapeutic success and we hope that larger studies provide more insight into how we can best leverage these agents in combating COVID-19 CRS.
Declarations:
Ethics approval and consent to participate: The IRB approved the use of biospecimens and data from COVID-19 patients, including those treated with tocilizumab or siltuximab. Informed consent was obtained as part of an existing IRB approved biospecimen protocol, in addition to a specific informed consent for the treatment.
Availability of data and material:
The datasets used and/or analyzed for this report are available from the corresponding author upon reasonable request. KSM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions:
Kapil S. Meleveedu was involved in drafting/revising the manuscript, analysis/interpretation of data, and contributed to the figures. John Miskovsky, Joseph Meharg, Abd Abdelrahman, and Richa Tandon were involved in critical review of the manuscript. Ashley E. Moody was involved in review/revising/submitting the manuscript. Priscilla Dasilva was involved in review of the manuscript. Gabrielle Masse was involved in data collection and review of the manuscript. Jason LaPorte was involved in performing assays, data acquisition, review of the manuscript, and contributed to figures. Abdul Saied Calvino, Greg Allen, Rabih El-Bizri, Todd Roberts, and Vincent Armenio were involved in critical review of the manuscript. Steven C. Katz was involved in drafting/revising the manuscript, analysis/interpretation of data, and contributed to figures.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cytox.2020.100035.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. The Journal of clinical investigation. 2020;130(5) doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diao B. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Frontiers in Immunology. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye, Z., et al., Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. European radiology, 2020: p. 1-9. [DOI] [PMC free article] [PubMed]
- 7.Cao J. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold, T., et al., Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medRxiv 2020. Google Scholar, 2020.
- 9.Ruan Q. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive care medicine. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D.W. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biology of Blood and Marrow Transplantation. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalli G. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet. Rheumatology. 2020 doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancio M. Emerging Trends in COVID-19 Treatment: Learning from Inflammatory Conditions Associated with Cellular Therapies. Cytotherapy. 2020 doi: 10.1016/j.jcyt.2020.04.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X. Proceedings of the National Academy of Sciences. 2020. Effective treatment of severe COVID-19 patients with tocilizumab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michot J.-M. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Annals of Oncology. 2020 doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Advances. 2020;4(7):1307. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alattar R. Tocilizumab for the Treatment of Severe COVID-19. Journal of Medical Virology. 2020 doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teachey D.T. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer discovery. 2016;6(6):664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude S.L. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer journal (Sudbury, Mass.) 2014;20(2):119. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteleone G., Sarzi-Puttini P.C., Ardizzone S. Preventing COVID-19-induced pneumonia with anticytokine therapy. The Lancet Rheumatology. 2020;2(5) doi: 10.1016/S2665-9913(20)30092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 22.Hirano T., Murakami M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020 doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGonagle D. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmunity reviews. 2020:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnaldez F.I. The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. Journal of ImmunoTherapy of Cancer. 2020 doi: 10.1136/jitc-2020-000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranucci M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. Journal of Thrombosis and Haemostasis. 2020 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, B., et al., Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? Journal of Autoimmunity, 2020: p. 102452. [DOI] [PMC free article] [PubMed]
- 27.Docherty, A.B., et al., Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv, 2020: p. 2020.04.23.20076042.
- 28.Bhatraju P.K. Covid-19 in critically ill patients in the Seattle region—case series. New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arentz M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. Jama. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmoudjafari Z. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group survey on chimeric antigen receptor T cell therapy administrative, logistic, and toxicity management practices in the United States. Biology of Blood and Marrow Transplantation. 2019;25(1):26–33. doi: 10.1016/j.bbmt.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. Journal of Experimental Medicine. 2020;217(6) doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.