Supplemental Digital Content is available in the text.
Keywords: acute hypoxemic respiratory failure, acute respiratory distress syndrome, coronavirus disease 2019, high-flow nasal oxygen
Abstract
Objectives:
An ongoing outbreak of coronavirus disease 2019 is spreading globally. Acute hypoxemic respiratory failure is the most common complication of coronavirus disease 2019. However, the clinical effectiveness of early high-flow nasal oxygen treatment in patients with coronavirus disease 2019 with acute hypoxemic respiratory failure has not been explored. This study aimed to analyze the effectiveness of high-flow nasal oxygen treatment and to identify the variables predicting high-flow nasal oxygen treatment failure in coronavirus disease 2019 patients with acute hypoxemic respiratory failure.
Design:
A multicenter, retrospective cohort study.
Setting:
Three tertiary hospitals in Wuhan, China.
Patients:
Forty-three confirmed coronavirus disease 2019 adult patients with acute hypoxemic respiratory failure treated with high-flow nasal oxygen.
Interventions:
None.
Measurements and Main Results:
Mean age of the enrolled patients was 63.0 ± 9.7 years; female patients accounted for 41.9%. High-flow nasal oxygen failure (defined as upgrading respiratory support to positive pressure ventilation or death) was observed in 20 patients (46.5%), of which 13 (30.2%) required endotracheal intubation. Patients with high-flow nasal oxygen success had a higher median oxygen saturation (96.0% vs 93.0%; p < 0.001) at admission than those with high-flow nasal oxygen failure. High-flow nasal oxygen failure was more likely in patients who were older (p = 0.030) and male (p = 0.037), had a significant increase in respiratory rate and a significant decrease in the ratio of oxygen saturation/Fio2 to respiratory rate index within 3 days of high-flow nasal oxygen treatment. In a multivariate logistic regression analysis model, male and lower oxygen saturation at admission remained independent predictors of high-flow nasal oxygen failure. The hospital mortality rate of the cohort was 32.5%; however, the hospital mortality rate in patients with high-flow nasal oxygen failure was 65%.
Conclusions:
High-flow nasal oxygen may be effective for treating coronavirus disease 2019 patients with mild to moderate acute hypoxemic respiratory failure. However, high-flow nasal oxygen failure was associated with a poor prognosis. Male and lower oxygenation at admission were the two strong predictors of high-flow nasal oxygen failure.
The coronavirus disease 2019 (COVID-19) has become the most serious public health emergency worldwide in the 21st century. The severe acute respiratory syndrome coronavirus 2 of COVID-19 is more infectious than the severe acute respiratory syndrome coronavirus and the Middle East respiratory syndrome coronavirus (1–3). Approximately 15–30% of COVID-19 patients rapidly progress to acute respiratory distress syndrome (ARDS) within 1–2 days of admission (4–8), and the mortality rate in the ARDS patients is currently as high as 74% (7). Considering the lack of specific drug treatment, organ support is currently the main treatment strategy; especially, respiratory support technology has been widely used in this COVID-19 outbreak (9, 10).
High-flow nasal oxygen (HFNO) is a novel noninvasive respiratory support modality that can deliver 60 L/min of gas flow and 0.21–1.0 of Fio2 through a special nasal cannula to patients (11, 12). Previous studies have shown that HFNO can reduce the rates of endotracheal intubation and 90-day mortality in patients with acute hypoxemic respiratory failure (AHRF) (13–15). Furthermore, recent studies (16, 17) have shown that HFNO may not be associated with increased aerosol dispersion with good interface fitting. Therefore, during the COVID-19 outbreak, HFNO is being commonly used for respiratory support in critically ill patients. In a single-center study by Yang et al (7), 63.5% of critically ill patients were treated with HFNO in the ICU. However, to our knowledge, the clinical efficacy and safety of early HFNO treatment in COVID-19 patients have not yet been explored. Therefore, we performed a multicenter, retrospective cohort study involving COVID-19 patients with AHRF treated with early HFNO to analyze the effectiveness of HFNO treatment and to identify the variables predicting HFNO failure.
MATERIALS AND METHODS
Study Design and Patients
This multicenter retrospective cohort study was performed at three tertiary hospitals in Wuhan city, China (Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology; Zhongnan Hospital of Wuhan University; and The Central Hospital of Wuhan) from February 15, 2020, to March 17, 2020. The inclusion criteria for HFNO cohort were as follows: patients with confirmed COVID-19, according to the World Health Organization (9) and Chinese official guideline; age more than 18 years; and treatment with HFNO within 14 days of admission. All patients were treated according to the standard protocols for antiviral, antibiotic, glucocorticoid, and Chinese medicine treatments.
The study was approved by the ethics committee of the participating hospitals (2020-21-K16). Written informed consent was waived due to the rapid emergence of this infectious disease.
HFNO Therapy and the Criteria for Treatment Failure
The clinical equipment used for HFNO treatment (OH-70C; Micomme Medical, Hunan, China; Hifent HUMID-BM; Shenyang RMS Medical Tech, Shenyang, China; and Airvo 2; Fisher & Paykel Healthcare, Auckland, New Zealand) in the three hospitals could be adjusted in terms of gas flow (8–60 L/min), Fio2 (0.21–1.0), and gas temperature (31–37°C). The main indication for HFNO treatment was the failure of conventional oxygen therapy (nasal cannula, mask, or nonrebreathing oxygen mask) in relieving respiratory distress and/or hypoxemia (ratio of Pao2 to Fio2 [PFR] < 300 mm Hg or more than 5 L/min of supplemental oxygen was required to maintain oxygen saturation [Spo2] > 93%). The initial gas flow rate was generally set to 40–50 L/min, the Fio2 was set to maintain Spo2 greater than 90%, and the temperature of the inhaled gas was adjusted according to the patient’s comfort and the dryness of the mouth and nose. To reduce the spread of aerosols during HFNO use, patients are instructed to wear surgical masks.
HFNO treatment was often continuous, and vital signs and breathing patterns were closely monitored. When it was difficult to alleviate respiratory distress symptoms or correct AHRF with HFNO, the attending physician and the patient together decided on selecting noninvasive positive pressure ventilation (NPPV) and invasive positive pressure ventilation (IPPV). The standard references for endotracheal intubation included the following: respiratory rate (RR) greater than 30 breaths/min; obvious accessary respiratory muscle activity or thoracoabdominal paradoxical breathing; PFR less than 120 mm Hg; progressive increase in Paco2; hemodynamic instability; and poor airway protection ability. HFNO failure was defined as upgrading respiratory support to NPPV or IPPV, or death after HFNO treatment.
Data Collection
We collected data on the following variables from the hospital electronic medical record systems: demographic characteristics (age, sex, and body mass index [BMI]); symptoms at admission (fever, fatigue, dyspnea, cough, and sputum); CT data (unilateral and bilateral lung lesions, ground-glass opacity, consolidation, and fibrosis); history of chronic diseases (hypertension, diabetes, cerebrovascular diseases, and malignant tumors); and changes in physiologic variables (vital signs and Spo2), the ratio of Spo2/Fio2 to RR (ROX) index, arterial blood gas variables (pH, Paco2, Pao2, bicarbonate, lactic acid, and PFR), and WBC and lymphocyte counts from day 1 (day of admission) to day 14. In addition, respiratory variables at various times after HFNO initiation during 72 hours and clinical outcomes (the rates of HFNO failure and endotracheal intubation, duration of HFNO treatment, length of hospital stay, and hospital mortality rate) were also recorded.
Statistical Analysis
Continuous variables are expressed as means ± sd or medians and interquartile ranges; categorical variables are expressed as frequencies or percentages. Continuous variables were compared between the HFNO success and failure groups using the t test (nonparametric tests were used for variables showing non-normal distribution). chi-square tests were used for comparing categorical variables. Variables associated with HFNO failure were assessed by means of a stepwise multivariate logistic regression analysis, and results are given as odds ratio (OR) with their 95% CIs. A two-sided p value of less than 0.05 was considered statistically significant.
RESULTS
From February 15, 2020, to March 17, 2020, a total of 290 confirmed COVID-19 patients were admitted, including 223 patients who had no ventilatory support, and 24 who received noninvasive or invasive mechanical ventilation (Fig. 1). Finally, a total of 43 patients (14.8%) fulfilling our eligibility criteria were included in the HFNO cohort. The mean age was 63.0 ± 9.7 years, female patients accounted for 41.9% (18 patients) of the cohort, and the mean BMI was 24.2 ± 2.5 kg/m2. Fever (90.7%), cough (72.1%), fatigue (55.8%), and dyspnea (53.5%) were the most common symptoms. Hypertension (39.5%) and diabetes (30.2%) were the most common comorbidities. The median duration from symptom onset to admission was 7.0 days (5.0–10.0 d). At admission, the RR and Spo2 were 24.2 ± 5.8 breaths/min and 95.0% (93.0–97.0%), respectively. Chest CT revealed ground-glass opacities in the bilateral lungs in 94.3% of patients (Table 1). As compared to intubated COVID-19 patients (13 patients) without going to HFNO first (Fig. 1), critically ill patients with HFNO treatment were associated with older age (mean 63.0 vs 53.9 yr; p = 0.040), higher oxygenation (Spo2: median 95% vs 90%; p = 0.006), fewer days from illness onset to admission (median 7.0 vs 10.0 d; p = 0.033), and lower hospital mortality (32.5% vs 66.7%; p = 0.048) (Supplement table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F734; and Fig. 1).
Figure 1.
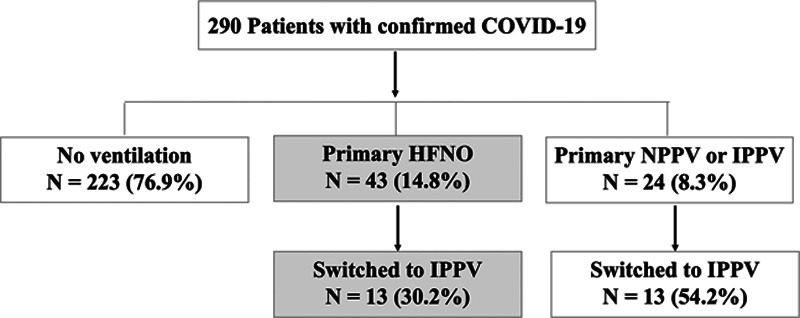
Flow chart of the study. COVID-19 = coronavirus disease 2019, HFNO = high-flow nasal oxygen, IPPV = invasive positive pressure ventilation, NPPV = noninvasive positive pressure ventilation.
TABLE 1.
Demographics, Symptoms, Physiology, Blood Test Results, and Clinical Outcomes Between High-Flow Nasal Oxygen Success and Failure on Hospital Admission
| Variables | All (n = 43) | HFNC Success Group (n = 23) | HFNC Failure Group (n = 20) | p |
|---|---|---|---|---|
| Age (yr), mean ± sd | 63.0 ± 9.7 | 60.0 ± 9.6 | 66.4 ± 8.9 | 0.030 |
| Female, n (%) | 18 (41.9) | 13 (56.5) | 5 (25.0) | 0.037 |
| Body mass index (kg/m2), mean ± sd | 24.2 ± 2.5 | 23.5 ± 1.8 | 24.9 ± 3.0 | 0.158 |
| Symptoms, n (%) | ||||
| Fever | 39 (90.7) | 20 (87.0) | 19 (95.0) | 0.610 |
| Cough | 31 (72.1) | 16 (69.6) | 15 (75.0) | 0.692 |
| Fatigue | 24 (55.8) | 13 (56.5) | 11 (55.0) | 0.920 |
| Dyspnea | 23 (53.5) | 9 (39.1) | 14 (70.0) | 0.043 |
| Sputum production | 19 (44.2) | 10 (43.5) | 9 (45.0) | 0.920 |
| Comorbidities, n (%) | ||||
| Hypertension | 17 (39.5) | 8 (34.8) | 9 (45) | 0.569 |
| Diabetes | 13 (30.2) | 5 (21.7) | 8 (40.0) | 0.193 |
| Smoking | 7/42 (16.7) | 3/23 (13) | 4/19 (21.1) | 0.682 |
| Alcohol | 3/42 (7.1) | 1/23 (4.3) | 2/19 (10.5) | 0.581 |
| Days from illness onset to admission (d), median (IQR) | 7.0 (5.0–10.0) | 6.0 (5.0–10.0) | 7.0 (6.5–9.5) | 0.238 |
| Vital signs | ||||
| Temperature (°C), mean ± sd | 37.6 ± 1.0 | 37.8 ± 0.8 | 37.4 ± 1.1 | 0.209 |
| Respiratory rate (breaths/min), mean ± sd | 24.2 ± 5.8 | 22.6 ± 4.3 | 25.9 ± 6.9 | 0.132 |
| Heart rate (beats/min), mean ± sd | 90.9 ± 13.6 | 90.2 ± 12.2 | 91.7 ± 15.3 | 0.731 |
| Mean blood pressure (mm Hg), mean ± sd | 92.4 ± 13.6 | 93.3 ± 11.2 | 91.4 ± 16.2 | 0.663 |
| Oxygen saturation (%), median (IQR) | 95.0 (93.0–97.0) | 96.0 (96.0–98.0) | 93.0 (90.5–94.5) | < 0.001 |
| Abnormalities on chest CT, n/total, n (%) | ||||
| Bilateral lesions | 33/35 (94.3) | 20/21 (95.2) | 13/14 (92.9) | > 0.999 |
| Ground-glass opacity | 33/35 (94.3) | 20/21 (95.2) | 13/14 (92.9) | > 0.999 |
| Patchy shadowing | 23/35 (65.7) | 11/21 (52.4) | 12/14 (85.7) | 0.07 |
| Arterial blood gas, mean ± sd | ||||
| pH | 7.50 ± 0.08 | 7.50 ± 0.06 | 7.50 ± 0.09 | 0.987 |
| Paco2 (mm Hg) | 31.3 ± 8.5 | 31.0 ± 6.6 | 31.5 ± 10.1 | 0.525 |
| Pao2 (mm Hg) | 79.0 ± 39.8 | 106.3 ± 46.0 | 57.8 ± 15.2 | 0.031 |
| Bicarbonate (mmol/L) | 22.2 ± 4.0 | 22.3 ± 4.1 | 22.1 ± 4.3 | 0.934 |
| Lactate (mmol/L) | 1.3 ± 0.6 | 1.2 ± 0.7 | 1.5 ± 0.5 | 0.483 |
| WBC count (× 109/L) | 7.2 ± 4.2 | 8.1 ± 5.3 | 6.4 ± 2.8 | 0.315 |
| Lymphocyte count (× 109/L) | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.7 ± 0.3 | 0.656 |
| Clinical outcomes | ||||
| Duration of high-flow nasal oxygen treatment (d), median (IQR) | 4.0 (2.0–7.0) | 5.0 (3.0–7.0) | 3.5 (1.5–6.5) | 0.229 |
| Length of stay in hospital (d), median (IQR) | 15.0 (9.0–28.0) | 22.5 (13.0–28.0) | 15.0 (8.0–26.0) | 0.387 |
| Endotracheal intubation, n (%) | 13 (30.2) | 0 (0.0) | 13 (65) | < 0.001 |
| Death, n (%) | 13/40 (32.5) | 0 (0.0) | 13/20 (65) | < 0.001 |
HFNC = high-flow nasal cannula, IQR = interquartile range.
HFNO Success Versus Failure
In the cohort, twenty patients (46.5%) experienced HFNO failure. HFNO failure was more likely in patients who were older (66.4 ± 8.9 vs 60.0 ± 9.6 yr; p = 0.03) and male (56.5% vs 25.0%; p = 0.037) (Table 1). Patients with HFNO success showed higher oxygenation levels (Spo2 96.0% [96.0–98.0%] vs 93.0% [90.5–94.5%]; p < 0.001) and fewer dyspnea symptom (39.1% vs 70.0%; p = 0.043) at admission than patients with HFNO failure (Table 1). Besides, patients with HFNO failure were associated with a significant increase in RR and a significant decrease in ROX index within the first 3 days of HFNO treatment (Fig. 2; and Supplement table 2, Supplemental Digital Content 2, http://links.lww.com/CCM/F735). After multivariate logistic regression analysis, male was independently associated with HFNO failure (adjusted OR, 6.948; 95% CI, 1.129–42.756; p = 0.037); a high Spo2 value at admission was a protective factor associated with HFNO failure (adjusted OR, 0.562; 95% CI, 0.384–0.823; p = 0.003) (Table 2).
Figure 2.
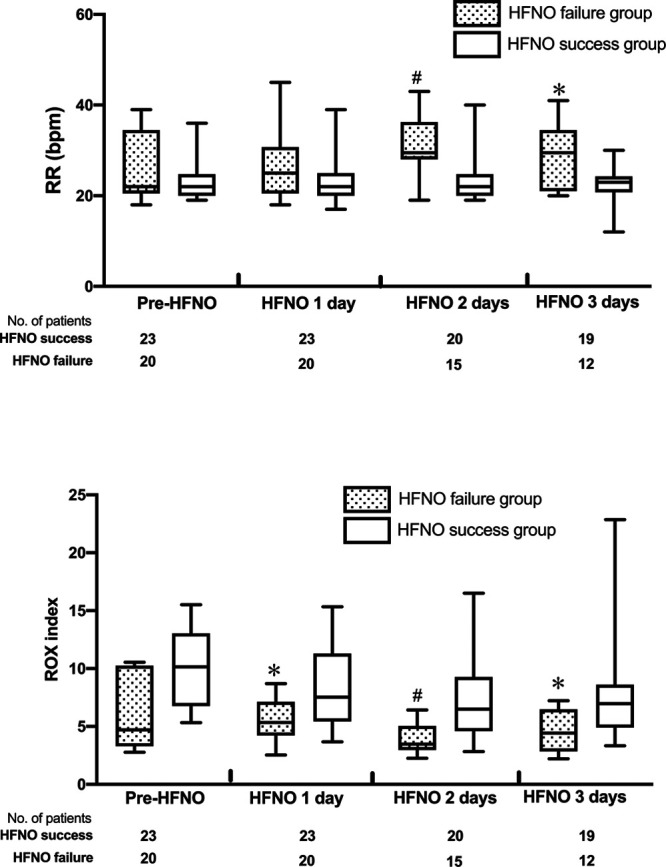
Box plots showing the changes of median respiratory rate (RR) and the ratio of oxygen saturation/Fio2 to RR (ROX) index (25–75th percentiles) within the first 3 d of high-flow nasal oxygen (HFNO) treatment between HFNO success group and HFNO failure group. *p < 0.05, #p < 0.001 between HFNO success group and HFNO failure group. bpm = breaths per minute.
TABLE 2.
Multivariate Logistic Regression Analysis of Factors Associated With High-Flow Nasal Oxygen Failure
| Risk Factorsa | OR (95% CI) | p |
|---|---|---|
| Gender | ||
| Female | Reference | |
| Male | 6.948 (1.129–42.756) | 0.037 |
| Oxygen saturation at admission (%) | 0.562 (0.384–0.823) | 0.003 |
OR = odds ratio.
aVariables entered in the multivariate logistic regression model were as follows: gender (female and male), age, oxygen saturation at admission, and the ratio of oxygen saturation/Fio2 to respiratory rate index on the first day of high-flow nasal oxygen treatment.
After median 3.5 hours (1.5–6.5 hr) of HFNO treatment, HFNO failure was observed in 20 patients (46.5%), of which 13 patients (30.2%) required endotracheal intubation, six patients (13.9%) later received NPPV treatment, and one patient died of arrhythmia and cardiac arrest after defecation. Owing to the rapid rate of respiratory deterioration in many patients, the results of pre-HFNO arterial blood gas analysis were available in only 12 patients; the mean Pao2/Fio2 was 122.3 ± 51.3 mm Hg. The median RR and Spo2 were 22.0 breaths/min (20.0–27.0 breaths/min) and 94% (92.0–95.5%), respectively, before HFNO treatment. In this HFNO cohort, the median duration from admission to the initial use of HFNO was 2.0 days (1.0–5.0 d), and the mean duration of HFNO treatment was 4.0 days (2.0–7.0 d).
Clinical Outcomes
The overall hospital mortality rate of the HFNO cohort was 32.5% (13/40 patients); however, the hospital mortality rates in patients with HFNO failure was 65% (13/20 patients), and the hospital mortality rates of HFNO failure patients with endotracheal intubation were 75% (nine of 12 patients), which was higher than that of patients intubated without HFNO treatment (66.7%, eight of 12 patients) (Supplement table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F734). The main causes of death were refractory hypoxia and shock. The median length of hospital stay was 15.0 days (9.0–28.0 d). Survivors were associated with younger age (60.7 ± 9.7 vs 69.9 ± 6.0 yr; p = 0.003), fewer dyspnea symptom (37.0% vs 76.9%; p = 0.018), higher Spo2 (96.0% [93.0–98.0%] vs 93.0% [91.0–95.0%]; p = 0.043), and longer length of hospital stay (25.1 ± 12.7 d vs 10.8 ± 4.7 d; p < 0.001) than nonsurvivors (Table 3).
TABLE 3.
Demographics, Physiology, and Blood Test Results Between Survivor and Nonsurvivor on Hospital Admission
| Variables | Survivor (n = 27) | Nonsurvivor (n = 13) | p |
|---|---|---|---|
| Age (yr), mean ± sd | 60.7 ± 9.7 | 69.9 ± 6.0 | 0.003 |
| Female, n (%) | 13 (48.1) | 2 (15.4) | 0.080 |
| Body mass index (kg/m2), mean ± sd | 23.7 ± 1.8 | 24.8 ± 3.5 | 0.375 |
| Symptoms, n (%) | |||
| Fever | 24 (88.9) | 13 (100) | 0.538 |
| Fatigue | 15 (55.6) | 8 (61.5) | 0.720 |
| Dyspnea | 10 (37.0) | 10 (76.9) | 0.018 |
| Cough | 21 (77.8) | 8 (61.5) | 0.451 |
| Sputum production | 11 (40.7) | 7 (53.8) | 0.435 |
| Comorbidities, n (%) | |||
| Hypertension | 10/26 (38.5) | 6/13 (46.2) | 0.736 |
| Diabetes | 8/27 (29.6) | 4/13 (30.8) | > 0.999 |
| Days from illness onset to admission (d), median (IQR) | 7.0 (5.0–10.0) | 7.0 (5.0–8.0) | 0.551 |
| Vital signs | |||
| Temperature (°C), median (IQR) | 37.7 (37.1–38.2) | 36.7 (36.6–38.5) | 0.611 |
| Respiratory rate (breaths/min), median (IQR) | 22.0 (20.0–24.0) | 24.0 (22.0–32.0) | 0.198 |
| Heart rate (beats/min), median (IQR) | 88.0 (84.0–98.0) | 99.0 (90.0–103.0) | 0.328 |
| Mean blood pressure (mm Hg), mean ± sd | 93.6 ± 11.6 | 90.9 ± 17.3 | 0.562 |
| Oxygen saturation (%), median (IQR) | 96.0 (93.0–98.0) | 93.0 (91.0–95.0) | 0.043 |
| WBC count (× 109/L), median (IQR) | 5.9 (3.1–8.2) | 6.2 (5.0–8.0) | 0.477 |
| Lymphocyte count (× 109/L), mean ± sd | 0.6 ± 0.3 | 0.7 ± 0.4 | 0.830 |
| Endotracheal intubation, n (%) | 3 (25) | 9 (75) | 0.001 |
| Length of stay in hospital (d), mean ± sd | 25.1 ± 12.7 | 10.8 ± 4.7 | < 0.001 |
| Duration of high-flow nasal oxygen treatment (d), mean ± sd | 4.8 ± 2.9 | 4.5 ± 3.0 | 0.719 |
IQR = interquartile range.
DISCUSSION
To our knowledge, this is the first study to evaluate the effectiveness and outcomes of HFNO in a cohort of 43 confirmed COVID-19 patients with AHRF. Following are the main results: 1) early HFNO may be an effective respiratory support modality for COVID-19 patients with mild to moderate AHRF; 2) failure of HFNO treatment indicates a poor prognosis; and 3) male and lower oxygenation at admission were independent predictors of HFNO failure.
HFNO has been increasingly used to avoid endotracheal intubation in patients with AHRF in recent years (18); however, its use has rarely been reported in patients with epidemic severe acute respiratory infection (19). In this HFNO cohort, the rate of intubation was 30.2%. These findings are similar to the results of the use of HFNO for AHRF due to other causes (13, 20–22). Frat et al (13) found that compared with standard oxygen therapy and NPPV, HFNO can effectively reduce the rate of tracheal intubation in patients with hypoxic ARF mainly caused by community-associated pneumonia (HFNO vs standard oxygen therapy and NPPV: 38% vs 47% and 50%). This may be related to the favorable physiologic effects of HFNO (11, 12), including low-level positive end-expiratory pressure (PEEP) to keep the alveoli open, washout of nasopharyngeal dead space to improve ventilation efficiency, improvement in breathing patterns, and enhancement of airway heating and humidification function. Chest CT (23) findings and pathology reports (24) of COVID-19 patients show that most patients show interstitial lung edema in the early course of the disease. Therefore, the low-level PEEP produced by HFNO can effectively expand the alveoli and improve lung ventilation and perfusion ratios, which avoids the exacerbation of hypoxemia.
During this unprecedented outbreak of COVID-19, many countries have recognized that human biological sex plays an important role affecting the prognosis of patients with COVID-19 (8, 25–27). Scully et al (25) reported that the case fatality rate of male patients was 1.7 times that of female patients (7.3% male vs 4.4% female; p < 0.0001) in 38 countries that provided sex-disaggregated data. In Wuhan, China, Xie et al (28) found that among 168 patients who died between January 21, 2020, and January 30, 2020, 75% were male patients. Besides, Mo et al (29) also showed that male patients were more prone to refractory cases (64.7% vs 44.3%; p = 0.011). In this study, we also found 75% of patients with HFNO failure were male. In a multivariate logistic regression analysis model, male remained an independent risk factor of HFNO failure. This significant difference of HFNO failure rate between men and women may partly be explained by sex biological factors (e.g., sex differences in immune responses and angiotensin-converting enzyme 2 receptors) and gender sociocultural factors (preexisting diseases [e.g., hypertension, diabetes, and chronic lung disease], higher risk behaviors [e.g., smoking and alcohol use], occupational exposure and so on) (25, 26).
Our study also showed that COVID-19 patients with HFNO failure have a poor prognosis, with a hospital mortality rate of 65%; this is also similar to the results of HFNO failure groups in other studies (30, 31). Therefore, accurate prediction of HFNO failure is the focus of attention. Lower oxygenation levels and higher RR at admission may suggest an increased risk of HFNO failure. Furthermore, increased RR after HFNO also provides a simple and reliable variable for predicting HFNO failure. Similar results have also been found in non-COVID-19 patients treated with HFNO (19, 20, 32, 33).
The ROX index is a simple index for predicting the risk of HFNO failure, proposed by Roca et al (31, 34). It is equal to the ratio of Spo2/Fio2 to RR, combining the patient’s oxygenation status and respiratory pattern. It has been confirmed that an ROX index greater than 4.88 after HFNO treatment indicates a lower risk of HFNO failure (31, 34). In this cohort, the ROX index in the HFNO failure group was continuously and significantly lower than that in the HFNO success group on the first 3 days after HFNO treatment. Therefore, the change trend of ROX index may be used as a simple bedside monitoring index in AHRF patients receiving HFNO treatment.
Patients with HFNO failure in this study showed a high mortality rate, especially those with endotracheal intubation who showed a rate as high as 75%; this is similar to the mortality rate in mechanically ventilated COVID-19 patients reported by recently published epidemiological studies (4–8). This may be related to the severity of illness and the delay in tracheal intubation (30, 31) in critically ill patients with COVID-19. In this study, patients with HFNO failure were endotracheally intubated after a median of 3.5 days (1.5–6.5 d) after HFNO treatment. Therefore, once HFNO treatment fails to improve gas exchange and ventilatory function, tracheal intubation should be performed as soon as possible.
This study has the following limitations. First of all, our study was a retrospective study because it is difficult to conduct a prospective controlled study during a rapidly progressing COVID-19 outbreak. In addition, the COVID-19 epidemic in Wuhan city has gradually been controlled, and recently, there have been fewer newly confirmed COVID-19 cases. However, the results of our study can be used as a basis for conducting prospective controlled studies in other countries where an outbreak is currently underway. Second, due to limited HFNO equipment in the sudden nature of the COVID-19 outbreak, there are obvious selection bias that HFNO may be often used for those more severe AHRF patients (e.g., Pao2/Fio2 122.3 ± 51.3 mm Hg of 12 patients with HFNO treatment). Therefore, it remains unclear whether HFNO treatment is more beneficial to early COVID-19 patients with mild hypoxic respiratory failure. Third, it was difficult to analyze many short-term physiologic variables and critical illness scores before and after HFNO treatment because of rapid progress of COVID-19, such as blood gas analysis results and the Sequential Organ Failure Assessment and the Acute Physiology and Chronic Health Evaluation scores. However, we included several clinically accessible variables (Spo2, the trend of ROX index and RR after HFNO treatment) that could possibly predict the risk of HFNO failure. Third, this study did not investigate aerosol dispersion during HFNO treatment in the ward. However, similar studies (16, 17) did not report an obvious increase of the spread of aerosol dispersion when HFNO use. Instructing the patients to wear surgical masks while using HFNO may have reduced the exhalation of aerosol droplets in our study.
CONCLUSIONS
Early HFNO may be an effective respiratory support modality for COVID-19 patients with mild to moderate AHRF and its use may reduce the need for tracheal intubation. However, failure of HFNO might be associated with higher hospital mortality rate. Male and lower oxygenation at admission were independent predictors of HFNO failure. Once HFNO failure and progressively impaired gas exchange, early intubation should be considered. Taken together in the context of the global COVID-19 outbreak, our results constitute a good basis for performing a prospective controlled study to further investigate the potential value of HFNO in COVID-19 patients with AHRF.
ACKNOWLEDGMENTS
We thank all medical staff involved in the treatment of patients with coronavirus disease 2019 in all participating centers, all patients and their families involved in the study, and Chen Liang, MHS, for her valuable contributions to the methodology of statistical analysis.
Supplementary Material
Footnotes
*See also p. 1704.
Drs. Xia, Zhang, Ni, Chen, Zhou, and Gao are contributed equally to this article.
Drs. Guo, Zhao, Hu, Cheng, and Zhan are joint corresponding authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
This study was supported by Zhejiang University special scientific research fund for coronavirus disease 2019 prevention and control (number 2020XGZX008); National Key Research and Development Program of China (number 2016YFC1304300); National Natural Science Foundation of China (number 81870072); Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (number 2018-I2M-1-003); and Nonprofit Central Research Institute Fund of CAMS (number 2019TX320006).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.World Health Organization. Coronavirus Disease (COVID-19) Outbreak. 2020. Available at: https://www.who.int. Accessed March 20, 2020
- 2.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020; 395:470–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeri NC, Shrestha N, Rahman MS, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned?. Int J Epidemiol. 2020; 49:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020; 395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected: Interim Guidance. 2020. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 13, 2020
- 10.Ñamendys-Silva SA. Respiratory support for patients with COVID-19 infection. Lancet Respir Med. 2020; 8:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spoletini G, Alotaibi M, Blasi F, et al. Heated humidified high-flow nasal oxygen in adults: Mechanisms of action and clinical implications. Chest. 2015; 148:253–261 [DOI] [PubMed] [Google Scholar]
- 12.Ischaki E, Pantazopoulos I, Zakynthinos S. Nasal high flow therapy: A novel treatment rather than a more expensive oxygen device. Eur Respir Rev. 2017; 26:170028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frat JP, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015; 372:2185–2196 [DOI] [PubMed] [Google Scholar]
- 14.Frat JP, Ragot S, Girault C, et al. ; REVA network. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: A post-hoc analysis of a randomised trial. Lancet Respir Med. 2016; 4:646–652 [DOI] [PubMed] [Google Scholar]
- 15.Coudroy R, Jamet A, Petua P, et al. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: An observational cohort study. Ann Intensive Care. 2016; 6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019; 53:1802339. [DOI] [PubMed] [Google Scholar]
- 17.Leung CCH, Joynt GM, Gomersall CD, et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: A randomized controlled crossover trial. J Hosp Infect. 2019; 101:84–87 [DOI] [PubMed] [Google Scholar]
- 18.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: A systematic review and meta-analysis. Intensive Care Med. 2019; 45:563–572 [DOI] [PubMed] [Google Scholar]
- 19.Rello J, Pérez M, Roca O, et al. ; CRIPS investigators. High-flow nasal therapy in adults with severe acute respiratory infection: A cohort study in patients with 2009 influenza A/H1N1v. J Crit Care. 2012; 27:434–439 [DOI] [PubMed] [Google Scholar]
- 20.Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: A prospective pilot study. Intensive Care Med. 2011; 37:1780–1786 [DOI] [PubMed] [Google Scholar]
- 21.Frat JP, Ragot S, Coudroy R, et al. ; REVA network. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Crit Care Med. 2018; 46:208–215 [DOI] [PubMed] [Google Scholar]
- 22.Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017; 151:764–775 [DOI] [PubMed] [Google Scholar]
- 23.Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology. 2020; 296:E15–E25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8:420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scully EP, Haverfield J, Ursin RL, et al. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020; 20:442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Global Health 50/50. Men, Sex, Gender and COVID-19. 2020. Available at: https://globalhealth5050.org/covid19/men-sex-gender-and-covid-19/. Accessed June 20, 2020
- 27.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020; 8:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J, Tong Z, Guan X, et al. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open. 2020; 3:e205619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020ciaa27032173725 [Google Scholar]
- 30.Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015; 41:623–632 [DOI] [PubMed] [Google Scholar]
- 31.Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019; 199:1368–1376 [DOI] [PubMed] [Google Scholar]
- 32.Papazian L, Corley A, Hess D, et al. Use of high-flow nasal cannula oxygenation in ICU adults: A narrative review. Intensive Care Med. 2016; 42:1336–1349 [DOI] [PubMed] [Google Scholar]
- 33.Messika J, Ben Ahmed K, Gaudry S, et al. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: A 1-year observational study. Respir Care. 2015; 60:162–169 [DOI] [PubMed] [Google Scholar]
- 34.Roca O, Messika J, Caralt B, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J Crit Care. 2016; 35:200–205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


