COVID-19 is caused by SARS-CoV-2, and typically manifests with systemic symptoms like fever and myalgia as well as respiratory symptoms including dry cough, dyspnoea and anosmia.1 Reports suggest that lineage B β-coronaviruses that are highly pathogenic to humans such as the SARS-CoV (2002) and SARS-CoV-2 (2019) can affect the liver and induce acute hepatitis.1 , 2 Herein, we report the cases of 2 patients who developed COVID-19 presenting as an acute acalculous cholecystitis.
The first case was an 84-year-old female patient who presented to our emergency department with symptoms and signs of urinary tract infection and fever (38.5°C) for 24 hours. Sepsis due to pyelonephritis was the initial diagnosis after a pathological urinalysis and positive urine culture. Blood tests revealed a slight cytolysis. Ceftriaxone was initiated alongside supportive care. On day 3, a right upper quadrant pain emerged, and on day 5, a positive Murphy sign was detected along with an increase in C-reactive protein, reaching 249.3 mg/l (Table S1). Ultrasonography revealed increased thickening of the gallbladder wall as well as peri-vesicular fluid. A thoraco-abdominal CT scan ruled out gallbladder perforation and showed a normal pulmonary parenchyma (Fig. 1 A). Metronidazole was added to the treatment and the patient underwent a laparoscopic cholecystectomy on day 8.
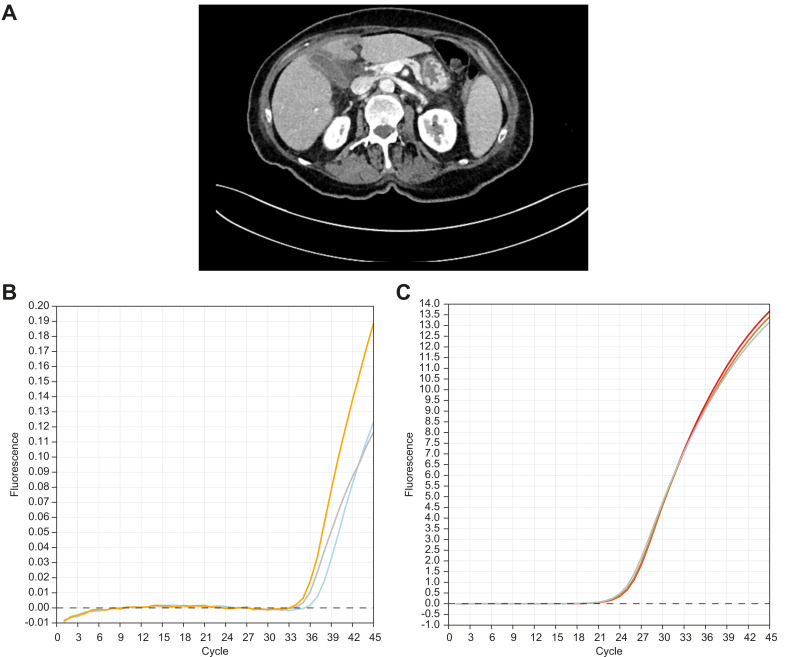
Fig. 1.
Radiological findings and SARS-CoV2 qRT-PCR of the gallbladder of patient one.
(A) Abdominal CT scan of the first patient showed signs of cholecystitis with peri-cholecystic fluid. qRT-PCR was performed on a gallbladder specimen to assess the presence of SARS-CoV-2. Standard protocols were used with the following primers: forward ACAGGTACGTTAATAGTTAATAGCGT reverse ATATTGCAGCAGTACGCACACA and a fluorescently labelled probe FAM-ACACTAGCCATCCTTACTGCGCTTCG-BBQ (complete protocol in supplementals) (B) The 3 samples from the gallbladder: A1 (blue), A2 (grey) and A3 (orange) were all positive for the presence of SARS-CoV-2 (experiment repeated 3 times with 2different extractions of RNA). (C) The RNA control was consistently positive (B1 [red], B2 [brown], B3 [grey]).
After extubation, the patient developed respiratory symptoms evolving into acute respiratory distress syndrome. Nasopharyngeal swabs confirmed the presence of SARS-CoV-2 RNA. The patient was transferred to the intensive care unit and passed away at postoperative day 5 from multiorgan failure. Histological analysis of the gallbladder did not demonstrate any inflammation but quantitative reverse transcriptase PCR (qRT-PCR) revealed the presence of SARS-CoV-2 in all 3 sampled regions of the gallbladder wall (Fig. 1B–C).
The second case concerned an 83-year-old man on dialysis for end-stage renal disease with type 2 diabetes, arterial hypertension and moderate aortic stenosis who was admitted with fever (38.3°C). No abdominal or respiratory symptoms were identified upon admission, and chest x-ray was normal. On day 5, the patient developed right upper quadrant pain with a positive Murphy sign, degradation of his inflammatory markers (C-reactive protein 209.9 mg/l, white blood cell count 19.5 G/L [Table S2]) and an increase in hepatic enzymes. Abdominal ultrasonography revealed a 4 mm thickening of the gallbladder wall, presence of peri-vesicular liquid, absence of gallstones and a radiologic Murphy sign. Conservative management with ceftriaxone and metronidazole therapy was initiated and led to a slow recovery. On day 6, the patient presented respiratory symptoms and a SARS-CoV-2 infection was confirmed.
SARS coronaviruses have a tropism to the lungs but also to the liver.1 Indeed, the intracellular entry of the virus occurs through interaction with the angiotensin-converting enzyme 2 receptor (ACE2) which is present in several tissues, including lungs and liver.1 In SARS-CoV autopsies, liver tissue exhibited different patterns of hepatocyte injuries, and viral RNA was found inside hepatocytes.3 Both SARS-CoV and SARS-CoV-2 are characterized by cytolysis with mild transaminase elevations.4 , 5 Moreover, ACE2 levels are higher in bile duct cells and Xu et al. suggested that liver injury induced by highly pathogenic SARS coronaviruses may be due to a direct harm to bile duct cells.5 COVID-19-related acalculous cholecystitis has been described in 2 case reports and was managed with surgery or gallbladder percutaneous drainage.6 , 7 Ying et al. performed RT-PCR of the bile after percutaneous drainage of the gallbladder in a patient with sludge and acute cholecystitis but did not detect SARS-CoV-2.8 Gallbladder epithelial cells are very similar to bile duct cells, express ACE2 and could be a target for SARS-CoV-2.9 RT-PCR of the formaldehyde fixed gallbladder of the first patient did not detect SARS-CoV-2 RNA with appropriate positive and negative controls (data not shown) but qRT-PCR testing confirmed the presence of the virus, indicating that SARS-CoV-2 was specifically present in the gallbladder. However, the significance of this finding to COVID-19 pathogenesis remains to be determined.
COVID-19 can present uniquely with digestive symptoms, without any respiratory manifestations, a longer time from onset to admission, and a worse prognosis.10 The 2 cases presented herein exhibited an evolving clinical picture of acalculous cholecystitis with radiological pattern of progressive wall thickening, perivesicular fluid and a positive radiological and clinical Murphy sign. Digestive symptoms preceded the classic respiratory manifestation of COVID-19. Hepatic COVID-19 manifestation in those patients might explain both the clinical picture that was induced by hepatitis and the radiological pattern of cholecystitis.
SARS-CoV-2 infection can mimic acalculous acute cholecystitis; viral RNA detection in the gallbladder indicates direct vesicular involvement, while the exact pathogenesis remains to be elucidated. Differential diagnosis can be challenging in this case and rapid testing for SARS-CoV-2, and potentially conservative management might make a difference to patient outcomes.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
ABa, KG, AP and SP collected the clinical data, ABo and TM processed and analysed the specimen, ABa, ABo and TM contributed to data analysis, ABa, KG, JM, CT and SP drafted the manuscript, ABa, KG, JM, AP, ABo, TM, CT, SP contributed to its critical revision, ABa, KG, JM, AP, ABo, TM, CT, SP approved the final version.
Ethics
For the first patient, consent for case publication was obtained from the patient's family and care representant as patient was deceased. For the second case, consent was directly obtained from the patient himself. All data, including radiology images were anonymized.
Conflict of interest
The authors have no conflict of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2020.08.020.
Supplementary data
References
- 1.Gavriatopoulou M., Korompoki E., Fotiou D., Ntanasis-Stathopoulos I., Psaltopoulou T., Kastritis E. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020 doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y., Korteweg C., McNutt M.A., Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu K.-L., Lu S.-N., Changchien C.-S., Chiu K.-W., Kuo C.-H., Chuah S.-K. Sequential changes of serum aminotransferase levels in patients with severe acute respiratory syndrome. Am J Trop Med Hyg. 2004;71:125–128. [PubMed] [Google Scholar]
- 5.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R., Domenico C., Rao S.D., Urgo K., Prenner S.B., Wald J.W. Novel coronavirus disease 2019 in a patient on durable left ventricular assist device support. J Card Fail. 2020;26:438–439. doi: 10.1016/j.cardfail.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruni A., Garofalo E., Zuccalà V., Currò G., Torti C., Navarra G. Histopathological findings in a COVID-19 patient affected by ischemic gangrenous cholecystitis. World J Emerg Surg. 2020;15:43. doi: 10.1186/s13017-020-00320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying M., Lu B., Pan J., Lu G., Zhou S., Wang D. COVID-19 with acute cholecystitis: a case report. BMC Infect Dis. 2020;20:437. doi: 10.1186/s12879-020-05164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong H., Yin B., Zhou H., Cai D., Ma B., Xiang Y. Loss of angiotensin-converting enzyme 2 promotes growth of gallbladder cancer. Tumour Biol. 2015;36:5171–5177. doi: 10.1007/s13277-015-3171-2. [DOI] [PubMed] [Google Scholar]
- 10.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.