Abstract
Background
we aimed to explore the relationship of acute kidney injury (AKI) with the severity and mortality of coronavirus disease 2019 (COVID-19).
Methods
A systematic literature search was conducted in PubMed, EMBASE, Scopus, Web of Science, MedRxiv Database. We compared the laboratory indicators of renal impairment and incidences of AKI in the severe versus non-severe cases, and survival versus non-survival cases, respectively.
Results
In 41 studies with 10,335 COVID-19 patients, the serum creatinine (sCr) in severe cases was much higher than that in non-severe cases (SMD = 0.34, 95% CI: 0.29–0.39), with a similar trend for blood urea nitrogen (BUN) (SMD = 0.66, 95%CI: 0.51–0.81), hematuria (OR = 1.59, 95% CI: 1.15–2.19), and proteinuria (OR = 2.92, 95% CI: 1.58–5.38). The estimated glomerular filtration rate decreased significantly in severe cases compared with non-severe cases (SMD = -0.45, 95% CI: −0.67– −0.23). Moreover, the pooled OR of continuous renal replacement therapy (CRRT) and AKI prevalence for severe vs. non-severe cases was 12.99 (95%CI: 4.03–41.89) and 13.16 (95%CI: 10.16–17.05), respectively. Additionally, 11 studies with 3759 COVID-19 patients were included for analysis of disease mortality. The results showed the levels of sCr and BUN in non-survival cases remarkably elevated compared with survival patients, respectively (SMD = 0.97, SMD = 1.49). The pooled OR of CRRT and AKI prevalence for non-survival vs. survival cases was 31.51 (95%CI: 6.55–151.59) and 77.48 (95%CI: 24.52–244.85), respectively.
Conclusions
AKI is closely related with severity and mortality of COVID-19, which gives awareness for doctors to pay more attention for risk screening, early identification and timely treatment of AKI.
Keywords: SARS-CoV-2, COVID-19, Severity, Mortality, Renal impairment, Acute kidney injury
Abbreviations: COVID-19, coronavirus disease 2019; sCr, serum creatinine; BUN, blood urea nitrogen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ARDS, acute respiratory distress syndrome; AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; SD, standard deviation; NOS, Newcastle-Ottawa scale; ACE2, angiotensin-converting enzyme 2; CRRT, continuous renal replacement therapy; PRISMA, preferred reporting items for systematic reviews and meta-analysis
1. Introduction
Coronavirus disease 2019 (COVID-19), a newly emerging acute respiratory disease, is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and causes substantial morbidity and mortality [1]. As of 12 June 2020, 7,519,566 COVID-19 cases have been confirmed and 419,447 people died from COVID-19 in more than 200 countries around the world. Most patients with COVID-19 are considered as non-severe patients and recover from this infection. However, the symptoms in about 10% of COVID-19 patients are severe and progress rapidly to critical conditions, including organ dysfunctions, such as acute respiratory distress syndrome (ARDS), acute cardiac injury, acute kidney injury (AKI) and even death [2].
Recently, several clinical studies have demonstrated that AKI was one of the most common complications in patients with SARS-CoV-2 infection. For example, in one retrospective study of 193 patients from Wuhan in China, Li et al. reported that proteinuria, hematuria, and elevated levels of blood urea nitrogen (BUN), as well as serum creatinine (sCr) were significantly associated with the death of COVID-19 patients [3]. In addition, an analysis of 355 inpatients in Wuhan showed that prevalence of AKI was 15.8% in admitted patients and 33.9% COVID-19 patients with AKI were died on mean 10.9 day after hospitalization [4]. However, the study of 116 hospitalized COVID-19 patients in Wuhan demonstrated that SARS-CoV-2 infection did not result in AKI [5]. A meta-analysis with large clinical samples is warranted to draw a reliable conclusion. Therefore, we performed the present meta-analysis to investigate the association of AKI with the severity and mortality of SARS-CoV-2 infection.
2. Methods
The systematic review and meta-analysis were performed according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and reported based on Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [6,7]. This meta-analysis has no protocol.
2.1. Search strategy
Articles published from December 2019 to 8 June 2020 in Pubmed, EMBASE, Web of Science, Scopes, and MedRxiv Database were searched. To identify all the articles displaying the renal impairment in COVID-19, we used the following terms alone or in combination for literature search: “SARS-CoV-2”, “COVID-19”, “2019-nCoV”, “nCoV”, “COVID-19”, “coronavirus”, “severe acute respiratory syndrome coronavirus 2”, “renal”, “kidney”, “acute kidney*”,“acute renal*”, “urology”, “urogential system”, “urea”, “urinalysis”, “creatinine”, “proteinuria”, “hematuria”, “blood urea nitrogen” and “serum creatinine”.
2.2. Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) subjects: adult inpatients diagnosed with COVID-19 according to the guidelines for the diagnosis and treatment of novel coronavirus disease; (2) clinical features: definite disease severity or mortality according to the guidelines for the diagnosis and treatment of novel coronavirus disease; (3) outcomes: COVID-19 patients with exact values of renal impairment indicators including BUN, sCr or estimated glomerular filtration rate (eGFR), and the incidences of hematuria, proteinuria, continuous renal replacement therapy (CRRT) and AKI.
Exclusion criteria included: (1) studies with special populations, such as children, elderly, pregnant women, transplant recipients and cancer patients; (2) case reports, reviews, letters, meta-analysis, guidelines, editorials and comments; (3) studies without the data of renal impairment indicators (eg.BUN, sCr or eGFR) or incidence of hematuria, proteinuria, CRRT and AKI for comparison between severe versus non-severe cases or survival versus non-survival cases; (4) sample size less than 20 patients. The flow chat of the study selection was drafted in accordance to the PRISMA principle.
2.3. Definitions
The degrees of COVID-19 severity were evaluated according to the the guidelines for the diagnosis and treatment of novel coronavirus disease. The clinical subgroups of disease severity were described as follows: (1) non-severe group: the clinical symptoms were mild, and there was no or mild imaging signs of pneumonia [8]; (2) severe group (any of the following conditions): I, shortness of breath with respiratory rate ≥30 bpm; II, finger SpO2 ≤ 93% at rest; III, ARDS or arterial partial pressure of oxygen/fraction of inspired oxygen ≤300 mmHg; IV, respiratory failure (requiring mechanical ventilation); V, shock; VI, other organ failure (requiring ICU monitoring and treatment) [9].
2.4. Data extraction and quality assessment
Two investigators worked independently to decide which studies should be included, and the disagreement was resolved by a third investigator. Data was extracted from selected studies including the first author's name, publication data, sex, average age, numbers of patients and study type. In addition, laboratory examinations of renal impairments including BUN, sCr, eGFR, proteinuria and hematuria, and incidence of AKI and CRRT were also extracted. The data shown as median and interquartile range was transformed into mean and standard deviation (SD) according to the formula below (http://www.math.hkbu.edu.hk/tongt/papers/median2mean.html). The prevalence of proteinuria, hematuria, CRRT and AKI as well as average means of BUN, sCr and eGFR were evaluated between severe and non-severe group or survival and non-survival group, respectively.
The quality of studies was evaluated according to the Newcastle-Ottawa scale (NOS) containing three aspects (selection, comparability and outcomes). Scores ranging from 0 to 9, and studies with the score ≥6 were considered as high quality studies.
2.5. Statistical analysis
All data was analyzed by the Review Manager meta-analysis software (version 5.4). The standardized mean differences (SMDs) and 95% confidence intervals (CIs) were calculated for continuous data. The odds ratios (ORs) and 95% CIs were calculated for dichotomous data. The magnitude of heterogeneity between different studies was tested using I 2 statistics. If there was no evidence of between studies heterogeneity (I 2 ≤ 50%), a fixed-effects model was used to calculate. Otherwise, a random-effects model was selected [10]. The Z score was tested for overall effect, with significance considered as P < .05. Publication bias was evaluated by funnel plot if the number of included studies >10.
3. Results
3.1. Study selections
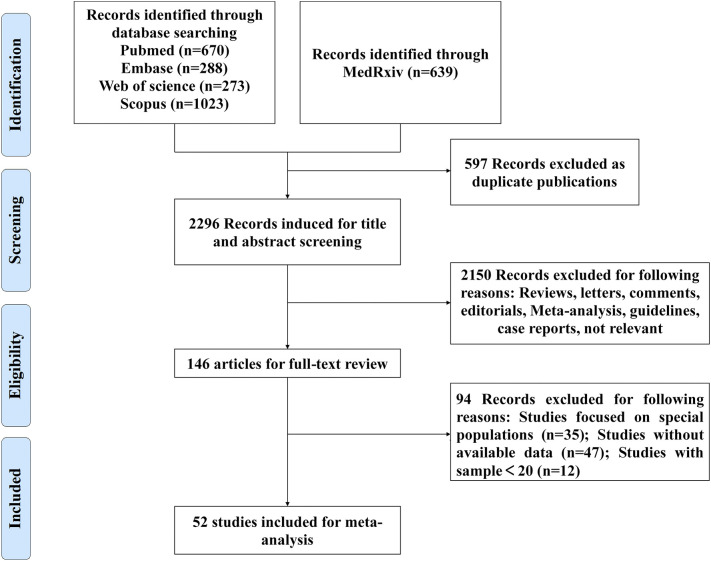
We searched a total of 2893 articles according to the search terms. Firstly, duplicated articles (n = 597) were excluded. After reviewing the titles and abstracts, case reports, reviews, letters, meta-analysis, editorials, guidelines, comments, not relevant studies and sample size less than 20 (n = 2150) were ruled out. 94 articles were excluded after thoroughly reviewing the full texts due to the following reasons: studies focused on special populations (n = 35); studies without available data (n = 47), studies with sample less than 20 (n = 12). Finally, 52 articles [[1], [2], [3], [4],9,[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]] with 14,094 patients were included in our meta-analysis. Fig. 1 showed the flow diagram of the studies selections.
Fig. 1.
Flowchart of study selection.
3.2. Study characteristics
As shown in Table 1 , most of studies were from China, and six studies were published from other countries [11,12,25,28,40,43]. Among of them, 41 studies with 10,335 patients were analyzed for the association of renal impairment with severity of COVID-19 [[2], [3], [4],9,[11], [12], [13], [14],[16], [17], [18],20,21,23,[26], [27], [28], [29], [30], [31], [32], [33], [34],[36], [37], [38], [39],[41], [42], [43], [44], [45], [46], [47],[49], [50], [51],[54], [55], [56], [57]]. In additions, 11 studies with 3759 patients reported the association of renal impairment with mortality of COVID-19 [1,15,19,22,24,25,35,40,48,52,53]. The incidence of AKI and CRRT during SARS-CoV-2 infections was evaluated between the severe versus non-severe cases or survival versus non-survival cases, respectively.
Table 1.
Characteristics of included studies.
| Study | Country | City | Type of study | Sample size, n | Male N.(%) | Age, years (mean (SD)/median(IQR)) | NOS score |
|---|---|---|---|---|---|---|---|
| Antinori S [11] | Italy | Milan | / | 35 | 26(74.3) | 63(51–69) | 6 |
| Argenziano MG [12] | US | New York | Retrospective | 850 | 511(60) | 63(50–75) | 6 |
| Bi QF [13] | China | Shenzhen | Retrospective | 420 | 200(47.6) | / | 7 |
| Cai QX [14] | China | Shenzhen | Retrospective | 298 | 145(48.66) | 47.5(33–61) | 7 |
| Cao M [16] | China | Shanghai | Cohort | 198 | 101(51.0) | 50.1(16.3) | 6 |
| Cao WL [17] | China | Xiangyang | Retrospective | 128 | 60(46.9) | / | 6 |
| Chen G [18] | China | Wuhan | Retrospective | 21 | 17(81) | 56.3(14.3) | 5 |
| Duan J [23] | China | Chongqin | Retrospective | 348 | 184(52.9) | / | 6 |
| Gong J [26] | China | Guangzhou, Wuhan | Retrospective | 189 | 88(46.6) | 49(35, 63) | 5 |
| Guan W [27] | China | Guangzhou | / | 1099 | 637(58) | 47(35–58) | 6 |
| Hong KS [28] | Korea | Daegu | Retrospective | 98 | 38(38.8) | 55.4(17.1) | 6 |
| Huang CL [30] | China | Wuhan | / | 41 | 30(73) | 49(41–58) | 6 |
| Huang H [31] | China | Guangzhou | Retrospective | 125 | 63(50.4) | 44.87(18.55) | 6 |
| Huang SP [32] | China | Shanghai | / | 415 | 217(52.3) | 44(30–61) | 5 |
| Huang YS [2] | China | Wuhan | Cohort | 223 | 126(56.5) | 62(49–70) | 6 |
| Hu L [29] | China | Wuhan | Retrospective | 323 | 166(51.4) | 61(23–91) | 7 |
| Jiang XF [33] | China | Wuxi | Retrospective | 55 | 27(49.1) | 45(27–60) | 7 |
| Liu R [37] | China | Wuhan | / | 119 | 40(33.61) | / | 8 |
| Li Z [3] | China | Wuhan, Chongqing | Retrospective | 193 | 95(49) | 57(46–67) | 7 |
| Pei GC [41] | China | Wuhan | Retrospective | 333 | 182(54.7) | 56.3(13.4) | 7 |
| PengYD [42] | China | Wuhan | Retrospective | 112 | 53(47.32) | 62(55–67) | 6 |
| Petrilli CM[43] | US | New York | Cross-sectional | 1999 | 1251(62.6) | 62(50–74) | 7 |
| Yan SJ [51] | China | Hainan | Retrospective | 168 | 81(48.2) | 51(36–62) | 5 |
| Rica R [21] | Spain | / | Cohort | 48 | 32(67) | 65.98(13.91) | 5 |
| Xu Y [50] | China | Wuhan | Retrospective | 69 | 35(50.7) | 57(43–69) | 6 |
| Wang DW [47] | China | Wuhan | Retrospective | 138 | 75(54.3) | 56(42–68) | 6 |
| Wan SX [46] | China | Chongqin | / | 135 | 72(53.3) | 47(36–55) | 5 |
| Wu CM [49] | China | Wuhan | Retrospective | 201 | 128(63.7) | 51(43–60) | 6 |
| Xu S [4] | China | Wuhan, Fuyang | Retrospective | 355 | 193(54.4) | / | 6 |
| Regina J [44] | Swiss | / | Retrospective | 200 | 120(60.0) | 70(55–81) | 6 |
| Shi PY [45] | China | Outside Wuhan | Retrospective | 134 | 65(48.5) | 46(34–58) | 6 |
| Yang QX [9] | China | Wuhan | Retrospective | 136 | 66(48.5) | 56(44–64) | 7 |
| Zhang GQ [54] | China | Wuhan | Retrospective | 221 | 108(48.9) | 55(39–66.5) | 6 |
| Zhang HZ [55] | China | Chongqin | Retrospective | 43 | 22(51.2) | / | 6 |
| Zhao XY [56] | China | Jingzhou | Retrospective | 91 | 49(53.8) | / | 6 |
| Zhou HF [57] | China | Wuhan | Retrospective | 178 | 72(40.4) | 47(35–61) | 6 |
| Chen X [20] | China | Hunan | / | 291 | 145(49.8) | 46(34, 59) | 6 |
| Ma KL [39] | China | Chongqing | Cohort | 84 | 48(57.1) | 48(42.3–62.5) | 5 |
| Liu JY [36] | China | Beijing | Prospective | 61 | 31(50.8) | 40(1–86) | 6 |
| Liu YL [38] | China | Wuhan | / | 109 | 59 (54.1) | 55(43–66) | 6 |
| Liu L [34] | China | Chongqin | Retrospective | 51 | 32(62.7) | 45(34–51) | 6 |
| Giacomelli A [25] | Italy | Milan | Cohort | 233 | 161(69.1) | 61(50–72) | 6 |
| Yang JK [52] | China | Wuhan | Cohort | 69 | 34(49.3) | 61(52–67) | 7 |
| Zhang F [53] | China | Wuhan | Retrospective | 48 | 33(68.8) | 70.58(13.38) | 6 |
| Chen T [19] | China | Wuhan | Retrospective | 274 | 171(62) | 62(44–70) | 7 |
| Deng Y [22] | China | Wuhan | Retrospective | 225 | 125(55.1) | / | 6 |
| Paranjpe I [40] | USA | New York | Retrospective | 2199 | 1293(58.8) | 65(54–76) | 6 |
| Zhou F [1] | China | Wuhan | Cohort | 191 | 119(62) | 56(46–67) | 6 |
| Cao JL [15] | China | Wuhan | Cohort | 102 | 53(52) | 54(37–67) | 6 |
| Fu L [24] | China | Wuhan | Cohort | 200 | 99(49.3) | / | 6 |
| Li KY [35] | China | Wuhan | Retrospective | 102 | 59(58) | 57(45–70) | 6 |
| Wang ZH [48] | China | Wuhan | Case-control | 116 | 65(56) | 61.1(51–69) | 8 |
SD, Mean difference; IQR, Interquartile range; NOS, Newcastle-Ottawa scale.
3.3. Association between AKI and mortality of COVID-19
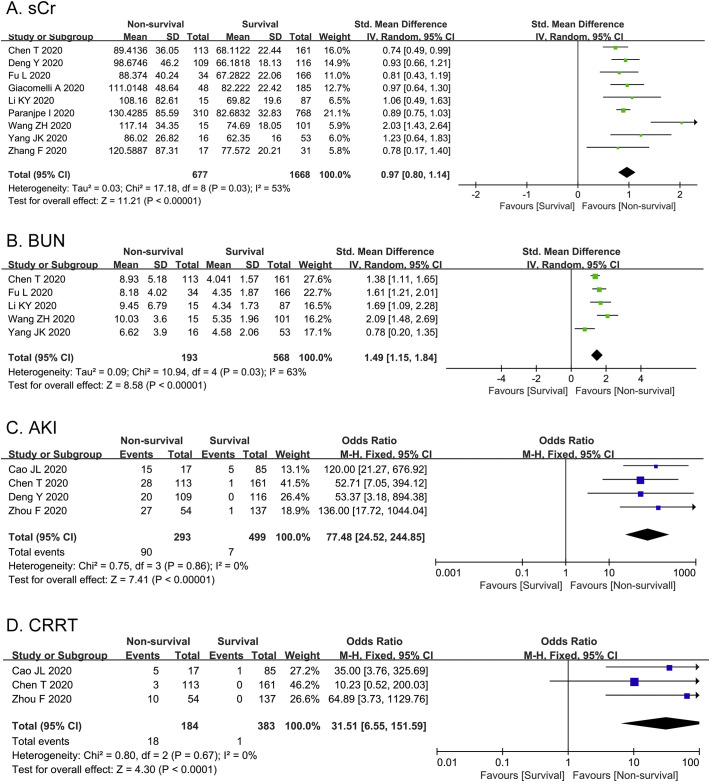
As shown in Fig. 2A, sCr was measured in nine studies among 2345 patients. The heterogeneity test of sCr was shown as I 2 = 53%, thus we applied the random-effects model for further investigation. The following results elucidated that sCr was significantly higher in non-survival group than that in survival group [SMD = 0.97, 95%CI (0.80, 1.14), Z = 11.21, P < 0.00001]. There was moderate statistical heterogeneity between the studies to evaluate BUN (I 2 = 63%). In Fig. 2B, the levels of BUN in five studies were remarkably elevated in non-survival group compared with survival group [SMD = 1.49, 95%CI (1.15, 1.84), Z = 8.58, P < 0.00001]. Furthermore, we compared the incidence of AKI between survival and non-survival group (Fig. 2C). The heterogeneity test of AKI was shown as I 2 = 0. Pooled analysis of four studies among 792 COVID-19 patients revealed that the incidence of AKI was statistically higher in non-survival group (30.72%) compared with survival group (1.4%) [OR 77.48, 95%CI (24.52, 244.85), Z = 7.41, P < 0.00001]. Additionally, 3 studies reported the application rate of CRRT in non-survival vs. survival group without heterogeneity (I 2 = 0). As shown in Fig. 2D, non-survival group had higher application rate of CRRT than survival group [OR = 31.51, 95% CI: 6.55 to 151.59, P < 0.0001].
Fig. 2.
Meta-analysis of prevalence of AKI and CRRT as well as two laboratory indexes of kidney injury. Forest plots represent the comparisons of the prevalence of AKI and CRRT and standard mean differences (SMD) in two laboratory indicators between non-survival and survival cases. A, sCr (serum creatinine, μmol/L); B, BUN (blood urea nitrogen, mmol/L); C, AKI (acute kidney injury); D, CRRT (continuous renal replacement therapy).
3.4. Correlation between AKI and severity of COVID-19
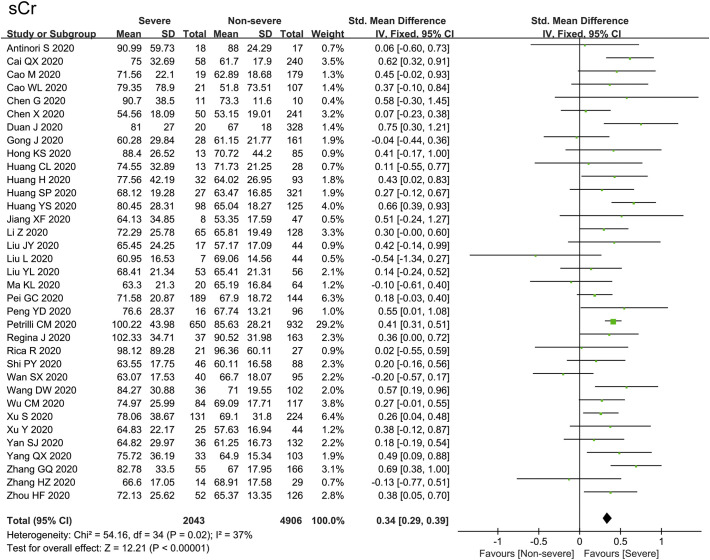
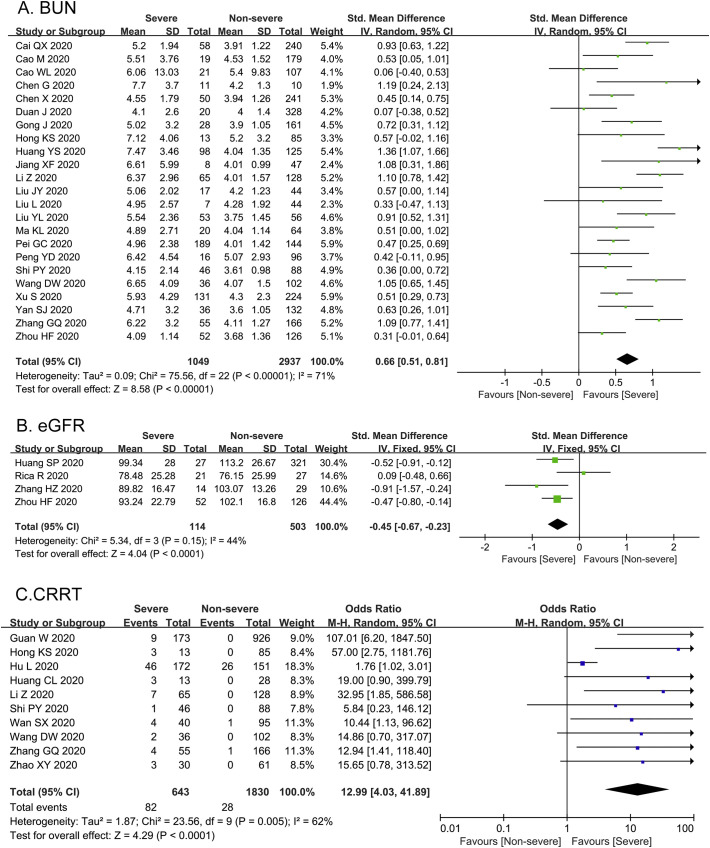
As illustrated in Fig. 3 , sCr was evaluated in 35 studies among 6949 patients, with no statistical heterogeneity (I 2 = 37%). 35 studies reported that the level of sCr was significantly increased in severe group compared with non-severe group [SMD = 0.34, 95%CI (0.29–0.39), Z = 12.21, P < 0.00001]. In Fig. 4A, the heterogeneity test of BUN in 23 studies was shown as I 2 = 71%, thus we applied the random-effects model for further investigation. Sensitivity analysis by removing one study each time suggested the results were robust. Subgroup analysis by the country of study, sample size, age, male percentage and quality score of the studies failed to resolve the obvious heterogeneity. The level of BUN in severe group was remarkably higher than that in non-severe group [SMD = 0.66, 95%CI (0.51–0.81), Z = 8.58, P < 0.00001]. As indicated in Fig. 4B, 4 studies reported the eGFR with no remarkable heterogeneity (I 2 = 44%). The eGFR decreased significantly in severe cases compared with non-severe cases [SMD = -0.45, 95% CI (−0.67− −0.23), Z = 4.04, P < 0.0001]. Additionally, 10 studies reported the application rate of CRRT with moderate heterogeneity (I 2 = 62%). As shown in Fig. 4C, the application rate of CRRT in severe group was significantly higher than that in non-severe group [OR = 12.99, 95% CI: 4.03 to 41.89, P < 0.0001].
Fig. 3.
Forest plot represents the comparisons of standard mean differences (SMD) in sCr between severe and non-severe cases.
Fig. 4.
Forest plots represent the comparisons of standard mean differences (SMD) in BUN and eGFR as well as the prevalence of CRRT between severe and non-severe cases. A, BUN (blood urea nitrogen, mmol/L); B, eGFR (estimated glomerular filtration rate, ml/min); C, CRRT (continuous renal replacement therapy).
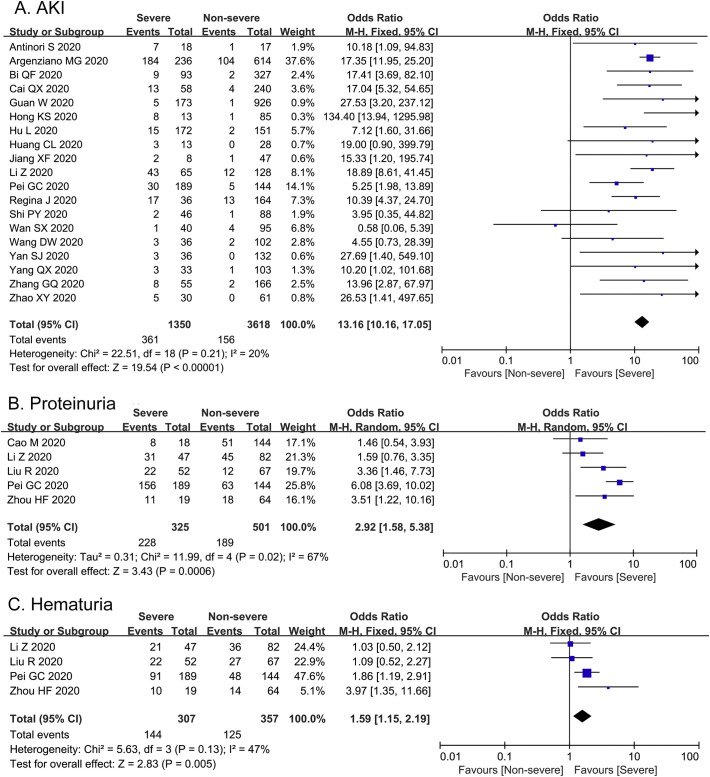
As severity of illness was related with complication in COVID-19, we also evaluated the incidence of AKI in severe and non-severe group (Fig. 5A). The heterogeneity test of AKI was shown as I 2 = 20%. 19 studies among 4968 COVID-19 patients reported that the incidence of AKI was shown to be 26.74% in severe group, which was significant higher than that in non-severe group (4.31%) [OR = 13.16, 95%CI (10.16–17.05), Z = 19.54, P < 0.00001].
Fig. 5.
Forest plots represent the comparisons of incidence of AKI and two clinical characteristics of kidney injury between severe and non-severe cases. A, AKI; B, Proteinuria; C, Hematuria.
As shown in Fig. 5B, based on the 5 studies with significant heterogeneity to evaluate proteinuria (I 2 = 67%), COVID-19 patients in severe cases had higher ratio of proteinuria than non-severe cases [OR = 2.92, 95% CI (1.58–5.38), Z = 3.43, P = 0.0006]. In addition, we also performed meta-analysis on the incidence of hematuria of 664 COVID-19 patients with no statistical heterogeneity among 4 studies (I 2 = 47%). The incidence of hematuria in severe group was statistically higher compared with non-severe group [OR = 1.59, 95% CI (1.15–2.19), Z = 2.83, P = 0.005] (Fig. 5C).
4. Discussion
Our meta-analysis including 14,094 subjects from 52 studies explored the potential relationship between renal impairment as well as AKI and the clinical outcome (severity and mortality) of COVID-19 patients. To our knowledge, this is the first systemic review and meta-analysis which evaluated the kidney function and prevalence of AKI between survival and non-survival cases. We found that the prevalence of AKI in non-survival cases was 30.72%, which was approximately 77.48-fold higher than that in survival cases. Furthermore, patients who died of COVID-19 displayed higher baseline of sCr and BUN as well as higher application rate of CRRT than the survival cases. Meanwhile, our results including severe and non-severe cases (41 studies, 10,335 patients) demonstrated that the overall rate of AKI in severe cases was 13.16-fold higher compared with non-severe cases. The levels of sCr and BUN were shown elevated, while eGFR was decreased in severe cases compared with non-severe cases. In addition, the average ratio of proteinuria, hematuria and CRRT were 2.92-fold, 1.59-fold and 12.99-fold in severe cases compared with those in non-severe cases, respectively.
Currently, the exact mechanism of renal impairment involved in COVID-19 remains unclear. One potential explanation is direct virus attack mediated via angiotensin-converting enzyme 2 (ACE2). RNA sequencing studies found that ACE2, the novel protein of coronavirus receptor, was highly expressed in proximal renal tubules, which could explain that the urinary analysis was obviously abnormal in COVID-19 patients [58]. Hence, early detection of urinary analysis is important for preventing the occurrence of AKI. In addition, hyper-activated immune response may be partly responsible for the development of kidney damage. Clinical studies have shown that the levels of inflammatory cytokines in severe patients are significantly increased compared with mild patients [30]. A recent biopsy pathology result of a COVID-19 patient with ARDS demonstrated that the numbers of CD4+ and CD8+ T cells in peripheral blood were greatly reduced, while T cells were excessively activated [59]. These above findings indicated that pathological waterfall-like cytokines storm caused by immune dysregulation may be involved in the occurrence and development of AKI and multiple organ dysfunctions. Additionally, patients with COVID-19, especially severe and critical cases, are prone to complications such as sepsis, shock, and hypovolemia, which could cause the occurrence or aggravation of AKI through excessive inflammatory responses, apoptosis, and mitochondrial stress [60]. Therefore, optimizing fluid volume and maintaining hemodynamic stability are crucial for severe COVID patients to ensure adequate and effective perfusion pressure of the kidney, which could prevent the occurrence or progression of AKI.
There are strengths of this meta-analysis. To the best of our knowledge, this is the first large meta-analysis which performed a pairwise comparison of kidney function indicators and prevalence of AKI in severe vs. non-severe or non-survival vs. survival cases, respectively. Secondly, we have included a large number of studies covering six countries, with patient population above fourteen thousand. Finally, our meta-analysis provides the awareness for clinicians to pay more attention for risk screening, early identification and timely treatment of AKI.
Our study also has several limitations. Firstly, although we firstly investigated the renal impairments in survival and non-survival cases, we did not analyze the laboratory changes in hematuria, proteinuria, and eGFR due to the lack of literatures. Secondly, we found moderate statistical heterogeneity in BUN levels. However, the heterogeneity could not be removed through subgroup analysis. Thirdly, another limitation of our analysis is that some articles provided median and interquartile ranges of values in sCr, BUN and eGFR. The mean and SD for these data were required for conversion based on the median and interquartile range, which might result in inaccuracy of values. Lastly, lots of drugs are nephrotoxic to cause the drug-related AKI, such as antibiotics, ACE inhibitors and nonsteroidal anti-inflammatory drugs. We were not sure whether clinical data were affected by drug side-effects.
In conclusions, our meta-analysis provides the further evidence that kidney impairment and AKI are susceptible to occur in COVID-19 patients with worse clinical outcome. The risk of AKI dramatically increased in severe COVID-19. Therefore, it is necessary to establish the early identification for AKI, such as dynamic monitoring urine analysis, renal function, and biomarker detections of renal injury, which should be helpful for improvement for prognosis of COVID-19 patients.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number: 81500064).
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y., Lyu X., Li D., et al. A cohort study of 223 patients explores the clinical risk factors for the severity diagnosis of COVID-19. medRxiv. 2020 [preprint] [Google Scholar]
- 3.Li Z., Wu M., Yao J., et al. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020 [preprint] [Google Scholar]
- 4.Xu S., Fu L., Fei J., et al. Acute kidney injury at early stage as a negative prognostic indicator of patients with COVID-19: a hospital-based retrospective analysis. medRxiv. 2020 [preprint] [Google Scholar]
- 5.Wang L., Li X., Chen H., et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.JPT Higgins, Thomas J., Chandler J., LT Cumpston M., Page M.J., Welch V.A., editors. Cochrane handbook for systematic reviews of interventions version 6.0. The Cochrane Collaboration; London, UK: July 2019. p. 2019.http://handbook.cochrane.org Available from: (Accessed June 2020) [Google Scholar]
- 7.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res Ed) 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W., Tao Z.W., Wang L., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Q., Xie L., Zhang W., et al. Analysis of the clinical characteristics, drug treatments and prognoses of 136 patients with coronavirus disease 2019. J Clin Pharm Ther. 2020;45(4):606–609. doi: 10.1111/jcpt.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 11.Antinori S., Cossu M.V., Ridolfo A.L., et al. Compassionate remdesivir treatment of severe COVID-19 pneumonia in intensive care unit (ICU) and non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol Res. 2020;158:104899. doi: 10.1016/j.phrs.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argenziano M.G., Bruce S.L., Slater C.L., et al. Characterization and clinical course of 1000 Patients with COVID-19 in New York: retrospective case series. medRxiv. 2020 doi: 10.1136/bmj.m1996. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi Q., Hong C., Meng J., et al. Characterizing clinical progression of COVID-19 among patients in Shenzhen, China: an observational cohort study. medRxiv. 2020 [preprint] [Google Scholar]
- 14.Cai Q., Huang D., Ou P., et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy Eur J Allergy Clin Immunol. 2020 doi: 10.1111/all.14309. [preprint] [DOI] [PubMed] [Google Scholar]
- 15.Cao J., Tu W.J., Cheng W., et al. Clinical features and short-term outcomes of 102 patients with Corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):748–755. doi: 10.1093/cid/ciaa243. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao M., Zhang D., Wang Y., et al. Clinical features of patients infected with the 2019 Novel Coronavirus (COVID-19) in Shanghai, China. medRxiv. 2020 [preprint] [Google Scholar]
- 17.Cao W., Shi L., Chen L., Xu X., Wu Z. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. medRxiv. 2020 [preprint] [Google Scholar]
- 18.Chen G., Wu D., Guo W., et al. Clinical and immunologic features in severe and moderate forms of Coronavirus Disease 2019. medRxiv. 2020 [preprint] [Google Scholar]
- 19.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clin Res Ed) 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Zheng F., Qing Y., et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv. 2020 [preprint] [Google Scholar]
- 21.de la Rica R., Borges M., Aranda M., et al. Low albumin levels are associated with poorer outcomes in a case series of COVID-19 patients in Spain: a retrospective cohort study. medRxiv. 2020 doi: 10.3390/microorganisms8081106. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng Y., Liu W., Liu K., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan J., Wang X., Chi J., et al. Correlation between the variables collected at admission and progression to severe cases during hospitalization among COVID-19 patients in Chongqing. J Med Virol. 2020 doi: 10.1002/jmv.26082. [ahead of online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu L., Fei J., Xiang H., et al. Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. medRxiv. 2020 [preprint] [Google Scholar]
- 25.Giacomelli A., Ridolfo A.L., Milazzo L., et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;104931 doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong J., Ou J., Qiu X., et al. A tool to early predict severe Corona Virus Disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong K.S., Lee K.H., Chung J.H., et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61(5):431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu L., Chen S., Fu Y., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa539. [ahead of online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H., Cai S., Li Y., et al. Prognostic factors for COVID-19 pneumonia progression to severe symptom based on the earlier clinical features: a retrospective analysis. medRxiv. 2020 doi: 10.3389/fmed.2020.557453. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S., Huang M., Li X., Zhang T., Lu H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio for predicting clinical outcomes in COVID-19. medRxiv. 2020 [preprint] [Google Scholar]
- 33.Jiang X., Tao J., Wu H., et al. Clinical features and management of severe COVID-19: a retrospective study in Wuxi, Jiangsu Province, China. medRxiv. 2020 [preprint] [Google Scholar]
- 34.Liu L., Gao J., Hu W., et al. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv. 2020 [preprint] [Google Scholar]
- 35.Li K., Chen D., Chen S., et al. Radiographic findings and other predictors in adults with COVID-19. medRxiv. 2020 doi: 10.1186/s12931-020-01411-2. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Liu Y., Xiang P., et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R., Ma Q., Han H., et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease. Clin Chem Lab Med. 2019:2020. doi: 10.1515/cclm-2020-0220. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y., Sun W., Li J., et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv. 2020 [preprint] [Google Scholar]
- 39.Ma K., Liu Z., Cao C., et al. COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv. 2020 [preprint] [Google Scholar]
- 40.Paranjpe I., Russak A., De Freitas J.K., et al. Clinical characteristics of hospitalized COVID-19 patients in New York City. medRxiv. 2020 [preprint] [Google Scholar]
- 41.Pei G., Zhang Z., Peng J., et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng Y.D., Meng K., Guan H.Q., et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(0) doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 43.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ (Clin Res Ed) 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regina J., Papadimitriou-Olivgeris M., Burger R., et al. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: an observational retrospective study. medRxiv. 2020 doi: 10.1371/journal.pone.0240781. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi P., Ren G., Yang J., et al. Clinical characteristics of imported and second-generation COVID-19 cases outside Wuhan, China: a multicenter retrospective study. medRxiv. 2020 doi: 10.1017/S0950268820002332. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wan S., Xiang Y., Fang W., et al. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z., Li H., Li J., et al. Elevated serum IgM levels indicate poor outcome in patients with coronavirus disease 2019 pneumonia: a retrospective case-control study. medRxiv. 2020 [preprint] [Google Scholar]
- 49.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Int Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y., Li Y., Zeng Q., et al. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. medRxiv. 2020 [preprint] [Google Scholar]
- 51.Yan S., Song X., Lin F., et al. Clinical characteristics of coronavirus disease 2019 in Hainan, China. medRxiv. 2020 [preprint] [Google Scholar]
- 52.Yang J., Jin J., Liu S., et al. Blood glucose is a representative of the clustered indicators of multi-organ injury for predicting mortality of COVID-19 in Wuhan, China. medRxiv. 2020 [preprint] [Google Scholar]
- 53.Zhang F., Yang D., Li J., et al. Myocardial injury is associated with in-hospital mortality of confirmed or suspected COVID-19 in Wuhan, China: a single center retrospective cohort study. medRxiv. 2020 [preprint] [Google Scholar]
- 54.Zhang G., Hu C., Luo L., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H., Wang X., Fu Z., et al. Potential factors for prediction of disease severity of COVID-19 patients. medRxiv. 2020 [preprint] [Google Scholar]
- 56.Zhao X., Xu X., Yin H., et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20(1) doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou H., Zhang Z., Fan H., et al. Urinalysis, but not blood biochemistry, detects the early renal-impairment in patients with COVID-19. medRxiv. 2020 doi: 10.3390/diagnostics12030602. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellomo R., Kellum J.A., Ronco C., et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]