Abstract
To determine whether gait and balance dysfunction are present in young urbanites exposed to fine particular matter PM2.5 ≥ annual USEPA standard, we tested gait and balance with Tinetti and Berg tests in 575 clinically healthy subjects, age 21.0 ± 5.7 y who were residents in Metropolitan Mexico City, Villahermosa and Reynosa. The Montreal Cognitive Assessment was also applied to an independent cohort n:76, age 23.3 ± 9.1 y. In the 575 cohort, 75.4% and 34.4% had abnormal total Tinetti and Berg scores and high risk of falls in 17.2% and 5.7% respectively. BMI impacted negatively Tinetti and Berg performance. Gait dysfunction worsen with age and males performed worse than females. Gait and balance dysfunction were associated with mild cognitive impairment MCI (19.73%) and dementia (55.26%) in 57/76 and 19 cognitively intact subjects had gait and balance dysfunction. Seventy-five percent of urbanites exposed to PM2.5 had gait and balance dysfunction. For MMC residents-with historical documented Alzheimer disease (AD) and CSF abnormalities, these findings suggest Alzheimer Continuum is in progress. Early development of a Motoric Cognitive Risk Syndrome ought to be considered in city dwellers with normal cognition and gait dysfunction. The AD research frame in PM2.5 exposed young urbanites should include gait and balance measurements. Multicity teens and young adult cohorts are warranted for quantitative gait and balance measurements and neuropsychological and brain imaging studies in high vs low PM2.5 exposures. Early identification of gait and balance impairment in young air pollution-exposed urbanites would facilitate multidisciplinary prevention efforts for modifying the course of AD.
Keywords: Alzheimer, Alzheimer continuum, Air pollution, Cognition, Gait, Balance, Berg, Nanoparticles, Dementia, Diesel, Mexico City, Mild cognitive impairment MCI, MoCA, Motoric cognitive risk syndrome MCR, PM 2.5, Tinetti, Young adults
Highlights
-
•
Exposure to air pollution is associated with gait and balance dysfunction.
-
•
Mexico City 575 urbanites 21.0 ± 5.7 y had abnormal Tinetti 75% and Berg 34%.
-
•
High risk of falls in 17% (Tinetti) and 5.7% (Berg) is of deep concern.
-
•
Gait and balance dysfunction are associated with MCI and dementia scores.
-
•
Gait and balance dysfunction suggest Alzheimer Continuum is in progress.
1. Introduction
Gait, equilibrium and postural disorders are linked to neurological and neurodegenerative pathologies (Dietz, 2013; Baker, 2018; Jahn et al., 2019; Jahn, 2019; Horak et al., 2016; Verghese et al., 2002; Dale et al., 2017; Waite et al., 2005; Ahmed et al., 2017; Gera et al., 2020; Fino et al., 2017; Finsterwalder et al., 2019; Chambers et al., 2020). Gait is a complex activity requiring the activation of the cerebral cortex, basal ganglia, cerebellum, brainstem, spinal cord and the musculoskeletal system (Takakusaki, 2013; Jahn et al., 2008; Mirelman et al., 2018; Armstrong, 1988; Orlovsky et al., 1999). Independent papers by Kaoru Takakusaki, Klaus Jahn and Anat Mirelman et al. (Takakusaki, 2013; Jahn et al., 2008; Mirelman et al., 2018), extensively discuss human locomotion and the key regions associated with gait and posture i.e., the mesencephalic locomotor region (MLR) and its projections to the pontomedullary reticular formation; the subthalamic locomotor region (SLR) and the cerebellar locomotor region (CLR). Jahn et al. (2008), studies in imaging human supraspinal locomotor brainstem and cerebellum are of particular interest in this paper given their clinical relevance and the connections of gait initiation and speed regulation pacemakers with anatomical structures such as the inter-fastigial cerebellum, midbrain tegmentum, cerebellar vermis and pontine reticular formation. Strikingly, motor imagery and actual movement activate not only premotor and supplementary motor areas, but also cingulate and parietal cortical areas (Jahn et al., 2008). The importance of postural control and cerebellar gait input has been put forward independently by Armstrong and Orlovsky (Armstrong, 1988; Orlovsky et al., 1999) and support the integration of widespread locomotor structures from proprioceptive, exteroceptive, visual, vestibular, cerebellar, dorsal brainstem and cortical input for gait initiation and modulation (Jahn et al., 2008). Thus, it is not a surprise gait pathology is described in the elderly especially in relation to neurodegenerative and dementia pathologies (Taylor and Close, 2018; Montero-Odasso and Perry, 2019; Sakurai et al., 2019a; Moretti et al., 2015; Leach et al., 2018; Cosentino et al., 2020; Mc Ardle et al., 2019; Tian et al., 2019). Associations between gray matter volume and gait parameters in mild cognitive impairment (MCI) patients (temporal lobe) and controls (frontal areas in healthy elderly), suggest a relationship between dementia-related pathology and gait dysfunction (Cosentino et al., 2020) that becomes clear in Alzheimer's disease (AD) patients when applying the Dual-Task Gait Performance testing (Cullen et al., 2019). Patients with dementia had significantly higher dual-task cost (DTC) in counting gait and naming animals conditions vs individuals with subjective cognitive impairment (SCI) and MCI (Cullen et al., 2019). Tian et al. (2019), have shown that greater rate of increase in lap time variability from a 400-m walk differentiates individuals who eventually develop MCI/AD from controls, supporting early pathology likely impacts the automaticity of walking.
Motoric cognitive risk syndrome (MCR) (Ayers and Verghese, 2019) characterized by subjective cognitive complaints and slow gait is common across the world with a prevalence of 9.7% (Verghese et al., 2014). Gait abnormalities are common in MCR and are associated with accelerated functional decline and gray matter atrophy in motor, insular and prefrontal cortex linked to the control aspects of gait such as motor planning and modulation (Blumen et al., 2019a). Muurling et al. (2020), have shown an association between gait specific measures related to pace and rhythm, cerebrospinal fluid (CSF) hyperphosphorylated tau and dementia. Muurling's work is key for this investigation given that Metropolitan Mexico City residents show evidence of Alzheimer's disease in 202/203 forensic autopsy cases age 25.36 ± 9.23 y, CSF Aβ1-42, BDNF, α-synuclein, and inflammatory markers are evolving in young urbanites showing underperformance in cognitive processes, and abnormal brainstem evoked potentials (Calderón-Garcidueñas et al., 2013,2016a, 2018a,b, 2019a,b,2020). These findings support a spectrum of cognitive, imaging and gait abnormalities in the elderly population and that gait abnormalities could indeed be an early indication of a high risk for MCI and dementia.
It is striking there are very few gait studies in young adults if we indeed recognize neurodegenerative processes take several decades to evolve (Jack et al., 2018, 2019) and if biologically defined AD is more prevalent that clinically defined AD (Jack et al., 2019). Heijnen and Rietdyk (2016) in a study of 94 undergraduate students found falls are the third leading cause of unintentional injuries for young adults ages 18-35 y. These young adults fell once out of every 18 perceived slips and trips – this is not a trivial problem for a population with no risk factors for diseases affecting gait and dynamic equilibrium.
Given these findings we are concerned young Metropolitan Mexico City (MMC) residents exposed to concentrations of fine particulate matter (PM2.5) above the United States Environmental Protection Agency (USEPA) standard and in conjunction with a historical documentation of Alzheimer pathology progressive changes (Calderón-Garcidueñas et al., 2018a) are showing the Montreal Cognitive Assessment (MoCA) scores in the published range of mild cognitive impairment and dementia (Calderón-Garcidueñas et al., 2019a, 2020). MMC clinically healthy children have shown abnormal findings in phoneme recognition, finger to nose dysmetria, gait deviation, Romberg's test positivity, fluid and crystallized cognition deficits and prefrontal white matter hyperintense lesions (Calderón-Garcidueñas et al., 2008a, 2011).
We hypothesized that Alzheimer neurodegenerative processes given rise to cognitive deficits, could also involve gait and balance dysfunction, particularly because we have extensively documented brainstem hyperphosphorylated tau, beta amyloid and alpha-synuclein in pediatric and young adults MMC forensic autopsies (Calderón-Garcidueñas et al., 2008b, 2018a).
The purpose of this study was to document gait and balance alterations and cognitive deficits in a cross sectional study of 575 clinically healthy adults, average age 21.03 ± 5.76 y residents in Mexican cities with concentrations of fine particulate matter PM2.5 above the USEPA annual standard. Our first aim was to score a selected balance scale: Berg (Berg et al., 1989, 1992) and a performance-gait and balance scale: Tinetti (Tinetti et al., 1986; Köpke and Meyer, 2006) in our young cohort. The second aim was to measure cognitive performance plus Tinetti and Berg in an independent cohort (n:76) to determine if an association between gait, balance and cognition deficits is present given the AD Continuum described in similar age MMC cohorts (Calderón-Garcidueñas et al., 2016a, 2018a). The MontrealCognitive Assessment version 7, translated into Spanish, was our selected instrument (Calderón-Garcidueñas et al., 2019a, 2020). MoCA covers several cognitive domains including episodic memory, language, attention, orientation, visuospatial and executive functions and has been validated in Mexican populations (Nasreddine et al., 2005; Torrens-Burton et al., 2020; Pugh et al., 2018; Julayanont et al., 2013; https://www.psychiatry.or, 2020).
Early identification of gait and balance impairment in young air pollution-exposed urbanites would facilitate multidisciplinary prevention efforts for modifying the course of AD.
2. Methods
2.1. Air quality data
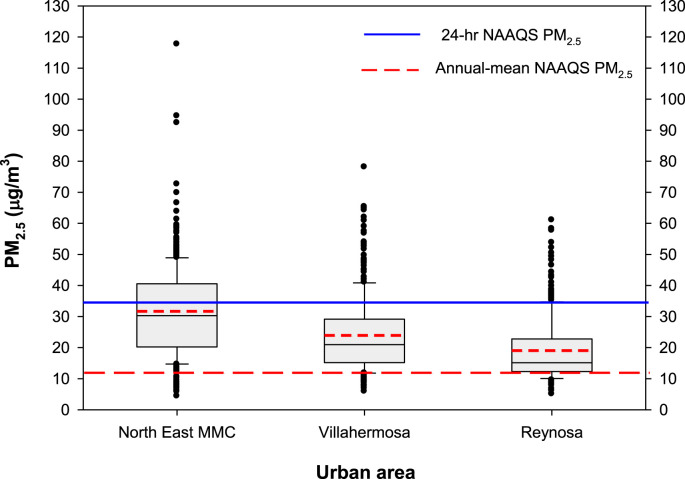
The Mexican urban areas selected were Metropolitan Mexico City (MMC), Villahermosa and Reynosa. Selection was based on geographic location, urban characteristics and stable air pollutant levels in the last 2 decades. The focus was on PM2.5 (≤2.5 μm particles) and we selected to illustrate their behavior for 2017 -the year previous to this clinical study-based on 24-hr and annual USEPA standards. Supplemental Table 1 shows a summary of the main characteristics of the study urban areas, sources of particulate matter and pertinent references. Fig. 1 shows PM2.5 24-hr ranges (box plots) for 2017 in the selected urban locations.
Fig. 1.
PM2.5 24-hr averages box plots at North East MMC, Villahermosa and Reynosa for 2017. NAAQS stands for National Ambient Air Quality Standards for the USEPA. The continuous line inside the box represents the 24 h median (35 μg/m3) and the dotted line the annual mean (12 μg/m3).
As shown in Fig. 1, the higher daily PM2.5 averages were registered in MMC. The 24-hr NAAQS was exceeded in MMC in nearly half of the days with maxima well above the recommended standard. The lower PM2.5 levels were registered in Reynosa and thus corresponded to the less polluted city. The three selected cities exceed the NAAQS annual PM2.5 standard (12 μ/m3) and the WHO guideline for PM2.5 annual average concentrations (10 μ/m3) (Supplementary Table 2).
2.2. Study population and demographics
Ethics approval was granted by the Universidad del Valle de Mexico Research Ethics Committee. All participants gave written consent prior to participation in the study. This is a cross sectional study of 575 participants average age of 21.0 ± 5.7 years, with 13.7 ± 2.4 years of formal education and average BMI of 25.7 ± 4.2 (Table 1 ). The clinically healthy participants fulfil the inclusion (negative family history of Alzheimer's or Parkinson's diseases, hospitalizations, chronic-degenerative diseases, head trauma, negative history of alcohol or light to moderate drinking according to the Dietary Guidelines for Americans, 2015–2020, negative tobacco consumption, and no prescribed or over the counter medications or illegal drugs, including marijuana) criteria. Subjects completed a baseline medical examination and were considered clinically healthy and representative of a Mexican socioeconomic middle class. Every participant was a lifelong resident of the targeted city. The assessment of gait and balance was done in a quiet, well-lit environment with no auditory or visual interference by Physiotherapy professors and trained senior students. Volunteers were instructed to wear comfortable shoes and non-restrictive clothes to the testing session. The clinical gait assessment included the observation of walking to detect clinically evident gait disturbances in the following categories: normal gait, ataxic gait, antalgic gait, cautious gait, frontal gait, hemiparetic gait, spastic gait and shuffling gait as per the Guidelines for gait assessment in the Canadian Consortium on neurodegeneration in aging (Cullen et al., 2018). Tinetti gait and balance (Tinetti et al., 1986; Köpke and Meyer, 2006) evaluations consisted of two parts. The first part is a static examination of standing that includes 13 items: standing position, ability to stand up and to resist external destabilizations. Each item is scored from 1 (normal) to 3 (abnormal). The second part is based on a gait observation with nine items, scored as 1 normal to 2. The gait score is 12 and the balance score is 16. The Berg Balance Scale (BBS) (Berg et al., 1989, 1992) consists of 14 items assessing the ability to stand up and to maintain standing position despite perturbations. Each item is scored from 0 (unable) to 4 (safely done) with a maximum score of 56.
Table 1.
Demographics in Metropolitan Mexico City (MMC), Villahermosa and Reynosa residents.
| Residency | # subjects | Gender F/M | Age | BMI | Tinetti Gait (12) |
Tinetti Balance (16) |
Tinetti Total (28) | Berg Total (56) |
MoCA (Calderón-Garcidueñas et al., 2019a) |
|---|---|---|---|---|---|---|---|---|---|
| MMC | 269 | 177/92 | 22.57 ± 7.69 | 25.51 ± 3.05 | 10.49 ± 1.31 | 12.23 ± 1.37 | 22.72 ± 2.19 | 52.17 ± 2.84 | 24.04 ± 2.72 |
| Reynosa | 56 | 45/11 | 19.84 ± 4.52 | 25.17 ± 5.80 | 11.14 ± 1.74 | 13.50 ± 1.04 | 24.64 ± 2.60 | 55.13 ± 1.74 | 23.53 ± 3.0 |
| Villahermosa | 250 | 156/94 | 19.66 ± 1.90 | 26.17 ± 4.96 | 8.63 ± 2.09 | 12.61 ± 2.01 | 21.24 ± 3.46 | 52.19 ± 7.70 | 22.64 ± 2.93 |
| All | 575 | 378/197 | 21.04 ± 5.76 | 25.76 ± 4.29 | 9.75 ± 1.99 | 12.52 ± 1.70 | 22.26 ± 3.03 | 52.47 ± 5.53 | 23.92 ± 2.82 |
2.3. MoCA administration and scoring
We had an independent clinically healthy cohort n:76, age 23.3 ± 9.1 y for the simultaneous administration of the Spanish version of the Montreal Cognitive Assessment (MoCA), Tinetti and Berg. MoCA assesses global cognitive function and contains 10 subtests (Nasreddine et al., 2005). MoCA scores were converted into 6 index scores based on the combinations used by Petersen (2004) and Julayanont et al. (2014) . Petersen original Delayed Recall Score plus VIS, EIS and LIS were also used (Petersen, 2004).
2.4. Statistical analysis
We first calculated the summary statistics of the Berg and Tinetti scores in the cohorts. Then, we tested the equality of mean scores of each meaningful pair. For the total Tinetti score we used ≥24 as of low risk of falling, 19–23 moderate risk and ≤18 as high risk. For the BBS we used 50–56 as normal, ≤49 abnormal (Berg et al., 1989,1992). We created three cohorts based on MoCA: Normal, MCI and D dementia. The cut-off points are based on total MoCA scores, normal ≥26, MCI 24–25, D ≤ 23. We tested the equality of mean scores of each meaningful pair. We calculated the index scores in each cohort as well. We also fit a multiple linear regression of the total Tinetti, Berg and MoCA score on Age, BMI, Gender, and Education years. We ran the regression models for each of the aforementioned groups. All statistical analyses were made using Excel and the statistical software ‘R’ (http://www.r-project.org/).
3. Results
3.1. Air pollution data
All 651 participants were residents in urban areas with concentrations of PM2.5 above the annual USEPA standard. MMC residents were exposed to high concentrations of PM2.5, including secondary aerosols and ozone for the last 15 years (Molina et al., 2019). Regular measurements of PM2.5 concentrations in MMC began in 2004 and the concentrations recorded along the years have shown minimal variations to the present time.
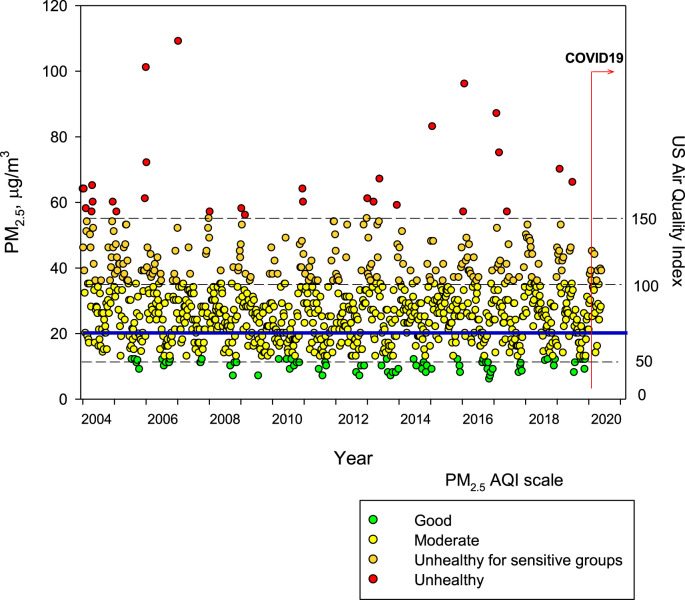
Fig. 2 includes the maximum PM2.5 24-hr averages observed up to April 11, 2020. Historically, the problem of air pollution in MMC (Velasco and Retama, 2017; Silva-Quiroz et al., 2019; Torres-Jardón and Sosa Núñez, 2018) has been associated with cars and buses using reformulated oxygenated gasolines and evaporative emissions from gas stations, fuel storage tanks and urban leakage of Liquefied Petroleum Gas from domestic cylinders. Poorly regulated heavy-duty diesel vehicles are the main emitters of NOx and PM2.5 with a high content in black carbon (SEDEMA. Secretaría del Medio Ambiente de la Ciudad de México, 2018). During several hours per day, particles with size less than 1 μm (PM1) constitute more than the 50% of the PM2.5 (Caudillo et al., 2020). PM1 includes ultrafine particles (UFP ≤100 nm). (Caudillo et al., 2020).
Fig. 2.
Trend of maximum of the maxima PM2.5 24-h average concentrations registered in all PM sampling MMC stations from January 2004 to April 2020 and their comparison against the WHO 24 h mean average (blue solid line) and the US Air Quality Index (AQI) right scale. The beginning of the COVID-19 pandemia February 28, 2020 in MMC is marked. Data correspond to measurements from the manual network of particulate matter of the SEDEMA under a 6-day sampling schedule. Source: http://www.aire.cdmx.gob.mx/default.php#(For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A critical component of MMC exposures are organic aerosols (OA) including primary and secondary aerosols (Molina et al., 2010). Villalobos-Pietrini and colleagues reported that benzo[ghi]perylene has been systematically the most abundant PPAH followed by indeno [1,2,3-c,d] pyrene: both are tracers of vehicular emissions (Villalobos-Pietrini et al., 2011). Metals are largely associated with industrial and mobile sources (Querol et al., 2008). Ozone is above current USEPA standard for South MMC residents (Velasco and Retama, 2017).
Thus, it is clear that MMC residents are exposed all year long to high concentrations of complex mixtures of air pollutants including metals and PAH as components of both, fine PM2.5 and ultrafine particles (UFP) and gaseous pollutants, including ozone and toxics such as benzene and acrolein (Molina et al., 2019; Velasco and Retama, 2017; Silva-Quiroz et al., 2019; Torres-Jardón and Sosa Núñez, 2018; SEDEMA. Secretaría del Medio Ambiente de la Ciudad de México, 2018; Caudillo et al., 2020; Molina et al., 2010; Villalobos-Pietrini et al., 2011; Querol et al., 2008).
3.2. Demographic, Tinetti and Berg results
We analyzed the results of both tests by residency (Table 1). The lowest score for Tinetti gait was in Villahermosa, a city with significant oil industry-associated air pollution, average low cognitive scores in the range of dementia and high BMI in young adults (Calderón-Garcidueñas et al., 2019a). The lowest score of Tinetti balance was for MMC residents. Reynosa residents, with the lowest air pollution concentrations had the highest scores for both tests. Table 2 shows the analysis of Tinetti and Berg scores in relation to gender, BMI and age. For MMC and Villahermosa residents, higher BMI had a negative statistical impact on Tinetti scores, including gait, balance and total scores. Males had lower Tinetti gait and total scores versus females, as their BMI increased. Across cohorts, BMI and age impacted negatively Tinetti and Berg scores. For the lowest polluted city, age, gender and BMI did not significantly impact Tinetti and Berg scores.
Table 2.
Tinetti and Berg results and the correlation with selected key variables in MMC and Villahermosa subjects. For Reynosa-the lowest polluted city-age, gender and BMI did not significantly impact on Tinetti and Berg scores.
| Variables | MMC | Villahermosa |
|---|---|---|
| BMI GENDER AGE |
↓ Tinetti gait p < 0.0001 ↓ Tinetti balance P < 0.0001 ↓ Tinetti Total score <0.0001 Males had ↓ T gait vs females p = 0.013 Males had ↓T Total vs females p = 0.040 No age effect |
↓ Tinetti gait p = 0.0020 ↓ Tinetti balance p = 0.00090 ↓ Tinetti Total score p = 0.00014 ↓ Berg 0.0044 No gender effect No age effect |
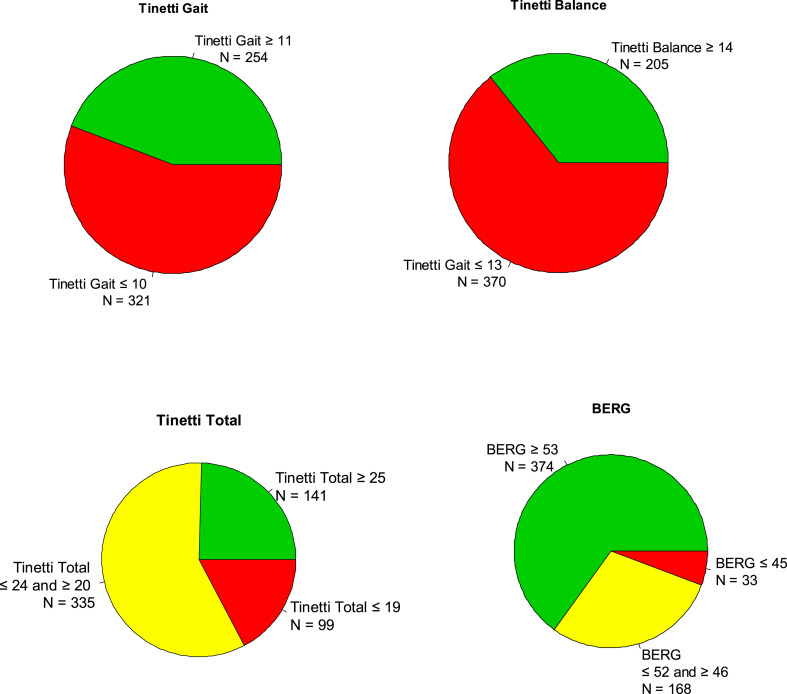
Fig. 3 shows the distribution of the 575 subjects based on their Tinetti and Berg scores: 75.47% n:434, had abnormal scores for Total Tinetti scores, including 99 subjects (17.21%) with high fall risk, while for Berg, 34.43% n:221 had abnormal scores with 33 subjects (5.73%) with high fall risk.
Fig. 3.
The distribution of the 575 subjects based on their Tinetti and Berg scores. Seventy-five per cent (n:434), had abnormal scores for Total Tinetti scores with 17% (n:99) with high fall risk. Thirty-four percent of subjects (n:221) had abnormal Berg scores and 5.73% (n:33), with high fall risk.
3.3. Tinetti, Berg and MoCA results
Table 3 shows 76 participants from the highest and the lowest polluted cities: MMC n:54, and Reynosa n:22 with Tinetti, Berg and MoCA studies. Their average age was 23.38 ± 9.1 y, with 14.11 ± 1.6 years of formal education and a BMI of 25.1 ± 4.6. There were no statistical differences between cohorts in Tinetti gait, while the differences were significant for Tinetti balance (p = 0.02) and Berg (p = 0.0008). The MoCA was on average 23.46 ± 2.68 with no statistical differences between the cohorts. In Table 4 we show the Cognitive Domain scores (Petersen, 2004; Julayanont et al., 2014), Tinetti and Berg scores in the 76 MMC and Reynosa subjects based on their MoCA scores: Normal cognition ≥26, MCI 24–25 and dementia ≤23. The lowest scores in Tinetti and Berg were seen in the 19 subjects (3 M, 16 F) with normal cognitive scores, age 24.47 ± 10.69 y and with 14.32 ± 1.38 y of formal education. In sharp contrast, as the subjects deteriorated towards dementia scores, EIS, LIS and VIS below the cut-off scores were recorded and the Tinetti gait score was lower vs the MCI group. Table 5 shows subjects with BMI ≥25 had significant statistical decrement in Tinetti gait p = 0.0217 with higher EIS scores and a similar relationship was recorded with higher Summary scores (p = 0.0075). Total Tinetti scores also showed a significant decrease in subjects with BMI≥25 and higher Summary Scores (p = 0.0221). Total Berg score decreased with increased AIS (p = 0.0258).
Table 3.
Demographics of 76 subjects in MMC and Reynosa with Tinetti, Berg and MoCA.
| Residency | # subjects | Gender F/M | Age | BMI | Tinetti Gait (12) |
Tinetti Balance (16) |
Tinetti Total (28) | Berg Total (56) |
MoCA |
|---|---|---|---|---|---|---|---|---|---|
| MMC | 54 | 34/20 | 24.33 ± 9.74 | 25.19 ± 3.39 | 10.19 ± 1.85 | 12.28 ± 1.52 | 22.46 ± 2.94 | 52.67 ± 3.79 | 23.48 ± 2.53 |
| Reynosa | 22 | 20/2 | 21.05 ± 7.01 | 24.86 ± 6.89 | 11.00 ± 1.72 | 13.18 ± 1.47 | 24.18 ± 3.06 | 54.77 ± 1.45 | 23.0 ± 2.67 |
| p value | – | – | 0.11 | 0.83 | 0.07 | 0.02 | 0.03 | 0.0008 | 0.47 |
| All | 76 | 54/22 | 23.38 ± 9.11 | 25.10 ± 4.63 | 10.42 ± 1.84 | 12.54 ± 1.55 | 22.96 ± 3.06 | 53.28 ± 3.42 | 23.46 ± 2.68 |
Table 4.
Cognitive Domains Index Scores (Petersen, 2004; Julayanont et al., 2014) data from 76 MMC and Reynosa subjects age 23.38 ± 9.11 y with 14.1 ± 1.6 y formal education.
| Cohort Groups based on MoCA total scores: Normal cognition MCI D |
Tinetti Gait Score (12) |
Tinetti Balance Score (16) |
Tinetti Total Score (28) |
Berg Total Score (56) |
EIS Total:13 CUTOFF SCORE 10.5 |
LIS Total:6 CUTOFF SCORE 5.5 |
VIS Total:7 CUTOFF SCORE 5.5 |
AIS Total:18 CUTOFF SCORE 16 |
Orientation Total:6 CUTOFF SCORE 5.5 |
Delay recall + EIS + VIS + LIS (Blumen et al., 2019a) Total:31 CUTOFF SCORE 24 |
|---|---|---|---|---|---|---|---|---|---|---|
| MoCA score ≥26 Normal Cognition 26.63 ± 0.76 3 M/16 F 24.47 ± 10.69 y 14.32 ± 1.38 yEdu n = 19 |
9.26 ± 2.51 | 12.16 ± 1.83 | 21.42 ± 4.10 | 52.63 ± 4.50 | 11.95 ± 0.97 | 5.58 ± 0.51 | 6.26 ± 0.65 | 17.26 ± 0.73 | 5.89 ± 0.32 | |
| MoCA Score 24-25 MCI 24.47 ± 0.52 4 M/11 F 20.93 ± 2.87 y 14.27 ± 1.67 yEdu n = 15 |
11.33 ± 0.90 | 12.73 ± 1.58 | 24.07 ± 1.94 | 53.20 ± 3.76 | 11.13 ± 1.64 | 5.47 ± 0.64 | 5.67 ± 1.11 | 16.53 ± 1.25 | 5.87 ± 0.35 | |
| MoCA score ≤23 D 21.45 ± 1.60 15 M/27 F 23.76 ± 9.79 y 14.33 ± 1.82 yEdu n = 42 |
10.62 ± 1.48 | 12.64 ± 1.41 | 23.26 ± 2.60 | 53.60 ± 2.71 | 9.95 ± 1.65 | 4.76 ± 0.79 | 5.19 ± 1.15 | 16.02 ± 1.41 | 5.64 ± 0.62 | |
| ALL 23.34 ± 2.56 22 M/54 F 23.38 ± 9.11 y 14.32 ± 1.67 n:76 |
10.42 ± 1.84 | 12.54 ± 1.55 | 22.96 ± 3.06 | 53.28 ± 3.42 | 10.68 ± 1.72 | 5.11 ± 0.79 | 5.55 ± 1.12 | 16.43 ± 1.33 | 5.75 ± 0.52 |
Executive Index Score (EIS) is the sum of Trail making, clock drawing, digit span forward and backward, letter A tapping, serial 7's subtraction, word fluency and abstraction.Language Index Scores (LIS): animal naming, sentence repetition and word fluency.Visuospatial Index Score (VIS): cube copy, clock drawing and animal naming. Attention Index Score (AIS): digit span forward and backward, letter A tapping, serial 7s subtraction, sentence repetition and Words Recalled in Both Immediate Recall Trials.The Orientation Index Score (OIS) includes all the Orientation items (0–6 points).Summary scores include: delay recall + EIS, VIS and LIS.
Table 5.
MoCA selected cognition index scores in MMC and Reynosa and the correlation with Tinetti and Berg scores.BMI ≥25 was the targeted value in Tinnetti.
| MoCA Cognition Index Scores |
Tinetti gait in subjects with BMI≥25 | Tinetti Total in subjects with BMI≥25 | Total Berg all subjects |
|---|---|---|---|
| EIS Increase | ↓ p = 0.0217 | ||
| AIS Increase | ↓ p = 0.0258 | ||
| Summary Score increases | ↓ p = 0.0075 | ↓ p = 0.0221 |
4. Discussion
Gait and balance dysfunction are present in ∼75% of 575 seemingly healthy young adults age 21.04 ± 5.76 y residing in Mexican cities with concentrations of fine particulate matter PM2.5 above the current USEPA annual standard. High risk of falls was detected in 17.2% and 5.7% of them using Tinetti and Berg instruments, respectively.
Young adults are showing higher fall risk and significant cognitive deficits in the absence of risk factors commonly associated with cognition impairment and dementia at younger ages (Kuruppu and Matthews, 2013). Outstandingly, these young adults with College education belong to the same socio-economic status as the 203 MMC subjects ≤40 y staged for AD with Alzheimer Continuum (Calderón-Garcidueñas et al., 2018a; Braak and Del Tredeci, 2015; Thal et al., 2002). In the forensic autopsy study (Calderón-Garcidueñas et al., 2018a), age and cumulative lifelong concentrations of PM2.5 were significant for developing NFT V. In the same autopsy cohorts, magnetic, combustion-derived nanoparticles are associated with early and progressive damage to the neurovascular unit, making the issue of nanoparticulate matter air pollution in MMC residents relevant to our discussion (González-Maciel et al., 2017). We have described the presence of magnetite nanoparticles (NPs) in frontal cortex and hearts of MMC subjects often associated with other non-endogenous metals (including platinum, cadmium, cerium) (Maher et al., 2016; Calderón-Garcidueñas et al., 2019c). Indeed, NPs (Calderón-Garcidueñas et al., 2019d) are abundant in MMC air and contain metals, including Fe, Pb or Zn (Adachi and Buseck, 2010). Villahermosa residents exposed mainly to -PM2.5 rich in PAHs emitted from the flaring of source gas and incineration of oil condensates from gas- and oil extraction activities and from uncontrolled biomass burning, are having the lowest Tinetti gait scores, while MMC residents, are worse in balance and are being exposed to primary and secondary PM2.5 associated with heavy duty diesel vehicles and the photochemical activity of the region. BMI and age are powerful variables for abnormal scores in both Tinetti and Berg and males are doing worse than females in MMC. Remarkably, we found in the 76 subjects with Berg, Tinetti and MoCA, statistical correlation between lower Tinetti scores and higher Executive Index Scores (EIS), a scenario reminiscent of the Motoric Cognitive Risk Syndrome (MCR)-without the subjective cognitive complaints-described in elderly cohorts (Ayers and Verghese, 2019; Verghese et al., 2014, 2019).
Our findings in the 575 young cohort with gait and balance abnormalities ought to be discussed on the bases of the extensive literature available in gait, mobility, cognition, Alzheimer and air pollution. First, there is no question about the high risk of falls in older people and the validity of paradigms linking shared brain pathways in mobility, falls and cognition (Verghese et al., 2019; Montero-Odasso et al., 2019; Tripathi et al., 2019; Mahoney et al., 2019; Rosso et al., 2017; Patience et al., 2019; Sakurai et al., 2019b; Bahureksa et al., 2017; Inzitari et al., 2019; Blumen et al., 2019b). Key to this discussion is the association between specific gray matter networks, normal pace walking speed, dual-task costs and different cognitive domains (Tripathi et al., 2019) and the strong evidence that shared neural systems responsible for gait speed and processing speed are indeed subjected to age and dementia-associated pathological changes (Blumen et al., 2019b). On the other hand, the extensive work on the Motoric Cognitive Risk Syndrome (MCR) defined as a pre-dementia syndrome characterized by subjective cognitive complaints and slow gait, the association between gait decline and mild cognitive impairment (MCI) and the cognitive and motor trajectories in their path to dementia are obligated topics in this discussion (Cohen and Verghese, 2019; Beauchet et al., 2016, 2017a; van der Leeuw et al., 2020; Sathyan et al., 2019a, 2019b; Sekhon et al., 2019; Snir et al., 2019; Montero-Odasso et al., 2018, 2020; Pieruccini-Faria et al., 2020; Sakurai et al., 2019c). Montero-Odasso et al., paper is critical (Montero-Odasso et al., 2018). The authors did a 5 y prospective longitudinal study of 154 adults ≥65 y without dementia at day 0 and defined motor and cognitive decline in those who progressed to dementia versus those who did not (Montero-Odasso et al., 2018). The authors concluded decline in serial measures of gait velocity, had a higher attributable risk for incident dementia than did cognitive decline alone and the highest risk for dementia was in subjects with a decline over time of both gait velocity and cognition (Montero-Odasso et al., 2018). Alterations in white matter (WM) radial diffusivity evaluated by diffusion tensor imaging (DTI) have shown an association with WM tracts participating in executive and visuospatial functions in MCI patients with fall history (Snir et al., 2019). This is very relevant to our neuropathological and electron microscopy MMC studies and the striking neurovascular unit (NVU) damage described by electron microscopy starting in pediatric ages (Calderón-Garcidueñas et al., 2008b, 2016b, 2018a, 2019d; González-Maciel et al., 2017). In our MMC forensic autopsy cohorts, the WM tract damage is not confined to supratentorial regions, it also involves the brainstem tracts and nuclei (Calderón-Garcidueñas et al., 2019b). Indeed, the significant alterations to the brainstem auditory evoked potentials are related to the damage combination of WM tracts and auditory nuclei, a finding evolving in MMC residents as their aged and one of our proposed non-invasive markers of Alzheimer Continuum (Calderón-Garcidueñas et al., 2019b; Jack et al., 2018). Metabolic and genetic factors also have to be considered when we analyzed motor and cognitive decline and the work of Sathyan et al. (2019b), precisely describing high BMI and waist circumference associated with high risk of MCR syndrome, an observation that is not a surprise in view of the literature linking insulin resistance, NVU abnormalities, endothelial dysfunction and certainly inflammation to the development of Alzheimer's disease (Calderón-Garcidueñas and de la Monte, 2017b; Pugazhenthi, 2017; Van Dyken and Lacoste, 2018; Kim et al., 2019). Ethnicity has to be included among the risk factors and Hispanics and particularly Mexican and Mexican-American individuals carry higher risk of cardiometabolic diseases and AD. (Raygor et al., 2019; Gonzales et al., 2019; Diniz et al., 2018). Slow gait and risk of cognitive impairment are also described in APOE4 carriers (Sakurai et al., 2019c), a finding relevant to our MMC populations carrying an allele 4 in20% and accelerating AD neuropathology staging in the first 2 decades of life (Calderón-Garcidueñas et al., 2018a).
The literature fully supports the association between MCI, gait dysfunction and fall risk, the work of Pieruccini-Faria et al. (2020), clearly shows the progression of gait dysfunction in up to 7 y of follow-up in 110 individuals ≥65 y with MCI. Slower gait speed and stride time variability were statistically significant in MCI patients.
The issue of variations in specific gray supratentorial areas in MCR older subjects is of key importance for our young cohorts with Alzheimer Continuum in progress (Calderón-Garcidueñas et al., 2018a), for one very important reason: the identification of specific gray anatomical areas involved in urbanites in the 2sd and 3rd decades will be a remarkable opportunity for intervention and follow-up. Blumen et al. (2019b), examined in 352 nondemented elderly subjects, structural brain correlates of gait speed, and their relationship to processing speed, executive function, and episodic memory. The authors identified gray matter volume covariance patterns associated with gait speed that included brainstem , precuneus, fusiform, motor, supplementary motor, and prefrontal (particularly ventrolateral prefrontal) cortex regions (Blumen et al., 2019b). Their findings are very relevant to MMC residents because the target areas identified in Blumen et al. work are indeed neuropathologically involved in the Alzheimer process i.e., hyperphosphorylated tau and beta amyloid plaques, in very young MMC children and young adults, including the brainstem (Calderón-Garcidueñas et al., 2018a).
In Doi et al. work (Doi et al., 2017) dual-task gait performance and gray matter volume measurements done in 560 elderly patients with MCI, clearly showed that an increased dual-task gait speed was associated with a gray matter increased volume in medial frontal gyrus, superior frontal gyrus, anterior cingulate, cingulate, precuneus, fusiform gyrus, middle occipital gyrus, inferior temporal gyrus and middle temporal gyrus. The authors also emphasized the brain substrates supporting dual-task gait performance in amnestic and non-amnestic MCI subtypes were different, a key finding potentially very relevant for our MMC populations with evolving cognitive domain deficits and significant differences in PM2.5 exposures and portals of entry of fine PM and nanoparticles to the brain (Calderón-Garcidueñas et al., 2016b, 2019a, 2019d, 2020; Maher, 2019; Gonet and Maher, 2019). Beauchet et al. (2017b), put forward a biologically plausible explanation for the correlation between greater subvolumes of the somatosensory cortex and hippocampus reported in fallers compared to non-fallers: a possible brain compensatory mechanism involving spatial navigation and integration of sensory information. Indeed, in explaining increased gain in the auditory pathway (measured as brainstem auditory evoked potentials) in MMC residents we strongly supported compensatory plasticity, neuroinflammation, and AD continuum as strong players in MMC residents (Calderón-Garcidueñas et al., 2019b). We have to fully agree with Beauchet et al. (2016), that the MCR-related smaller global and regional gray matter volumes involving premotor and prefrontal cortices, could be used to predict cortical neurodegenerative changes. Based on our MRI/MRS studies in MMC residents, we also are of the opinion volumetric changes in hippocampus (Rosso et al., 2017) and entorhinal cortex (Sakurai et al., 2019b) are not early findings and we have a higher likelihood of detecting MRS changes, as shown in MMC young residents (Calderón-Garcidueñas et al., 2015a, 2015b).
The key question to be asked for Metropolitan Mexico City residents is why are we seen gait and balance dysfunction in seemingly healthy young adults 21.04 ± 5.76 y in the setting of cognitive deficits involving 55% of the population age 23.9 ± 2.8 y and MoCA dementia scores (≤23) in 22.38 ± 7.7 y olds (Calderón-Garcidueñas et al., 2019a, 2020)? These are MMC residents with abnormal brainstem auditory evoked potentials (Calderón-Garcidueñas et al., 2019b) and those with extensive olfactory bulb neurovascular unit damage with evidence of both Alzheimer and Parkinson's diseases (Calderón-Garcidueñas et al., 2017a, 2018c). Cognitive deficits in young MMC subjects progressively targeted Visuospatial, Executive, Language, and Memory domains, body mass index (BMI) impacted total scores negatively and age drove down Executive, Visuospatial, and Language index scores (Calderón-Garcidueñas et al., 2019a, 2020). Are the MMC residents gait dysfunction and the cognitive deficits at a young age a surprise? No. Is the presence of normal cognition subjects and significant lower scores in Tinetti and Berg another surprise? No, because the knowledge associated with the extensive MCR literature points towards different patterns of motoric and cognitive effects. In fact, we strongly support that we are likely detecting the very early stages of significant gait dysfunction (motoric) with still intact cognitive performance. Potential interpretations of such observations in heavily PM2.5 and nanoparticles exposed individuals could include:
-
1
Significant brainstem and cerebellar involvement directly disrupting centers mapped out as gait initiation and speed regulation pacemakers (Jahn et al., 2008) before quantifiable supratentorial involvement. We have documented brainstem Htau in MMC babies along with early alpha-synuclein in several brainstem nuclei, thus brainstem markers of AD and PD are present very early in heavily exposed PM residents (Calderón-Garcidueñas et al., 2008b, 2011, 2017a, 2018c, 2019b, 2019d, 2020).
-
2.
Beauchet et al. (2017b), biological explanation for a possible brain compensatory mechanism has to be entertained. Indeed, compensatory plasticity, neuroinflammation, and AD continuum are playing a role in the auditory pathway increased gain over time in MMC residents (Calderón-Garcidueñas et al., 2019b).
-
3
The portal of entry of fine PM and nanoparticles (NPs) ought to be considered. The inhalation respiratory portal is important, however the gastrointestinal/neuroenteric portal could also be extraordinary important in some people. The GI system includes an extensive biological barrier, but ingestion of NPs will impact the microbiota and mucus and an altered intestinal homeostasis is an expected outcome. NPs taken through the GI pathway can access the brainstem directly, through the vagus nerves (Calderón-Garcidueñas et al., 2017a; Fröhlich and Roblegg, 2016; Lundquist and Artursson, 2016; Gillois et al., 2018).
-
4
The concentrations and peak exposures to NPs, their chemical composition, the NPs protein corona changes as they cross the NVU and the complexity of NPs interaction with soluble proteins and key organelles determine the specific cell and organelle target in the brain (Calderón-Garcidueñas et al., 2019d, 2019e; Fröhlich and Roblegg, 2016; Lundquist and Artursson, 2016; Mercier-Bonin et al., 2018).
-
5
We have commented before that oxidative, endoplasmic reticulum and mitochondrial stress, and a faulty complex protein quality control are at the core of Alzheimer and Parkinson's diseases and NPs mechanisms of action and toxicity are strong candidates for early development and progression of both fatal diseases (Calderón-Garcidueñas et al., 2019d, 2019e).
Thus, the combination of gait, balance and cognition deficits seen in MMC subjects are likely the result of complex interactions between portals of entry, NPs chemical composition, protein interactions, etc., and an ultimate cellular damage in target brain cells. Ben-Avraham et al., meta-analysis work on the contribution of genetic variation to gait speed in older individuals (Ben-Avraham et al., 2017) is indeed very relevant: gait speed is a polygenic complex trait in five major networks and synaptic function and neuronal development pathways are critical.
We strongly support that in highly exposed PM2.5 populations, clinical gait assessment and quantitative gait parameters should be included in their neuropsychological assessment, along BAEPs, olfaction and cognition tests. The use of a computerized walkway and the inclusion of key variables for gait (pace, rhythm and variability), stride length, swing time, stride length variability, swing time variability along balance testing ought to be included.
The concept of Alzheimer's disease as defined by the National Institute on Aging and Alzheimer's Association Research Framework (Jack et al., 2018): Alzheimer's disease is defined by its underlying pathologic processes that can be documented by postmortem examination or in vivo by biomarkers, is a welcome biologic construct enabling researchers to add variables to the frame work aimed to an accurate characterization and understanding of the sequence of events that lead to cognitive impairment. The fact that biologically defined Alzheimer disease is more prevalent than clinically defined probable Alzheimer disease at any age is a crucial piece of information (Jack et al., 2019), one that obligates us to use every potential early marker to identify subjects in the early stages of AD and defining early gait and balance abnormalities is part of the strategy.
4.1. Advantages and shortcomings of this study relative to other studies
A major advantage of our research design is the access to Mexican subjects with similar socioeconomic status, formal education years, nutrition patterns, etc., enabling us to rule out the possibility that these key variables will modify our results across different urban areas. We selected healthy young individuals and we have a detailed description of AD pathology in 203 consecutive MMC forensic autopsies, ages 11 months to 40 years with no extra-neural pathology, allowing us to put forward direct correlations with the MoCA results in MMC residents and indirectly with residents across the country (Calderón-Garcidueñas et al., 2018a, 2019a, 2020). The study has shortcomings. Our major gap is the lack of funding to purchase a computerized walkway and to do APOE genotyping that will allow us to identify subjects with earlier stages of AD. (Calderón-Garcidueñas et al., 2018a)
5. Summary
The association between gait and balance dysfunction with MCI and dementia scores in cohorts of 23 y olds supports AD Continuum and we need to contemplate an early development of a Motoric Syndrome in the 19 adults with gait dysfunction and normal cognition. We strongly support Alzheimer's disease is preventable in the scenario of air pollution. As such, we enthusiastically endorse screening for gait and balance abnormalities as part of the AD research frame in PM2.5 exposed young adults, children and teens.
Credit author statement
Lilian Calderón-Garcidueñas, Formal analysis and interpretation of data, writing, drafting and revising the manuscript, study Supervision and coordination, funding. Ana Karen Torres-Solorio, analysis and interpretation of data, writing, drafting and revising the manuscript, Randy J. Kulesza, analysis and interpretation of data, writing, drafting and revising the manuscript air pollution sections writing, drafting and revising the manuscript, Luis Oscar González-González, acquisition of data, study Supervision and coordination, Formal analysis and interpretation of data. Ricardo Torres-Jardon air pollution section writing,drafting and revising the manuscript. All other authors acquisition of data, study Supervision and coordination and revising the manuscript.
Declaration of competing interest
None of the authors have any conflict of interest.
Acknowledgements
This work was supported by SEP- CONACYT project 255956 G7 CB-2015-01.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2020.110087.
Contributor Information
Research Universidad del Valle de México UVM Group:
Martha Morales-Aguirre, Silvia Ramírez-Sánchez, Nora Vacaseydel-Aceves, Sylvia Carrillo-Cortez, Félix Márquez-Celedonio, Jorge Roura-Velasco, Joaquín Vázquez-Cruz, David Benítez-Varela, Rocío Ríos-Mendoza, Cynthia Lizbeth López-Morales, Ana Laura Garduza-Hernández, Miguel Angel Zamora-Ramón, José Francisco Arévalo-Campos, Loraine Viveros-Castillo, Jesús Acopa-Tobias, Karen Ramos-Bastard, Juan Torres-Montalvo, Isabel Godínez-Cerón, María Erika Hernández-Sánchez, Rubén Alberto Tiburcio-Bonilla, Natalia Acevedo-Ramírez, Luis Enrique Apango-González, Kevin Barbosa-Moreno, Andrea Cabrera-Peña, Betsy Angélica Carmona-González, Alfredo Castro-Reyes, Carlos Enrique Crespo-Pérez, Uriel Froylan Curiel-Espejel, Ricardo Gómez-Flores, Brenda Janet Lazcano-Hernández, Mariana Marcelino-Macedo, Andrea Martínez-Espinoza, Natalia Edith Mata-Villanueva, Guadalupe David Medrano-López, Ilse Montes-Porras, Laura Abigail Moreno-Espinosa, Jennifer Guadalupe Pérez-Osorio, Valeria Rentería-Rodríguez, Jimena Romero-Cruz, Abdi Soria-Villa, Jorge Velásquez-Romero, Griselda García-Alonso, José Manuel Vega-Riquer, Francisco Javier Olmos-García, Teresa de Jesús Cano-Montoya, Adriel Aguilar-Flores, Vasti Cilos-García, and Rafael Brito-Aguilar
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adachi K., Buseck P.R. Hosted and free-floating metal-bearing atmospheric nanoparticles in Mexico City. Environ. Sci. Technol. 2010;44:2299–2304. doi: 10.1021/es902505b. [DOI] [PubMed] [Google Scholar]
- Ahmed M.M., Mosalem D.M., Alfeeli A.K. Relationship between gait parameters and postural stability in early and late Parkinson's disease and visual feedback-based balance training effects. Open Access Maced J Med Sci. 2017;5:207–214. doi: 10.3889/oamjms.2017.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D.R. The supraspinal control of mammalian locomotion. J. Physiol. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers E., Verghese J. Gait dysfunction in motoric cognitive risk syndrome. J Alzheimer Dis. 2019;71:S95–S103. doi: 10.3233/JAD-181227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahureksa L., Najafi B., Saleh A. The impact of mild cognitive impairment on gait and balance: a systematic review and meta-analysis of studies using instrumented assessment. Gerontology. 2017;63:67–83. doi: 10.1159/000445831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.M. Gait disorders. Am. J. Med. 2018;131:602–607. doi: 10.1016/j.amjmed.2017.11.051. [DOI] [PubMed] [Google Scholar]
- Beauchet O., Allali G., Annweiler C. Association of motoric cognitive risk syndrome with brain volumes: results from the GAIT study. J Gerontol A Biol Sci Med Sci. 2016;71(8):1081–1088. doi: 10.1093/gerona/glw012. [DOI] [PubMed] [Google Scholar]
- Beauchet O., Allali G., Sekhon H. Guidelines for assessment of gait and reference values for spatiotemporal gait parameters in older adults: the biomathics and Canadian gait consortiums initiative. Front. Hum. Neurosci. 2017;11:353. doi: 10.3389/fnhum.2017.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O., Launay C.P., Barden J. Biomathics and Canadian gait Consortium. Association between falls and brain subvolumes: results from a cross-sectional analysis in healthy older adults. Brain Topogr. 2017;30:272–280. doi: 10.1007/s10548-016-0533-z. [DOI] [PubMed] [Google Scholar]
- Ben-Avraham D., Karasik D., Verghese J. The complex genetics of gait speed: genome-wide meta-analysis approach. Aging (Albany NY) 2017;9:209–246. doi: 10.18632/aging.101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K., Wood-Dauphinee S., Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother. Can. 1989;41:304–310. [Google Scholar]
- Berg K., Maki B.E., Williams J.I. Clinical and laboratory measures of postural balance in an elderly population. Arch. Phys. Med. Rehabil. 1992;73:1073–1080. [PubMed] [Google Scholar]
- Blumen H.M., Allali G., Beauchet O. Gray matter volume covariance network associated with the motoric cognitive risk syndrome: a multicohort MRI study. J Gerontol A Biol Sci Med Sci. 2019;74:884–889. doi: 10.1093/gerona/gly158. [DOI] [PubMed] [Google Scholar]
- Blumen H.M., Brown L.L., Habeck C. Gray matter volume covariance patterns associated with gait speed in older adults: a multi-cohort MRI study. Brain Imaging Behav. 2019;13:446–460. doi: 10.1007/s11682-018-9871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Del Tredeci K. The preclinical phase of the pathological process underlying sporadic Alzheimer's disease. Brain. 2015;138:2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., de la Monte S.M. Apolipoprotein E4, gender, body mass index, inflammation, insulin resistance, and air pollution interactions: recipe for Alzheimer's disease development in Mexico city young females. J Alzheimers Dis. 2017;58:613–630. doi: 10.3233/JAD-161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Mora-Tiscareño A., Ontiveros E. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cognit. 2008;68:117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Solt A.C., Henríquez-Roldán C. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain-barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., D'Angiulli A., Kulesza R.J. Air pollution is associated with brainstem auditory nuclei pathology and delayed brainstem auditory evoked potentials. Int. J. Dev. Neurosci. 2011;29:365–375. doi: 10.1016/j.ijdevneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Cross J.V., Franco-Lira M. Brain immune interactions and air pollution: macrophage inhibitory factor (MIF), prion cellular protein (PrP(C)), interleukin-6 (IL-6), interleukin 1 receptor antagonist (IL-1Ra), and interleukin-2 (IL-2) in cerebrospinal fluid and MIF in serum differentiate urban children exposed to severe vs. Low air pollution. Front. Neurosci. 2013;7:183. doi: 10.3389/fnins.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Mora-Tiscareño A., Melo-Sánchez G. A critical proton MR spectroscopy marker of Alzheimer's disease early neurodegenerative change: low hippocampal NAA/Cr ratio impacts APOE ɛ4 Mexico city children and their parents. J Alzheimers Dis. 2015;48:1065–1075. doi: 10.3233/JAD-150415. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Mora-Tiscareño A., Franco-Lira M. Decreases in short term memory, IQ, and altered brain metabolic ratios in urban apolipoprotein ε4 children exposed to air pollution. J Alzheimers Dis. 2015;45:757–770. doi: 10.3233/JAD-142685. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Avila-Ramírez J., Calderón-Garcidueñas A. Cerebrospinal fluid biomarkers in highly exposed PM2.5 urbanites: the risk of Alzheimer's and Parkinson's diseases in young Mexico city residents. J Alzheimers Dis. 2016;54:597–613. doi: 10.3233/JAD-160472. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Reynoso-Robles R., Vargas-Martínez J. Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer's disease. Environ. Res. 2016;146:404–417. doi: 10.1016/j.envres.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Reynoso-Robles R., Pérez-Guillé B. Combustion-derived nanoparticles, the neuroenteric system, cervical vagus, hyperphosphorylated alpha synuclein and tau in young Mexico City residents. Environ. Res. 2017;159:186–201. doi: 10.1016/j.envres.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., de la Monte S. Apolipoprotein E4, Gender, Body Mass Index, Inflammation, Insulin Resistance, and Air Pollution Interactions: Recipe for Alzheimer's Disease Development in Mexico City Young Females. J Alzheimers Dis. 2017;58:613–630. doi: 10.3233/JAD-161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Gónzalez-Maciel A., Reynoso-Robles R. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤ 40 years of age. Environ. Res. 2018;164:475–487. doi: 10.1016/j.envres.2018.03.023. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Mukherjee P.S., Waniek K. Non-phosphorylated tau in cerebrospinal fluid is a marker of Alzheimer's disease continuum in young urbanites exposed to air pollution. J Alzheimers Dis. 2018;66:1437–1451. doi: 10.3233/JAD-180853. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., González-Maciel A., Reynoso-Robles R. Alzheimer's disease and alpha-synuclein pathology in the olfactory bulbs of infants, children, teens and adults ≤ 40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ. Res. 2018;166:348–362. doi: 10.1016/j.envres.2018.06.027. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Mukherjee P.S., Kulesza R.J. Research Universidad del Valle de México UVM Group. Mild Cognitive Impairment and Dementia Involving Multiple Cognitive Domains in Mexican Urbanites. J Alzheimers Dis. 2019;68:1113–1123. doi: 10.3233/JAD-181208. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Kulesza R.J., Mansour Y. Increased gain in the auditory pathway, Alzheimer's disease continuum, and air pollution: peripheral and central auditory system dysfunction evolves across pediatric and adult urbanites. J Alzheimers Dis. 2019;70:1275–1286. doi: 10.3233/JAD-190405. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., González-Maciel A., Mukherjee P.S. Combustion- and friction-derived magnetic air pollution nanoparticles in human hearts. Environ. Res. 2019;176:108567. doi: 10.1016/j.envres.2019.108567. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., González-Maciel A., Kulesza R.J. Air pollution, combustion and friction derived nanoparticles, and Alzheimer's disease in urban children and young adults. J Alzheimers Dis. 2019;70:343–360. doi: 10.3233/JAD-190331. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Reynoso-Robles R., González-Maciel A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: the culprit of Alzheimer and Parkinson's diseases. Environ. Res. 2019;176:108574. doi: 10.1016/j.envres.2019.108574. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Torres-Jardón R., Kulesza R.J. Alzheimer disease starts in childhood in polluted Metropolitan Mexico City. A major health crisis in progress. Environ. Res. 2020;183:109137. doi: 10.1016/j.envres.2020.109137. (Review) [DOI] [PubMed] [Google Scholar]
- Calderón-GarcidueñasL L., de la Monte, Salcedo S., Peralta O. Nanoparticle size distributions in Mexico City. Atmospheric Pollution Research. 2020;11:78–84. [Google Scholar]
- Chambers N.E., Lanza K., Bishop C. Pedunculopontine nucleus degeneration contributes to both motor and non-motor symptoms of Parkinson's disease. Front. Pharmacol. 2020;10:1494. doi: 10.3389/fphar.2019.01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.A., Verghese J. Gait and dementia. Handb. Clin. Neurol. 2019;167:419–427. doi: 10.1016/B978-0-12-804766-8.00022-4. [DOI] [PubMed] [Google Scholar]
- Cosentino E., Palmer K., Della Pietà C. Association between gait, cognition, and gray matter volumes in mild cognitive impairment and healthy controls. Alzheimer Dis. Assoc. Disord. 2020 Jan 21 doi: 10.1097/WAD.0000000000000371. [DOI] [PubMed] [Google Scholar]
- Cullen S., Montero-Odasso M., Bherer L. Canadian gait and cognition network. Guidelines for gait assessments in the Canadian Consortium on neurodegeneration in aging (CCNA) Can Geriatr J. 2018;21:157–165. doi: 10.5770/cgj.21.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen S., Borrie M., Carroll S. Are cognitive subtypes associated with dual-task gait performance in a clinical setting? J Alzheimers Dis. 2019;71(s1):S57–S64. doi: 10.3233/JAD-181196. [DOI] [PubMed] [Google Scholar]
- Dale M.L., Horak F.B., Wright W.G. Impaired perception of surface tilt in progressive supranuclear palsy. PloS One. 2017;12(3) doi: 10.1371/journal.pone.0173351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V. Gait disorders. Handb. Clin. Neurol. 2013;110:133–143. doi: 10.1016/B978-0-444-52901-5.00012-5. [DOI] [PubMed] [Google Scholar]
- Diniz B.S., Fisher-Hoch S., McCormick J. The association between insulin resistance, metabolic variables, and depressive symptoms in Mexican-American elderly: a population-based study. Int. J. Geriatr. Psychiatr. 2018;33:e294–e299. doi: 10.1002/gps.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T., Blumen H.M., Verghese J. Gray matter volume and dual-task gait performance in mild cognitive impairment. Brain Imaging Behav. 2017;11:887–898. doi: 10.1007/s11682-016-9562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino P.C., Peterka R.J., Hullar T.E. Assessment and rehabilitation of central sensory impairments for balance in mTBI using auditory biofeedback: a randomized clinical trial. BMC Neurol. 2017;17(1):41. doi: 10.1186/s12883-017-0812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterwalder S., Wuehr M., Gesierich B. Minor gait impairment despite white matter damage in pure small vessel disease. Ann Clin Transl Neurol. 2019;6:2026–2036. doi: 10.1002/acn3.50891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich E., Roblegg E. Oral uptake of nanoparticles: human relevance and the role of in vitro systems. Arch. Toxicol. 2016;90:2297–2314. doi: 10.1007/s00204-016-1765-0. [DOI] [PubMed] [Google Scholar]
- Gera G., Fling B.W., Horak F.B. Cerebellar white matter damage is associated with postural sway deficits in people with multiple sclerosis. Arch. Phys. Med. Rehabil. 2020;101:258–264. doi: 10.1016/j.apmr.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Gillois K., Lévêque M., Théodorou V. Mucus: an underestimated gut target for environmental pollutants and food additives. Microorganisms. 2018;6(2):E53. doi: 10.3390/microorganisms6020053. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonet T., Maher B.A. Airborne, vehicle-derived Fe-bearing nanoparticles in the urban environment: a review. Environ. Sci. Technol. 2019;53:9970–9991. doi: 10.1021/acs.est.9b01505. [DOI] [PubMed] [Google Scholar]
- Gonzales M.M., Durazo-Arvizu R.A., Sachdeva S. Associations of insulin resistance with cognition in individuals without diagnosed diabetes: results from the hispanic community health study/study of latinos. Diabetes Res. Clin. Pract. 2019;150:38–47. doi: 10.1016/j.diabres.2019.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maciel A., Reynoso-Robles R., Torres-Jardón R. Combustion-derived nanoparticles in key brain target cells and organelles in young urbanites: culprit hidden in plain sight in Alzheimer's disease development. J Alzheimers Dis. 2017;59:189–208. doi: 10.3233/JAD-170012. [DOI] [PubMed] [Google Scholar]
- Heijnen M.J., Rietdyk S. Falls in young adults: perceived causes and environmental factors assessed with a daily online survey. Hum. Mov. Sci. 2016;46:86–95. doi: 10.1016/j.humov.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Horak F.B., Mancini M., Carlson-Kuhta P. Balance and gait represent independent domains of mobility in Parkinson disease. Phys. Ther. 2016;96:1364–1371. doi: 10.2522/ptj.20150580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.psychiatry.org/psychiatrists/practice/dsm
- Inzitari M., Metti A., Rosano C. Qualitative neurological gait abnormalities, cardiovascular risk factors and functional status in older community-dwellers without neurological diseases: the Healthy Brain Project. Exp. Gerontol. 2019;124:110652. doi: 10.1016/j.exger.2019.110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Bennett D.A., Blennow K. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Therneau T.M., Weigand S.D. Prevalence of biologically vs clinically defined alzheimer spectrum entities using the national Institute on aging-alzheimer's association research Framework. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2019.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K. The aging vestibular system: dizziness and imbalance in the elderly. Adv. Oto-Rhino-Laryngol. 2019;82:143–149. doi: 10.1159/000490283. [DOI] [PubMed] [Google Scholar]
- Jahn K., Deutschländer A., Stephan T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39:786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Jahn K., Freiberger E., Eskofier B.M. Balance and mobility in geriatric patients : assessment and treatment of neurological aspects. Z. Gerontol. Geriatr. 2019;52:316–323. doi: 10.1007/s00391-019-01561-z. [DOI] [PubMed] [Google Scholar]
- Julayanont P., Phillips N., Chertkow H. Montreal cognitive assessment (MoCA): concept and clinical review. In: Larner A., editor. Cognitive Screening Instruments. Springer; London: 2013. pp. 111–151. [Google Scholar]
- Julayanont P., Brousseau M., Chertkow H. Montreal cognitive assessment memory index score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer's disease. J. Am. Geriatr. Soc. 2014;62:679–684. doi: 10.1111/jgs.12742. [DOI] [PubMed] [Google Scholar]
- Kim S.E., Lee J.S., Woo S. Sex-specific relationship of cardiometabolic syndrome with lower cortical thickness. Neurology. 2019;93(11):e1045–e1057. doi: 10.1212/WNL.0000000000008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpke S., Meyer G. The Tinetti test: babylon in geriatric assessment. Z. Gerontol. Geriatr. 2006;39:288–291. doi: 10.1007/s00391-006-0398-y. [DOI] [PubMed] [Google Scholar]
- Kuruppu D.K., Matthews B.R. Young-onset dementia. Semin. Neurol. 2013;33:365–385. doi: 10.1055/s-0033-1359320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J.M., Mancini M., Kaye J.A. Day-to-Day variability of postural sway and its association with cognitive function in older adults: a pilot study. Front. Aging Neurosci. 2018;10:126. doi: 10.3389/fnagi.2018.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist P., Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016;106(Pt B):256–276. doi: 10.1016/j.addr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Maher B.A. Airborne magnetite- and iron-rich pollution nanoparticles: potential neurotoxicants and environmental risk factors for neurodegenerative disease, including Alzheimer's disease. J Alzheimers Dis. 2019;71:361–375. doi: 10.3233/JAD-190204. [DOI] [PubMed] [Google Scholar]
- Maher B.A., Ahmed I.A., Karloukovski V. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2016;113:10797–10801. doi: 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney J.R., Cotton K., Verghese J. Multisensory integration predicts balance and falls in older adults. J Gerontol A Biol Sci Med Sci. 2019;74:1429–1435. doi: 10.1093/gerona/gly245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Ardle R., Galna B., Donaghy P. Do Alzheimer's and Lewy body disease have discrete pathological signatures of gait? Alzheimers Dement. 2019;15:1367–1377. doi: 10.1016/j.jalz.2019.06.4953. [DOI] [PubMed] [Google Scholar]
- Mercier-Bonin M., Despax B., Raynaud P. Mucus and microbiota as emerging players in gut nanotoxicology: the example of dietary silver and titanium dioxide nanoparticles. Crit. Rev. Food Sci. Nutr. 2018;58:1023–1032. doi: 10.1080/10408398.2016.1243088. [DOI] [PubMed] [Google Scholar]
- Mirelman A., Shema S., Maidan I. Gait. Handb Clin Neurol. 2018;159:119–134. doi: 10.1016/B978-0-444-63916-5.00007-0. [DOI] [PubMed] [Google Scholar]
- Molina L.T., Madronich S., Gaffney J.S. An overview of the MILAGRO 2006 Campaign: Mexico City emissions and their transport and transformation. Atmos. Chem. Phys. 2010;10:8697–8760. [Google Scholar]
- Molina L.T., Velasco E., Retama A. Experience from integrated air quality management in the Mexico city metropolitan area and Singapore. Atmosphere. 2019;10(9):512. doi: 10.3390/atmos10090512. [DOI] [Google Scholar]
- Montero-Odasso M., Perry G. Gait disorders in Alzheimer's disease and other dementias: there is something in the way you walk. J Alzheimers Dis. 2019;71(s1):S1–S4. doi: 10.3233/JAD-190790. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M., Speechley M., Muir-Hunter S.W. Canadian gait and cognition network. Motor and cognitive trajectories before dementia: results from gait and brain study. J. Am. Geriatr. Soc. 2018;66:1676–1683. doi: 10.1111/jgs.15341. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M., Almeida Q.J., Bherer L. Canadian gait and cognition network. Consensus on shared measures of mobility and cognition: from the Canadian Consortium on neurodegeneration in aging (CCNA) J Gerontol A Biol Sci Med Sci. 2019;74:897–909. doi: 10.1093/gerona/gly148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M., Ismail Z., Camicioli R. Alzheimer disease, biomarkers, and clinical symptoms-quo vadis? JAMA Neurol. 2020 Feb 3 doi: 10.1001/jamaneurol.2019.4959. [DOI] [PubMed] [Google Scholar]
- Moretti R., Cavressi M., Tomietto P. Gait and apathy as relevant symptoms of subcortical vascular dementia. Am J Alzheimers Dis Other Demen. 2015;30:390–399. doi: 10.1177/1533317514550329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muurling M., Rhodius-Meester H.F.M., Pärkkä J. Gait disturbances are associated with increased cognitive impairment and cerebrospinal fluid tau levels in a memory clinic cohort. J Alzheimer Dis. 2020 Jun 24 doi: 10.3233/JAD-200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Orlovsky G.N., Deliagina T.G., Grillner S. Oxford UP; Oxford: 1999. From Mollusc to Man. (Neuronal control of locomotion) [Google Scholar]
- Patience J., Lai K.S.P., Russell E. Relationship between mood, thinking, and walking: a systematic review examining depressive symptoms, executive function, and gait. Am. J. Geriatr. Psychiatr. 2019;27:1375–1383. doi: 10.1016/j.jagp.2019.07.007. [DOI] [PubMed] [Google Scholar]
- Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Pieruccini-Faria F., Sarquis-Adamson Y., Anton-Rodrigo I. Mapping associations between gait decline and fall risk in mild cognitive impairment. J. Am. Geriatr. Soc. 2020;68:576–584. doi: 10.1111/jgs.16265. Epub 2019 Dec 17. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S. Metabolic syndrome and the cellular phase of Alzheimer's disease. Prog Mol Biol Transl Sci. 2017;146:243–258. doi: 10.1016/bs.pmbts.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Pugh E.A., Kemp E.C., van Dyck C.H. Alzheimer's disease neuroimaging initiative. Effects of normative adjustments to the montreal cognitive assessment. Am. J. Geriatr. Psychiatr. 2018:S1064–S7481. doi: 10.1016/j.jagp.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querol X., Pey J., Minguillón M.C. PM speciation and sources in Mexico during the MILAGRO-2006 Campaign. Atmos. Chem. Phys. 2008;8:111–128. [Google Scholar]
- Raygor V., Abbasi F., Lazzeroni L.C. Impact of race/ethnicity on insulin resistance and hypertriglyceridaemia. Diabetes Vasc. Dis. Res. 2019;16:153–159. doi: 10.1177/1479164118813890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso A.L., Verghese J., Metti A.L. Slowing gait and risk for cognitive impairment: the hippocampus as a shared neural substrate. Neurology. 2017;89:336–342. doi: 10.1212/WNL.0000000000004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai R., Watanabe Y., Osuka Y. Overlap between apolipoprotein Eε4 allele and slowing gait results in cognitive impairment. Front. Aging Neurosci. 2019;11:247. doi: 10.3389/fnagi.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai R., Bartha R., Montero-Odasso M. Entorhinal cortex volume is associated with dual-task gait cost among older adults with MCI: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2019;74:698–704. doi: 10.1093/gerona/gly084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai R., Watanabe Y., Osuka Y. Overlap between apolipoprotein Eε4 allele and slowing gait results in cognitive impairment. Front. Aging Neurosci. 2019;11:247. doi: 10.3389/fnagi.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan S., Ayers E., Gao T. Frailty and risk of incident motoric cognitive risk syndrome. J Alzheimers Dis. 2019;71(s1):S85–S93. doi: 10.3233/JAD-190517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan S., Wang T., Ayers E. Genetic basis of motoric cognitive risk syndrome in the Health and Retirement Study. Neurology. 2019;92(13):e1427–e1434. doi: 10.1212/WNL.0000000000007141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedema Secretaría del Medio Ambiente de la Ciudad de México . Ciudad de México; 2018. Inventario de Emisiones de la Ciudad de México 2016. Dirección General de Gestión de la Calidad del Aire, Dirección de Programas de Calidad del Aire e Inventario de Emisiones. Sept. [Google Scholar]
- Sekhon H., Launay C.P., Chabot J. Motoric cognitive risk syndrome: could it Be defined through increased five-times-sit-to-stand test time, rather than slow walking speed? Front. Aging Neurosci. 2019;10:434. doi: 10.3389/fnagi.2018.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Quiroz R., Rivera A.L., Ordoñez P. Atmospheric blockages as trigger of environmental contingencies in Mexico City. Heliyon. 2019;5(7) doi: 10.1016/j.heliyon.2019.e02099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snir J.A., Bartha R., Montero-Odasso M. White matter integrity is associated with gait impairment and falls in mild cognitive impairment. Results from the gait and brain study. Neuroimage Clin. 2019;24:101975. doi: 10.1016/j.nicl.2019.101975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov. Disord. 2013;28:1483–1491. doi: 10.1002/mds.25669. [DOI] [PubMed] [Google Scholar]
- Taylor M.E., Close J.C.T. Dementia. Handb. Clin. Neurol. 2018;159:303–321. doi: 10.1016/B978-0-444-63916-5.00019-7. [DOI] [PubMed] [Google Scholar]
- Thal D.R., Rüb U., Orantes M. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Tian Q., Resnick S.M., Studenski S.A. Lap time variability from a 400-m walk is associated with future mild cognitive impairment and Alzheimer's disease. J. Am. Med. Dir. Assoc. 2019;20:1535–1539. doi: 10.1016/j.jamda.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti M.E., Williams T.F., Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am. J. Med. 1986;80:429–434. doi: 10.1016/0002-9343(86)90717-5. [DOI] [PubMed] [Google Scholar]
- Torrens-Burton A., Hanley C.J., Wood R. Lacking pace but not precision: age-related information processing changes in response to a dynamic attentional control task. Brain Sci. 2020 June 19;10(6):E390. doi: 10.3390/brainsci10060390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Jardón R. Políticas públicas y su efecto en la calidad del aire de la Zona Metropolitana de la Ciudad de México. In: Sosa Núñez G.S., editor. Tranversalidad de la Política del Aire en México. Instituto de Investigaciones Dr. José María Luis Mora. México, DF; 2018. pp. 43–74. [Google Scholar]
- Tripathi S., Verghese J., Blumen H.M. Gray matter volume covariance networks associated with dual-task cost during walking-while-talking. Hum. Brain Mapp. 2019;40:2229–2240. doi: 10.1002/hbm.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Leeuw G., Ayers E., Blankenstein A.H. The association between pain and prevalent and incident motoric cognitive risk syndrome in older adults. Arch. Gerontol. Geriatr. 2020;87:103991. doi: 10.1016/j.archger.2019.103991. [DOI] [PubMed] [Google Scholar]
- Van Dyken P., Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front. Neurosci. 2018;12:930. doi: 10.3389/fnins.2018.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco E., Retama A. Ozone's threat hits back Mexico City. Sustainable Cities and Society. 2017;31:260–263. [Google Scholar]
- Verghese J., Lipton R.B., Hall C.B. Abnormality of gait as a predictor of non-Alzheimer's dementia. N. Engl. J. Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- Verghese J., Annweiler C., Ayers E. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83:718–726. doi: 10.1212/WNL.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J., Wang C., Bennett D.A. Motoric cognitive risk syndrome and predictors of transition to dementia: a multicenter study. Alzheimers Dement. 2019;15:870–877. doi: 10.1016/j.jalz.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos-Pietrini R., Amador-Muñoz O., Valle-Hernández B. Chapter 17. Organic compound in airborne particles and their genotoxic effects in Mexico city. In: Mazzeo A., editor. Air Quality Monitoring, Assessment and Management, Nicolás. 2011. pp. 345–378. IntechOpen. [Google Scholar]
- Waite L.M., Grayson D.A., Piguet O. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J. Neurol. Sci. 2005;89–93:229–230. doi: 10.1016/j.jns.2004.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.