The COVID-19 pandemic and post-surge planning highlights the significance of preoperative optimization in a way previously unrecognized in the perioperative period. Published protocols have largely emphasized planning for the intraoperative period1,2 with less attention afforded to reporting the design, implementation, and scaling of preoperative considerations and testing for long-term safety strategy. We present 2 transformative and sustained changes to our preoperative assessment process initiated during the COVID-19 surge that were pivotal to our success in resuming elective surgeries. These preoperative interventions are the “overnight” transition to 100% virtual clinic appointments and the widespread implementation of multiple COVID-19 testing pathways. The improvements were accomplished by redeployment of preoperative clinic staff to a work-at-home model, moving to preoperative video visits, incorporating universal COVID-19 screening, and the development and refinement of previously non-existent preoperative respiratory pathogen testing protocols.
In the post-COVID pandemic era, federal, state, and county regulators and national medical societies have established principles to resuming elective surgery. These center around evaluation of timing of reopening based on local cases, availability of COVID-19 testing in the facility, supply of personal protective equipment (PPE), and prioritization of cases and scheduling.3,4 When the California ban on elective surgery was lifted on April 23, 2020, Stanford Health Care (SHC) and Lucille Packard Children's Hospital had already drafted preliminary plans for resuming elective procedures. Our health system's thoughtful and preemptive perioperative preparation was based on rapid iterations from multidisciplinary teams that had commenced a month earlier when we ceased performing elective cases in mid-March.
APPROACH TO ACCELERATED IMPLEMENTATION OF TELEHEALTH VISITS AND PREOPERATIVE TESTING PROTOCOLS
Transition to Telehealth Platform for all Preoperative Evaluations
Our SHC Interventional Platform (IP) provides the umbrella structure for patients presenting for any surgery or procedure, including out-of-operating room, cardiac catheterization, interventional radiology, and endoscopic procedures. The IP team also works closely with our Anesthesia Preoperative Evaluation Clinic, which consists of patient assessment by nurse practitioners or anesthesiology residents, with medical direction provided by 1 supervising attending anesthesiologist for 6 clinic sites.
Before the COVID-19 pandemic, APEC conducted >700 video visits a month through a telehealth platform, comprising 27% of our total preoperative visits. Our shelter-in-place mandate instituted in mid-March provided the impetus to rapidly pivot to an almost total telehealth model and enabled nearly all APEC nurse practitioners to work from remote locations. Within one business day, APEC converted all in-person visits to an entirely virtual platform through completion of remote access training and the delivery of laptop computers for staff to ensure a seamless go-live implementation (Fig. 1 a).
FIGURE 1.

(A) Ratio of in-person to telehealth preoperative anesthesia visits pre- and post-implementation. (B) Prioritization criteria for COVID-19 preoperative testing. (DOS: day of surgery). (C) Protocol for COVID-19 preoperative testing of outpatients. (D) Preoperative protocol for low-risk procedures with moderate sedation. (E) Protocol for COVID-19 preoperative testing of inpatients. (F) COVID-19 testing workflow optimization improvements. (G) Preoperative COVID-19 testing order sets. (H) Status of preoperative COVID-19 tests on OR dashboard.
FIGURE 1 (Continued).
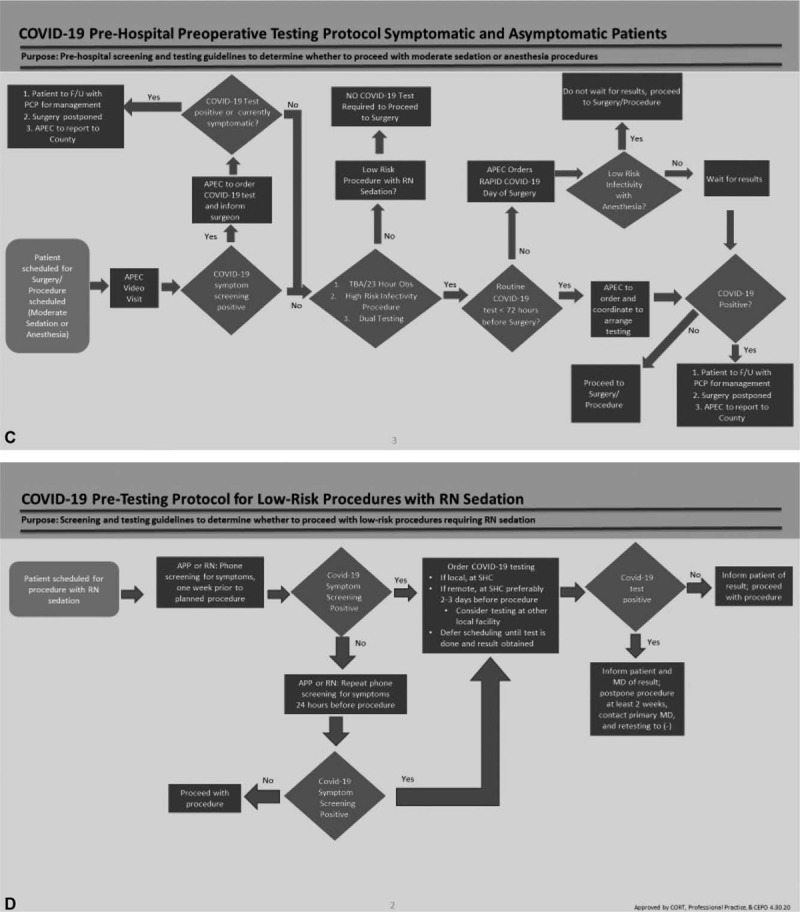
(A) Ratio of in-person to telehealth preoperative anesthesia visits pre- and post-implementation. (B) Prioritization criteria for COVID-19 preoperative testing. (DOS: day of surgery). (C) Protocol for COVID-19 preoperative testing of outpatients. (D) Preoperative protocol for low-risk procedures with moderate sedation. (E) Protocol for COVID-19 preoperative testing of inpatients. (F) COVID-19 testing workflow optimization improvements. (G) Preoperative COVID-19 testing order sets. (H) Status of preoperative COVID-19 tests on OR dashboard.
FIGURE 1 (Continued).
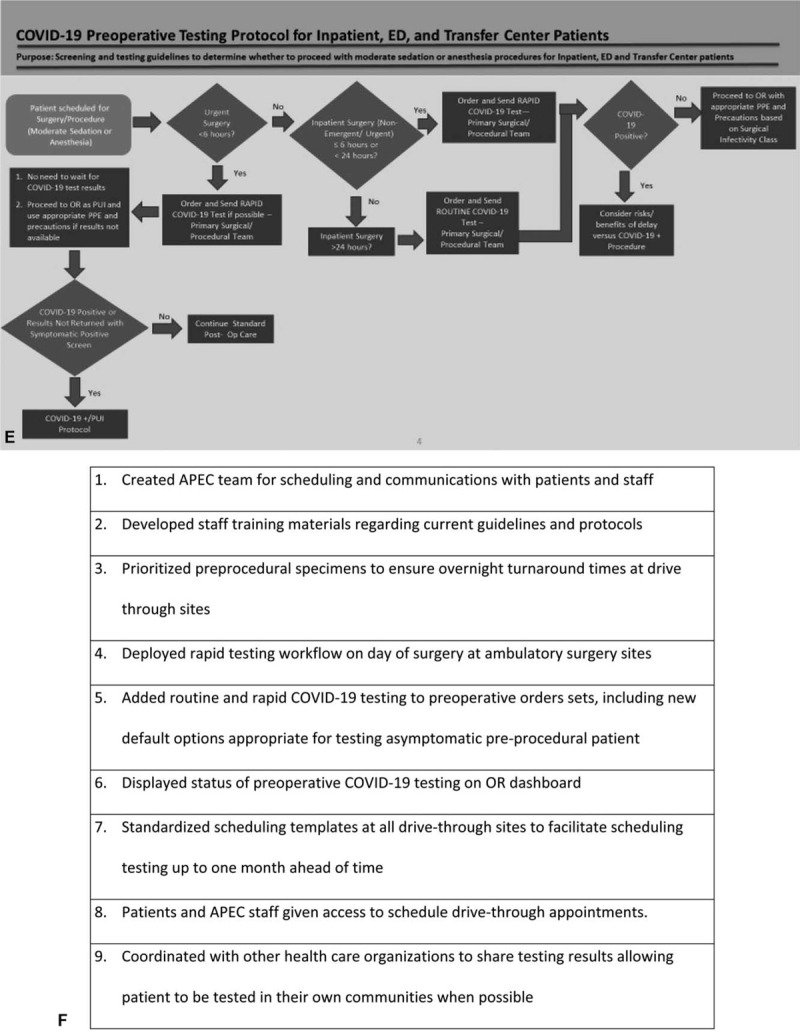
(A) Ratio of in-person to telehealth preoperative anesthesia visits pre- and post-implementation. (B) Prioritization criteria for COVID-19 preoperative testing. (DOS: day of surgery). (C) Protocol for COVID-19 preoperative testing of outpatients. (D) Preoperative protocol for low-risk procedures with moderate sedation. (E) Protocol for COVID-19 preoperative testing of inpatients. (F) COVID-19 testing workflow optimization improvements. (G) Preoperative COVID-19 testing order sets. (H) Status of preoperative COVID-19 tests on OR dashboard.
FIGURE 1 (Continued).
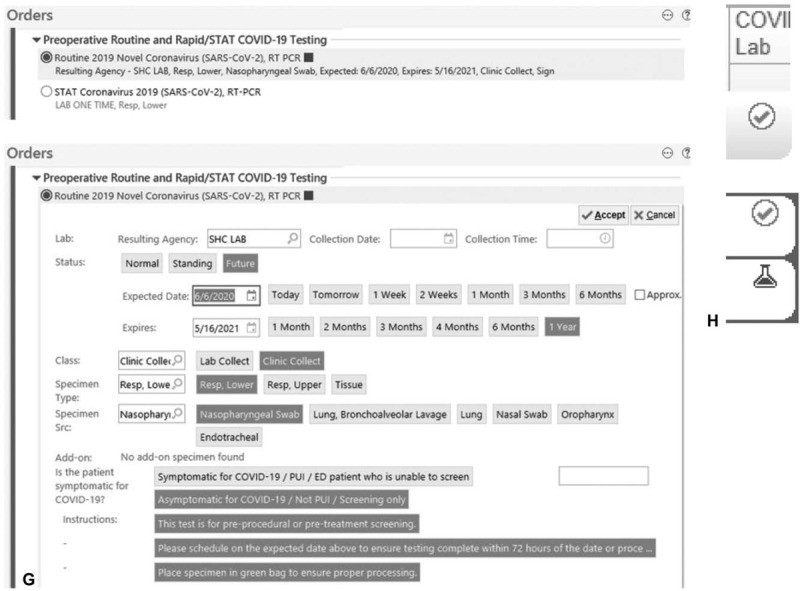
(A) Ratio of in-person to telehealth preoperative anesthesia visits pre- and post-implementation. (B) Prioritization criteria for COVID-19 preoperative testing. (DOS: day of surgery). (C) Protocol for COVID-19 preoperative testing of outpatients. (D) Preoperative protocol for low-risk procedures with moderate sedation. (E) Protocol for COVID-19 preoperative testing of inpatients. (F) COVID-19 testing workflow optimization improvements. (G) Preoperative COVID-19 testing order sets. (H) Status of preoperative COVID-19 tests on OR dashboard.
Since the transition to accommodate the shelter-in-place mandate, we conducted >3500 telehealth visits. Post implementation, only several patients were seen in person. Reasons to evaluate in person were to auscultate cardiac murmurs and for the inability for a video visit for 1 elderly patient. Preoperative laboratory tests, EKGs, and additional diagnostic tests were coordinated at the patient's primary care offices in some instances, but predominantly deferred until the day of surgery. Staff made every attempt to obtain prior medical records and testing results, limiting the need for new testing. Efforts were also made not to use commercial labs for standard preoperative testing to minimize patient travel and maximize social distancing. Anecdotal reports showed patients and APEC staff felt safer with virtual visits, as this provided reduced exposure to potential transmission and preserved personal protective equipment (PPE). They expressed satisfaction at the opportunity to continue their prescheduled appointments and employment during the shelter-in-place mandate.
Rapid Implementation of Preoperative COVID-19 Testing Protocols
Before implementation, we had to overcome several barriers related to COVID-19 testing prioritization and availability. Through coordination with surgery, anesthesiology, and primary care departments, we obtained consensus to test asymptomatic patients when limited RT-PCR testing resources were restricted to symptomatic patients in a small pilot. At that time, little data existed to support preoperative COVID-19 testing. The second hurdle was to coordinate testing at multiple drive-through sites in the 3-day window before surgery, to minimize exposure to patients and staff and to ensure the tests were resulted before surgery. Third was the challenge of rapidly iterating the protocols to a dynamic situation and communicating evolving changes effectively to the clinical team. Finally, before the shelter-in-place mandate, only selected groups of patients scheduled for surgery underwent advanced preoperative evaluation before sedation and anesthesia. The COVID-19 pandemic revealed the need for establishing consistent standards for preoperative assessment of any patient undergoing sedation and anesthesia. As a result, all patients, regardless of anesthesia or sedation class, were evaluated virtually by the APEC staff, which also allowed for universal COVID-19 symptom screening.
A pilot program was conducted where preoperative testing was performed for all high-risk patients for aerosol production with consensus approval from department chairs and perioperative leadership. Guiding principles for preoperative screening were developed, stratifying the high-risk patients (Fig. 1 B), including those having aerodigestive procedures, thoracic surgeries, otolaryngology, and head and neck procedures. Patients undergoing surgeries deemed to pose a higher risk of transmission due to prolonged intraoperative exposure of aerosol with the use of powered instrumentation in the airway received dual testing. Same-day testing was offered to patients undergoing urgent procedures and outpatients where geographic or physical limitations precluded testing before the day of surgery. All inpatients would undergo testing before any procedures needing monitored anesthesia care, given the possible need for bag-mask ventilation.
During the pilot, results from the “routine” (RT-PCR) test were obtained within 24 to 48 hours, compared to 2 to 6 hours for the “rapid” (Cepheid, Xpert) test. At the end of the 2-week pilot, we were able to offer widespread testing availability and a turnaround time of 12 hours for routine tests and <2 hours for the rapid test. We quickly developed multiple preoperative pathways for outpatients, inpatients, day of surgery, and low-risk procedures with moderate sedation (Fig. 1 C-F). Two key decision nodes in the protocols were deciding when to order routine versus rapid tests and whether the results were needed before surgery based on the infectivity risk and procedure urgency.
Protocol for COVID-19 Preoperative Testing of Outpatients
The APEC staff coordinated the outpatient pathway through virtual visits with the patients, communication with the drive-through testing sites, and follow-up with the virology laboratory for all test results (Fig. 1 C). If the screening was positive, APEC staff ordered a COVID-19 test. If the patient was symptomatic or had a positive test, surgery was postponed, and the patient was referred to their primary care physician. Depending on test results, the patient proceeded with the protocol based on risk of infectivity and type of hospital stay post-procedure. If patients were unable to complete drive-through testing due to a physical or geographic limitation or hardship, then day of surgery testing was scheduled. Asymptomatic outpatients presenting for low-risk procedures with nurse administered moderate sedation were not tested unless they had a positive screening (Fig. 1 D).
Protocol for COVID-19 Preoperative Testing of Inpatients
In our inpatient pathway (Fig. 1 E), all patients were tested before procedures. Although patients undergoing emergent and urgent cases received the same day test, procedures were not delayed for test results, and they proceeded with appropriate PPE and precautions. At the time of the pilot, we had a limited supply of Cepheid tests used on our same day pathway, and procedures that could wait 24 hours had routine tests sent. All other inpatient procedures received a same day test.
Post-Surge Preoperative Assessment and Testing Workflow Stabilization
As we transitioned to post-surge planning, we continued to adapt and optimize our COVID-19 preoperative pathways. New operational challenges in this phase included scaling universal preoperative testing and virtual visits to all patients while rescheduling the significant backlog of elective procedures, incorporating new patients, and continuing remote work policies for clinic staff. Multiple improvements were made to efficiently accommodate 150 daily preoperative virtual visits and COVID-19 tests (Fig. 1 F). These enhancements included: prioritization and standardization given to tests drawn for preoperative assessment from drive-through sites, creating small team dedicated to scheduling preoperative COVID-19 tests and follow-up of results, adding COVID-19 testing orders to preoperative order sets (Fig. 1 G), and displaying testing status updates on OR dashboards (Fig. 1 H). These interventions have led to the successful resumption of surgical volume to close to 90% of prepandemic levels within 2 weeks.
DISCUSSION
Thoughtful preoperative assessment has been shown to be cost-effective in reducing case cancellations on the day of surgery and in optimizing perioperative care.5–7 We anticipate the unparalleled emergence of COVID-19 will alter the future of perioperative medicine, with increased emphases on preoperative virtual visits and screening and testing pathways for COVID-19 and other possible viral pathogens. Before post-surge planning, preoperative reports were limited to recommending fever screening and using PPE for in-person preoperative visits.8 Recent medical specialty society recommendations have proposed universal symptom screening and further evaluation and testing based on a population risk assessment.1,2,9 Our multidisciplinary IP team rapidly implemented and expanded an innovative preoperative telehealth platform and widespread testing pathways using an accelerated plan-do-study-act (PDSA) model. Robust preoperative viral respiratory pathogen testing was previously non-existent. Our experiences during the COVID-19 pandemic represent the evolving changes with technology and the strong impetus to develop pragmatic preoperative testing pathways and virtual assessment to provide protection to patients, health care workers, and the community.
Several barriers and lessons emerged during implementation. Earlier challenges to video visit adoption became more evident including enrolling patients into our electronic health portal, patients’ lack of adequate internet access or usable electronic devices, and access to interpreter services. Patients previously deemed too complex for virtual visits now had no in-person option for preoperative assessment. Vital signs such as blood pressure, oxygen saturation, and heart rate were rarely assessed during the virtual visit. Consistent with published studies on telehealth visits,10 our patient experience surveys showed strong acceptance for virtual preoperative appointments.
Challenges with preoperative testing included resolving demographic and financial hardship in accessing testing sites, increasing access to tests performed outside of SHC, and assessing patient requests for day of surgery rapid testing exceptions. The inability to test certain patients, either due to patient refusal or other contraindication, poses ethical concerns. Clinician agreement on the appropriate preoperative testing windows continues to evolve as testing availability and accuracy improves, best-practice patterns emerge from increased preoperative testing, and as our local prevalence changes over time. Additional considerations in the post-surge COVID-19 era include increasing access to telehealth and testing in underserved areas or for vulnerable patient populations, assessing efficacy of telehealth versus in-person evaluations on clinical outcomes, establishing sustainable reimbursement models for virtual health visits, and expanding options to obtain reliable remote physical examination and vital signs.11
Our multipronged approach led to successful and widespread implementation at our health system with universal preoperative virtual assessments and accessible COVID-19 testing for all patients presenting for any procedural intervention. These protocols may provide guidance when determining emerging best practice COVID-19 pathways for preoperative optimization during the post-surge era while ensuring patient and health care provider safety.
Acknowledgments
The authors gratefully acknowledge the many faculty and staff at Stanford Health Care, the Lucille Packard Children's Hospital, and the Stanford School of Medicine for their efforts during the COVID-19 pandemic. The authors would also like to acknowledge the efforts of Drs. Mary Hawn, Ronald Pearl, and Denny Lund for their leadership during this time.
Footnotes
Disclosure: This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no conflicts of interest.
Reprints: Reprints be available from the authors.
REFERENCES
- 1. Wick, EC, Pierce, L, Conte MC, et al. Operationalizing the operating room: ensuring appropriate surgical care in the era of COVID-19. Ann Surg, published ahead of print. Available at: https://journals.lww.com/annalsofsurgery/Documents/Operationalizing%20the%20Operating%20Room.pdf. Accessed May 14, 2020. [Google Scholar]
- 2. Muret-Wagstaff SL, Collins JS, Mashman DL, et al. In situ simulation enables operating room agility in the COVID-19 pandemic. Ann Surg, published ahead of print. Available online: https://journals.lww.com/annalsofsurgery/Documents/In%20Situ%20Simulation.pdf. Accessed May 14, 2020. [Google Scholar]
- 3. American College of Surgeons. Local resumption of elective surgery guidance. Am Coll Surg. Available at: https://www.facs.org/covid-19/clinical-guidance/resuming-elective-surgery. Accessed May 14, 2020. [Google Scholar]
- 4. American College of Surgeons. COVID-19: Joint Statement: Roadmap for Resuming Elective Surgery after COVID-19 Pandemic. Available at: https://www.facs.org/covid-19/clinical-guidance/roadmap-elective-surgery. Accessed May 14, 2020. [Google Scholar]
- 5.Argo JL, Vick CC, Graham LA, et al. Elective surgical case cancellation in the Veterans Health Administration system: identifying areas for improvement. Am J Surg 2009; 198:600–606. [DOI] [PubMed] [Google Scholar]
- 6.Ferschl MB, Tung A, Sweitzer B, et al. Preoperative clinic visits reduce operating room cancellations and delays. Anesthesiology 2005; 103:855–859. [DOI] [PubMed] [Google Scholar]
- 7.Seim AR, Fagerhaug T, Ryen SM, et al. Causes of cancellations on the day of surgery at two major university hospitals. Surg Innov 2009; 16:173–180. [DOI] [PubMed] [Google Scholar]
- 8. Greenland JR, Michelow MD, Wang L, et al. COVID-19 Infection: Implications for Perioperative and Critical Care Physicians. Published online ahead of print April 24, 2020. Anesthesiology. Available at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=ovftu&NEWS=N&AN=00000542-900000000-96235. Accessed May 14, 2020. [Google Scholar]
- 9. ASA and APSF Joint Statement on Perioperative Testing for the COVID-19 Virus. April 29, 2020. Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2020/04/asa-and-apsf-joint-statement-on-perioperative-testing-for-the-covid-19-virus. Accessed May 14, 2020. [Google Scholar]
- 10.Bridges KH, McSwain JR, Wilson PR. To infinity and beyond: the past, present, and future of tele-anesthesia. Anesth Analg 2020; 130:276–284. [DOI] [PubMed] [Google Scholar]
- 11.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med 2020; 382:1679–1681. [DOI] [PubMed] [Google Scholar]


