Supplemental Digital Content is available in the text.
Keywords: cerebrovascular disorders, demography, odds ratio, pandemics, risk factors
Abstract
Background and Purpose:
Coronavirus disease 2019 (COVID-19) evolved quickly into a global pandemic with myriad systemic complications, including stroke. We report the largest case series to date of cerebrovascular complications of COVID-19 and compare with stroke patients without infection.
Methods:
Retrospective case series of COVID-19 patients with imaging-confirmed stroke, treated at 11 hospitals in New York, between March 14 and April 26, 2020. Demographic, clinical, laboratory, imaging, and outcome data were collected, and cases were compared with date-matched controls without COVID-19 from 1 year prior.
Results:
Eighty-six COVID-19–positive stroke cases were identified (mean age, 67.4 years; 44.2% women). Ischemic stroke (83.7%) and nonfocal neurological presentations (67.4%) predominated, commonly involving multivascular distributions (45.8%) with associated hemorrhage (20.8%). Compared with controls (n=499), COVID-19 was associated with in-hospital stroke onset (47.7% versus 5.0%; P<0.001), mortality (29.1% versus 9.0%; P<0.001), and Black/multiracial race (58.1% versus 36.9%; P=0.001). COVID-19 was the strongest independent risk factor for in-hospital stroke (odds ratio, 20.9 [95% CI, 10.4–42.2]; P<0.001), whereas COVID-19, older age, and intracranial hemorrhage independently predicted mortality.
Conclusions:
COVID-19 is an independent risk factor for stroke in hospitalized patients and mortality, and stroke presentations are frequently atypical.
In December 2019, coronavirus disease 2019 (COVID-19)—the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—first appeared as a respiratory illness in Wuhan, China.1 Since then, it has become a global pandemic, infecting at least 15.8 million people worldwide, including >4.1 million people in the United States.2 SARS-CoV-2 infection has protean presentations and clinical courses, ranging from asymptomatic carriers3 to critical illness with acute respiratory distress syndrome and multiorgan failure secondary to an accentuated immune response.4–6 Hypercoagulability may also develop, resulting in venous and arterial thromboembolic disease, including stroke.7–12
To date, only 13 stroke patients from China,9 7 patients from the United Kingdom,10 and 37 patients from the United States11,12 have been described in the literature. We present the largest series to date of COVID-19 patients who either presented with stroke or had cerebrovascular complications while hospitalized for COVID-19.
Methods
This is a retrospective case series of COVID-19 patients concurrently diagnosed with stroke, admitted to 11 Northwell Health hospitals in New York City and Long Island between March 14 and April 26, 2020. Our institutional review board approved this study as minimal risk, waiving informed consent. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Inclusion required (1) confirmed SARS-CoV-2 infection by polymerase chain reaction testing of nasopharyngeal swab samples and (2) concurrent stroke diagnosis, defined as stroke symptom onset during COVID-19 illness or onset of COVID-19 symptoms or SARS-CoV-2 polymerase chain reaction positivity within 14 days of stroke symptom onset. Only imaging-confirmed cases were included. Historical controls without COVID-19 were comprised of all stroke patients admitted 1 year earlier, between the same dates, to the same hospitals.
Data obtained from retrospective chart review and Get With The Guidelines-Stroke and COVID-19 databases included demographic, clinical, laboratory, and outcome measures. Additional details may be found in Materials in the Data Supplement. Clinical outcome was measured by discharge disposition, including death. Case neuroimaging data including brain computed tomography or magnetic resonance imaging and angiographic findings were attained by independent neuroradiologist review.
Statistical Analysis
A bivariate analysis of demographic, clinical, and outcome variables comparing COVID-19 stroke patients to controls was performed using χ2. A logistic regression model was created to determine independent risk factors for in-hospital stroke and mortality. Statistical significance was considered for P<0.05. All statistical analyses were done in SAS v9.4 (SAS Institute).
Results
In the study period, 10 596 COVID-19 patients were hospitalized (mean [range] percent hospital admissions with COVID-19, 43.3% [29.8%–64.2%]), and of these, 86 (0.8%; mean age [range], 67.4 [25–94] years; 44.2% women) met inclusion with imaging-confirmed infarctions (83.7%) or exclusive intracranial hemorrhage (16.3%). In the ischemic subgroup, 20.8% had associated brain hemorrhage, including hemorrhagic transformation (n=6) and simultaneous hemorrhage and infarction (n=9). Multivascular territory infarction (45.8%) was most common. Of ischemic patients with noninvasive angiography (38.9%), 57.1% had extracranial or intracranial large vessel occlusion (Figure).
Figure.
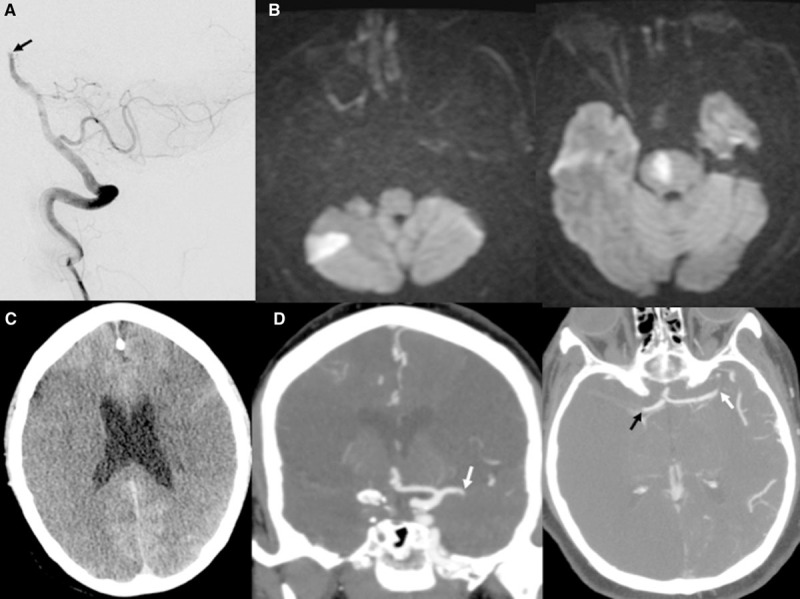
Coronavirus disease 2019 (COVID-19)–associated large vessel occlusion. A, Angiogram displaying distal basilar artery occlusion (black arrow) in a 54-y-old man. B, Post-thrombectomy, magnetic resonance imaging diffusion shows right inferior cerebellar and paramedian pontine infarctions. C, Computed tomography (CT) head showing bilateral middle cerebral artery infarctions in a 62-y-old woman with. D, CT angiogram demonstrating occlusions of proximal left middle cerebral artery (white arrows) and terminal right internal carotid artery and middle cerebral artery (black arrow).
Fifty-eight patients (67.4%) presented with nonfocal deficits. Encephalopathy was the most common presenting symptom (n=41), followed by seizures, generalized weakness, falls, and dizziness. Many had substantial COVID-19 complications while hospitalized, including pneumonia (88.4%), hypoxia (69.8%), and end-organ dysfunction (67.4%), with frequent critical-care admission (51.2%) and mechanical ventilation (44.0%). Of 45 patients testing positive for COVID-19 after stroke onset, most had mild COVID-19 symptoms (n=23) or were asymptomatic (n=13). Stroke onset during hospitalization for COVID-19 occurred in 41 patients (47.7%). The majority of these were diagnosed by brain imaging for encephalopathy (n=23), while only 9 had sudden-onset focal deficits.
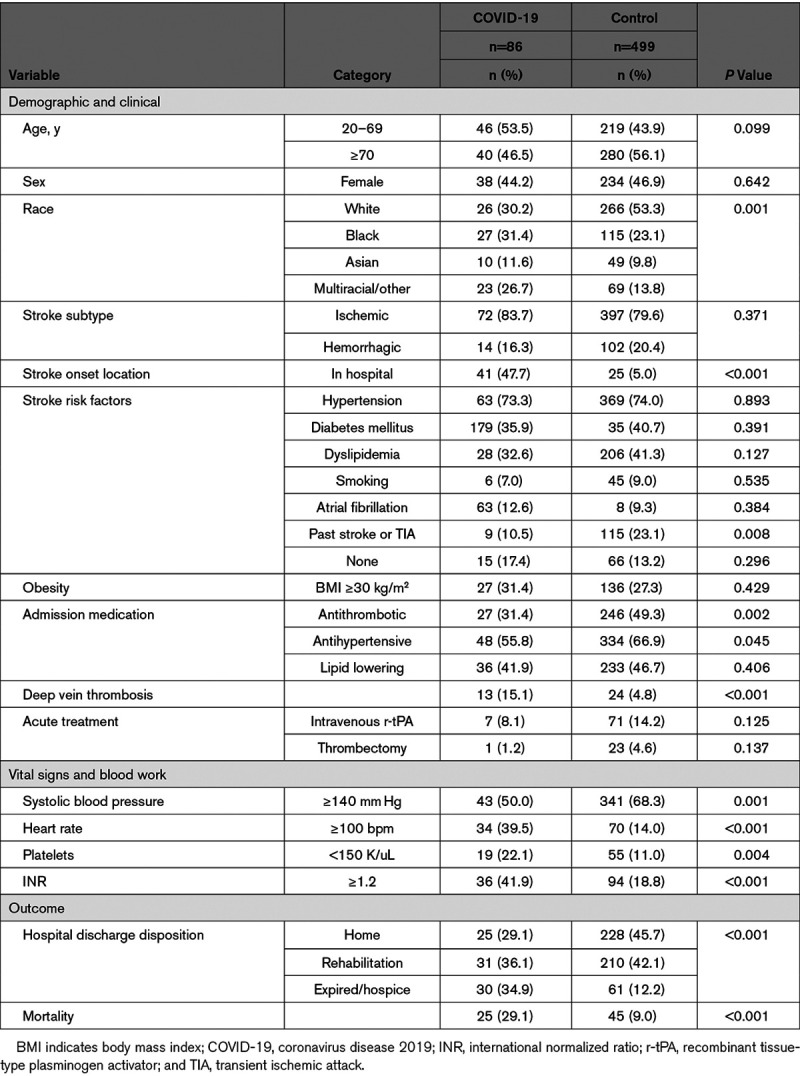
Table 1 compares demographic, clinical, laboratory, and outcome variables of COVID-19 stroke patients and controls (n=499). No significant differences were found in terms of age or sex. Black and multiracial minorities were significantly more common in the COVID-19 cohort (58.1% versus 36.9%; P=0.001). COVID-19 patients were significantly more likely to have stroke while hospitalized (47.7% versus 5.0%; P<0.001) and had significantly less frequent cerebrovascular history (10.5% versus 23.1%; P=0.008) and preadmission antithrombotic and antihypertensive use. More frequent admission tachycardia, thrombocytopenia, international normalized ratio elevation, and deep vein thrombosis were observed in COVID-19 patients. COVID-19 patients were significantly more likely to die (P<0.001), with 29.1% all-cause mortality.
Table 1.
Bivariate Analysis Comparing Stroke Patients With and Without COVID-19
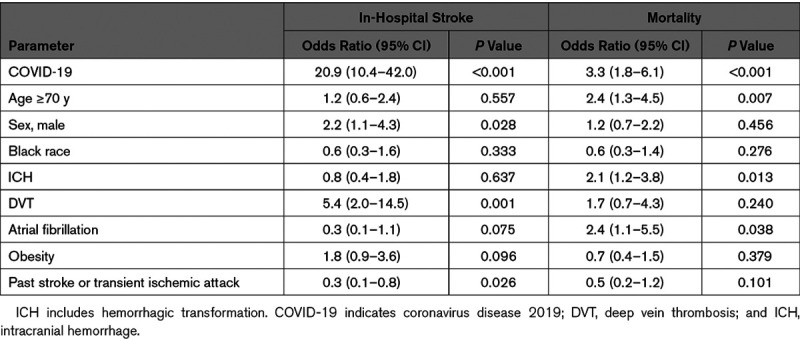
Multivariable logistic regression results are presented in Table 2. COVID-19 was the strongest independent predictor of stroke in hospitalized patients. Other risk factors included male sex and deep vein thrombosis. Cerebrovascular history predicted decreased in-hospital stroke risk. COVID-19, age ≥70 years, atrial fibrillation, and any intracranial hemorrhage, including hemorrhagic transformation, were independent risk factors for mortality.
Table 2.
Independent Risk Factors for In-Hospital Stroke and Mortality
Discussion
A major finding of our study was the frequency of in-hospital stroke onset, accounting for 48% of the COVID-19 cohort compared with 5% of controls. As COVID-19 was the strongest independent predictor of in-hospital stroke onset, a causal association between COVID-19 and stroke is suggested and further supported by the absence of cerebrovascular history and evidence of coagulopathy specific to COVID-19 stroke patients. This high in-hospital stroke rate was found by others12 and may reflect the acuity of COVID-19 patients hospitalized during the pandemic peak.
A significant racial disparity was noted with Black and multiracial minorities being the majority (58%) of the COVID-19 stroke cohort. In a consecutive series of 5700 COVID-19 patients hospitalized in the same system, 52% were Black/multiracial.4 Therefore, this overrepresentation may result from the higher prevalence of COVID-19 in these populations. Inequalities in risk factor modification, healthcare access, socioeconomics, diet, and genetic predilections may also contribute.
The majority of COVID-19 patients presented with nonfocal deficits (67.4%), predominantly encephalopathy, ranging from confusion to coma. In previous studies, stroke chameleons accounted for up to 22% of stroke patients, and altered mental status is the most common admitting diagnosis.13 COVID-19 patients were also more likely to die, with 29% in-hospital mortality. Although not directly comparable given the methodological differences between studies, this rate is higher than the 21% COVID-19 mortality rate separately reported by our health system.4 COVID-19 was the strongest independent risk factor for mortality. While we were unable to differentiate stroke-specific mortality from the independent lethality of COVID-19, our excess mortality reflects an even worse prognosis for COVID-19–associated stroke.
Our study has several strengths and limitations. Numerous factors likely resulted in undercounting the COVID-19 stroke population. While inclusion of only patients with imaging-confirmed stroke is a strength, many potential stroke patients with normal initial brain imaging did not undergo repeat imaging and were not included. In addition, SARS-CoV-2 polymerase chain reaction–negative stroke patients with other biomarkers suggesting infection were excluded. Given the false negative rate of COVID-19 testing,14 the true burden of COVID-19–related cerebrovascular complications may be underestimated. Nevertheless, our overall COVID-19 stroke rate of 0.8% is similar to the 0.9% rate reported by others.12 By including historical controls, we were able to demonstrate that COVID-19 is an independent risk factor for stroke, specifically in-hospital onset, and avoided contaminating our control group with false negative COVID-19 patients (see the Data Supplement for details). Yet, by not including a contemporaneous comparison group, we were unable to control for differences in health access and hospital staffing during the pandemic. To our knowledge, ours is the largest series to date of COVID-19 stroke patients and includes outcome for all patients.
In conclusion, COVID-19 is a strong independent risk factor for stroke in hospitalized patients, and stroke in COVID-19 portends increased mortality. COVID-19–related stroke is more frequent among racial minorities, and presentations are often atypical, without focal deficits, in multivascular territories, and with concomitant hemorrhages. Recognition is, therefore, critical, since prompt diagnosis may impact management.
Sources of Funding
None.
Disclosures
None.
Supplemental Materials
Additional Methods and Discussion
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
For Sources of Funding and Disclosures, see page e231.
This manuscript was sent to Marc Fisher, Senior Consulting Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.031265.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. ; China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020382727–733doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. doi: 10.1016/S1473-3099(22)00434-0. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html - /bda7594740fd40299423467b48e9ecf6. Accessed July 25, 2020. [DOI] [PMC free article] [PubMed]
- 3.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; Northwell COVID-19 Research Team Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 20203232052–2059doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 20208475–481doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 20203951033–1034doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 202018844–847doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao W, Li T. COVID-19: towards understanding of pathogenesis. Cell Res 202030367–369doi: 10.1038/s41422-020-0327-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Wang M, Zhou Y, Chang J, Xian Y, Mao L, Hong C, Chen S, Wang Y, Wang H, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Lancet. 2020 doi: 10.1136/svn-2020-000431. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=355002510. Accessed April 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humpries F, Jäger HR, Losseff NA, Perry RJ, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 202091889–891doi: 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York Healthcare System. Stroke 2020512002–2011doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arch AE, Weisman DC, Coca S, Nystrom KV, Wira CR, III, Schindler JL. Missed ischemic stroke diagnosis in the emergency department by emergency medicine and neurology services. Stroke 201647668–673doi: 10.1161/STROKEAHA.115.010613 [DOI] [PubMed] [Google Scholar]
- 14.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020296e32–e40doi: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


