Abstract
Objective:
To determine the outcomes of patients undergoing tracheostomy for COVID-19 and of healthcare workers performing these procedures.
Background:
Tracheostomy is often performed for prolonged endotracheal intubation in critically ill patients. However, in the context of COVID-19, tracheostomy placement pathways have been altered due to the poor prognosis of intubated patients and the risk of transmission to providers through this highly aerosolizing procedure.
Methods:
A prospective single-system multi-center observational cohort study was performed on patients who underwent tracheostomy after acute respiratory failure secondary to COVID-19.
Results:
Of the 53 patients who underwent tracheostomy, the average time from endotracheal intubation to tracheostomy was 19.7 days ± 6.9 days. The most common indication for tracheostomy was acute respiratory distress syndrome, followed by failure to wean ventilation and post-extracorporeal membrane oxygenation decannulation. Thirty patients (56.6%) were liberated from the ventilator, 16 (30.2%) have been discharged alive, 7 (13.2%) have been decannulated, and 6 (11.3%) died. The average time from tracheostomy to ventilator liberation was 11.8 days ± 6.9 days (range 2–32 days). Both open surgical and percutaneous dilational tracheostomy techniques were performed utilizing methods to mitigate aerosols. No healthcare worker transmissions resulted from performing the procedure.
Conclusions:
Alterations to tracheostomy practices and processes were successfully instituted. Following these steps, tracheostomy in COVID-19 intubated patients seems safe for both patients and healthcare workers performing the procedure.
Keywords: aerosol-generating procedures, COVID-19, intubation, invasive mechanical ventilation, personal protective equipment, SARS-CoV-2, tracheostomy
Respiratory decompensation with the need for mechanical ventilation is the hallmark of severe novel coronavirus 2019 (COVID-19) caused by the virus SARS-CoV-2. In such cases, patients develop acute respiratory distress syndrome (ARDS) with the resultant need for mechanical ventilation.
We have previously reported evidence-based recommendations for tracheotomy in intubated COVID-19 patients.1 In the available literature at the time, intubated patients exhibited a poor prognosis with few surviving to extubation.2,3 This has been corroborated by data from the United States with an 88% mortality rate for patients who are placed on the ventilator.4 A small subset of patients demonstrated prolonged invasive mechanical ventilation for as long as 31 days which suggested that some of these patients may benefit from a tracheotomy to facilitate ventilator weaning.5 Studies have also shown that most patients no longer exhibit viral shedding by 21 days from symptom onset, though viral detection may not truly reflect the patient's infectious potential.6–9
For these reasons, we determined that avoidance of tracheostomy before 21 days of endotracheal intubation was prudent, offering only to patients with anticipated ventilator liberation survival. We acknowledged that some patients may warrant tracheostomy earlier than 21 days for reasons such as pulmonary toilet or need for sedation weaning. Others have similarly recommended tracheostomy no sooner than 2–3 weeks after intubation with similar criteria, despite the fact that long-term laryngeal problems may result from longer periods of intubation.10–13
In patients warranting tracheostomy, appropriate personal protective equipment (PPE) is critical to mitigate transmission during this aerosol-generating procedure. Other principles of limiting the number of personnel present, performing the procedure in a negative pressure room, and novel procedural modifications to minimize aerosolization also help to achieve this goal. Others have published similar recommendations that follow these guidelines.14–18
To reduce aerosolization during the procedure, we suggested open surgical tracheostomy may be safer compared to a standard percutaneous dilational tracheostomy (PDT) technique which is supported by others.16 However, based on more recent experience, adjustments can be made to the PDT procedure that reduce aerosolization time to an amount similar to the open technique.19
Here, we describe our early experience with tracheostomy in the COVID-19 patient population and discuss findings in our initial cohort of patients with respect to both patient and provider outcomes. As this is the first available data in the literature on this topic, we hope to further refine existing guidelines based on this emerging evidence.
METHODS
Institutional Review Board exemption was obtained from the University of Pennsylvania. A single-system multi-center prospective nonrandomized cohort study of patients with COVID-19 undergoing tracheostomy was performed including 5 hospitals within the University of Pennsylvania Health System. Data including demographic information, past medical history, admission information, presence of ARDS, duration of ventilator requirement, tracheostomy procedure details, complications, length of stay, and disposition information was collected from the electronic health record and stored in REDCap.
Co-morbidities were defined as conditions identified by the Centers for Disease Control and Prevention as high-risk factors for severe illness from COVID-19.20 Ventilator liberation was defined as the first full 24-hour period without ventilator assistance. Additionally, information on healthcare worker (HCW) and hospital factors, such as the type of PPE used, number of personnel, and presence of COVID-19 infection were collected. An interim analysis of the data was performed. Statistical analysis was performed via 2-tailed independent t-test for equal variances. A Pearson correlation coefficient was calculated using RStudio where applicable.
RESULTS
To date, tracheostomy has been performed in 53 COVID-19 patients with acute respiratory failure (Table 1). Patients undergoing the procedure were predominantly male (33 patients, 62%) reflecting findings of higher disease severity in males in the literature.21 The average age was 62.0 years ± 14.3 years (range 23.5–81.7 years). 81% of patients had at least 1 comorbidity. Thirteen patients were white (25%) and 23 were black (43%) which not only correlates with the demographic makeup of our institution's catchment area but also reflects national trends of a higher disease prevalence amongst racial minority groups.22
TABLE 1.
Demographic Characteristics of Patients With COVID-19 Who Underwent Tracheotomy
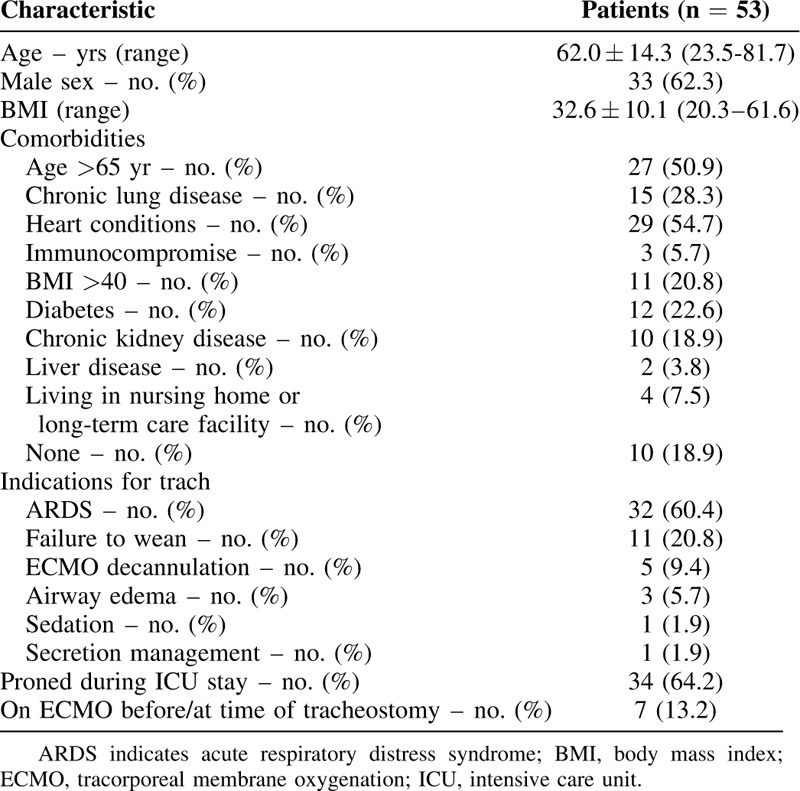
ARDS was the most common indication for tracheostomy (32 patients, 60%), followed by failure to wean ventilation without ARDS (11 patients, 21%), extracorporeal membrane oxygenation (ECMO) decannulation (5 patients, 9%), persistent airway edema (3 patients, 6%), need for secretion management (1 patient, 2%), and need for sedation management (1 patient, 2%) (Table 2). Thirty-four patients (64.2%) had been proned previously as part of their ARDS management but none were proned in the 24 hours before tracheostomy.
TABLE 2.
Indications for Tracheostomy and the Relationship to Time From Intubation and Time to Ventilator Liberation
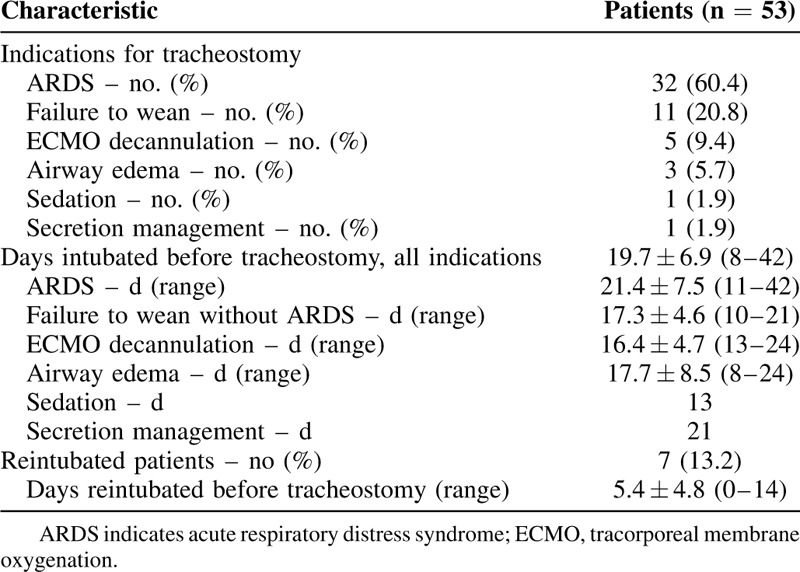
The average time of intubation before tracheostomy, defined as the time from first intubation to tracheostomy, was 19.7 days ± 6.9 days, with a range of 8–42 days. There was no significant difference in time of intubation before tracheostomy between indications. Seven patients failed extubation and required reintubation before tracheostomy. In this group, the average time between reintubation and tracheostomy was 5.4 days ± 4.8 days (range 0–14 days).
Tracheostomies were performed by 11 different attending surgeons amongst 5 hospitals. Institution-specific standard perioperative antibiotic prophylaxis was given in all cases. All patients were fully paralyzed to minimize coughing and aerosolization during airway manipulation. Open tracheostomy and PDT were both performed depending on surgeon preference and expertise. A total of 29 PDTs and 24 open surgical tracheostomies were performed (Table 3). All open tracheostomies utilized a Björk flap, with the exception of 2 revision tracheostomies in patients with a distant history of tracheostomy and subsequent decannulation for unrelated reasons. Björk flaps were created whereas the endotracheal tube (ETT) balloon was more distal in the airway. Before pulling the ETT proximally and inserting the tracheostomy, positive pressure ventilation was disconnected or paused. Ventilation was resumed when the tracheostomy tube cuff was inflated and the ventilator circuit was connected.
TABLE 3.
Tracheostomy Procedural and Technical Considerations
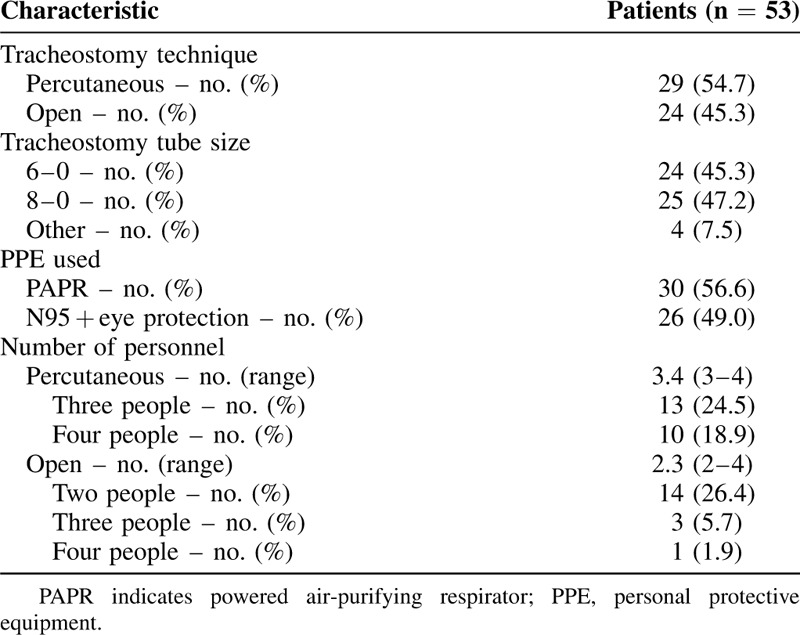
Slight variations in technique for PDT was observed. Bronchoscopic assistance was used in all PDT cases utilizing disposable bronchoscopes. In 19 of the 29 PDT cases, aerosolization was minimized by disconnecting the ETT from the ventilator whereas the bronchoscope was in the ETT. Ventilation was not resumed until the tracheostomy tube cuff was inflated and the ventilator circuit was connected. In the other 10 cases, the bronchoscope was placed alongside the ETT and the cuff was repositioned so that it was below the tracheal entry site whereas ventilation was continued.19 Ventilation was suspended when the circuit was switched from the ETT to the tracheostomy tube and resumed thereafter.
Thirty patients (56.6%) were liberated from the ventilator after tracheostomy. The average time between tracheostomy and ventilator liberation was 11.8 ± 6.9 days (range 2–32 days). Of these patients, the average time of intubation before tracheostomy was 17.5 ± 4.9 days (range 8–30 days). There was a weak positive correlation between time from intubation to tracheostomy and time from tracheostomy to ventilator liberation (Fig. 1, R2 = 0.14, P = 0.04). Fourteen patients (26.4%) have had their tracheostomy tube downsized and 7 patients (13.2%) have been decannulated. The average time to decannulation after tracheostomy was 16.6 ± 5.0 (11–24) days. Nineteen patients (35.8%) remain on some level of ventilatory support.
FIGURE 1.
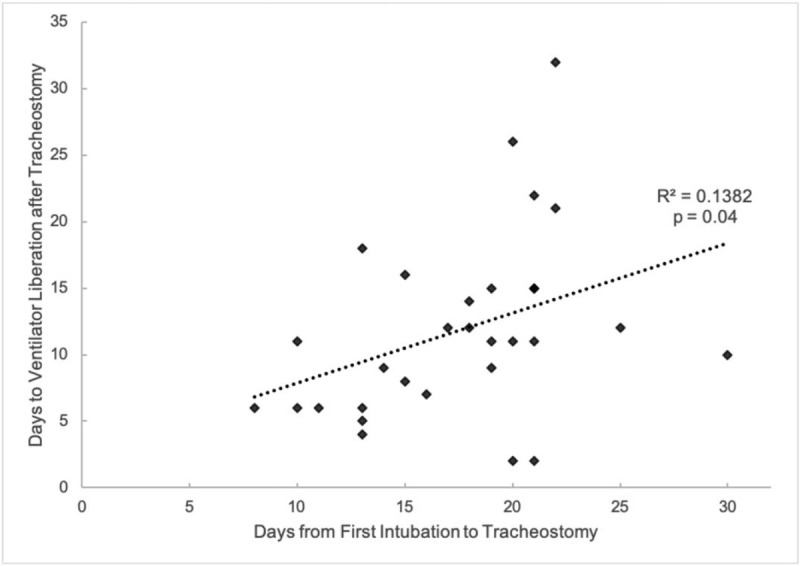
Correlation between time from first intubation to tracheostomy and time from tracheostomy to ventilator liberation P = 0.04.
At the time of manuscript preparation, 16 patients (30.2%) patients have been discharged alive (Figure 2). Of these patients, the average duration of intubation before tracheostomy was 17.4 ± 5.4 days (range 10–30 days) and the average hospital length of stay was 37.2 ± 9.7 days (range 18–51 days). Minor complications were reported in 2 patients (3.8%), including cellulitis (1 patient) and bleeding (1 patient). Six patients (11.3%) died after tracheostomy. Of patients who died, the average duration of intubation before tracheostomy was 22.7 ± 7.1 days (range 13–34 days) and the average time from tracheostomy to death was 15.7 ± 9.2 days (range 1–26 days).
FIGURE 2.

Patient reuniting with family after 31-d hospitalization involving endotracheal intubation, open surgical tracheostomy, ventilator liberation, tracheostomy decannulation, and discharge home. (Permission for use granted by all individuals in photo).
No transmissions to HCWs occurred during any procedure. Airborne, contact, and droplet precaution-level PPE were worn by all HCW in the room during the procedure. A powered air-purifying respirator was used in 30 cases (56.6%) and an appropriately fitted N95 was used in 26 cases (49.0%). For PDTs, 3 personnel members were present in the room in 13 cases (24.5%) and 4 personnel members were present in 10 cases (18.9%). For open tracheostomies, 2 personnel members were present in the room for 14 cases (26.4%), 3 personnel members were present in 3 cases (5.7%), and 4 personnel members were present in 1 case (1.9%). All procedures, except 1 done in the operating room, were performed at the bedside in a negative pressure room.
DISCUSSION
This study is one of the first to describe early outcomes of tracheotomy patients with COVID-19. It is also the largest series in the literature describing a variety of surgical techniques. Angel et al demonstrated that a modified PDT technique could be safely performed with proper PPE and reported no transmission to HCWs as a result of the procedure.23 Of note, patients were eligible for PDT as early as 5 days after intubation. They demonstrated a ventilator liberation rate of 33%, a decannulation rate of 8%, and a mortality rate of 7%. Other smaller series have similarly reported no HCW transmission from involvement in tracheostomy procedures.24–27
Herein, we have reported not only on excellent outcomes with regard to HCW safety during COVID tracheostomies, but also on promising early outcomes for COVID-19 patients who have undergone tracheostomy, with higher rates of ventilator liberation and decannulation than reported in comparably sized studies.23 In our series, the average time from intubation to tracheostomy was slightly earlier than the originally anticipated 21 days per standard recommendations, but consistent with global recommendations and in line with trends in non-COVID critical care practice.10 Several factors have likely led to this shift in our practice, including a number of patients who required tracheostomy for reasons other than ARDS. We had expected that resource utilization might become a factor, but in our institution preparedness has avoided the need to do tracheostomy solely for that reason.
Our local experience has also demonstrated that laryngeal edema often presents after extubation in COVID-19 patients often leading to stridor and the need for reintubation immediately after planned extubation. In many, these are difficult reintubations due to significant edema in patients who were originally straightforward intubations. Similar experiences have been reported by others.28 At our institution, this has also led to the creation of a strict extubation planning protocol for extubation candidates, with the recommendation for tracheostomy in patients with multiple failed cuff leak tests despite peri-extubation steroid treatment.
Use of ECMO has also led to earlier time to tracheostomy, with an average time of intubation before tracheostomy of 16.4 days. Though the role of ECMO in COVID-19 patients is uncertain,29,30 several institutions have utilized it for cases of severe ARDS with refractory hypoxemia.31,32 Standard practice at many institutions, including our own, is to perform tracheostomy after decannulation from ECMO to minimize work of breathing and optimize ventilator liberation.33 Though the sample size is small, early tracheostomy in these patients may be beneficial, as 4 of the 5 post-ECMO patients in our series have been liberated from the ventilator at the time of manuscript preparation.
In our series, there was a weak positive correlation between pre-tracheostomy intubation time and post-tracheostomy ventilator dependence. Patients who underwent earlier tracheostomy tended to achieve earlier ventilator liberation compared to patients who underwent later tracheostomy. Though there may be confounding factors including a selection bias of healthier patients for earlier tracheostomy and the delay of tracheostomy in patients with questionable prognoses, these results offer preliminary support for earlier tracheostomy.
For these reasons, it is reasonable to consider early tracheostomy for the appropriately selected patient, even before 14 days, if the patient has favorable respiratory dynamics and earlier tracheostomy would directly facilitate their recovery in terms of improved secretion management, ventilator synchrony, and decreased need for sedation.34 For patients who are simply critically ill on the ventilator with ARDS, waiting 14–21 days before offering tracheostomy still remains the standard recommendation. Additionally, before consideration of tracheostomy, a goals of care discussion with the primary team and the patient's family regarding prognosis and expected benefit from the procedure should occur.35 Of the 6 patients who died in our series, all had complex past medical histories or hospital courses. As with any critically ill patient, if prognosis seems poor regardless of time of intubation, it may be prudent to recommend against tracheotomy if this would ultimately not impact outcome.
Both percutaneous and open techniques were performed, utilizing different methods to minimize aerosolization without major complications. Patients should be fully paralyzed for the procedure. Ventilation should be paused or disconnected whenever instrumentation of the airway occurs below the cuff during PDT. Ventilation may be continued in techniques where the cuff is inflated and below the tracheal entry point. Although a percutaneous technique without a bronchoscope was not performed, the technique remains feasible in the appropriately selected patient with favorable anatomy as an alternative method for minimizing aerosolization. However, providers must be cognizant of the risk of being unable to confirm cuff and ETT position, even with the adjunct of ultrasound.
Approximately equal numbers of size 8-0 and 6-0 tracheostomy tubes have been used, varying depending on expected potential therapeutic bronchoscopy. In our experience, utilizing a larger sized tube has not led to decreased air leakage or aerosolization. The appropriately sized tracheostomy tube should be used based on the anticipated need for extensive toilet bronchoscopy, composition of secretions, and available bronchoscope sizing. In the absence of an extensive need for specialized bronchoscopy, smaller tracheostomy devices can reduce the risk of subsequent tracheal stenosis.36
Based on this series, with careful patient selection, tracheostomy seems to be safe in this patient population. Thus far, with 30 patients liberated from the ventilator, 16 discharged alive, and 7 decannulated, patient outcomes after tracheostomy seem optimistic compared to other series where the intubated COVID-19 patient's prognosis is extremely poor.2,3
This data also suggests that tracheostomies can be safe for HCWs performing the procedure, though it should be noted that the incubation period for those who have performed tracheostomy in our center has not yet been completed at the time of manuscript preparation. Nevertheless, all providers passed symptom-based screenings and daily temperature checks and thus did not meet institutional criteria for SARS-CoV-2 testing. The initial recommendation of avoiding tracheostomy sooner than 21 days was intended to balance the expected prognosis of the patient with the risk to the provider. This assumed a likely PPE scarcity and that most patients would no longer be infectious by 21 days of intubation. When PPE is readily available and appropriately donned and doffed, the risk to personnel can be effectively mitigated. Additionally, variations in PPE use across providers and hospital centers in our series suggests that both powered air-purifying respirators and an appropriately fitted N95 mask with eye protection afford adequate protection against SARS-CoV-2 transmission.
The risk to providers may be reduced even further with the increased availability of rapid COVID testing. Preoperative testing of COVID-19 patients to determine viral clearance may help guide the decision of when to perform a tracheostomy if PPE availability were to become a concern, especially in light of recent studies suggesting patients with severe disease have an even more prolonged duration of viral shedding.37 Though not yet the protocol at our institution, testing of proceduralists should be considered a priority as asymptomatic HCW testing begins worldwide.
Additionally, we have shown that the procedure can be safely performed while minimizing the number of HCWs present in the room during the aerosolizing portion of the procedure. For open surgical tracheostomies, as few as 2 people, the surgeon and the respiratory therapist, are present in the room during the aerosolizing portion of the procedure and the nurse administering medications may leave the room before this point. For PDT, 1 additional person is needed to operate the bronchoscope.
The importance of communication between services and the integration of a multidisciplinary team despite differences in technique cannot be overemphasized. At our institution, the development of tracheostomy guidelines in COVID-19 patients was built on the foundation of an established airway safety committee. From the conception of the guidelines to the ongoing analysis of data, a collaborative effort with the involvement of surgical and medical intensivists, general surgeons, otolaryngologists, anesthesiologists, interventional pulmonologists, nurses, and respiratory therapists is paramount to the continued refinement of our practice in an evidence-based way.
This further allows for a high degree of responsiveness to the needs of the critical care teams primarily managing these patients. Our COVID-19 tracheostomy task force coordinates tracheostomy planning in an organized fashion to share rapidly, to discuss, and to assess options including PPE variations and procedural experiences across a network of hospitals. Moreover, each entity in our health system has a clinician tracheostomy champion, and an identified backup team, who works with the primary critical care team and the ultimate procedure provider to minimize variation.
This study is limited by the inability to randomize patients to tracheostomy or continued intubation. This may introduce bias supporting tracheostomy for COVID-related intubation due to the pre-selection of patients with a high chance of recovery. Factors such as pre-tracheostomy ventilator settings and peri-procedural hypoxia play a role in ventilator liberation and mortality and should be investigated in future studies to further delineate candidacy for tracheostomy. Additionally, though this is one of the first studies on outcomes after tracheostomy in COVID-19 and one of the largest series of patients receiving tracheostomy for any similar disease outbreak, it is limited by a relatively small sample size. Additional patients are needed to determine statistical significance of the data. Further cohort analysis is needed to elucidate any potential benefit from tracheostomy in this population and determine the optimal timing of this procedure.
CONCLUSIONS
This study is one of the first to describe outcomes for a cohort of patients who underwent tracheostomy after intubation for COVID-19. Our early experience demonstrates that tracheostomy can be performed in a way that maximizes safety of the surgeon while achieving the desired outcome for the patient. Earlier tracheostomy, before 14–21 days, may be warranted for select patients. Further assessment of outcomes after tracheostomy in this patient population is required.
Acknowledgments
The authors would like to acknowledge all members of the COVID-19 Tracheostomy Task Force who were instrumental in the development of our institutional guidelines. From otorhinolaryngology: Michael Kohanski. From general surgery: Daniel Holena and Patrick Reilly. From nursing: Christine Aiello, Julianne Jablonski, Melissa Kavanagh, Stacie Neefe, and Rebecca Stamm. From respiratory therapy: Steven Gudowski.
Footnotes
Tiffany N. Chao and Sean P. Harbison are co-first authors.
Joshua H. Atkins and Christopher H. Rassekh are co-senior authors.
The authors declare no conflict of interests.
REFERENCES
- 1. Chao TN, Braslow BM, Martin ND, et al. Tracheostomy in ventilated patients with COVID-19. Ann Surg. Epub ahead of print, doi: 10.1097/SLA.0000000000003956. [Google Scholar]
- 2.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA [Internet]. 2020 [cited 2020 Apr 25]. Available at: https://jamanetwork.com/journals/jama/fullarticle/2765184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Ji, Fan H, Zhang L, et al. Retrospective analysis of clinical features in 101 death cases with COVID-19 [Internet]. Intensive Care Crit Care Med; 2020 [cited 2020 Apr 20]. Available at: http://medrxiv.org/lookup/doi/10.1101/2020.03.09.20033068. [Google Scholar]
- 6.Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020; 80:e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian G-Q, Chen X-Q, Lv D-F, et al. Duration of SARS-CoV-2 viral shedding during COVID-19 infection. Infect Dis Lond Engl 2020; 52:511–512. [DOI] [PubMed] [Google Scholar]
- 8.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miles BA, Schiff B, Ganly I, et al. Tracheostomy during COV-SARS-CoV-2 pandemic: recommendations from the New York Head and Neck Society. Head Neck [Internet] [cited 2020 Apr 20];n/a(n/a). Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/hed.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parker NP, Schiff BA, Fritz MA, et al. Tracheotomy recommendations during the COVID-19 pandemic [Internet]. Am Acad Otolaryngol-Head Neck Surg. 2020 [cited 2020 Apr 18]. Available at: https://www.entnet.org/content/tracheotomy-recommendations-during-covid-19-pandemic. [Google Scholar]
- 12. Takhar A, Walker A, Tricklebank S, et al. Recommendation of a practical guideline for safe tracheostomy during the COVID-19 pandemic. Eur Arch Otorhinolaryngol [Internet]. 2020 [cited 2020 Apr 22]. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koshkareva Y, Gaughan JP, Soliman AMS. Risk factors for adult laryngotracheal stenosis: a review of 74 cases. Ann Otol Rhinol Laryngol 2007; 116:206–210. [DOI] [PubMed] [Google Scholar]
- 14. Jacob T, Walker A, Mantelakis A, et al. A framework for open tracheostomy in COVID-19 patients. Clin Otolaryngol [Internet]. [cited 2020 Apr 18];n/a(n/a). Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/coa.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pichi B, Mazzola F, Bonsembiante A, et al. CORONA-steps for tracheotomy in COVID-19 patients: a staff-safe method for airway management. Oral Oncol 2020; 105:104682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tay JK, Khoo ML-C, Loh WS. Surgical considerations for tracheostomy during the COVID-19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngol-Head Neck Surg 2020; Epub ahead of print, doi:10.1001/jamaoto.2020.0764. [DOI] [PubMed] [Google Scholar]
- 17. Vargas M, Servillo G. Improving staff safety during tracheostomy in COVID-19 patients. Head Neck [Internet]. [Cited 2020 Apr 18];n/a(n/a). Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/hed.26163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhong Y, Xiao H, Varvares MA. How to avoid nosocomial spread during tracheostomy for Covid-19 patients. Head Neck [Internet]. [Cited 2020 Apr 18];n/a(n/a). Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/hed.26167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rafeq S. Personal Correspondence to ARH and CTH. 2020. [Google Scholar]
- 20. CDC. Coronavirus disease 2019 (COVID-19) [Internet]. Cent. Dis. Control Prev. 2020 [cited 2020 Apr 18]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/underlying-conditions.html. [Google Scholar]
- 21.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020; Epub ahead of print, doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyer O. Covid-19: black people and other minorities are hardest hit in US. BMJ 2020; 369:m1483. [DOI] [PubMed] [Google Scholar]
- 23.Angel L, Kon ZN, Chang SH, et al. Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann Thorac Surg 2020; Epub ahead of print, doi: 10.1016/j.athoracsur.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turri-Zanoni M, Battaglia P, Czaczkes C, et al. Elective tracheostomy during mechanical ventilation in patients affected by COVID-19: preliminary case series from Lombardy, Italy. Otolaryngol Neck Surg 2020; Epub ahead of print, doi: 10.1177/0194599820928963. [DOI] [PubMed] [Google Scholar]
- 25. Broderick D, Kyzas P, Baldwin AJ, et al. Surgical tracheostomies in COVID-19 patients: a multidisciplinary approach and lessons learned. Oral Oncol [Internet]. 2020 [cited 2020 May 15]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7196417/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Huang Q, Niu X, et al. Safe and effective management of tracheostomy in COVID-19 patients. Head Neck 2020; Epub ahead of print, doi: 10.1002/hed.26261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botti C, Lusetti F, Castellucci A, et al. Safe tracheotomy for patients with COVID-19. Am J Otolaryngol 2020; Epub ahead of print, doi: 10.1016/j.amjoto.2020.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGrath BA, Wallace S, Goswamy J. Laryngeal oedema associated with COVID-19 complicating airway management. Anaesthesia [Internet]. [cited 2020 Apr 18];n/a(n/a). Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/anae.15092. [DOI] [PubMed] [Google Scholar]
- 29.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA 2020; 323:1245–1246. [DOI] [PubMed] [Google Scholar]
- 30. Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care [Internet]. 2020 [cited 2020 Apr 20]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7118619/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taniguchi H, Ogawa F, Honzawa H, et al. Veno-venous extracorporeal membrane oxygenation for severe pneumonia: COVID-19 case in Japan. Acute Med Surg [Internet]. 2020 [cited 2020 Apr 20];7. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7161844/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeng Y, Cai Z, Xianyu Y, et al. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Crit Care 2020;24(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe T, Madotto F, Pham T, et al. Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Crit Care 2018; 22:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mattioli F, Fermi M, Ghirelli M, et al. Tracheostomy in the COVID-19 pandemic. Eur Arch Otorhinolaryngol [Internet]. 2020 [cited 2020 Apr 22]. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fausto J, Hirano L, Lam D, et al. Creating a palliative care inpatient response plan for COVID19 - the UW medicine experience. J Pain Symptom Manage [Internet]. 2020 [cited 2020 Apr 20]. Available at: http://www.sciencedirect.com/science/article/pii/S0885392420301767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Yiu Y, Merrill T, et al. Risk factors for posttracheostomy tracheal stenosis. Otolaryngol Neck Surg 2018; 159:698–704. [DOI] [PubMed] [Google Scholar]
- 37. Zhou B, She J, Wang Y, et al. The duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis [Internet]. [Cited 2020 Apr 20]. Available at: https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa451/5821307. [DOI] [PMC free article] [PubMed] [Google Scholar]


