Molecular alterations are increasingly being used to distinguish tumors with similar histopathologic features but distinct clinical characteristics. Among IDH-wildtype and H3-wildtype (IDH-wt/H3-wt) diffuse gliomas is an important, though uncommon, group of tumors presenting in children and young adults. These demonstrate an indolent clinical behavior, unlike IDH-mutant diffuse gliomas and the IDH-wt/H3-wt diffuse gliomas of later life [2, 3]. This group of ‘pediatric-type’ diffuse gliomas has a relatively restricted range of genetic alterations, which are distinct from those in ‘adult-type’ IDH-wt/H3-wt diffuse gliomas and involve FGFR1, BRAF, MYB and MYBL1 [2, 4]. Following the 2016 update to the World Health Organization (WHO) classification of CNS tumors, entities delineated by combinations of histopathologic and genetic characteristics and with distinctive clinical profiles have been presented in a series of papers from the cIMPACT-NOW group. These new entities include the ‘pediatric-type’ diffuse gliomas [3]. In order for uncommon genetically defined entities to be considered for inclusion in the WHO classification, it is important to collect as much information as possible about their clinicopathologic profiles. In this correspondence with electronic supplementary data (Suppl. Table), we present the clinicopathologic and molecular features of 46 gliomas with a MYB or MYBL1 alteration diagnosed at St. Jude Children’s Research Hospital.
Patients were primarily young children (median age, 5 years; range, 0 – 26 years). No gender predilection was observed (male 52.2%; female 47.8%). Tumors involved cerebral cortex (58.7%), cerebral white matter and/or deep gray nuclei (26.1%), or brain stem (15.2%). Cortical tumors, centrally located tumors, and brain stem tumors commonly presented with epilepsy, symptoms of raised intracranial pressure, and cranial nerve deficits, respectively. Outcome data were available for 37 patients (follow-up, 1 – 226 months; median, 28 months; IQ range, 1–140 months). The 10-year progression-free survival and overall survival rates were 89.6% (95%, CI 77.0 – 100%) and 95.2% (95%, CI 86.6 – 100%), respectively. No histopathologic or genetic feature was associated with outcome.
Pre-operative MR images were available for 23 patients (cerebral - 8, deep gray nuclei - 8, and brain stem - 7). Tumors were T1 iso-intense to hypo-intense and showed mixed signal or hyperintensity on T2/FLAIR. There was no contrast enhancement or restricted diffusion, except in one case with faint, diffuse contrast enhancement. Cortical tumors frequently had a lobulated appearance, resembling dysembryoplastic neuroepithelial tumors, while those involving more caudal sites were diffuse and poorly-defined. However, all tumors had some features of an infiltrative pattern (Suppl. Fig.1).
All tumors showed low-grade histopathologic features, being characterized by bland nuclei and rare or absent mitotic activity. They were broadly classified as diffuse astrocytoma (DA, n=11) or angiocentric glioma (AG, n=35), but AGs consistently demonstrated entrapment of CNS parenchyma, with areas that resembled a DA (Suppl. Fig.2), and subtle angiocentricity was found in 10/11 tumors classified as DA. In 6/11 DAs, tumor cells barely raised the cell density of infiltrated parenchyma, as described for isomorphic diffuse glioma [1]. Subpial condensation of tumor cells was frequent, but perineuronal satellitosis, vascular endothelial proliferation, and necrosis were not identified. All tumors showed a similar immunophenotype (Suppl. Fig.3); tumor cells expressed GFAP, showed variable reactivity for MAP2, and were immunonegative for SOX10. Infiltrating tumor cells in both DAs and AGs generally expressed OLIG2, while perivascular tumor cells were OLIG2-negative. A dot-like intracytoplasmic pattern of EMA expression was observed in all tumors.
Molecular alterations were interrogated by immunohistochemistry (n=46), RNA sequencing (n=29), interphase fluorescence in situ hybridization (n=24), or PCR-based sequencing (n=11). All tumors had alterations involving MYB (n=44) or MYBL1 (n=2). None had alterations in IDH1, IDH2, TP53, ATRX, or histone H3 genes. Two had a concomitant BRAF:p.V600E mutation (Suppl. Table). MYB and MYBL1 were fused with various gene partners (Figure 1a). Two tumors with MYBL1 alterations were DAs. Molecular alterations did not show any clear association with clinical parameters.
Figure 1.
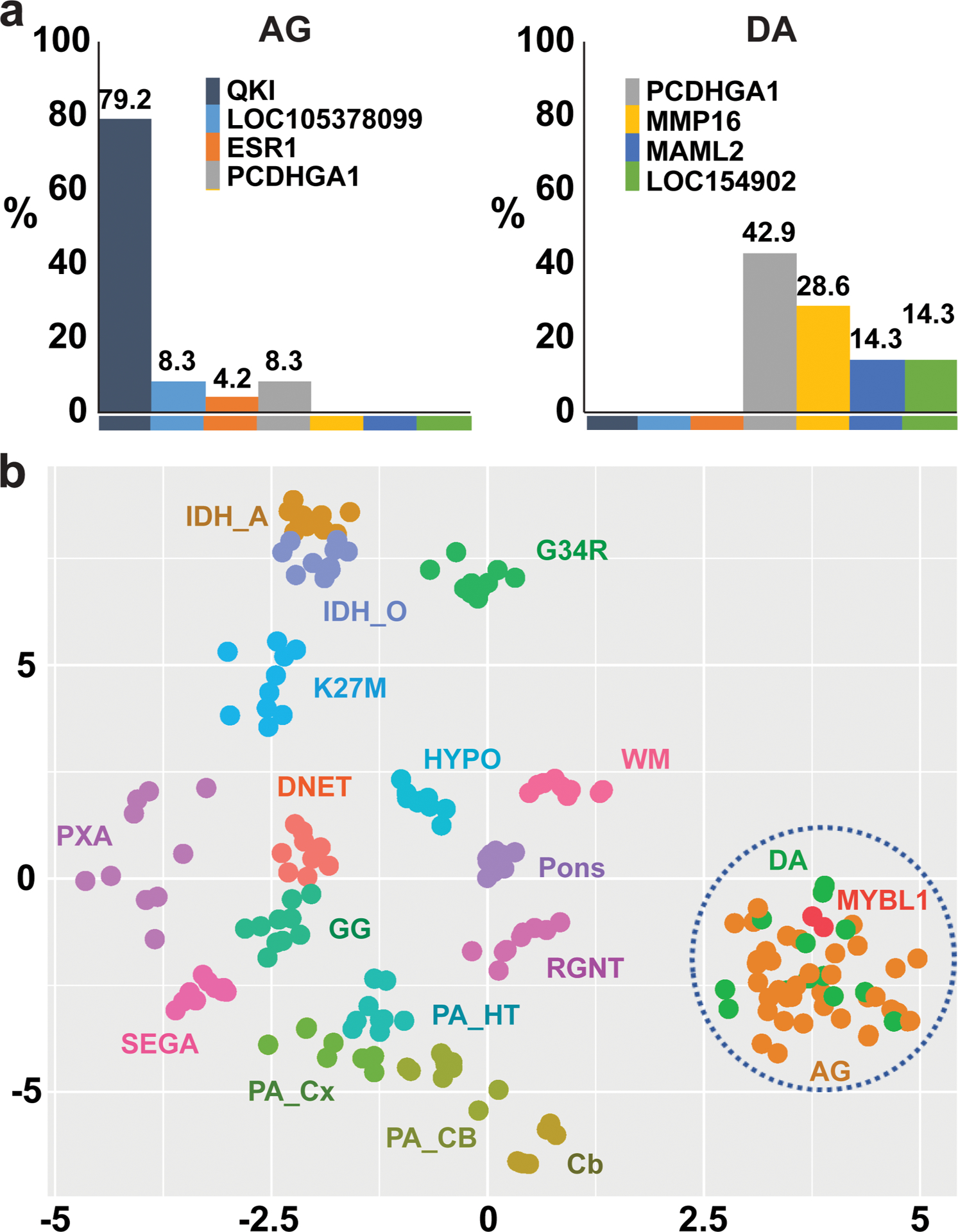
(a) Proportions of AGs or DAs with specific MYB or MYBL1 gene fusion partners. (b) t-SNE analysis of genomic DNA methylome of MYB/MYBL1-altered diffuse gliomas. AG: MYB-altered angiocentric glioma, Cb: cerebellum, DA: MYB-altered diffuse astrocytoma, DNET: dysembryoplastic neuroepithelial tumor, G34R: H3F3A G34R-mutant high-grade glioma, GG: ganglioglioma, HYPO: hypothalamus, IDH_A: IDH-mutant astrocytoma, IDH_O: IDH-mutant and 1p/19q-codeleted oligodendroglioma, K27M: H3 K27M-mutant diffuse midline glioma, PA_CB: cerebellar pilocytic astrocytoma, PA_Cx: cortical pilocytic astrocytoma, PA_HT: hypothalamic pilocytic astrocytoma, PXA: pleomorphic xanthoastrocytoma, RNGT: rosette-forming glioneuronal tumor, SEGA: subependymal giant cell astrocytoma, WM: cerebral white matter.
Genomic DNA methylome profiling and t-distributed stochastic neighbor embedding (t-SNE) analysis were undertaken on 35 tumors, with 12 CNS tumor types and normal CNS tissues in a reference series (Figure 1b). By t-SNE analysis, MYB/MYBL1-altered gliomas formed a single cluster, regardless of histopathologic diagnosis or anatomic location. This result, alongside overlapping clinical, neuro-imaging, and histopathologic characteristics, supports the proposal that MYB/MYBL1-altered gliomas represent a single disease entity with a range of clinical and pathologic characteristics.
In summation, we have provided clinicopathologic, genetic, and epigenetic data on 46 MYB/MYBL1-altered gliomas. The data demonstrate significant overlap on these parameters, with implications for tumor classification.
Supplementary Material
Supplementary Figure 1. Representative axial T2-weighted MRI scans from the study cohort. AG: angiocentric glioma, DA003A diffuse astrocytoma, IDG: isomorphic diffuse glioma
Supplementary Figure 2. (a & b) Representative hematoxylin and eosin (H&E)-stained section of angiocentric glioma (AG). Its infiltrative nature is revealed by entrapment of cerebral cortical pyramidal neurons (b). (c &d) Representative H&E-stained section of diffuse astrocytoma (DA) and isomorphic diffuse glioma (IDG). (e) A diffusely infiltrative component resembling DA is a common finding in AG (tumor #4).
Supplementary Figure 3. Angiocentric glioma (AG) and diffuse astrocytoma (DA) showed a similar immunophenotype. Infiltrating tumor cells in both AG and DA expressed OLIG2, while perivascular tumor cells were OLIG2-negative. A dot-like cytoplasmic pattern of EMA reactivity was observed in all tumors.
References
- 1.Blumcke I, Luyken C, Urbach H, Schramm J, Wiestler OD (2004) An isomorphic subtype of long-term epilepsy-associated astrocytomas associated with benign prognosis. Acta Neuropathol 107: 381–388 Doi 10.1007/s00401-004-0833-3 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, et al. (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372: 2481–2498 Doi 10.1056/NEJMoa1402121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison DW, Hawkins C, Jones DTW, Onar-Thomas A, Pfister SM, Reifenberger G, Louis DN (2019) cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF(V600E) mutation. Acta Neuropathol 137: 683–687 Doi 10.1007/s00401-019-01987-0 [DOI] [PubMed] [Google Scholar]
- 4.Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, Tang B, Haupfear K, Punchihewa C, Easton J, et al. (2016) Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 131: 833–845 Doi 10.1007/s00401-016-1539-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative axial T2-weighted MRI scans from the study cohort. AG: angiocentric glioma, DA003A diffuse astrocytoma, IDG: isomorphic diffuse glioma
Supplementary Figure 2. (a & b) Representative hematoxylin and eosin (H&E)-stained section of angiocentric glioma (AG). Its infiltrative nature is revealed by entrapment of cerebral cortical pyramidal neurons (b). (c &d) Representative H&E-stained section of diffuse astrocytoma (DA) and isomorphic diffuse glioma (IDG). (e) A diffusely infiltrative component resembling DA is a common finding in AG (tumor #4).
Supplementary Figure 3. Angiocentric glioma (AG) and diffuse astrocytoma (DA) showed a similar immunophenotype. Infiltrating tumor cells in both AG and DA expressed OLIG2, while perivascular tumor cells were OLIG2-negative. A dot-like cytoplasmic pattern of EMA reactivity was observed in all tumors.


