Abstract
Purpose
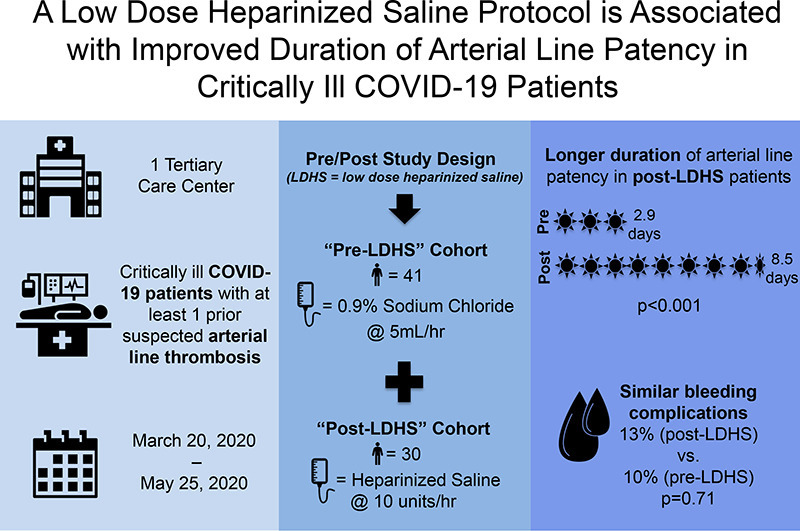
Critically ill patients with Coronavirus Disease 2019 (COVID-19) have high rates of line thrombosis. Our objective was to examine the safety and efficacy of a low dose heparinized saline (LDHS) arterial line (a-line) patency protocol in this population.
Materials and Methods
In this observational cohort study, patients ≥18 years with COVID-19 admitted to an ICU at one institution from March 20–May 25, 2020 were divided into two cohorts. Pre-LDHS patients had an episode of a-line thrombosis between March 20–April 19. Post-LDHS patients had an episode of a-line thrombosis between April 20–May 25 and received an LDHS solution (10 units/h) through their a-line pressure bag.
Results
Forty-one patients (pre-LDHS) and 30 patients (post-LDHS) were identified. Baseline characteristics were similar between groups, including age (61 versus 54 years; p = 0.24), median Sequential Organ Failure Assessment score (6 versus 7; p = 0.67) and systemic anticoagulation (47% versus 32%; p = 0.32). Median duration of a-line patency was significantly longer in post-LDHS versus pre-LDHS patients (8.5 versus 2.9 days; p < 0.001). The incidence of bleeding complications was similar between cohorts (13% vs. 10%; p = 0.71).
Conclusions
A LDHS protocol was associated with a clinically significant improvement in a-line patency duration in COVID-19 patients, without increased bleeding risk.
Keywords: COVID-19, Critical illness, Heparin, Thrombosis, Vascular access devices
Abbreviations: COVID-19, Coronavirus Disease 2019; DVT, deep vein thrombosis; PE, pulmonary embolism; ICU, intensive care unit; LDHS, low dose heparinized saline; SOFA, Sequential Organ Failure Assessment; PTT, partial thromboplastin time; INR, international normalized ratio; CRP, C-reactive protein; CRNMB, clinically relevant non-major bleeding; IQR, interquartile range
Graphical abstract
Highlights
-
•
Critically ill COVID-19 patients have frequent arterial line (a-line) thrombosis.
-
•
A low dose heparinized saline (LDHS) a-line patency protocol was examined.
-
•
Two a-line thrombosis cohorts were identified: “post-LDHS” and “pre-LDHS”.
-
•
A-line patency was longer in post- vs. pre-LDHS patients (8.5 vs. 2.9 d; p < 0.001).
-
•
Bleeding complications were similar between groups (13% vs. 10%, p = 0.7).
1. Introduction
Critically ill patients with Coronavirus Disease 2019 (COVID-19) have been observed to have high rates of thrombotic complications, including deep vein thrombosis (DVT), pulmonary embolism (PE), mesenteric ischemia, and line thrombosis [[1], [2], [3], [4], [5]]. To support the surge of COVID-19 admissions at our institution, a dedicated procedure team was established to place central, arterial, and temporary dialysis lines across multiple intensive care units (ICUs) [4]. In the ICU setting, one of the major challenges identified was frequent arterial line (a-line) thrombosis, limiting arterial access options during prolonged ICU stays [4]. In response, a low dose heparinized saline (LDHS) protocol was developed and implemented to increase the duration of a-line patency in patients with COVID-19.
Outcomes are mixed on the success of similar LDHS protocols implemented to improve a-line duration in non-COVID-19 patients. While some randomized-controlled trials report improved a-line patency in medical ICU and pediatric patients [[6], [7], [8]], several studies found no clinical effect [[9], [10], [11], [12]], and these protocols have not been widely implemented. Study populations and dosages varied between studies, and thus it was unclear whether an LDHS protocol would benefit critically ill patients with COVID-19. The objective of this study was to evaluate the effectiveness and safety of a LDHS protocol in critically ill patients with COVID-19. Specifically, we sought to: 1) Compare the duration of a-line patency in pre-LDHS versus post-LDHS patients, and 2) Compare bleeding complications between the two groups.
2. Materials and methods
2.1. Study design and patient selection
An observational cohort study of adult patients (≥18 years) with COVID-19 admitted to an ICU at the Massachusetts General Hospital in Boston, MA between March 20, 2020 and May 25, 2020 was performed. The LDHS protocol was developed and implemented across the ICUs on April 20, 2020 as a quality improvement initiative in response to frequent a-line thrombosis, with a multi-disciplinary team of individuals from pharmacy, nursing, anesthesia, surgery, and hematology. This protocol used heparin 2 units/mL in a 0.9% sodium chloride solution, which was administered as a continuous infusion through the a-line pressure bag at 10 units/h in lieu of 0.9% sodium chloride at 5 mL/h. Patients could be initiated on the protocol if they had an a-line complicated by documented thrombosis or a thrombus at multiple arterial access sites, limiting line placement options. While all patients who met these criteria were candidates for the protocol, it was initiated at the discretion of the ICU attendings. Contraindications to the protocol included history of heparin-induced thrombocytopenia (HIT), documented heparin allergy, ongoing hemorrhage, or if it was otherwise deemed clinically inappropriate by the attending physician (e.g. history of intracranial hemorrhage).
All patients with COVID-19 who received the LDHS protocol during their ICU admission (April 20 – May 25, 2020) were included and referred to as the “post-LDHS” cohort. These patients were compared to a cohort of “pre-LDHS” patients with COVID-19, who were identified using the same criteria but were admitted in the 30 days prior to institutional implementation of the LDHS protocol (March 20 – April 19, 2020).
This study was approved by the Institutional Review Board, with the requirement for informed consent waived.
2.2. Variables collected and analyzed
Patient characteristics were gathered through chart review, including age, sex, body mass index (BMI), smoking status (current, former, or never), past medical history of thrombosis (DVT, PE, myocardial infarction, ischemic stroke, or other arterial thrombosis), risk factors for thrombosis (antiphospholipid syndrome, factor V Leiden, protein C deficiency, protein S deficiency, prothrombin gene G20210A mutation, antithrombin deficiency, pregnancy, obesity, diabetes, oral contraceptive use, malignancy, and peripheral vascular disease), Sequential Organ Failure Assessment (SOFA) score on the day of observation period start, and history of recent hospitalization or surgery in 30 days prior to ICU admission. Systemic administration of anticoagulation (sometimes referred to as “therapeutic anticoagulation”) at any point during the observation period was recorded, along with the specific agent that was used, the indication for anticoagulation, and the target anti-Xa or PTT level associated. Baseline coagulation laboratory values were also recorded [international normalized ratio (INR), partial thromboplastin time (PTT), fibrinogen, anti-Xa level, platelet count, C-reactive protein (CRP), D-Dimer, and ferritin level]. If lab data were missing, this was excluded from the analysis.
2.3. Initiation of data collection
Data collection began for each patient with the index a-line. The “index a-line” was the a-line in place at protocol start in post-LDHS patents, and the a-line placed following the first a-line thrombosis event in pre-LDHS patients. The observation time was defined as the duration of LDHS administration in post-LDHS patients, and the total duration from index a-line placement to final a-line removal in pre-LDHS patients. For each group, the total number of a-lines placed prior to the index a-line and the location of the index a-line were documented, along with all a-lines replaced or re-wired during the observation period.
2.4. Study outcomes
The primary outcome was duration of index a-line patency, measured from initiation of the LDHS protocol (post-LDHS patients) or placement of index a-line (pre-LDHS patients) until an endpoint of documented a-line thrombosis requiring a-line re-wiring or replacement. If there were no thrombotic episodes requiring a-line re-wiring or replacement, the duration of time was censored at LDHS protocol discontinuation, permanent a-line removal, or patient death. In order to identify episodes of a-line thrombosis, chart review was performed looking for re-wiring or replacement, preceded by documentation of one of the following: 1) Severe arterial waveform dampening that did not improve with flushing or repositioning, 2) Inability to withdraw blood from the catheter, and/or 3) Catheter malfunction requiring replacement with documented thrombosis on bedside ultrasound of the vessel. If an a-line was replaced, but a reason could not be identified, this was included as a positive event. A-line removal for other reasons (i.e. infection, accidental dislodgement, kinking, etc.), were excluded from the primary outcome analysis. The secondary outcome was incidence of bleeding complications, divided into major bleeding, clinically relevant non-major bleeding (CRNMB), and minor bleeding, as defined by the International Society for Thrombosis and Hemostasis [13]. Development of HIT with a positive heparin-PF4 antibody test was also recorded in the post-LDHS patients.
2.5. Statistical analysis
Descriptive statistics were performed and reported as median and interquartile range (IQR), or numbers and percentages as appropriate. Categorical data were evaluated using the Chi-square or Fisher's exact test, as appropriate, and continuous variables were evaluated with Wilcoxon rank sum test. The median duration of index a-line patency was initially calculated in each group using a combined endpoint including the first of: a-line thrombosis, permanent a-line removal, or death. Kaplan-Meier methodology was used to compare differences in a-line patency duration between the groups. In this analysis, failure was defined as a-line thrombosis, and all patients were censored at the 20-day time point. Additional censoring events included permanent removal of the a-line for reasons other than thrombosis, or death while the a-line was still patent. A log rank test was performed to compare the Kaplan Meier curves of the two groups. Cox proportional hazard regression analysis was conducted to define independent predictors of arterial line thrombosis, adjusting for the following variables: age (categorized as 18–39 years, 40–64 years, and 65+ years), sex, obesity (BMI < 30 kg/m2 vs. BMI ≥ 30 kg/m2), history of thrombosis, presence of the most common thrombotic risk factors (diabetes, malignancy, and smoking status), recent surgery (within 30 days), SOFA score (as a continuous variable), index arterial line location, total arterial lines prior to index arterial line, systemic anticoagulation use, and LDHS protocol use. A two-sided p-value of ≤0.05 was used to denote statistical significance. All statistical analyses were performed using STATA® release 14.2 (StataCorp LLC, College Station, TX, USA). GraphPad Prism 8 (GraphPad Software, LLC, San Diego, CA) was used for Kaplan Meier figures.
3. Results
3.1. Patient characteristics
A total of 71 patients were included, with 30 patients in the post-LDHS cohort, and 41 patients in the pre-LDHS cohort. Baseline characteristics of both cohorts are described in Table 1 . Compared to pre-LDHS patients, post-LDHS patients were similar in age (median 61 vs. 54 years; p = 0.24), male sex (60% vs. 61%; p = 1), presence of thrombotic risk factors (57% versus 66%; p = 0.47), SOFA (median score 6 vs. 7; p = 0.67), and receipt of systemic anticoagulation during the study period (47% vs. 32%, p = 0.32). Among patients on systemic anticoagulation, the most common indications were clotting arterial lines (N = 4 pre-LDHS vs. N = 4 post-LDHS), suspected PE (N = 4 pre-LDHS vs. N = 4 post-LDHS), DVT (N = 3 pre-LDHS vs. N = 1 post-LDHS), and clotting the renal replacement circuit (N = 1 pre-LDHS vs. N = 3 post-LDHS), and there was no significant difference between these indications for anticoagulation between the pre-LDHS and post-LDHS cohorts (p = 0.64). Four of these patients had more than one indication for anticoagulation. The majority of patients in each group were on heparin (92% pre-LDHS vs. 86% post-LDHS, p = 0.59). The most common target range used for patients on systemic heparin was 0.3–0.7 IU/mL among patients in pre-LDHS and post-LDHS cohorts (67% vs. 83%, p = 0.38). No patients in the post-LDHS group had a past medical history of thrombosis compared to five patients in the pre-LDHS group who specifically reported a history of DVT (N = 2), PE (N = 2), and ischemic stroke (N = 1) (p = 0.07). Of those patients who received prophylactic anticoagulation during the study period, there was no significant difference in the agent chosen (subcutaneous enoxaparin vs. subcutaneous heparin) between the pre- and post-LDHS groups (38% heparin versus 55% heparin; p = 0.28). Table 2 shows all baseline laboratory values stratified by systemic anticoagulation status. Baseline coagulation labs were similar between groups with the exception of ferritin levels, which were significantly higher in the post-LDHS patients versus pre-LDHS patients [median (IQR), 1625 μg/L (860–2917) vs. 896 μg/L (475–1299), respectively; p = 0.02].
Table 1.
Patient characteristics.
| Patient characteristicsa | Pre-LDHS patients (n = 41) | Post-LDHS patients (n = 30) | pvalue |
|---|---|---|---|
| Age (years) | 54 (46, 68) | 61 (53, 68) | 0.24 |
| Male, n (%) | 25 (61) | 18 (60) | 1 |
| BMI ≥30 kg/m2, n (%) | 17 (41) | 17 (57) | 0.24 |
| Smoker, n (%) | 0.32 | ||
| Never smoker | 30 (73) | 18 (60) | |
| Current smoker | 5 (12) | 3 (10) | |
| Past smoker | 6 (15) | 9 (30) | |
| History of thrombosis, n (%) | 5 (12) | 0 (0) | 0.07 |
| Patients with thrombosis risk factors, n (%) | 27 (66) | 17 (57) | 0.47 |
| Obesity | 19 (46) | 14 (47) | |
| Diabetes | 16 (39) | 10 (33) | |
| Malignancy | 5 (12) | 2 (7) | |
| Hospitalized in 30 days prior to admission, n (%) | 0 (0) | 1 (3) | 0.42 |
| Surgery in 30 days prior to ICU admission, n (%) | 1 (2) | 2 (7) | 0.57 |
| Systemic anticoagulation, n (%) | 13 (32) | 14 (47) | 0.32 |
| Heparin | 12 (92) | 12 (86) | |
| Enoxaparin | 1 (8) | 2 (14) | |
| SOFA scoreb | 7 (5,9) | 6 (3,10) | 0.67 |
| Arterial lines prior to index arterial line | 1 (1,1) | 1 (1,2) | 0.30 |
| Arterial line placed by procedure service, n (%) | 27 (66) | 24 (80) | 0.19 |
| Location of index arterial line, n (%) | |||
| Radial | 38 (93) | 26 (87) | 0.45 |
| Femoral | 2 (5) | 2 (7) | 1 |
| Brachial | 1 (2) | 1 (3) | 1 |
| Axillary | 0 (0) | 1 (3) | 0.42 |
| Baseline laboratory values | |||
| INR | 1.2 (1.1, 1.2) | 1.2 (1.1, 1.3) | 0.88 |
| PTT (seconds) | 34 (30, 40) | 36 (31, 44) | 0.29 |
| Fibrinogen (mg/dL) | 716 (632, 855) | 744 (606, 883) | 0.87 |
| Anti-Xac (IU/mL) | 0.66 (0.53, 0.79) | 0.46 (0.23, 0.59) | 0.14 |
| Platelets (K/uL) | 287 (214, 348) | 279 (198, 360) | 0.99 |
| CRP (mg/L) | 158 (139, 285) | 146 (120, 190) | 0.16 |
| D-Dimer (ng/mL) | 2396 (1685, 5061) | 2667 (1259, 4371) | 0.74 |
| Ferritind (mcg/L) | 896 (475, 1299) | 1625 (860, 2917) | 0.02 |
Abbreviations: BMI = Body Mass Index; ICU = Intensive Care Unit; IQR = Interquartile Range; LDHS = Low Dose Heparinized Saline; SOFA = Sequential Organ Failure Assessment.
Data presented as median (interquartile range) unless otherwise stated.
At protocol start for heparin patients and at index arterial line for pre-LDHS patients.
Patients on systemic enoxaparin versus heparin often had different anti-Xa goals.
Statistically significant at p < 0.05.
Table 2.
Baseline coagulation laboratory values, stratified by systemic anticoagulation status.
| Lab valuea | Systemic anticoagulation |
No systemic anticoagulation |
||||
|---|---|---|---|---|---|---|
| Pre-LDHS patients (n = 13) | Post-LDHS patients (n = 14) | p value | Pre-LDHS patients (n = 28) | Post-LDHS patients (n = 16) | p value | |
| INR | 1.15 (1.1, 1.2) | 1.2 (1.1 1.3) | 0.46 | 1.2 (1.1, 1.3) | 1.2 (1.1, 1.2) | 0.61 |
| PTT (seconds) | 35.5 (31.5, 55) | 39.9 (33.9, 52.9) | 0.49 | 33.7 (29.3, 39.1) | 33 (31, 38) | 0.93 |
| Fibrinogen (mg/dL) | 691 (643, 855) | 650 (579, 840) | 0.49 | 749 (605, 854) | 864 (632, 927) | 0.33 |
| Anti-Xab (IU/mL) | 0.66 (0.53, 0.79) | 0.46 (0.23, 0.59) | 0.25 | – | – | – |
| Platelets (K/uL) | 268 (236, 322) | 262 (168, 311) | 0.53 | 297 (196, 349) | 325 (205, 441) | 0.46 |
| CRP (mg/L) | 167 (137.5, 238.2) | 145 (129, 190) | 0.87 | 150 (144, 289) | 147 (67, 205) | 0.16 |
| D-Dimer (ng/mL) | 5211 (3184, 5801) | 2464 (1290, 3982) | 0.07 | 1927 (1391, 3075) | 3289 (925, 4607) | 0.40 |
| Ferritin (mcg/L) | 1254 (973, 1574) | 1862 (581, 3011) | 0.54 | 726 (412, 1261) | 1403 (920, 2629) | 0.009 |
Abbreviations: CRP = C-Reactive Protein; INR = International Normalized Ratio; IQR = Interquartile Range; LDHS = Low Dose Heparinized Saline; PTT = Partial Thromboplastin Time.
Data presented as median (interquartile range) unless otherwise specified.
Patients on systemic enoxaparin versus heparin often had different anti-Xa goals.
All data for baseline characteristics were complete except for lab values, which were reported only when collected and had missing data as follows: INR (post-LDHS 4 missing; pre-LDHS 9 missing), PTT (post-LDHS 6 missing; pre-LDHS 13 missing), fibrinogen (post-LDHS 15 missing; pre-LDHSs 17 missing), Anti-Xa (post-LDHS 23 missing; pre-LDHS 39 missing), platelets (post-LDHS 0 missing; pre-LDHS 1 missing), CRP (post-LDHS 12 missing; pre-LDHS 11 missing), D-Dimer (post-LDHS 2 missing; pre-LDHS 4 missing), and ferritin (post-LDHS 5 missing; pre-LDHS 7 missing). All patients were followed to the first event of the following: death, ICU discharge, or the 20-day time point.
3.2. Outcomes
Median duration of a-line patency, bleeding complications, and deaths are indicated in Table 3 , stratified by systemic anticoagulation status. Median duration of a-line patency across all patients in the post-LDHS cohort was 8.5 days (IQR 4.8, 14.7) compared with 2.9 days (IQR 0.7, 6.2) in the pre-LDHS cohort (p < 0.001). Among patients on systemic anticoagulation, median (IQR) duration of a-line patency was 13.9 days (9.3, 18.3) in the post-LDHS group compared to 4.1 days (1, 10.1) in the pre-LDHS group (p < 0.001). Among patients not on systemic anticoagulation, the median duration of arterial line patency was 4.9 days in the post-LDHS group and 2.2 days in the pre-LDHS group (p = 0.03). Given the risk of confounding by the five patients in the pre-LDHS group with a past history of thrombosis, a sensitivity analysis was performed with these five patients excluded. In this analysis, the significant effect was maintained, with a median (IQR) duration of a-line patency of 8.5 days (4.8, 14.7) in the post-LDHS cohort versus 3 days (1.05, 6.2) in the pre-LDHS cohort (p < 0.001). Similar effects were retained when stratified by systemic anticoagulation status.
Table 3.
Outcomes.
| Outcome | Systemic anticoagulation |
No systemic anticoagulation |
||||
|---|---|---|---|---|---|---|
| Pre-LDHS patients (n = 13) | Post-LDHS patients (n = 14) | p value | Pre-LDHS patients (n = 28) | Post-LDHS patients (n = 16) | p value | |
| Time to eventa (days), median (IQR) | 4.1 (1.0, 10.1) | 13.9 (9.3, 18.3) | <0.001 | 2.2 (0.7, 6.1) | 4.9 (3.3, 8.5) | 0.03 |
| Bleeding complications, n (%) | 2 (15.4) | 3 (21.4) | 1 | 2 (7.1) | 1 (6.3) | 1 |
| Major | 1 (7.7) | 0 (0) | – | 1 (3.6) | 0 (0) | – |
| CRNMB | 1 (7.7) | 3 (21.4) | – | 0 (0) | 0 (0) | – |
| Minor | 0 | 0 (0) | – | 1 (3.6) | 1 (6.3) | – |
| Death, n (%) | 4 (30.8%) | 2 (14.3%) | 0.38 | 6 (21.4%) | 4 (25.0%) | 1 |
Abbreviations: CRNMB = Clinically Relevant Non-Major Bleeding; IQR = Interquartile range; LDHS = Low dose heparinized saline.
“Event” in this situation includes arterial line thrombosis, discontinuation of arterial line when it was no longer needed, or patient death.
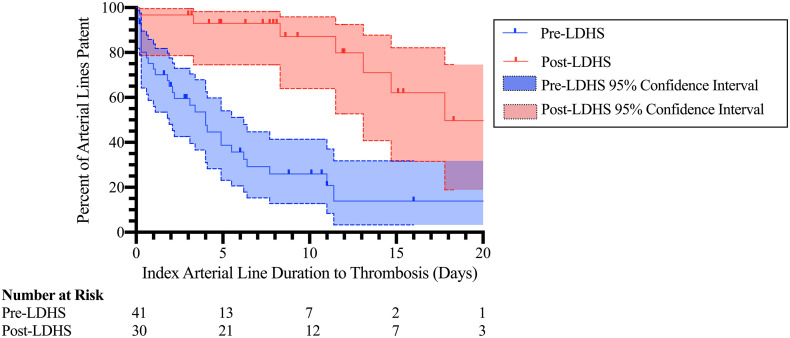
Kaplan Meier survival curves comparing the probability of a-line patency in the post-LDHS group to the pre-LDHS group over time were significantly different by log rank test (p < 0.0001) (Fig. 1 ). Additionally, at the five-day time point, 93% (95% CI 75%–98%; 7 censored) of patients in the post-LDHS cohort still had a patent index a-line, compared with 39% (95% CI 23%–54%; 4 censored) of pre-LDHS patients.
Fig. 1.
Kaplan Meier curves demonstrating the duration of index arterial line patency in patients receiving the low dose heparin protocol compared with pre-LDHS patients.
On Cox proportional hazard regression analysis, independent predictors of a-line thrombosis were obesity and not being on the LDHS protocol (Table 4 ). Obese patients (BMI ≥ 30 kg/m2) had more than twice the risk of a-line thrombosis than patients with BMI < 30 kg/m2 (hazard ratio 2.28; 95% CI 1.07–4.84; p = 0.03). Post-LDHS patients had an 87% thrombosis risk reduction compared to pre-LDHS patients (hazard ratio 0.13, 95% CI 0.05–0.34; p < 0.001). Systemic anticoagulation did not significantly decrease the risk of index a-line thrombosis (hazard ratio 0.61; 95% CI 0.27–1.38; p = 0.23).
Table 4.
Cox proportional hazard regression model for predictors of index arterial line thrombosis.
| Variables | Hazard ratio | 95% Confidence interval | p value |
|---|---|---|---|
| Age | |||
| 18–39 years | Ref | – | – |
| 40–64 years | 0.41 | 0.14–1.25 | 0.12 |
| 65+ years | 1.19 | 0.28–5.08 | 0.81 |
| Female | 1.09 | 0.47–2.53 | 0.84 |
| BMI | |||
| <30 kg/m2 | Ref | – | – |
| ≥30 kg/m2a | 2.28 | 1.07–4.84 | 0.03 |
| Smoker | |||
| Never smoker | Ref | – | – |
| Current smoker | 1.01 | 0.26–3.90 | 0.99 |
| Past smoker | 0.71 | 0.21–2.42 | 0.58 |
| Personal history of thrombosis | 1.13 | 0.32–3.96 | 0.84 |
| Diabetes | 0.71 | 0.30–1.67 | 0.43 |
| Malignancy | 1.09 | 0.31–3.87 | 0.89 |
| Surgery in 30d prior to ICU admission | 6.98 | 0.69–70.9 | 0.10 |
| SOFA scoreb | 1.05 | 0.89–1.24 | 0.54 |
| Number of arterial lines prior to index arterial line | 1.18 | 0.64–2.19 | 0.59 |
| Index arterial line located in radial artery | 2.79 | 0.56–13.8 | 0.21 |
| Systemic anticoagulation | 0.61 | 0.27–1.38 | 0.23 |
| Low dose heparin protocola | 0.13 | 0.05–0.34 | <0.001 |
Abbreviations: BMI = Body Mass Index; LDHS = low dose heparinized saline; Ref = Reference group; SOFA = Sequential Organ Failure Assessment.
Statistically significant at p < 0.05.
At protocol start for post-LDHS patients and at index arterial line for pre-LDHS patients.
3.3. Bleeding complications and coagulation markers
The incidence and severity of bleeding complications, stratified by systemic anticoagulation and post-LDHS versus pre-LDHS group, are available in Table 3. While there were no major bleeding complications in the post-LDHS group, three CRNMB complications (all of which occurred in patients receiving systemic anticoagulation) did occur. Among pre-LDHS patients, two patients had major bleeding complications. One patient on systemic anticoagulation developed a small intraparenchymal hemorrhage. Another patient (not on systemic anticoagulation) developed pulsatile bleeding around a femoral arterial line requiring removal and manual pressure, in addition to bleeding around a temporary dialysis catheter site, requiring four units of packed red blood cells over several days. All bleeding complications and their management are described in detail in Table 5 .
Table 5.
Bleeding complication details and management.
| Complication type | Pre-LDHS patients |
Post-LDHS patients |
||
|---|---|---|---|---|
| Details | Management | Details | Management | |
| Major bleeding | Small intraparenchymal hemorrhagea | Discontinuation of systemic anticoagulation | None | None |
| Pulsatile bleeding around femoral arterial line and oozing around a temporary dialysis catheter site | Removal and manual pressure on femoral site, transfusion of four units of packed red blood cells total | |||
| Clinically relevant non-major bleeding | Femoral arterial catheter accidentally dislodgeda | Manual pressure, transfusion of one unit of packed red blood cells | Diffuse oozing from lines and tracheostomya | Discontinuation of systemic anticoagulation |
| Diffuse oozing from lines and peripheral intravenous linea | Discontinuation of systemic anticoagulation | |||
| Renal replacement equipment malfunction leading to blood loss in tubinga | Transfusion of one unit of packed red blood cells | |||
| Minor bleeding | Small groin hematoma | No intervention | Small groin hematoma | No intervention |
Abbreviations: LDHS = low dose heparinized saline.
Patient on systemic anticoagulation.
There were no significant differences in coagulation labs before versus after LDHS protocol initiation in post-LDHS patients, including INR, PTT, or platelet count (Table 6 ). None of the patients in the post-LDHS group developed HIT.
Table 6.
Coagulation laboratory values before and after low dose heparinized saline protocol initiation.
| Lab valuea | No systemic anticoagulation (n = 16) |
Systemic anticoagulation (n = 14) |
||||
|---|---|---|---|---|---|---|
| Before | After | p value | Before | After | p value | |
| INR | 1.2 (1.1, 1.3) | 1.2 (1.1, 1.4) | 0.29 | 1.2 (1.1, 1.2) | 1.2 (1.1, 1.2) | 0.69 |
| PTT (seconds) | 40 (34, 53) | 48 (31, 76) | 0.07 | 33 (31, 38) | 32 (29, 38) | 0.45 |
| Fibrinogen (mg/dL) | 650 (579, 840) | 735 (648, 803) | 0.38 | 864 (632, 927) | 731 (640, 892) | 0.22 |
| Anti-Xab (IU/mL) | 0.46 (0.23, 0.59) | 0.62 (0.28, 0.9) | 1 | – | – | – |
| Platelets (K/uL) | 262 (168, 311) | 207 (168, 315) | 0.23 | 325 (205, 441) | 317 (190, 447) | 0.61 |
| CRP (mg/L) | 145 (129, 190) | 149 (133, 288) | 0.18 | 147 (67, 205) | 145 (127, 257) | 1 |
| D-Dimer (ng/mL) | 2464 (1290, 3982) | 2732 (1971, 5951) | 1 | 3289 (925, 4607) | 2716 (1458, 5271) | 1 |
| Ferritin (mcg/L) | 1862 (581, 3011) | 2475 (1265, 3525) | 0.75 | 1404 (920, 2629) | 880 (665, 1547) | 0.11 |
Abbreviations: CRP = C-Reactive Protein; INR = International Normalized Ratio; IQR = Interquartile Range; PTT = Partial Thromboplastin Time.
Data presented as median (interquartile range) unless otherwise specified.
Patients on systemic enoxaparin versus heparin often had different anti-Xa goals.
4. Discussion
In critically ill patients with COVID-19, a LDHS protocol was associated with increased duration of a-line patency in patients with a history of a-line thrombosis. Over 90% of post-LDHS a-lines were still patent at day 5, compared to less than 40% of catheters in the pre-LDHS group. There were no significant differences in coagulation labs after LDHS initiation, nor were there increased bleeding complications in the post-LDHS cohort.
The issue of arterial access is particularly important for critically ill patients admitted with COVID-19. Across multiple studies, median ICU length of stay for COVID-19 patients is over two weeks, with patients spending a median of 10–16 days on the ventilator, and 28–47% of patients requiring prone positioning [[14], [15], [16]]. Frequent arterial blood gas measurements during prone positioning are required to guide ventilator management, determine timing of supination, and indicate need for extracorporeal membrane oxygenation. However, prone positioning also limits arterial access to the upper extremities. As such, utilization of a LDHS protocol may ensure a-line access during prone positioning, where access options are limited and maintaining access is critical.
Patients with COVID-19 initiated on the LDHS protocol had a significantly longer median duration of a-line patency compared to pre-LDHS patients, both among patients on systemic anticoagulation and those not on systemic anticoagulation. The LDHS protocol was an independent predictor of a-line patency duration, whereas the use of systemic anticoagulation was not. We speculate that continuous administration of the LDHS protocol directly at the a-line catheter tip was an important driver of this effect.
The predominant mechanism of a-line thrombosis is intravascular thrombosis immediately proximal to the catheter tip [17]. Local mechanisms of endothelial damage have been proposed to explain catheter-associated thrombosis, including shear stress from guidewire advancement and catheter flushing, fibrin accumulation due to local turbulent flow, and local trauma on the vessel wall by the catheter [[18], [19], [20], [21], [22]]. In COVID-19 patients, mechanisms proposed to explain increased thrombosis include direct infection of endothelial cells by virus, immune-mediated endotheliitis, systemic hypercoagulability, and a severe inflammatory state mimicking disseminated intravascular coagulopathy [23,24]. Systemic anticoagulation has been proposed to mitigate thrombotic risk in these patients, although this remains untested, and the bleeding risk of systemic anticoagulation may outweigh the benefits for line thrombosis alone.
Prior to the COVID-19 outbreak, a number of studies have examined the use of LDHS protocols in ICU patients to improve a-line patency, with mixed results [[6], [7], [8], [9], [10], [11], [12],25,26]. The proposed mechanism of the LDHS protocol is infusing the anticoagulant directly to the site of potential clot formation proximal to the catheter tip. In a randomized-controlled trial of 300 pediatric ICU patients, patients who received sodium chloride 0.9% had more than three times the risk of a-line thrombosis versus patients who received the LDHS protocol at 2–5 units/h [7]. In a multicenter, randomized-controlled trial of over 5000 adult ICU patients, a LDHS protocol was associated with increased a-line patency, along with longer catheters (>2 in.), systemic anticoagulation, femoral catheter placement, and male sex [26]. Conversely, in adult cardiac surgery patients, a LDHS infusion was not associated with an increased rate of patency [12]. A Cochrane review committee in 2014 deemed the evidence limited and biased, and were unable to perform a meta-analysis [25]. This body of evidence has led to heterogeneous protocol use, often varying within hospitals by dose and patient population. While doses varied between studies, the Cochrane authors did conclude that while lower doses had varied effectiveness, one study did show significantly increased patency duration with a dose of 4 units/mL (run continuously for a total of 12 units/h) [6]. We believe that using a dose of 10 units/h and targeting therapy to patients with COVID-19 who had already thrombosed at least one a-line, our selected population was likely at higher risk of a-line thrombosis, and more likely to benefit from treatment.
There are several limitations of this study. First, this was an observational study that depended on chart review. We used proxies of thrombosis including a-line dampening and inability to draw back, as ultrasound evidence was inconsistently documented. Severe a-line dampening and inability to withdraw blood have both been described as appropriate proxies of thrombosis in previous randomized-controlled trials of a-line patency, even in the absence of ultrasound confirmation [8]. In one study, 84% of patients with either an overdampened wave form, sluggish backflow, inability to withdraw blood or inability to flush were found on bedside ultrasound to have intravascular thrombus adjacent to the catheter tip [17]. Furthermore, arterial catheter type and placement technique (e.g. “through-and-through” insertion technique, micropuncture, etc.) were not included, due to inconsistent documentation. That said, in a study comparing the risk of thrombosis in radial arteries cannulated using a “through-and-through” methodology (where the access needle is advanced through the back wall of the artery) compared to those cannulated directly into the artery showed no difference in patency [27]. Finally, a-line re-wires were also not always documented and these events may not have been fully captured despite review of nursing, procedure, and provider notes to confirm as many procedures as possible.
To the authors' knowledge, this is the first study to evaluate the implementation of a LDHS protocol in patients with COVID-19 who developed a-line thrombosis. The protocol was implemented across previously established ICUs along with “surge” ICUs (developed in response to the surge of critically ill patients with COVID-19), highlighting its streamlined approach and ease of use. Furthermore, we did not observe any major bleeding complications in the LDHS cohort. While a study of cardiac surgery patients found a small but statistically significant increase in activated clotting time (ACT) and PTT with LDHS [12], in our study, there were no differences in coagulation markers (ACT was not followed).
As hospitals continue to care for critically ill patients with COVID-19 now and in the future, there are multiple benefits to a low-risk intervention that prolongs a-line patency and limits the need for invasive procedures. For institutions faced with equipment and medication shortages during high-volume admission periods, implementation of the LDHS protocol in patients with COVID-19 who develop a-line thrombosis has the potential to maintain a-line patency while reducing the need for systemic anticoagulation. The results of this study suggest that a LDHS protocol is a safe and effective therapeutic option to implement more broadly in patients with COVID-19 who develop a-line thrombosis.
5. Conclusions
A LDHS protocol is associated with an increased duration of a-line patency in critically ill patients with COVID-19, without an increased risk of bleeding complications. Given the protocol's success in patients who have already demonstrated thrombosis, further prospective investigation is required to determine if this protocol could benefit a broader population of critically ill patients with COVID-19, before development of initial a-line thrombosis.
Acknowledgments
Acknowledgments
We appreciate the multidisciplinary assistance from the pharmacy, ICU, nursing, hematology, surgery, and anesthesia departments, who all supported the implementation of this quality improvement effort and its evaluation.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
Dr. Jarone Lee is on the Scientific Advisory Board of the Butterfly Network, Inc. (unrelated to this work), and has the research grants from the following funding bodies (also unrelated to this work): DOD/MTEC, NIH, Nihon-Kohden, Beckman Coulter. Dr. Rachel Rosovsky is a consultant for Bristol Myers Squibb and Janssen (unrelated to this work). The remaining authors have disclosed that they do not have any conflicts of interest.
References
- 1.Kaafarani H.M.A., El Moheb M., Hwabejire J.O., Naar L., Christensen M.A., Breen K. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020 doi: 10.1097/sla.0000000000004004. Published online ahead of print April, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albutt K., Luckhurst C., Alba G., El Hechi M., Mokhtari A., Breen K. Design and impact of a COVID-19 multidisciplinary bundled procedure team. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004089. (Published online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haoudar A., Kantri A., Ziati J., Aidaoui K., Elkettani C. Arterial and vein related catheters thrombosis in a patient with COVID-19: a case report. Thromb Res. 2020 (Published online ahead of print) [Google Scholar]
- 6.Clifton G.D., Branson P., Kelly H.J., Dotson L.R., Record K.E., Phillips B.A. Comparison of normal saline and heparin solutions for maintenance of arterial catheter patency. Heart Lung J Crit Care. 1991;20:115–118. [PubMed] [Google Scholar]
- 7.De Neef M., Heijboer H., Van Woensel J.B.M., De Haan R.J. The efficacy of heparinization in prolonging patency of arterial and central venous catheters in children: a randomized double-blind trial. Pediatr Hematol Oncol. 2002;19:553–560. doi: 10.1080/08880010290097404. [DOI] [PubMed] [Google Scholar]
- 8.Everson M., Webber L., Penfold C., Shah S., Freshwater-Turner D. Finding a solution: heparinised saline versus normal saline in the maintenance of invasive arterial lines in intensive care. J Intensive Care Soc. 2016;17:284–289. doi: 10.1177/1751143716653763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziyaeifard M., Alizadehasl A., Aghdaii N., Sadeghi A., Azarfarin R., Masoumi G. Heparinized and saline solutions in the maintenance of arterial and central venous catheters after cardiac surgery. Anesthesiol Pain Med. 2015;5 doi: 10.5812/aapm28056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Cotillo M., Grané N., Llavoré M., Quintana S. Heparinized solution vs. saline solution in the maintenance of arterial catheters: a double blind randomized clinical trial. Intensive Care Med. 2008;34:339–343. doi: 10.1007/s00134-007-0886-6. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni M., Elsner C., Ouellet D., Zeldin R. Heparinized saline versus normal saline in maintaining patency of the radial artery catheter. Can J Surg. 1994;37:37–42. [PubMed] [Google Scholar]
- 12.Xiong J., Pan T., Jin H., Xie X., Wang Y., Wang D. A comparison of heparinised and non-heparinised normal saline solutions for maintaining the patency of arterial pressure measurement cannulae after heart surgery. J Cardiothorac Surg. 2019;14:39. doi: 10.1186/s13019-019-0860-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaatz S., Ahmad D., Spyropoulos A.C., Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 14.Ziehr D.R., Alladina J., Petri C.R., Maley J.H., Moskowitz A., Medoff B.D. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202004-1163LE. Published online April 29, 2020 ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/nejmoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleury Y., Arroyo D., Couchepin C., Robert-Ebadi H., Righini M., Lobrinus J.A. Impact of intravascular thrombosis on failure of radial arterial catheters in critically ill patients: a nested case-control study. Intensive Care Med. 2018;44:553–563. doi: 10.1007/s00134-018-5149-1. [DOI] [PubMed] [Google Scholar]
- 18.Staniloae C.S., Mody K.P., Sanghvi K., Mindrescu C., Coppola J.T., Antonescu C.R. Histopathologic changes of the radial artery wall secondary to transradial catheterization. Vasc Health Risk Manag. 2009;5:527–532. doi: 10.2147/vhrm.s5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedford R. Long-term radial artery cannulation: effects on subsequent vessel function. Crit Care Med. 1978;6:64–67. doi: 10.1097/00003246-197801000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Kaye J., Heald G., Morton J., Weaver T. Patency of radial arterial catheters. Am J Crit Care. 2001;10:104–111. [PubMed] [Google Scholar]
- 21.Cosemans J.M.E.M., Angelillo-Scherrer A., Mattheij N.J.A., Heemskerk J.W.M. The effects of arterial flow on platelet activation, thrombus growth, and stabilization. Cardiovasc Res. 2013;99:342–352. doi: 10.1093/cvr/cvt110. [DOI] [PubMed] [Google Scholar]
- 22.Galbusera M., Zoja C., Donadelli R., Paris S., Morigi M., Benigni A. Fluid shear stress modulates von Willebrand factor release from human vascular endothelium. Blood. 1997;90:1558–1564. doi: 10.1182/blood.v90.4.1558.1558_1558_1564. [DOI] [PubMed] [Google Scholar]
- 23.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 doi: 10.1111/jth.14850. Published online April 17, 2020 ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson-Malt S., Malt G.N., Farquhar V., Greer W. Heparin versus normal saline for patency of arterial lines. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD007364.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evaluation of the effects of heparinized and nonheparinized flush solutions on the patency of arterial pressure monitoring lines: the AACN Thunder Project. By the American Association of Critical-Care Nurses. Am J Crit Care. 1993;2:3–15. doi: 10.4037/ajcc1993.2.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Jones R.M., Hill A.B., Nahrwold M.L., Bolles R.E. The effect of method of radial artery cannulation on postcannulation blood flow and thrombus formation. Anesthesiology. 1981;55:76–78. doi: 10.1097/00000542-198107000-00016. [DOI] [PubMed] [Google Scholar]