Abstract
Research on how insect immunity changes with age as insects develop within an instar, or larval developmental stage, is limited and contradictory. Insects within an instar are preparing for the next developmental stage, which may involve changes in morphology or habitat. Immunity may also vary accordingly. To determine how immunity varies in the fifth instar, we tested humoral immune responses, antimicrobial peptide activity, and phenoloxidase activity using the tobacco hornworm, Manduca sexta. We determined that while M. sexta have more robust antimicrobial peptide and phenoloxidase responses at the beginning of their fifth instar, this did not translate into better survival of bacterial infection or lower bacterial load in the hemolymph. We also determined that M. sexta injected with bacteria early in the fifth instar experience lower growth rates and longer development times than caterpillars of the same age injected with sham. This could indicate a shift in energy allocation from growth and development to metabolically costly immune responses. Because of the importance of insects as pests and pollinators, understanding how immunity varies throughout development is critical.
Introduction
Insects are numerous, diverse, and able to survive a plethora of pathogens despite their lack of antibody-mediated immunity. Insects have a robust innate immune system that is divided into two branches, cell mediated and humoral (Tanaka and Yamakawa 2011; Satyavathi et al. 2014). Cell-mediated immune responses are driven by circulating hemocytes, immune cells that perform phagocytosis, nodule formation, and encapsulation (Strand 2008; Tanaka and Yamakawa 2011; Satyavathi et al. 2014). The humoral branch is driven by both hemocytes and the fat body, an organ responsible for metabolic and immune functions. Activation of humoral immunity results in antimicrobial peptide (AMP) production and phenoloxidase (PO) activation (Jiang et al. 2010; Demas and Nelson 2011). AMPs kill pathogens by altering their transcriptional activity or cell membrane structures, whereas PO results in the production of cytotoxic molecules and melanin (Kanost et al. 2004; Tanaka and Yamakawa 2011).
Insect immune responses can be plastic with development (Eleftherianos et al. 2008; Tian et al. 2010). One of the most common model organisms for studying insect immunity is Manduca sexta (tobacco hornworm), which develops in five stages, or instars, throughout its larval life cycle (Reinecke et al. 1980). Many studies have characterized transcription of AMPs within the fifth instar of M. sexta (Yu and Kanost 1999; Jiang et al. 2004; Beetz et al. 2008; Eleftherianos et al. 2008; Tanaka and Yamakawa 2011), but only a few studies have identified how the overall insect immune responses change within that instar (e.g., Eleftherianos et al. 2008). However, there are conflicting results among these studies. Some results have indicated that cell-mediated and humoral immune responses were more robust in the beginning of the final instar of M. sexta (Eleftherianos et al. 2008) and Bombyx mori (silk worm; Tian et al. 2010). In direct contrast, others have shown that humoral immune responses were more robust at the end of the final instar of Galleria mellonella (wax moth; Benesova et al. 2009) and Ostrinia furnacalis (Asian corn borer; Lu et al. 2006). Although these contradictory results could be due to species differences, the timing of the development of immune systems remains unclear. In addition, none of these studies identifies precisely when immune responses start to change during development.
Insect immunity encompasses many separate physiological responses that work together to clear an animal of infection. When faced with an infection, animals may respond with resistance, tolerance, or a combination of both. Tolerance is characterized by the survival of animals despite a heavy pathogen load, while resistance is characterized by survival and a low pathogen load (Moreno-García et al. 2014). Resistance is mediated by effective immune responses to lower the pathogen load (Tanaka and Yamakawa 2011; Satyavathi et al. 2014). The field of ecoimmunology is moving toward using functional assays, such as survival and pathogen load, to more accurately determine immunocompetency rather than using individual indexes of immune responses (Demas and Nelson 2011). Individual indexes of immune responses, like AMP and PO activity, do not always positively correlate with survival of bacterial infection (Adamo 2004). However, since the consensus in ecoimmunology to use functional assays was reached, few studies have determined how survival of infection or bacterial load changes with development. Although Eleftherianos et al. (2008) observed increases in susceptibility to bacterial infection in M. sexta later in the fifth instar, more studies are needed to test how functional immunity varies with development.
Immune responses are a metabolically costly physiological function (Freitak et al. 2003; Ardia et al. 2012; Catalan et al. 2012). If an animal is perturbed with an immune challenge such as a bacterial infection, normal allocation of energy toward processes like growth, development, metabolism, and reproduction may be disrupted, since resources are limited (Stearns 1992). Delayed growth and development are often the energetic costs of an immune challenge in many insects, including M. sexta and Acheta domesticus (house cricket; Adamo et al. 2007; Bascuñán-García et al. 2010; Diamond and Kingsolver 2011). On the basis of life-history theory, predictions can be made about how animals will respond to perturbations like an immune challenge, but no studies have investigated how energy allocations may vary with age on an immune challenge in insect larvae.
In this study, we used several assays to test three main hypotheses. First, to test the hypothesis that humoral immune responses vary with development, we measured AMP and PO activity in M. sexta on each day of the fifth instar (days 0–4). We predicted that AMP and PO activity would decrease with each day of development within the fifth instar. Second, to test the hypothesis that functional immunity varies with development, we assessed bacterial load and survival of infection throughout fifth-instar development in M. sexta. On the basis of the predicted outcomes for hypothesis 1, we also predicted that bacterial load would increase and survival would decrease during fifth-instar development. Third, to test the hypothesis that changes in energy allocation on infection vary with development, we measured growth rate and development time of infected M. sexta on each day of the fifth instar (days 0–4). We predicted that growth rate would decrease and development time would increase in infected early-fifth-instar caterpillars.
Material and Methods
Animal Care
Manduca sexta larvae (Carolina Biological, Burlington, NC) were reared at 25°C under a 16L: 8D photoperiod with ad lib. access to an artificial, wheat germ–based diet, as described elsewhere (Vishnuvardhan et al. 2013). Manduca sexta were observed daily to determine the age within an instar, indicated by the presence or absence of head capsules. Head capsule formation indicates the last day of each instar, while head capsule slippage marks the first day of each instar (day 0). The end of the fifth instar is indicated by cessation of feeding and visualization of the dorsal heart, typically day 4 of the fifth instar (Reinecke et al. 1980). The following life stage is prepupal formation, in which caterpillars have a lighter pigmentation, a moist cuticle, and increased activity (Reinecke et al. 1980).
Micrococcus lysodeikticus Preparation
Lyophilized M. lysodeikticus (Sigma-Aldrich, St. Louis, MO) used in the AMP activity assay were prepared at a concentration of 10 mg/mL in Manduca saline buffer (MSB; 4 mM NaCl, 40 mM KCl, 18 mM MgCl2.6H2O, 3 mM CaCl2).
Bacterial Cultures
Escherischia coli and Stenotrophomonas maltophilia were grown to stationary phase in Luria broth (LB), and the optical densities of the bacterial suspensions were measured using a spectrophotometer at 600 nm (Beckman Coulter, Indianapolis, MN). The bacterial suspensions were centrifuged at 4,000 g for 15 min. Resulting pellets were washed with phosphate-buffered saline (PBS; 137 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) and centrifuged again at 4,000 g for 15 min. The final E. coli bacterial pellet was resuspended in PBS at a concentration of 1.00 × 106 colony-forming units (CFU) per 10 μL. The final S. maltophilia bacterial pellet was resuspended in 1.66 mL of PBS. Then, 10 μL of bacterial dilutions (10−1–10−5) in PBS were plated on LB agar, and bacterial colonies were counted 24 h later to determine the concentration of bacteria injected into each caterpillar.
Injections
Regardless of injected treatment and age of M. sexta, the injection method was the same. Larvae were cooled on ice for 5 min and surface sterilized with 75% ethanol. A 25-μL syringe (Hamilton, Reno, NV) with a 26-gauge needle was filled with 10 μL of control buffer or bacterial suspension. The needle was inserted subcutaneously into the hemocoel of the caterpillar between the first and second prolegs. Once an injection was completed, the injection site was monitored for potential leaking of hemolymph, but no caterpillars exhibited leaking. Caterpillars were then transferred to individual containers with ad lib. access to food and monitored daily.
According to standards in developmental insect immune studies, caterpillars were given the same amount of bacteria, regardless of body mass (Hung and Boucias 1996; Zerofsky et al. 2005; Beetz et al. 2008; Eleftherianos et al. 2008). To validate this approach for our study, we conducted a preliminary experiment comparing the immune responses of caterpillars of similar ages and body mass that were given either the standard dose or a mass-corrected dose of bacteria. Younger (end of fourth instar) and older (day 2 of fifth instar) caterpillars were injected with either 10 μL of heat-killed M. lysodeikticus (10 mg/mL in MSB) or 10 μL of a mass-corrected dose. We corrected the dose on the basis of the average mass of a fifth-instar day 0 caterpillar by using the following correction factor: (caterpillar mass mg × 10 mg)/1,100 mg. Twenty-four hours after injection, hemolymph was collected and prepared for hemocyte count by diluting 10 μL into 90 μL of PBS for a 1:10 dilution. The hemolymph solution was vortexed, and 10 μL was placed on a hemocytometer. Hemocytes were counted at × 40 magnification, and hemocyte concentration (hemocytes/mL hemolymph) and total hemocyte counts (hemocytes/insect) were calculated. Because we found no significant difference between young and old caterpillars in their responses to standard or mass-corrected bacterial doses, we used the standard dose for all our experiments (fig. 1; effect of dose, F1,8 = 0.6, P = 0.46).
Figure 1.
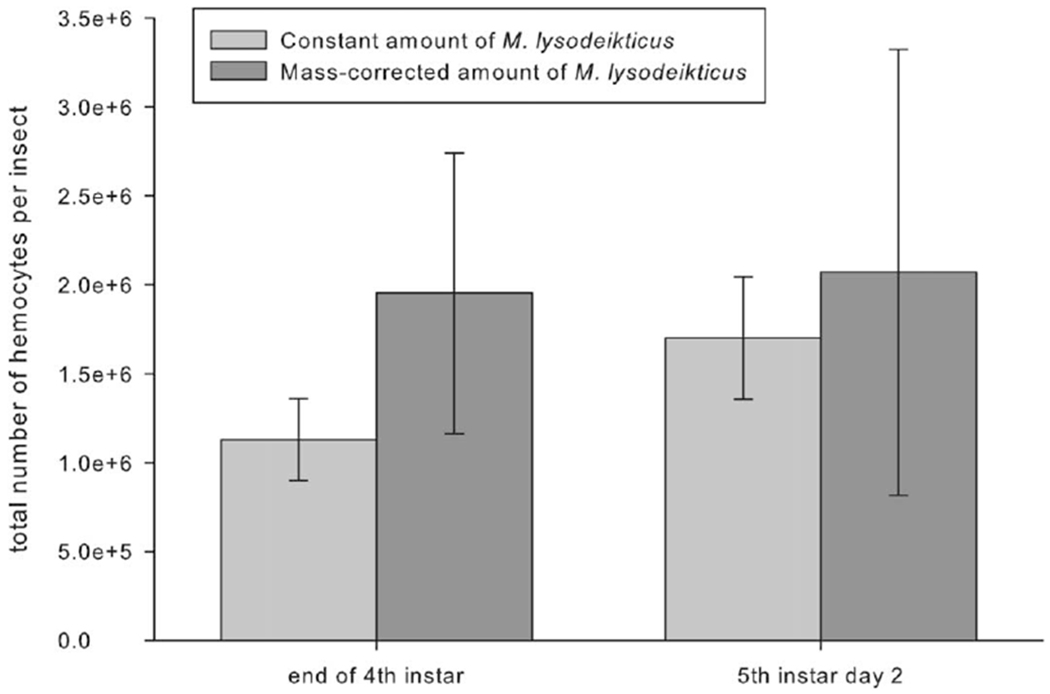
Total hemocyte counts for Manduca sexta larvae hemolymph collected 24 h after injection. Caterpillars were injected at fourth-instar head capsule or on day 2 of the fifth instar with either a constant amount of 10 μL of a Micrococcus lysodeikticus solution at a concentration of 10 mg/mL Manduca saline buffer or a mass-corrected concentration. Both age groups showed no statistically significant difference between constant and mass-specific bacterial injections (P > 0.05, two-way ANOVA; n = 3 for all treatment groups). Error bars indicate SEM.
Caterpillars for AMP assays were injected with 10 mg of M. lysodeikticus in 10 μL of Manduca saline. Caterpillars for the PO activity assay were injected with 10 μL of Manduca saline. Caterpillars for bacterial load, growth rate, and development time assays were injected with 1.00 × 106CFU of E. coli in 10 μL of PBS. Caterpillars for the mortality rate from the S. maltophilia assay were injected with varying amounts of bacteria (see “Mortality Assay” for details).
Hemolymph Collection
Hemolymph was collected 12 h after E. coli injection in the bacterial load assay and 24 h after M. lysodeikticus injection in the AMP and PO activity assays. Manduca sexta larvae were cooled on ice for 5 min and surface sterilized with 75% ethanol before sample collection. To collect hemolymph, the first right proleg was cut with a sterilized razor blade, and hemolymph was expelled into a 1.5-mL Eppendorf tube on ice. The hemolymph for the bacterial load assay was used immediately, while the hemolymph for the AMP and PO activity assays was stored at −20°C.
AMP Activity Assay
To determine the activity of the AMPs produced as a part of the humoral immune response, a traditional zone-of-inhibition assay was performed (Hoffmann et al. 1981). Caterpillars from each day of the fifth instar (days 0–4) were injected with 10 μL of lyophilized M. lysodeikticus at a concentration of 10 mg/mL to induce the production of AMPs. Hemolymph was collected 24 h later. AMPs were isolated by centrifugation at 7,000 g for 5 min to separate hemocytes from hemolymph. The plasma layer was collected and heated at 95°C for 5 min and spun again at 13,400 g for 10 min. AMPs remain in the supernatant. Wells (3 mm in diameter) were carved into LB agar plates embedded with DH5α E. coli. Five microliters of AMPs from each caterpillar (n = 6) was loaded into the wells in duplicate. The plates were incubated at 37°C for 1 h and then at 25°C overnight. The next morning, the plates were photographed with a ruler for calibration to measure the zone of inhibition, the diameter around the well where the AMPs inhibited bacterial growth. Using ImageJ (ver. 1.45K; Rasband 1997-2011), the diameter of the area lacking bacteria was measured vertically, horizontally, and diagonally across the cleared area. Then, using the oval selection tool, the circumference of the zone of inhibition was measured, and from that a diameter was calculated. These four measures of diameter were averaged for each well to yield one zone of inhibition per well. The zone of inhibition for each caterpillar was divided by the average hemolymph volume collected from the appropriate caterpillar age to account for increases in hemolymph volume during fifth-instar development.
PO Activity Assay
Caterpillars from each day of the fifth instar (days 0–4) were injected with 10 μL of Manduca saline to induce the production of PO. Hemolymph was collected 24 h later and centrifuged at 7,000 g at 4°C for 10 min to separate the hemocytes from the hemolymph plasma. A 10-μL fraction of the hemolymph plasma was mixed for 1 h with E. coli lipopolysaccharide (Sigma-Aldrich), an effective pro-PO to PO activator (Laughton and Siva-Jothy 2010). The reaction was started by adding 20 mM 4-methylcatechol (Sigma-Aldrich). The conversion of 4-methylcatechol to colorimetric quinone by PO was marked by a change in absorbance (at 490 nm), which was measured every 15 min for 1 h at room temperature by means of a microplate reader (Bio-Rad, Hercules, CA). The change in absorbency over time (mOD/min) is directly related to PO activity (Eleftherianos et al. 2008). Slopes from each hemolymph sample were calculated and compared. PO activity for each caterpillar was divided by the average hemolymph volume collected from the appropriate caterpillar age to account for increases in hemolymph volume during fifth-instar development.
Bacterial Load Assay
Caterpillars from days 0, 2, and 4 of the fifth instar were injected with 1.00 × 106 CFU of E. coli or 10 μL of PBS. Hemolymph was collected 12 h later, and the concentration of bacteria was determined via standard colony counts after serial dilution. We assumed that the proportion of bacteria adhering to tissues was similar among days. Thus, this measurement of load indicates the amount of circulating bacteria.
Growth Rate Assay
Caterpillars from each day of the fifth instar (days 0–4) were injected with 1.00 × 106 CFU of E. coli or 10 μL of PBS. Caterpillars were weighed 0, 4, and 8 h after injection. The rate of change in growth over 8 h after injection was calculated to represent growth rate. The growth rate over the first 8 h after injection was chosen because the fifth-instar day 4 animals develop into prepupae 16 h after injection. Prepupae normally lose weight and were thus not included in the growth rate calculations.
Development Time Assay
Caterpillars from each day of the fifth instar (days 0–4) were injected with 1.00 × 106 CFU of E. coli or 10 μL of PBS. Caterpillars were observed twice daily for signs of reaching the prepupal life stage. The number of days it took for caterpillars to reach prepupal formation from the day of fifth-instar ecdysis was recorded to yield development time.
Mortality Assay
Caterpillars from days 0, 2, and 4 of the fifth instar were injected with between 6.85 × 107 and 1.72 × 108 CFU of S. maltophilia. Caterpillars were assessed for mortality from the time of injection until 48 h after injection. Caterpillars unable to right themselves after being placed on their dorsum were considered dead. Observations of mortality were made hourly for 8 h after injection and then 10,14, 18, 22, 24, 26, 30, 42, 46, and 48 h after injection.
Data Analysis
Statistical analyses were performed using IBM SPSS software (ver. 21). To analyze hemocyte counts, we used two-way ANOVA. For the PO activity assay, bacterial load assay, growth rate assay, and development time assay, an ANOVA test was used to obtain F values, and the Bonferroni post hoc test was used to detect differences between treatment groups. Because the AMP activity data were not normally distributed, the Kruskal-Wallis test was used to obtain a χ2 value, and the Tamhane post hoc test was used to detect differences between treatment groups. For the mortality assays, the Cox regression test was used to obtain a χ2 value, and the Kaplan-Meier post hoc test was used to detect differences between treatment groups. A probability value <0.05 indicates statistical significance. Data are expressed as mean ± SEM throughout.
Results
AMP activity peaked at the beginning of the fifth instar (day 0) and decreased significantly on subsequent days during the fifth instar (days 1–4), approximately halving AMP activity with each consecutive day (fig. 2; χ2 = 41.22, P<0.05, Kruskal-Wallis; n = 6 for all treatment groups). PO activity also peaked at the beginning of the fifth instar (day 0). PO activity in hemolymph extracted from fifth-instar day 0 larvae was twofold higher than that in hemolymph from day 1 or 2 larvae, fivefold higher than that in hemolymph from day 3 larvae, and eightfold higher than that in hemolymph from day 4 larvae (fig. 3; F6,14 = 17.823, P < 0.05, ANOVA; n = 3 for all treatment groups).
Figure 2.

Antimicrobial peptide (AMP) activity throughout the fifth instar in Manduca sexta. Manduca sexta from each day of the fifth instar (days 0–4) were injected with dried Micrococcus lysodeikticus, and AMP activity was assessed 24 h later. Bars with different letters are significantly different from one another (χ2 = 41.22, P < 0.05, Kruskal-Wallis). Error bars indicate SEM.
Figure 3.

Phenoloxidase (PO) activity throughout the fifth instar in Manduca sexta. Manduca sexta from each day of the fifth instar (days 0–4) were injected with Manduca saline, and PO activity was assessed 24 h later. Asterisks indicate values that are significantly lower than those on day 0, and plus signs indicate values that are significantly lower than those on day 1 (F6,14 = 17.823, P < 0.05, ANOVA). Error bars indicate SEM. mOD/min = change in absorbency over time.
Hemolymph from fifth-instar day 0 caterpillars had about 25-fold higher Escherischia coli bacterial loads than hemolymph from fifth-instar day 4 caterpillars (fig. 4; F2, 53 = 4.107, P < 0.05, ANOVA; n = 10 for all treatment groups). Hemolymph from Manduca sexta injected with PBS did not grow any bacteria (data not shown). Manduca sexta were also challenged with a lethal dose of Stenotrophomonas maltophilia on varying days within the fifth instar to examine differences in survival kinetics. Manduca sexta injected with S. maltophilia as fifth-instar day 0 larvae died twice as quickly as caterpillars injected as fifth-instar day 4 larvae (fig. 5; fifth instar day 0 vs. day 2: χ2 = 7.442, P < 0.05, Kaplan-Meier; fifth instar day 0 vs. 4: χ2 = 9.751, P < 0.005, Kaplan-Meier; fifth instar day 2 vs. 4: χ2 = 8.310, P < 0.005; n = 10 for all treatment groups). Regardless of age on injection, 100% of M. sexta survived PBS injection.
Figure 4.
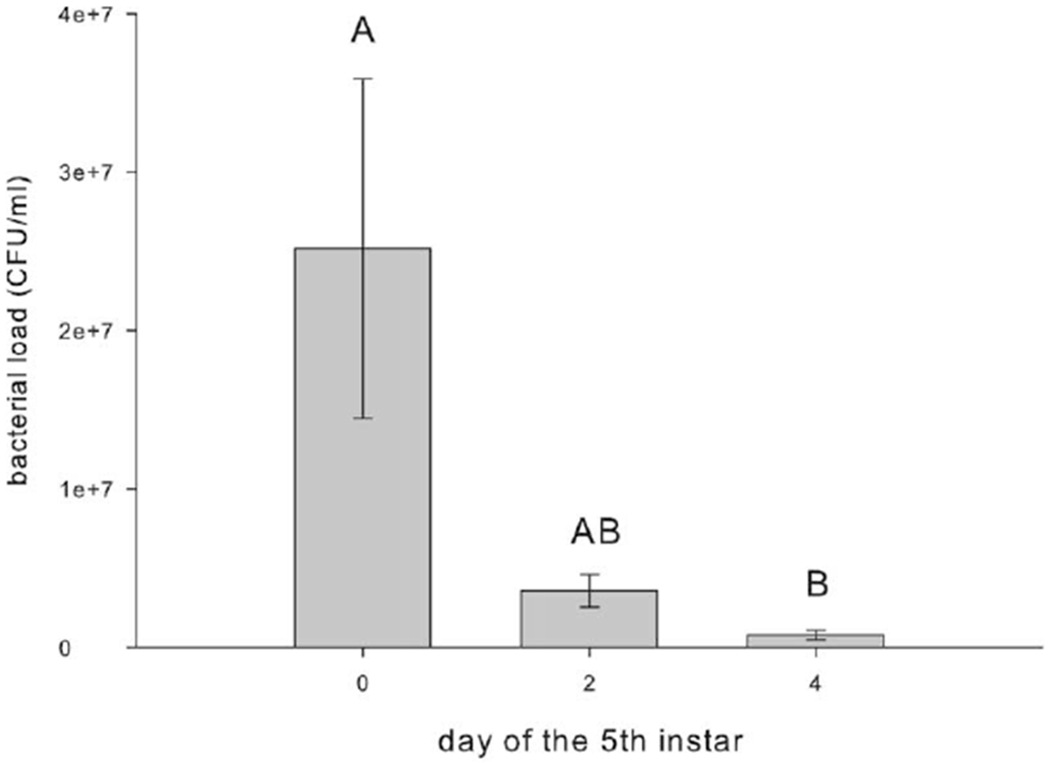
Bacterial load of Manduca sexta injected with Escherischia coli on days 0, 2, and 4 of the fifth instar. Hemolymph was collected 12 h after injection, and bacterial growth was assessed. Bars with the same letter are not significantly different from one another (F2,53 = 4.107, P < 0.05, ANOVA). Error bars indicate SEM. CFU = colony-forming unit.
Figure 5.
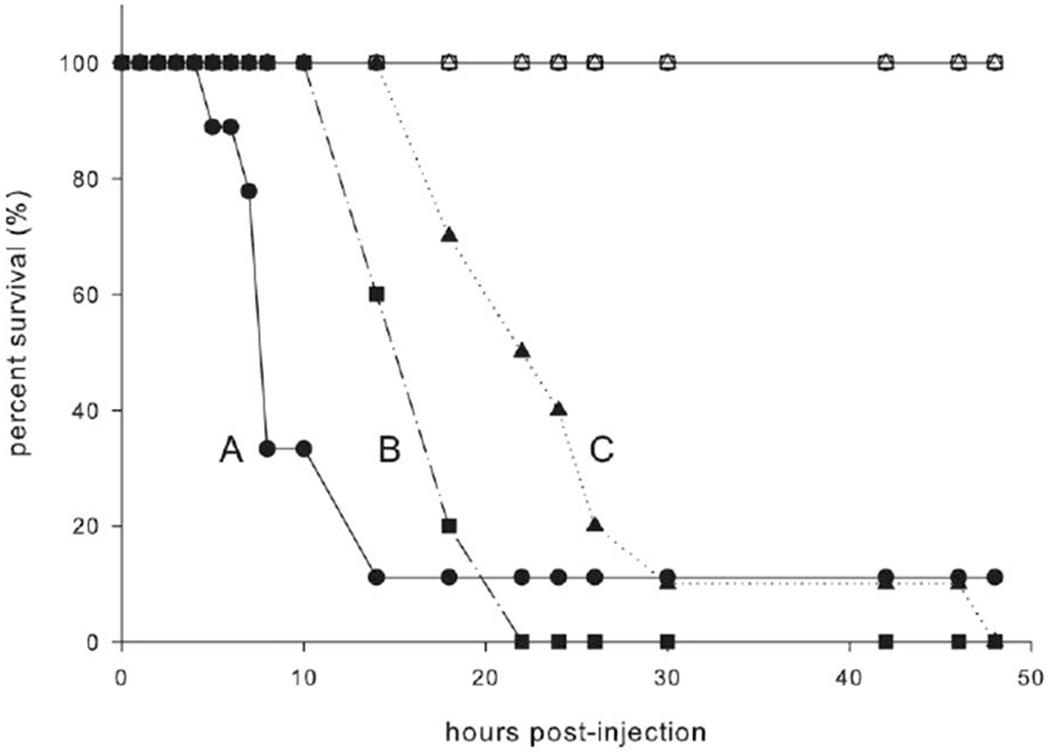
Survival of Stenotrophomonas maltophilia infection by Manduca sexta injected on days 0,2, and 4 of the fifth instar. Caterpillars were checked hourly for 8 h and then every 4 h for signs of life. Phosphate-buffered saline-injected caterpillars are represented by open symbols, and S. maltophilia-injected caterpillars are represented by filled symbols. Fifth-instar day 0 caterpillars are represented by circles, fifth-instar day 2 caterpillars are represented by squares, and fifth-instar day 4 caterpillars are represented by triangles. Lines with different letters are significantly different from one another (χ2 = 57.691, P < 0.05, Cox regression).
Injection with E. coli significantly delayed growth in caterpillars that were early in the instar (fig. 6; F1,87 = 32.328, P < 0.05, ANOVA; n = 10 for all treatment groups). Differences n growth rates depended on treatment injection (PBS or E. coli) and existed only in M. sexta injected as fifth-instar day 0, 1, and 2 larvae. Manduca sexta injected with PBS as fifth-instar day 0 or 1 larvae had fourfold higher growth rates during the first 8 h after injection than caterpillars of the same age injected with E. coli. Manduca sexta injected with PBS as fifth-instar day 2 larvae had twofold higher growth rates than caterpillars of the same age injected with E. coli. The delayed development also affected the time to reach the prepupal stage, but the effect differed depending on the age of the caterpillar at injection (fig. 7; F1,85 = 74.420, P < 0.05, ANOVA; n = 10 for all treatment groups). Manduca sexta injected with E. coli as fifth-instar day 0 larvae took twice as long to reach the prepupal stage as caterpillars injected with PBS. Regardless of the day on which the fifth-instar caterpillars were injected, those injected with PBS reached the prepupal stage in about 5 ± 0.1 d.
Figure 6.
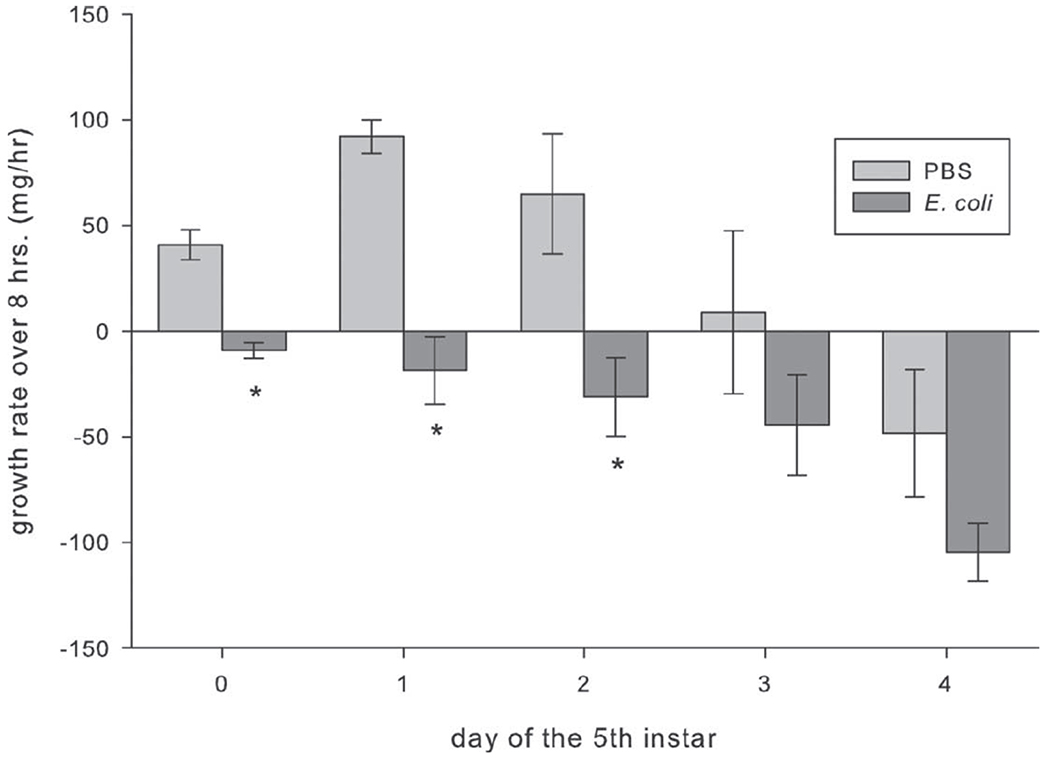
Growth rates of Manduca sexta injected with either phosphate-buffered saline (PBS) or Escherischia coli on each day of the fifth instar (days 0–4). Caterpillars were weighed immediately, 4 h, and 8 h after infection. Asterisks indicate values that are significantly lower than those for the PBS control for the given age (F1,87 = 32.328, P < 0.05, ANOVA). Error bars indicate SEM.
Figure 7.
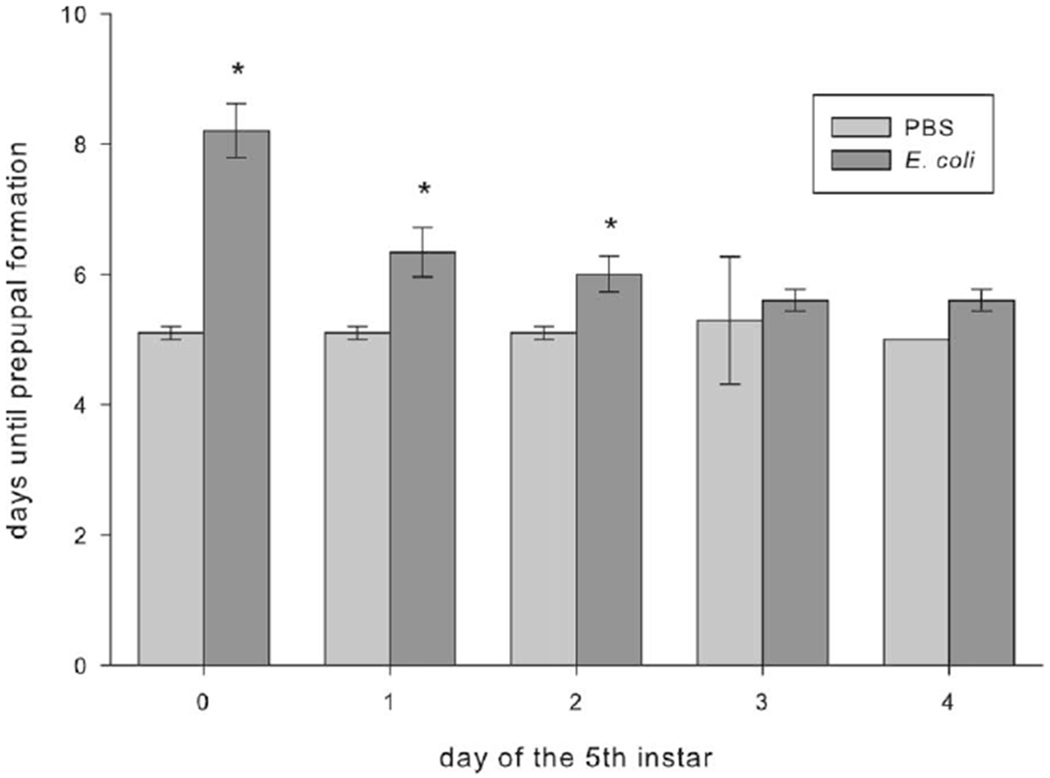
Development times of Manduca sexta larvae injected with either phosphate-buffered saline (PBS) or Escherischia coli on each day of the fifth instar (days 0–4). Larvae were observed for signs of prepupal formation twice daily. Asterisks indicate values that are significantly higher than those for the PBS control for the given age (F1,85 = 74.420, P < 0.05, ANOVA). Error bars indicate SEM.
Discussion
Our results concur with previous findings that humoral immune responses, AMP activity, and PO activity peak at the beginning of the fifth instar in insects (figs. 2, 3; Eleftherianos et al. 2008; Tian et al. 2010). Supporting our results, Eleftherianos et al. (2008) determined that Manduca sexta larvae from early during the fifth instar had more robust cell-mediated (phagocytosis and nodule formation) and humoral (AMP gene expression and PO activity) immune responses to Photorhabdus luminescens infection than animals from later within the fifth instar (Eleftherianos et al. 2008). In another study, Tian et al. (2010) determined that Bombyx mori had decreased AMP activity against Staphylococcus aureus and Escherischia coli as they age within the fourth and final instars. Our data provide a unique perspective on this topic because ours is the first study to track every day of fifth-instar M. sexta, specifically determining humoral immune response decline after just a single day in the fifth instar. Although many physiological changes occur in the fifth and final instar, it is striking that such drastic changes in humoral immunity occur in as short of a time span as 1 d. This emphasizes the need to control for age in experimental design. Scientists should also use caution when interpreting and integrating information from various physiological studies using different ages of insects. Manduca sexta in the fifth instar are used most often in insect physiology studies because of the large amount of tissue that can be isolated from them. Even comparing studies using the same M. sexta instar may not be enough to provide an accurate portrayal of the field of M. sexta immunity as a whole.
Although the body of research is limited, our results clarify how humoral immunity varies with development in insects. Our results provide evidence for development-related declines in humoral immunity in direct contrast to what others have observed. PO activity peaked at the end of the seventh and final instar of Galleria mellonella (Benesova et al. 2009) and the fifth and final instar of Ostrinia furnacalis (Lu et al. 2006). Immune function against E. coli increased until the last developmental stage before adult onset of Tachycineta bicolor (Stambaugh 2009). As larval Lepidoptera are voracious crop pests, identification of the particularly vulnerable stages of larval immunity could allow for better biological control methods. Farmers would be able to apply pesticides at a targeted time when larval Lepidoptera are most vulnerable. The opposite could be true for other animals, especially endangered populations. Because infectious diseases could be a contributing factor to fluctuations in populations (MacPhee and Greenwood 2013), finding a point in development where treatments would be most effective would be beneficial.
From an ecological perspective, it is still unclear why there would be such drastic declines in immunity during the fifth instar. It could be beneficial for caterpillars to have robust immune responses immediately after molting into the fifth instar, before the cuticle has fully sclerotized. Our alternative hypothesis relies on the life-history theory changes in energy allocation during immune challenge. We hypothesize that energy is already allocated toward storage for pupation at the end of the fifth instar and is not available for immune responses. From a mechanistic perspective, the driving force behind development-related changes in immunity within the fifth instar remains unclear. Many changes occur within an instar that could affect immunity, including the level of juvenile hormone (JH). JH, a developmental hormone that prevents early molting, peaks early and decreases within an instar until molting (Nijhout 1974; Baker 1987). JH analog applied topically to early-wandering fourth-instar silkworms, B. mori, resulted in a dramatic increase in gene expression for several AMPs, including cecropin, morincin, lebocin, and neucin, and the gloverin-like proteins 1 and 2 (Tian et al. 2010). In addition, JH analog application increased AMP activity against both E. coli and S. aureus in a traditional zone-of-inhibition assay (Tian et al. 2010). Although JH is immunostimulatory in larval B. mori, it is unclear how JH might be involved in immunity in M. sexta.
Alternatively, the changes in growth may be an adaptive response from the host, mediated by the insect cytokine growth-blocking peptide (GBP). Hayakawa (1995) and Noguchi et al. (2003) showed that armyworms, Pseudaletis separata, display elevated levels of GBP when parasitized by the wasp, Cotesia kariyai. The release of GBP on infection has multiple benefits for the host, such as creating a higher JH-concentrated environment, which could possibly increase AMP activity (Tian et al. 2010), and delaying growth, which would provide more time for healing before molting. GBP causes increased gene expression of dopa decarboxylase, which produces the enzyme necessary to convert dopa into dopamine (Noguchi et al. 2003). Elevated levels of dopamine in the hemolymph and nerve cord delay both growth and metamorphosis (Hayakawa 1995; Noguchi et al. 2003) and repress JE esterase synthesis (Hayakawa 1995; Noguchi et al. 2003), keeping JH levels high and perhaps delaying molting (Nijhout 1974; Baker 1987). Elevated levels of dopamine also cause a paralysis effect, resulting in reduced feeding and delayed growth (Hayakawa 1995). A better understanding of the roles played by JH and GBP in modulating development-related changes in humoral immunity is clearly necessary.
Interestingly, our results show that although M. sexta have more robust AMP and PO activity earlier in the fifth instar, they also have 25-fold higher E. coli bacterial loads and die twice as quickly after Stenotrophomonas maltophilia infection, indicating ineffective immune responses (figs. 4, 5). The disparity between our results for the individual insect immune responses (AMP and PO activity) and the functional immune assays (bacterial load and survival) is puzzling because of the assumption that active immune responses will clear an infection. A possible explanation for this disparity is varying AMP production across ages, resulting in different responses to infection. Although the body of research is limited, there are some reports of this phenomenon in the literature. For example, total PO activity and baseline lysozyme-like activity did not predict survival of Serratia marcescens, Serratia liquefaciens, or Bacillus cereus infection in male crickets Gryllus texensis (Adamo 2004).
The ability to survive an infection depends on a vast number of immune responses working together, which does not translate into all immune responses with peak activity on an immune challenge. There could be trade-offs even within an individual’s immune response. For example, increased encapsulation and PO activity was associated with lower lysozyme activity in Tenebrio molitor (mealworm beetle) and Acheta domesticus (Ardia et al. 2012). Lysozyme-like activity was also negatively correlated with hemocyte density and PO activity in Spodoptera littoralis (African cotton leafworm; Cotter et al. 2004). Animals assessed with only certain immune assays may appear to have greater immune capacity. However, unassessed immune responses may be equally important in clearing an animal of infection. For example, perhaps the unmeasured cell-mediated immune responses at the end of the fifth instar are more active and effective than humoral immune responses at clearing E. coli and S. maltophilia infections. In support of this hypothesis, mass-corrected total circulating hemocytes were significantly higher at the end of the fifth instar in M. sexta, possibly indicating more inducible cell-mediated responses than earlier in the fifth instar (Beetz et al. 2008). Our results emphasize the importance of shifting ecoimmunology research toward functional immune assays. Because the immune response to infection is so complex, measuring all aspects of the immune response is difficult. Performing bacterial load and survival assays in conjunction with specific immune responses assays can improve our interpretation of results.
Our results also indicate that M. sexta from early in the fifth instar injected with E. coli exhibit lower growth rates and longer development times than caterpillars of the same age injected with sham (figs. 6, 7). This could indicate a shift in energy allocation from growth and development to metabolically costly immune responses. As indicated with sham-injected caterpillars, growth rates in M. sexta normally decline with fifth-instar development and represent a change in energy allocation from juvenile growth to storage for pupation (Sears et al. 2012). Our results are consistent with previous studies in other insect-pathogen systems. For example, M. sexta injected with S. marcescens had lower growth rates than caterpillars injectedwith sham (Adamo et al. 2007), and M. sexta with higher levels of encapsulation of Sephadex beads had slower growth rates (Diamond and Kingsolver 2011). Acheta domesticus had lower body masses on immune challenge with nylon compared with control crickets (Bascuñán-García et al. 2010). Immune responses are indeed metabolically costly. At times, if the cost is considered too high, energy could be allocated to other responses that are more beneficial (Krams et al. 2014; Moreno-García et al. 2014). Encapsulation of thread was positively correlated with increased metabolic rates measured as CO2 emission rates in T. molitor, A. domesticus, Cotinis nitida (June bug), and Periplaneta americana (American cockroach; Ardia et al. 2012). Pieris brassicae pupae (white cabbage butterfly) challenged with nylon also showed increases in metabolic rate (Freitak et al. 2003). To provide further support, even M. sexta fed high-quality food had higher levels of encapsulation than those fed a regular diet (Diamond and Kingsolver 2011). Although there is plenty of evidence for changes in energy allocation during an immune challenge, other mechanisms (e.g., sickness-induced anorexia) may better explain infection-induced declines in growth rates and development times.
Sickness-induced anorexia is a puzzling paradox because it seems counterintuitive for animals to cease feeding upon infection, as it is when they most need energy to combat it. However, the observation of sickness-induced anorexia is pervasive among diverse taxa and has been observed in M. sexta in response to infection with S. marcescens (Adamo et al. 2007). The cessation of feeding during the response to bacterial infection is associated with lower growth rates (Adamo et al. 2007) and may be the cause of our observed decreases in growth rate during E. coli infection in early-fifth-instar M. sexta. In addition, we anecdotally observed that M. sexta injected with E. coli do not consume as much food as caterpillars injected with sham. However, it is unclear whether this is an age-dependent response, since M. sexta infected later in the fifth instar did not experience changes in growth rate and development time compared with sham-injected caterpillars. It would be interesting to investigate whether M. sexta injected later in the fifth instar show any long-term effects of infection. For example, even though M. sexta infected later in the fifth instar do not show changes in growth rate or development time, perhaps their survival through pupation, longevity, or fecundity may be affected.
When reviewing the data as a whole, it is important to note that as animals age, they may undergo a reconfiguration of the immune response from resistance, fighting off an infection, to tolerance, surviving despite a high bacterial load. According to Moreno-García et al. (2014), insects could decide between resisting or tolerating a pathogen by sensing the levels of possible damage. If the damage were sensed to be minimal or below the damage threshold, it would be tolerated. If it were above the threshold, an immune response would activate for resistance. However, if the resistance was predicted to be ineffective, all energy would be put toward reproduction (Krams et al. 2014; Moreno-García et al. 2014). In T. molitor, this type of terminal investment toward reproduction causes an increase in attractiveness, ensuring reproduction while costing it survival (Krams et al. 2014). Perhaps if M. sexta injected later in the fifth instar sensed the damage to be minimal, a reconfiguration to tolerance would be possible. The opposite is also plausible; if damage was sensed to be too high, energy could have been allocated toward pupation and reproduction.
We are the first to identify insect immune response decline even within a single day of development in the fifth instar, which is crucial information for scientists and farmers alike. Scientists should recognize that they should not overlook age as a confounding factor in experimental design. Lepidopteran larvae are notorious crop pests, particularly during the fifth instar, when they are getting ready for pupation. Our results will help us understand their physiology and possibly identify vulnerable life stages of insects. We also encourage the use of functional immune assays to help interpret immune response data because immune response data may not always be an accurate depiction of an animal’s defensive state. Although we did not measure energy variations or reserves, we speculate that the vulnerable life stage of insects could be caused by changes in how energy is allocated throughout development.
Acknowledgments
DH5-α Escherischia coli used in the growth rate and development time assays were generously donated by Dr. Birgit Pruess. We thank Taylor Lundquist and Eric DeRocher for help with sample collection and Dr. Michael Kanost for training on the antimicrobial peptide activity assay. We also thank the three anonymous reviewers for their thorough critiques of the manuscript. This research was funded, in part, by the National Science Foundation (grants NSF-CHE 1062701 and NSF IOS-0953297 to K.J.G.), the National Center for Research Resources (grant NIH P20RR015566), grant NSF EPSCoR EPS-0447679, and a Grant-in-Aid of Research to K.B. from the National Academy of Sciences, administered by Sigma Xi, the Scientific Research Society.
Footnotes
This paper was submitted in response to a call for papers for a Focused Issue on “Developmental Physiology.”
Literature Cited
- Adamo SA 2004. Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J Insect Physiol 50: 209–216. [DOI] [PubMed] [Google Scholar]
- Adamo SA, Fidler TL, and Forestell CA. 2007. Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain Behav Immun 21:292–300. [DOI] [PubMed] [Google Scholar]
- Ardia D, Gantz J, Schneider B, and Strebel S. 2012. Costs of immunity in insects: an induced immune response increases metabolic rate and decreases antimicrobial activity. Funct Ecol 26:732–739. [Google Scholar]
- Baker FC, Tsai LW, Reuter CC, and Schooley DA. 1987. In vivo fluctuation of JH, JH acid, and ecdysteroid titer, and JH esterase activity during development of fifth stadium Manduca sexta. Insect Biochem Mol Biol 17:989–996. [Google Scholar]
- Bascuñán-García AP, Lara C, and Córdoba-Aguilar A. 2010. Immune investment impairs growth, female reproduction and survival in the house cricket, Acheta domesticus. J Insect Physiol 56:204–211. [DOI] [PubMed] [Google Scholar]
- Beetz S, Holthusen TK, Koolman J, and Trenczek T. 2008. Correlation of hemocyte counts with different developmental parameters during the last larval instar of the tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol 67: 63–75. [DOI] [PubMed] [Google Scholar]
- Benesova J, Dobes P, and Hyrsl P. 2009. Developmental changes in phenol-oxidizing activity in the greater wax moth Galleria mellonella. Bull Insectology 62:237–243. [Google Scholar]
- Catalan T, Wozniak A, Niemeyer H, Kalergis A, and Bozinovic F. 2012. Interplay between thermal and immune ecology: effect of environmental temperature on insect immune response and energetic costs after an immune challenge. J Insect Physiol 58:310–317. [DOI] [PubMed] [Google Scholar]
- Cotter SC, Kruuk LE, and Wilson K. 2004. Costs of resistance: genetic correlations and potential trade-offs in an insect immune system. J Evol Biol 17:421–429. [DOI] [PubMed] [Google Scholar]
- Demas G and Nelson R. 2011. Ecoimmunology. Oxford University Press, New York. [Google Scholar]
- Diamond SE and Kingsolver JG. 2011. Host plant quality, selection history and trade-offs shape the immune responses of Manduca sexta. Proc R Soc B 278:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftherianos I, Baldwin H, Ffrench-Constant RH, and Reynolds SE. 2008. Developmental modulation of immunity: changes within the feeding period of the fifth larval stage in the defence reactions of Manduca sexta to infection by Photorhabdus. J Insect Physiol 54:309–318. [DOI] [PubMed] [Google Scholar]
- Freitak D, Ots I, Vanatoa A, and Horak P. 2003. Immune response is energetically costly in white cabbage butterfly pupae. Proc R Soc B 270:S220–S222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y 1995. Growth-blocking peptide: an insect biogenic peptide that prevents the onset of metamorphosis. J Insect Physiol 41:1–6. [Google Scholar]
- Hoffmann D, Hultmark D, and Boman H. 1981. Insect immunity: Galleria mellonella and other Lepidoptera have cecropia-P9-like factors active against gram negative bacteria. Insect Biochem 11:537–548. [Google Scholar]
- Hung SY and Boucias DG. 1996. Phenoloxidase activity in hemolymph of naive and Beauveria bassiana-infected Spodoptera exigua larvae. J Invertebr Pathol 67:35–40. [Google Scholar]
- Jiang H, Ma CC, Lu ZQ, and Kanost MR. 2004. β-1,3-Glucan recognition protein-2 (βGRP-2) from Manduca sexta: an acute-phase protein that binds β-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem Mol Biol 34:89–100. [DOI] [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, and Kanost M. 2010. Immunity in lepidopteran insects. Adv Exp Med Biol 708:181–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Jiang HB, and Yu XQ. 2004. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev 198:97–105. [DOI] [PubMed] [Google Scholar]
- Krams IA, Krama T, Moore FR, Kivleniece I, Kuusik A, Freeberg TM, Mänd R, Rantala MJ, Daukšte J, and Mand M. 2014. Male mealworm beetles increase resting metabolic rate under terminal investment. J Evol Biol 27: 541–550. [DOI] [PubMed] [Google Scholar]
- Laughton AM and Siva-Jothy MT. 2010. A standardised protocol for measuring phenoloxidase and prophenoloxidase in the honey bee, Apis mellifera. Apidologie 42:140–149. [Google Scholar]
- Lu J-F, Hu J, and Fu W-J. 2006. Levels of encapsulation and melanization in two larval instars of Ostrinia furnacalis Guenee (Lep., Pyralidae) during simulation of parasitization by Macrocentrus cingulum Brischke (Hym., Braconidae). J Appl Entomol 130:290–296. [Google Scholar]
- MacPhee RDE and Greenwood AD. 2013. Infectious disease, endangerment, and extinction. Int J Evol Biol 2013: 571939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-García M, Condé R, Bello-Bedoy R, and Lanz-Mendoza H. 2014. The damage threshold hypothesis and the immune strategies of insects. Infect Genet Evol 24:2533. [DOI] [PubMed] [Google Scholar]
- Nijhout HF and Williams CM. 1974. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol 61:493–501. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Tsuzuki S, Tanaka K, Matsumoto H, Hiruma K, and Hayakawa Y. 2003. Isolation and characterization of a dopa decarboxylase cDNA and the induction of its expression by an insect cytokine, growth-blocking peptide in Pseudaletia separata. Insect Biochem Mol Biol 33: 209–217. [DOI] [PubMed] [Google Scholar]
- Rasband WS 1997-2011. ImageJ software. http://imagej.nih.gov/ij/ Accessed March 11, 2012.
- Reinecke JP, Buckner JS, and Grugel SR. 1980. Life cycle of laboratory-reared tobacco hornworms, Manduca sexta, a study of development and behavior, using time-lapse cinematography. Biol Bull 158:129–140. [Google Scholar]
- Satyavathi VV, Minz A, and Nagaraju J. 2014. Nodulation: an unexplored cellular defense mechanism in insects. Cell Signal 26:1753–1763. [DOI] [PubMed] [Google Scholar]
- Sears KE, Kerkhoff AJ, Messerman A, and Itagaki H. 2012. Ontogenetic scaling of metabolism, growth, and assimilation: testing metabolic scaling theory with Manduca sexta larvae. Physiol Biochem Zool 85:159–173. [DOI] [PubMed] [Google Scholar]
- Stambaugh TR 2009. Development of the innate immune response in nestline tree swallows (Tachycineta bicolor). McNair Sch J 13:11. [Google Scholar]
- Stearns S 1992. The evolution of life histories. Oxford University Press, New York. [Google Scholar]
- Strand MR 2008. The insect cellular immune response. Insect Sci 15:1–14. [Google Scholar]
- Tanaka H and Yamakawa M. 2011. Regulation of the innate immune responses in the silkworm, Bombyx mori. Invertebr Surviv J 8:56–69. [Google Scholar]
- Tian L, Guo EE, Diao YP, Zhou S, Peng Q, Cao Y, Ling EJ, and Li S. 2010. Genome-wide regulation of innate immunity by juvenile hormone and 20-hydroxyecdysone in the Bombyx fat body. BMC Genomics 11:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnuvardhan S, Ahsan R, Jackson K, Iwanicki R, Boe J, Haring J, and Greenlee KJ. 2013. Identification of a novel metalloproteinase and its role in juvenile development of the tobacco hornworm, Manduca sexta (Linnaeus). J Exp Biol 320B:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XQ and Kanost MR. 1999. Developmental expression of Manduca sexta hemolin. Arch Insect Biochem Physiol 42: 198–212. [DOI] [PubMed] [Google Scholar]
- Zerofsky M, Harel E, Silverman N, and Tatar M. 2005. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4:103–108. [DOI] [PubMed] [Google Scholar]


