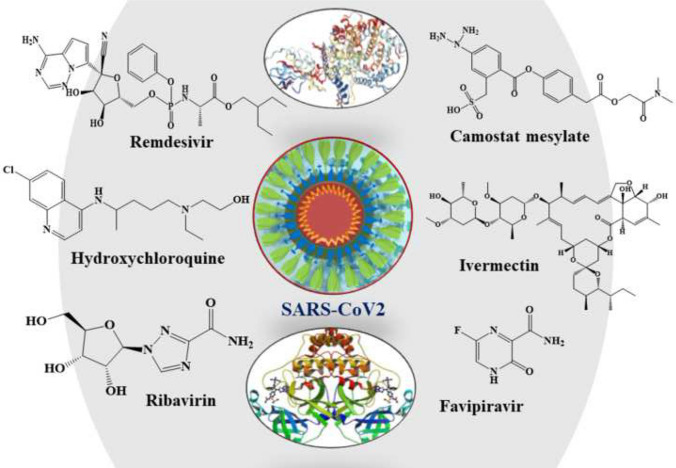
Abstract
Abstract
After the 1918 Spanish Flu pandemic caused by the H1N1 virus, the recent coronavirus disease 2019 (COVID-19) brought us to the time of serious global health catastrophe. Although no proven therapies are identified yet which can offer a definitive treatment of the COVID-19, a series of antiviral, antibacterial, antiparasitic, immunosuppressant drugs have shown clinical benefits based on repurposing theory. However, these studies are made on small number of patients, and, in majority of the cases, have been carried out as nonrandomized trials. As society is running against the time to combat the COVID-19, we present here a comprehensive review dealing with up-to-date information of therapeutics or drug regimens being utilized by physicians to treat COVID-19 patients along with in-depth discussion of mechanism of action of these drugs and their targets. Ongoing vaccine trials, monoclonal antibodies therapy and convalescent plasma treatment are also discussed. Keeping in mind that computational approaches can offer a significant insight to repurposing based drug discovery, an exhaustive discussion of computational modeling studies is performed which can assist target-specific drug discovery.
Graphic abstract
Keywords: Computational, Coronavirus, COVID-19, Drug, SARS-CoV-2, Vaccine
Introduction
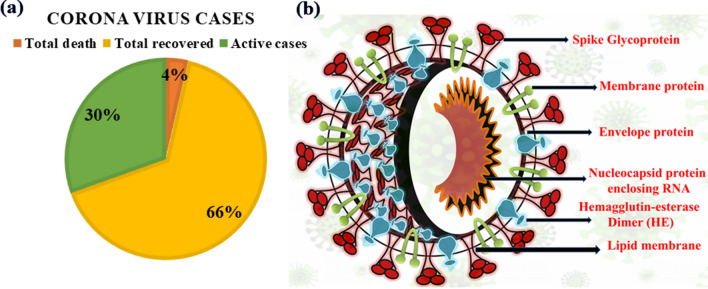
A form of pulmonary disease was first reported in China from a city called Wuhan in the Hubei Province on December 31, 2019 [1]. The deadly disease was later termed as COVID-19 by the World Health Organization (WHO) on February 11, 2020. The identified causative novel coronavirus (2019-nCoV) is termed as severe acute respiratory syndrome-related coronavirus SARS-CoV-2 as it shares around 79.6% of genome similarity with SARS-CoV which also previously emerged in China during 2002–2003 [2]. With the announcement of COVID-19 as ‘Global Pandemic’ by WHO on March 11, 2020, SARS-CoV-2 has eventually affected 212 countries and territories around the world and 2 international conveyances. As of August 13, 2020, 20,881,635 cases have been confirmed with 748,503 deaths and 13,771,549 recovery cases, while among the active cases, 6,297,028 cases are in mild condition and 64,555 cases in a serious or critical condition [3] (Fig. 1a).
Fig. 1.
a The global trend of COVID-19 reported death, recovered and active cases till August 13, 2020; b structure of SARS-CoV-2
The literature reported seven coronaviruses (CoVs) that are known to cause human disease where the strains 229E (α-CoV), HKU1 (β-CoV), OC43 (β-CoV) and NL63 (α-CoV) caused mild infections of the upper respiratory tract in humans [4]. On the contrary, other two strains SARS-CoV (occurring in 2002–2003) and MERS-CoV (Middle East respiratory syndrome occurring in 2012) and the newly identified SARS-CoV-2 belonging to β-CoV have caused serious health threat and fatality [5]. The present scenario and available pathophysiology specify that SARS-CoV-2 is highly transmittable and contagious than its progenitor affecting not only the respiratory system but also the gastrointestinal system, central nervous system, kidney, heart and liver leading to multiple organ failure [6]. The SARS-CoV-2 spike S glycoprotein has 72% identical sequence with human SARS with a unique furin-like cleavage site, which is absent in other SARS-like CoVs [7]. The Cryo-EM structural evidence has revealed that SARS-CoV-2 has 10–20 times higher binding affinity to the ACE2 receptor than SARS-CoV which may lead to higher transmission and contagiousness [8]. Therefore, blocking of the isolated viral S protein at its host receptor region and/or binding within the S protein-ACE2 interface are the two most important strategies to design probable drugs for COVID-19 (Fig. 1b) [9]. The SARS-CoV-2 virus replicates via multiple processes after entering into the host cell, and the proteins associated with these replication steps are the principal targets to treat the infected patients by blocking the viral replication. The replication-associated proteins are [10]:
Translation of genomic RNA,
Proteolysis of the translated polyprotein with viral 3C-like proteinase,
Replication of genomic RNA with the viral replication complex which comprises 3′-to-5′ exonuclease, RNA-dependent RNA polymerase (RdRp), endoRNAse and helicase, 2′-O-ribose methyltransferase,
Assembly of viral components.
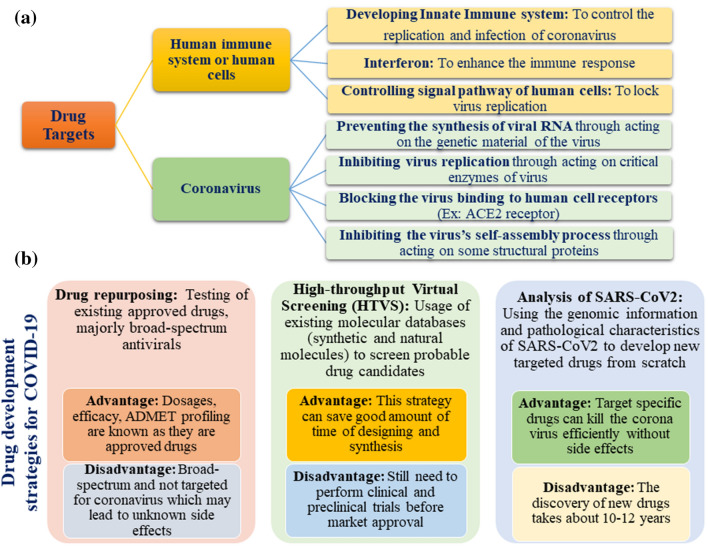
Along with the above-mentioned targets, the most commonly employed drug targets and drug discovery strategies employed all over the world right now are illustrated in Fig. 2.
Fig. 2.
a Possible drug targets and b drug development strategies to fight COVID-19
Presently, there is no specific treatment or approved drugs available to treat COVID-19. In most of the active cases, physicians are relying on symptom-based treatment for mild cases and primarily oxygen therapy (if required, with ventilator support) for critically ill patients. A set of approved marketed drugs like hydroxychloroquine (HCQ) [11, 12], chloroquine (CLQ) [12], combination of HCQ and azithromycin [13], remdesivir [14], lopinavir [15] and ritonavir [15] are being evaluated for the infection treatment; their clinical trials are also going on in different pharmaceutical industries. A series of new vaccines are also under clinical trial such as mRNA-1273 [16], Ad5-nCoV [17] and ChAdOx1 nCoV19 [18] along with existing Bacillus Calmette–Guerin (BCG) vaccine [19, 20] to check its efficiency in COVID-19. Due to severity and contagious nature of the SARS-CoV-2, researchers are exploring multiple in silico approaches and artificial intelligence [21–26] with the aim of identifying target-specific and potent therapeutic agents to speed up the discovery process. The RCSB protein data bank (PDB) (www.rcsb.org) has already deposited around 110 protein crystal structures associated with SARS-CoV-2 and COVID-19 to allow understanding important structural binding sites which can be explored in rational designing of small molecules.
The current review discusses the most updated and probable drug candidates which are being experimentally used to treat patients in different parts of the world. Also, their possible targets and pharmacological mechanisms of action which might not be clear in many cases and their pathophysiology along with the details about the status of convalescent plasma treatment and ongoing vaccine trials are discussed. We have compiled up-to-date in silico studies providing information related to computational tools, employed protein crystal structure used in the study followed by probable future drug candidates evolved from the repurposed virtual screening (VS) study employing docking, molecular dynamics (MD) and homology modeling. Therefore, the details related to SARS-CoV-2 transmission, protein structures, epidemiology, disease spectrum, diagnosis and testing are not discussed here at all as they have already been discussed in multiple literatures and separate reviews [1, 2, 4–7, 27–29]. The present review is significantly different from the other recently published ones on a similar topic in that it covers and gives emphasis on the in silico modeling studies in search of drugs against COVID-19. Thus, this paper provides an important source for the knowledge about possible drug candidates and vaccines along with their targets and pathophysiology information.
Investigational drugs or combination of drugs against COVID-19 up-to-date
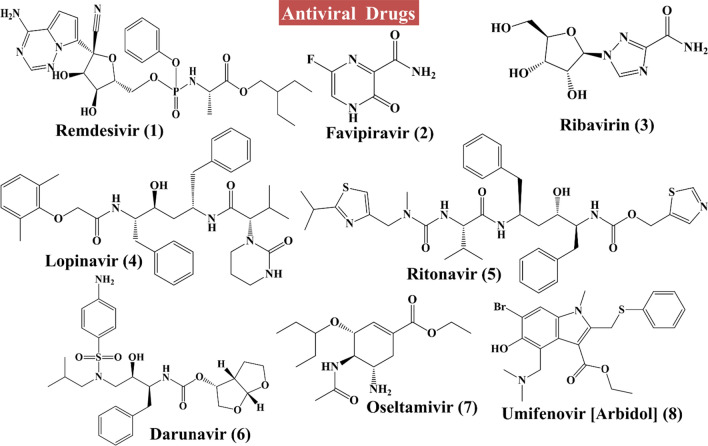
At the time of writing this article, there are no clinically approved drugs or vaccine available for the treatment of COVID-19 [30]. However, there are many drugs under trial for the treatment of COVID-19 (chemical structures 1–19 in Figs. 3, 4, 5) including angiotensin II type I receptor (AT1R) blockers, antiviral drugs, antimalarial drugs, interferon, IL-6 inhibitors, corticosteroids, ascorbic acid, some antibacterial antibiotics, etc. An up-to-date list of drugs under trials against COVID-19 with their targets, mechanisms of action, the developer companies or institutions, uses and recent status are tabulated in Table 1. Several industries and research institute are also trying to develop miscellaneous drugs and/or therapeutics agents, followed by investigation of the effectiveness of combination of drugs listed under Box 1. Among these tabulated drugs, most effective ones are discussed here. Mechanisms of different categories of drugs used in COVID-19 patients on various stages of SARS-CoV-2 life cycle are schematically depicted in Fig. 6.
Fig. 3.
Structures of antivirals to combat COVID-19 (Compounds 1–8)
Fig. 4.
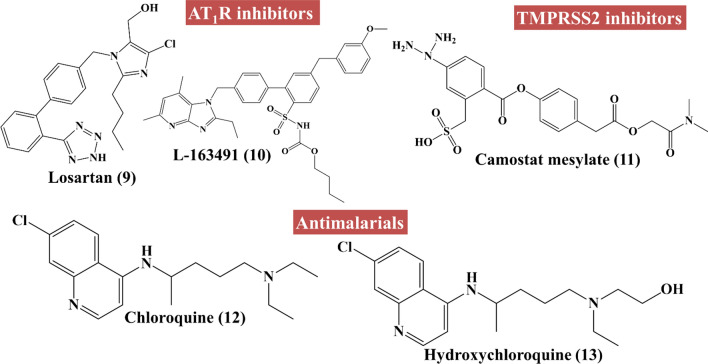
Structures of angiotensin II type I receptor (AT1R) inhibitors (Compounds 9–10), type 2 transmembrane serine protease (TMPRSS2) inhibitors (Compound 11) and antimalarials (Compounds 12–13) to combat COVID-19
Fig. 5.
Structures of miscellaneous drugs to combat COVID-19 (Compounds 14–19)
Table 1.
Drug candidates under trial against COVID-19 with probable targets, mechanism of action, pathophysiology, application and current status [11, 12, 14, 30, 40, 43, 45, 53, 55, 57–88]
| Drug candidates under trial | Company | Targets | Mechanism | Application | Current status against COVID-19 |
|---|---|---|---|---|---|
| Remdesivir [14, 57–59] (antiviral: nucleoside analog) | Gilead Sciences | RdRp |
(1) Inhibit the RdRps (2) Premature termination of viral RNA transcription |
(1) Broad-spectrum antiviral (2) In vitro activity against coronaviruses (under trial) (3) Reduces pulmonary pathology (4) Tried in Ebola virus too |
Phase-III clinical trials are underway in both China and the USA |
| Favipiravir [favilavir or Avigan] [60, 61] (antiviral) | Fujifilm Toyama Chemical | RdRp |
(1) Purine nucleoside leading to erroneous viral RNA synthesis (2) Inhibiting the RdRp |
Viral infection | Yet to be approved by the US FDA for COVID-19 |
| Ribavirin [58, 62, 63] (antiviral: guanine derivative) | RdRp | Inhibit RNA polymerization |
(1) RSV infection, hepatitis C, some viral hemorrhagic fevers (2) In case of COVID-19, this drug is tried in combination with IFN-α or lopinavir/ritonavir |
Performed two trials: NCT04254874, 2/5/20; ChiCTR2000029308, 1/23/20) | |
| Lopinavir/ritonavir [Kaletra] [45, 57, 64] (antiviral: HIV protease inhibitor) | AbbVie |
(1) Coronavirus main protease 3CLpro (2) Papain-like protease PLpro |
(1) Inhibits 3CLpro and PLpro (viral protease). Bind to Mpro, a key enzyme for coronavirus replication |
(1) Approved drug for HIV infection (2) WHO has mentioned it as an agent that can be tried for COVID-19 (3) It can also be used in combination with Interferon alpha or Ribavirin (4) Powerful CYP3A4 inhibitor (monitor for drug interactions) |
(1) At least three trials are going on (e.g., ChiCTR2000029603, 2/6/20) (2) European Society of Intensive Care Medicine (ESICM) and Critical Care Medicine (SCCM) Surviving Sepsis Campaign recommended the routine use of lopinavir; ritonavir in critically ill adults |
| Darunavir/darunavir + cobicistat [Prezcobix] [65] (antiviral) | Shanghai public health clinical center | HIV-1 Protease |
(1) HIV-1 Protease inhibitor (2) CYP3A inhibitor |
(1) HIV infection (2) Studied as a possible treatment for SARS-CoV-2 |
Clinical trials are underway |
| Oseltamivir [66] (antiviral) | Neuraminidase enzyme | Inhibits neuraminidase enzyme in influenza | Used in influenza patient. This drug is used for repurposing only in combination with other drugs like ASC09F and ritonavir | No trials on COVID-19 yet. Due to lack of suitable control group in the studies, definite evidence of efficacy is questionable | |
| Umifenovir [Arbidol] [38, 39] (antiviral) | Pharmstandard |
(1) ACE 2 (2) Viral spike glycoprotein |
(1) Interrupts the attachment of viral envelope protein to host cells thus prevents viral entry to the target cell (2) Inhibits viral-host cell fusion |
(1) An antiviral treatment for influenza infection used in Russia and China (2) Proposed reduction of cytokine storm |
At present, there are no clinical data to support either starting or discontinuing ACEi/ARBs on any patients with COVID-19 |
| L-163491 [67] | Angiotensin AT1/AT2 receptor | Partial antagonist of AT1 receptor and partial agonist of AT2 receptor | To treat coronavirus-induced lung injury | Yet to be determined | |
| Losartan [34] | University of Minnesota | Angiotensin II type 1 receptor (AT1R) | Inhibit AT1 receptor | Reduce organ failure | Phase-I clinical trials. |
| Camostat mesylate [40] | Serine protease TMPRSS2 |
(1) Inhibit TMPRSS2 (2) Able to block SARS-CoV-2 entry to the host cells |
Treats SARS-CoV-2 infection of lung cells | A clinically approved TMPRSS2 inhibitor, was able to block SARS-CoV-2 infection of lung cells | |
| Chloroquine [Aralen] [11, 12, 14, 43, 45] (antimalarial) | Sanofi (Aralen) | Endosome/ACE2 |
(1) Inhibits viral DNA and RNA polymerase, viral protein glycosylation, virus assembly, new virus particle transport and virus release (2) Inhibits fusion of the virus to the receptor, thus prevent the entry into the host cell (3) Also acts by preventing the sialic acid containing glycoprotein and gangliosides (key binding factors along the respiratory tract) intermediated attachment to the S protein |
(1) CLQ has been proven effective in treating coronavirus in China (2) Treating pneumonia patients with SARS-CoV-2 infection |
Approved for clinical trial against COVID-19 |
| Hydroxychloroquine [Plaquenil] [11, 12, 44] (antimalarial) | Sanofi (Plaquenil); Mylan, Teva, Novartis, Bayer, Rising Pharmaceuticals (generics) | Endosome/ACE2 |
(1) Hampers viral replication (2) Inhibits viral DNA and RNA polymerase, Viral protein glycosylation, virus assembly, new virus particle transport, and virus release (3) May also involve ACE2 cellular receptor inhibition (4) Acts by inhibiting fusion of the virus to the host cells thus prevents the entry to the host cells (5) Immunomodulation of cytokine release (6) Also acts by inhibiting the sialic acid containing glycoprotein and gangliosides (key binding factors along the respiratory tract) that intermediate binding to the S protein |
Used to treat COVID-19 disease. This drug has also immunomodulating properties | At present, one of the most highlighted drug against COVID-19 |
| Baricitinib [68] | Concert Pharmaceuticals, Inc., USA | Janus kinase inhibitor (JAK) | Affects the inflammatory processes |
(1) Approved drug for rheumatoid arthritis (2) Used to treat acute respiratory disease in COVID-19 patients |
Eli Lilly has announced plans to conduct a clinical trial of baricitinib (Olumiant) |
| Azithromycin [13] (Macrolide antibacterial) | Pfizer | 50S subunit of the bacterial ribosome |
(1) Their direct effects on viral clearance are not clear (2) Downregulates inflammatory responses and decreases the excessive cytokine release related with respiratory viral infections (3) Immunomodulatory properties in pulmonary inflammatory disorders |
(1) Prevents bacterial superinfection (2) Used as adjunct therapy due to immunomodulatory properties |
Combination with HCQ showed promising activity against COVID-19 Pfizer has announced positive clinical trial data performed in France for this drug along with HCQ |
| Nitazoxanide [58] | Materno-Perinatal Hospital of the State of Mexico | Pyruvate:ferredoxin oxidoreductase (PFOR) enzyme | Inhibits viral protein expression | Used to treat various infections caused by helminth, protozoa and virus | Phase-IV clinical trial (NCT04341493) is going on using Nitazoxanide and combination of Nitazoxanide and HCQ |
| Tocilizumab [atlizumab] [69, 70] (immunosuppressant drug) | Roche (as Actemra) | Interleukin-6 (IL-6) receptor | Acts by inhibiting IL-6-mediated signaling |
Used to treat severe disease in COVID-19 patients Initial analysis suggests that tocilizumab may have a clinical advantage as an adjunctive therapy |
Roche launched a Phase-III trial (COVACTA) to assess tocilizumab’s efficiency |
| Ivermectin [53] (antiparasitic agent) | Biomedicine Discovery Institute, Australia | HIV-1 integrase protein (IN) and the importin (IMP) α/β1 heterodimer | Inhibits nuclear transport | Broad-spectrum antiviral |
Phase-III clinical trial in dengue patients Need to design further study to check the worthiness in SARS-CoV-2 treatment |
| Mavrilimumab [71, 72] (monoclonal antibody) | Kiniksa Pharmaceuticals | Granulocyte macrophage colony stimulating factor (GM-CSF) receptor alpha | Showed antagonistic activity of GM-CSF signaling by binding to the α-subunit of the GM-CSF receptor (GM-CSFRα) | Kiniksa reported that as of 31st March 2020 the patients treated with this drug experienced early resolution of fever and enhanced oxygenation within 1–3 days and also reported that none of the patients required mechanical ventilation as of that time | A consortium of US academic sites plans to initiate parallel prospective, interventional studies with mavrilimumab in patients with pneumonia and hyper-inflammation caused by SARS-Cov-2. Kiniksa also reported that they are engaging with FDA about the path forward for potential |
| Lenzilumab [73, 74] (monoclonal antibody) | Humanigen | GM-CSF | This drug neutralizes GM-CSF which is a crucial cytokine in the initiation of cytokine storm | Have a defensive consequence against cytokine release syndrome (CRS) associated with CAR-T therapy. It can aid cytokine-mediated immunopathology of lung injury and acute respiratory distress syndrome (ARDS) | On 2 April 2020, FDA authorized the use of lenzilumab in COVID-19 patients under an eIND application. Humanigen is planning a Phase-III clinical trial with lenzilumab for the prevention of ARDS in patients with pneumonia caused by SARS-CoV-2 |
| Leronlimab [75] (monoclonal antibody) | CytoDyn (as PRO 140) | Humanized IgG4 monoclonal antibody | Blocks the CCR5 co-receptor on the surface of immune cells like CD4 cells |
(1) Leronlimab in combination with carboplatin can be used for the treatment of CCR5-positive metastatic triple-negative breast cancer. It is also used in antiretroviral therapy (HAART) in HIV (2) This drug can increase the immune response in patients with CRS from respiratory distress |
FDA authorized the use of leronlimab in COVID-19 patients under an eIND. It has been showed that patients treated under the eIND have a lower level of cytokine storm and lower levels of IL-6 and TNF-alpha |
| Gimsilumab [76] monoclonal antibody | Roivant | GM-CSF | Inhibits GM-CSF | Used as anti-inflammatory drug | Phase-I clinical trial |
| Sarilumab [77] (anti-rheumatic drug) (monoclonal antibody) | Sanofi and Regeneron (as Kevzara) | IL-6 receptor | IL-6 receptor antagonist | Used to reduce the inflammatory response in the lungs associated with the COVID-19 patients who develop ARDS | A Phase-II/III trial (NCT04327388) of 400 patients sponsored by Sanofi. Regeneron is currently underway in the USA. A second, Phase-II/III trial is being conducted in Italy, Spain, Germany, France, Canada and Russia also |
| Aviptadil [78] (analog of vasoactive intestinal polypeptide) | NeuroRx and Relief Therapeutics | IL-6 |
(1) IL-6 inhibitor (2) Reduction of inflammatory cytokines |
(1) Used for the treatment of inflammation produced by cytokines (2) Used in ARDS (3) Erectile dysfunction |
Phase-II clinical trials |
| Siltuximab [Sylvant] [79] (monoclonal antibody) | EUSA Pharma | IL-6 | IL-6 inhibitor | To treat Acute Respiratory Distress Syndrome as a result of Covid-19 |
EUSA Pharma initiates the study of siltuximab to treat Covid-19 patients Based on the clinical data, the company reported that 16 patients using siltuximab were stable or had improved disease at the interim analysis |
| Camrelizumab [AiRuiKa] [80] (monoclonal antibody) | South East University, China | Programmed cell death 1 (PD-1) | Programmed cell death 1 (PD-1) inhibitor |
(1) Classical Hodgkin lymphoma (2) Treat pneumonia (3) Sepsis |
Phase-II clinical trials (NCT04268537) |
| Eculizumab [Soliris] [81] (monoclonal antibody) | Alexion | Binds terminal complement protein C5 | Modulates the activity of the distal complement preventing the formation of the membrane attack complex |
(1) In severe Pneumonia (2) In ARDS |
Plan for Phase-II clinical trials |
| Bevacizumab [Avastin] [82] | Qilu Hospital of Shandong University | Vascular endothelial growth factor (VEGF) | Inhibit vascular endothelial growth factor (VEGF) | May be used as a promising drug for acute lung injury (ALI) and/or ARDS in COVID-19 through suppression of pulmonary edema |
Under clinical trial (NCT04275414) Bevacizumab, an anti-VEGF drug, approved by the FDA on February 26, 2004 and widely used in clinical oncotherapy |
| CD24Fc [83] | OncoImmune | Recombinant fusion protein |
(1) It targets a novel immune pathway checkpoint and modulates immune response through binding to Danger-Associated Molecular Patterns (DAMPS) and sialic acid-binding immunoglobulin-type lectins (Siglecs) (2) It also acts by reducing multiple inflammatory cytokines |
Used for the treatment of graft-versus-host disease (GVHD) in leukemia patients receiving hematopoietic stem cell transplantation | OncoImmune is planning a Phase-III clinical trial against COVID-19 patients with absolute lymphocyte counts ≤ 800/mm in peripheral blood (NCT04317040) |
| Colchicine [84] | Montreal Heart Institute | Tubulin | Tubulin disruption | Used as anti-inflammatory agents | Phase-III clinical trials for CIVID-19 |
| SNG001 [85] | Synairgen Plc | Antiviral protein interferon-beta 1a (IFN-β), a natural antiviral produced in lungs during viral lung infections | Delivers extra IFN-β direct to the lungs, correcting this deficiency and counteracting viral strategies to evade the host’s immune defenses by inhibiting natural IFN-β production | Used to treat severe lower respiratory tract illness caused by cold and flu infections when they spread to the lungs | Phase-II clinical trials |
| COVID-19 convalescent plasma [55] (Immunoglobulin) | Mount Sinai | Passive antibody therapy; possible sources of antibody for SARS-CoV-2 are human convalescent sera | Plasma collected from recovered COVID-19 patients that may contain antibodies to SARS-CoV-2 | In China, a case series of 5 patients suffering from SARS-CoV-2 and ARDS treated with convalescent plasma showed better clinical status | Clinical trials are going on to assess the use of COVID-19 convalescent plasma to treat patients with COVID-19 infections |
| Tissue plasminogen activator (tPA) [86] [alteplase] (anti-clotting drug) | Researchers at Beth Israel Deaconess Medical Center (BIDMC) | Serine protease | It catalyzes the conversion of plasminogen to plasmin | Used to treat COVID-19 induced ARDS. Used for heart attacks or stroke | Under clinical trial. A recent report suggested that the use of this drug could reduce deaths among the COVID-19 patients with ARDS |
| Corticosteroids [87] | Beijing Chao Yang Hospital, China | Cytokines | Modulating a variety of cytokines involved in the inflammatory response | May be used to treat ARDS | Not indicated in treating SARS-CoV-2 as per available evidence. Might prolong viral shedding |
| Ascorbic acid [88] | University of Palermo | T-lymphocytes |
(1) Recent studies showed that ascorbic acid (vitamin C) clearly affects the development and maturation of T-lymphocytes, natural Killer cells (NK cells) involved in the immune response to viral agents. It also contributes to the remodulation of cytokine network responsible for systemic inflammatory syndrome by inhibiting ROS production (2) It blocks the expression of intercellular adhesion molecule 1 (ICAM-1) and activation of NF Kappa B that are involved in inflammatory, neoplastic, and apoptotic processes by the inhibition of TNF alpha |
Effective against COVID-19 pneumonia | Under clinical trials (NCT04323514) |
Box 1.
Miscellaneous therapeutics under investigation for COVID-19
| TAK-888 (antibodies from recovered COVID-19 patients developed by Takeda; preclinical stage), REGN3048-3051 (antibodies from mice developed by Regeneron; Phase-I preclinical stage), Thymosin (PD-1 blocking antibody developed by Southeast University, China, which is under Phase-II clinical trial), antibodies from recovered COVID-19 patients (companies like Celltrion, Kamada, Vir Biotech/WuXi Biologics/Biogen, Lilly/Ab-Cellera, Swiftscale Biologics, Erasmus MC/Utrecht University, and AstraZeneca are trying to develop), Galidesivir (developed by BioCryst Pharmaceuticals; preclinical stage), Combination of ebastine, lopinavir and interferon alpha (developed by Wuhan Red Cross Hospital; clinical stage), Combination of Ganovo and danoprevir (hepatitis C virus NS3 protease inhibitor), Ritonavir and interferon (approved in China to treat hepatitis C, developed by Ascletis; clinical stage), ASC09 (HIV protease inhibitor developed by Ascletis Pharma; clinical stage), Truvada combination of Emtricitabine and Tenofovir (both are HIV-1 nucleoside analog reverse transcriptase inhibitors developed by Gilead/Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital; clinical stage), Xofluza (polymerase acidic endonuclease inhibitor developed by Roche; clinical stage), Azvudine (reverse transcriptase inhibitor developed by Henan Provincial People’s Hospital; clinical stage), Washed microbiota transplantation (The Second Hospital of Nanjing Medical University; clinical stage), Jakafi/Jakavi (Ruxolitinib in combination with mesenchymal stem cells) (Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology/Incyte Corp; clinical stage), Peginterferon alfa-2b (PegIntron, Sylatron, IntronA; clinical stage), Novaferon (Zhejiang University Medical School), Interferon (Zhejiang University Medical School), Ifenprodil (NP-120) (an NDMA receptor glutamate receptor antagonist targeting Glu2NB developed by Algernon Pharmaceuticals: preclinical stage), APN01 (a physiological formulation of recombinant soluble human ACE2 developed by University of British Columbia/Apeiron Biologics; clinical stage), Brilacidin (a defensin mimetic developed by Innovation Pharmaceuticals; preclinical stage), BXT-25 (a glycoprotein developed by Bioxytran; preclinical stage), Peptides (developed by CEL-SCI; preclinical stage), Gilenya (fingolimod) (developed by Fujian Medical University/Novartis; clinical stage), A number of synthesized nanoviricide drug candidates (developed by different industries like Novartis; preclinical stage), Scanning compounds to repurpose (Janssen Pharmaceutical Companies, Novartis, Merck, Pfizer, Materia Medica/Cyclica, Enanta Pharmaceuticals, Southwest Research Institute, Takeda), RNA-based treatment like RNAi–testing 150 RNAis (Sirnaomics; preclinical), siRNA candidates (Vir Biotech/Alnylam Pharmaceuticals; preclinical stage), Ampligen (AIM ImmunoTech/National Institute of Infectious Diseases in Japan; preclinical stage), OT-101 (a TGF-Beta antisense drug candidate developed by Mateon Therapeutics; preclinical stage), Cell-based therapies like PLX cell product (placenta-based cell therapy developed by Pluristem Therapeutics/BIH Center for Regenerative Therapy/Berlin Center for Advanced Therapies; preclinical stage), Mesenchymal stem cells (numerous trials with Chinese research sponsors; clinical stage), Ryoncil (Remestemcel-L) (allogenic mesenchymal stem cells developed by Mesoblast; preclinical stage). Dexamethasone (Dextenza, Ozurdex, others) (University of Oxford; Phase-II/III) |
Under parenthesis companies and research institutes along with stage of development are mentioned
Fig. 6.
Mechanisms of action of different categories of drugs used in COVID-19 patients acting on various stages of the SARS-CoV-2 life cycle
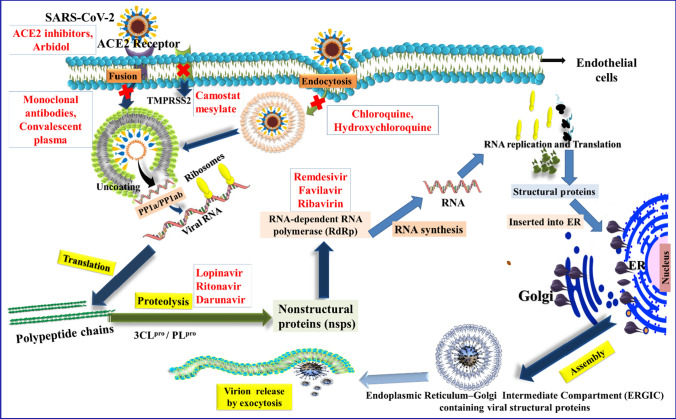
The entry of SARS-CoV-2 to the host cell can occur in two ways, i.e., either via plasma membrane fusion or via endosomes (endocytosis blockers: CLQ and HCQ) (Fig. 6). In both ways, spike proteins (S1, S2) of SARS-CoV-2 mediate attachment to the membrane of a host cell and engage angiotensin-converting enzyme 2 (ACE2) as the entry receptor. Inhibitors like convalescent plasma, monoclonal antibodies bind to the spike glycoprotein, thus preventing the viral entry. When virions are taken up into endosomes, the spike protein can be activated by the cellular serine protease TMPRSS2 in close proximity to the ACE2 receptor, which initiates fusion of the viral membrane with the plasma membrane. Camostat mesylate inhibits the TMPRSS2 receptor. The plasma membrane fusion entry is less likely to trigger host cell antiviral immunity and therefore more efficient for viral replication. After the viral RNA is released into the host cell, polyproteins are translated. The coronavirus genomic RNA encodes non-structural proteins that have a critical role in the synthesis of viral RNA and structural proteins which are important for virion assembly. First, polyproteins are translated and cleaved by some of proteases like 3CLpro, PLpro, etc. (lopinavir, ritonavir and darunavir act as inhibitors of this step) to form RNA replicase-transcriptase complex. The non-structural protein RdRp is responsible for replication of structural protein RNA. (Remdesivir, favilavir and ribavirin act as inhibitors of this enzyme). Structural proteins S1, S2, envelope and membrane are translated by ribosomes that are bound to the endoplasmic reticulum (ER) and presented on its surface as preparation of virion assembly. The nucleocapsids (N) remain in cytoplasm and are assembled from genomic RNA. They fuse with the virion precursor which is then transported from the ER through the Golgi apparatus to the cell surface via small vesicles. The mature virions are then released from the infected cell through exocytosis, and then, they search another host cell (Fig. 6).
Angiotensin II type I receptor (AT1R) blockers
SARS-CoV-2 has transmembrane spikes (S protein). The spikes attached to the lipid membrane of the coronavirus recognize a host cell to attach and infect it with its viral RNA. The attachment of the coronavirus S protein to angiotensin converting enzyme 2 (ACE2) at its cellular binding site promotes the entry into human cells. The S protein contains two subunits such as an N-terminal S1 subunit which is responsible for receptor-virus binding and a C-terminal S2 subunit which is responsible for the fusion of virus with cell membrane [31, 32]. The S1 subunit has two domains such as a receptor-binding domain (RBD) and an N-terminal domain (NTD). At the time of infection, at first coronavirus binds to the human cell through interaction between the cell ACE2 receptor and S1-RBD of coronavirus. As a result, conformational changes in the S2 subunit are triggered followed by virus-cell fusion and entry into the target cell. ACE2 is a natural protein present in the lungs and the intestine (epithelial cells), and in the heart and the kidneys (endothelial cells). ACE2 regulates the blood pressure by converting angiotensin molecules. It was found that the coronavirus which caused the SARS outbreak in 2002 also binds to the same ACE2 molecule, but in case of SARS-CoV-2, the binding affinity is 10 to 20 times more on human cells than the spike from the SARS virus of 2002, which makes it a suitable target for COVID-19. Due to the high affinity of this virus to human cells, it is spread without any difficulty from one person to another than the earlier virus [33].
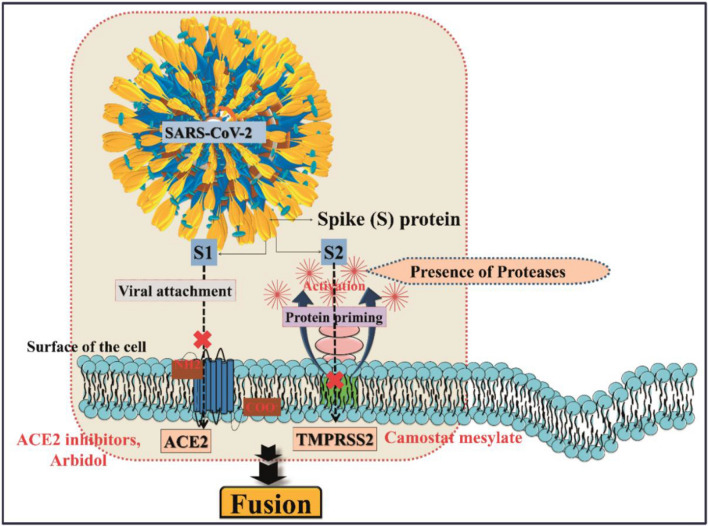
The entry of SARS-CoV-2 to the host cells via binding with ACE2 enzyme leads to ACE2 down-regulation. As a result, angiotensin II is produced excessively by the correlated enzyme ACE1, while a lower amount of ACE2 is not capable of transforming it to the vasodilator heptapeptide angiotensin 1–7. Thus, expression of higher ACE2 resulting from frequently medicating COVID-19 patients with AT1R blockers may resist them against acute lung injury. This can be described by following two complementary mechanisms: (1) blocking the excessive angiotensin II-mediated AT1R activation caused by the viral infection, (2) upregulation of ACE2 decreasing angiotensin II production by ACE and enhancing the production of the vasodilator angiotensin 1–7 [34]. The role of ACE2 to enter the coronavirus into the host cell and mechanism of action of ACE2 inhibitors to control the COVID-19 are depicted in Fig. 7.
Fig. 7.
The role of ACE2 receptor for the entry of coronavirus into the host cell, and mechanism of action of ACE2 inhibitors and TMPRSS2 inhibitors to control COVID-19
The SARS-CoV-2 uses the S protein to facilitate viral entry into the host cells. The pathogen S protein consists of two subunits S1 and S2, of which “S1” allows entry of pathogen and binding of S protein to ACE2 (cellular receptor) (Fig. 7). In addition, the entry requires S protein priming by cellular proteases, which is responsible for cleavage of S protein. After this, “S2” subunit employs fusion of viral and cellular membranes. SARS-CoV-2 engages ACE2 as the entry receptor that can be blocked by ACE2 inhibitors and Arbidol, and it employs the cellular serine protease TMPRSS2 for S protein priming which is inhibited by camostat mesylate (Fig. 7). Conclusively, SARS-CoV-2/ACE2 interface occurs in the molecular level, and the efficiency of ACE2 usage is a key determinant of SARS-CoV-2 transmissibility.
Although it is widely accepted that coronavirus enters into the host cell through the ACE2 receptor, due to limited number of studies, it is yet to establish how ACE2, AT1 and AT2 receptors exert their activities in coronavirus-induced diseases [35, 36]. Thus, ACE2 inhibitors and AT1 receptor antagonists [e.g., L-163491 as a partial antagonist of AT1 receptor and partial agonists of AT2 receptor; losartan, valsartan, irbesartan, candesartan cilexetil, telmisartan, and eprosartan (FDA-approved AT1 receptor blockers)] may be used as important drug candidates to control lung injury of COVID-19 patients [34]. The binding of viral S protein with its receptor ACE2 on host cells followed by viral endocytosis into the cells may also be a possible drug target. For example, the broad-spectrum antiviral drug Arbidol recently entered the clinical trial for the treatment of SARS-CoV-2 which may act by inhibiting virus-host cell fusion, thus preventing the viral entry into host cells against influenza virus [37–39].
Camostat mesylate
The serine protease TMPRSS2 produced by the host cells plays a key role for cell entry of coronaviruses by S protein priming to the receptor ACE2 binding in human cells (Fig. 7). A recent study shows that camostat mesylate, a clinically approved inhibitor of TMPRSS2 (responsible for S protein priming), has been able to block SARS-CoV-2 infection of lung cells. Thus, this drug may be a potential drug candidate for COVID-19 [40].
Remdesivir
Remdesivir, a nucleoside analog and a monophosphoramidate prodrug of remdesivir-triphosphate (RDV-TP) developed by Gilead Sciences Inc. (USA), was previously tried for the Ebola virus disease, and it showed promising effects in MERS and SARS. It acts by inhibiting RNA-dependent RNA polymerases (RdRp). Incorporation of this drug into nascent viral RNA chain causes premature termination. For incorporation of remdesivir-TP into nascent viral RNA chains, it competes with adenosine-triphosphate. After incorporation into the viral RNA at position i, remdesivir-triphosphate arrests RNA synthesis at position i + 3. Due to the incorporation of 3 additional nucleotides after RDV-TP, it does not cause instant chain termination because these three additional nucleotides may protect the inhibitor from excision by the viral 3′–5′ exo-ribonuclease activity (Fig. 6). Recent reports showed that the EC90 value of remdesivir against COVID-19 in VeroE6 cells was 1.76 µM, half-cytotoxic concentration (CC50) was greater than 100 µM, and the selective index (SI) was greater than 129.87, suggesting that its working concentrations are likely to be achieved in nonhuman primate (NHP) [14, 41]. This drug is also able to inhibit virus infection proficiently in human liver cancer Huh-7 cells sensitive to COVID-19. A recent case study revealed that treatment with remdesivir improved the clinical condition of the first patient infected by SARS-CoV-2 in the USA [42]. A recent in vitro data showed that remdesivir and chloroquine (CQ) phosphate are capable of inhibiting SARS-CoV-2 infection [14]. Remdesivir is currently being studied in Phase-III clinical trials against SARS-CoV-2 in Wuhan, China, as on February 4, 2020, and in the USA.
Chloroquine/hydroxychloroquine
Chloroquine (CLQ) and hydroxychloroquine (HCQ) have received deep attention because of positive results from some small studies. An antimalarial drug, CLQ, has recently been reported to have potential in vitro activity against SARS-CoV-2. CLQ protects from viral infection by enhancing endosomal pH (making the environment unfavorable) which is required for virus-cell fusion. This drug may also block viral infection by inhibiting viral enzymes or processes like viral DNA and RNA polymerases, virus assembly, new virus particle transport, immunomodulation of cytokine release and virus release. CLQ also affects the glycosylation process of ACE2 (as discussed earlier that to enter the host cell, the viral S protein binds with this receptor) [11, 14, 43, 44]. Besides this mechanism, a recent report [12] showed that this drug also acts by inhibiting the sialic acid containing glycoprotein and gangliosides (act as primary attachment factors along the respiratory tract) mediated attachment to the S protein which is the first step for viral replication. In the NTD of the S protein of SARS-CoV-2, a ganglioside-binding site was recognized. The antimalarial drug CLQ was found to be a probable blocker of the S–ganglioside interaction. Thus, this drug may be used to fight pathogenic coronaviruses especially SARS-CoV-2 which is responsible for COVID-19. A detailed mechanism of action of CLQ and HCQ against SARS-CoV-2 is illustrated in Fig. 8. A recent report showed that the EC90 value of CLQ against the SARS-CoV-2 in VeroE6 cells was 6.9 µM, which may be clinically achievable. Although specific data are not available, this drug is able to inhibit the exacerbation of pneumonia patients with SARS-CoV-2 infection.
Fig. 8.
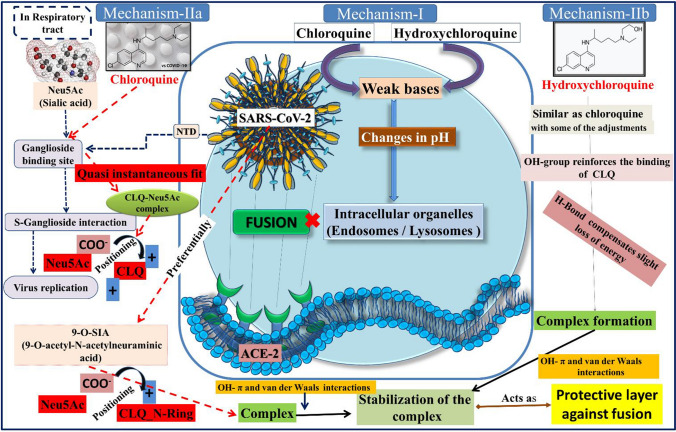
Schematic representation of different mechanistic pathways of CLQ and HCQ against SARS-CoV-2
Figure 8 explains two possible mechanisms of CLQ and HCQ against SARS-CoV-2. The mechanism 1 is that CLQ and its derivative HCQ are weak bases, which can raise the pH of acidic intracellular organelles, such as endosomes/lysosomes, essential for membrane fusion. On the other hand, mechanism-2 explains the entry of SARS-CoV-2 into the host cells also depends upon sialic acid (Neu5Ac) containing glycoproteins and gangliosides that act as the key binding factors along the respiratory tract. A ganglioside-binding site at the N-terminal domain (NTD) of the S glycoprotein of SARS-CoV-2 was recognized, and CLQ was found to be a possible blocker of the S–ganglioside interaction which occurs in the first step of the viral replication cycle (i.e., attachment to the surface of respiratory cells, intermediated by the S protein) [12]. The interaction was augmented by placing the negative charge of the carboxylate anion of Neu5Ac and one of the two positive charges of CLQ. SARS-CoV-2 especially interacted with 9-O-acetyl-N-acetylneuraminic acid (9-O-SIA). In this case, the carboxylic acid group of the sialic acid interacted with the cationic group of the nitrogen-containing ring of CLQ. The formed complex of CLQ and 9-O-SIA was further stabilized by OH-π and van der Waals interactions. Next, the complex developed from HCQ was very close to that obtained from CLQ, although numerous conformational adjustments happened for the period of the simulations. Interestingly, the –OH group of HCQ reinforced the binding of CLQ to Neu5Ac via formation of a hydrogen bond. The formed complex of CLQ-OH and 9-O-SIA will be stabilized again like CLQ to form a protective layer against fusion of the SARS–CoV-2.
HCQ is a hydroxy derivative of CLQ, which can block the viral infection by a similar mechanism as chloroquine; thus, this drug may also be a potential candidate against SARS-CoV-2. This drug is less toxic (~ 40%) than chloroquine in animals. It was found that there were seven clinical trials registered as on February 23, 2020, in the Chinese Clinical Trial Registry (http://www.chictr.org.cn), for using HCQ to treat COVID-19. The in vitro results suggested that this drug can efficiently inhibit SARS-CoV-2 infection. Based on an in vitro report, it was suggested that HCQ may be more potent than CLQ to treat COVID-19 [11, 44]. (EC50 values for CLQ > 100 µM at 24 h and 18.01 µM at 48 h; EC50 values for HCQ were 6.25 µM at 24 h and 5.85 µM at 48 h). Due to the relatively low selectivity index (SI) of HCQ, it requires cautious designing and conducting of clinical trials to attain resourceful and safe control of the SARS-CoV-2 infection.
Favipiravir or Favilavir (Avigan)
A purine nucleoside presently labeled as Avigan, developed by Fujifilm Toyama Chemical of Japan, has recently been approved for Phase-III clinical trial (March 31, 2020) for the COVID-19 patients. This drug is approved for manufacturing and sale in Japan for the treatment of influenza as an antiviral. In case of Influenza virus, it selectively inhibits RNA polymerase which is essential for viral replication when human cells are infected. It is believed that this drug may be effective for the treatment of COVID-19 as SARS-CoV-2 uses same enzyme (RNA polymerase) for replication and classification into the same type of single-stranded RNA virus like influenza. Thus, this drug acts by inhibiting the RdRp leading to inaccurate viral RNA synthesis (Fig. 6). This drug is recommended by the director of the China National Center for Biotechnology Development under the Ministry of Science and Technology to treat COVID-19. Italy has also approved the drug to treat COVID-19 cases. Due to the effectiveness of this drug against COVID-19, it is being mass-produced as generic version in China [45, 46].
Ritonavir and lopinavir (Kaletra)
These drugs are approved HIV-1 protease inhibitors, used in combination with other anti-retroviral drugs to treat HIV-1 infection in both adults and pediatric patients who is older than 14 days. Coronaviruses encode either two or three protease enzymes like papain-like proteases (PLpro), a serine-type protease, the main protease, or Mpro which cleave the polyproteins into non-structural polyproteins (nsps). These nsps are essential for viral RNA synthesis. Ritonavir and lopinavir act by inhibiting these protease enzymes. The mechanisms of action of these drugs suppressing coronavirus activity [47] are depicted in Fig. 6. A combination of these drugs is recommended in Italy to treat COVID-19 patients. Several trials are going on worldwide using these drugs or in combination of other drugs. A collaborative research from China and the UK conducted a clinical trial to examine the effectiveness of a combination of these two drugs against COVID-19 which was published in the New England Journal of Medicine (NEJM) [15]. The output of their trial did not provide any significant benefit in the patients with COVID-19. They suggest that “future trials in patients with severe illness may help to confirm or exclude the possibility of a treatment benefit.” Among those trials, two trials are investigating against pneumonia caused by COVID-19. One trial is conducted in the Tongji Hospital, Wuhan, China, using lopinavir–ritonavir against Arbidol hydrochloride (influenza drugs) and oseltamivir (NCT04255017). In South Korea, a comparative study of lopinavir–ritonavir against HCQ in patients with mild cases of COVID-19 (NCT04307693) was made. The two arms of the WHO SOLIDARITY trial are lopinavir–ritonavir alone and in combination with interferon-beta [48].
Ivermectin
Ivermectin is an FDA-approved broad-spectrum antiparasitic drug, which recently showed in vitro antiviral activity against SARS-CoV-2. It acts by inhibiting the interaction between HIV-1 integrase protein (IN) and the importin (IMP) α/β1 heterodimer which is responsible for IN nuclear import [49, 50]. Therefore, (IMP) α/β1 is unable to bind to the viral protein and preventing it from entering the nucleus, thus inhibiting HIV-1 replication [49, 50]. As a result, inhibition of the antiviral responses is reduced leading to a normal, more efficient antiviral response.
Monoclonal antibodies
The trial of potential monoclonal antibody-based therapy against COVID-19 is going on by using the previous knowledge on the neutralizing monoclonal antibodies (nMAb) against similar coronaviruses such as SARS-CoV and MERS-CoV. Monoclonal antibodies targeting the vulnerable sites of trimeric spike (S) glycoproteins on the viral surface which are responsible for the entry to the host cell are increasingly being recognized as a promising class of drugs against COVID-19. Potential neutralizing monoclonal antibodies mainly targeting the receptor-interaction domain at S1 subunit ultimately disabled cell–receptor interactions [51–53]. The detailed mechanism of action of this class of drugs is depicted in Fig. 9. Recently, several monoclonal antibodies, namely tocilizumab (atlizumab), mavrilimumab, lenzilumab, leronlimab, gimsilumab, sarilumab, siltuximab (Sylvant), camrelizumab (AiRuiKa), eculizumab (Soliris), etc., are being tried to investigate their potency against COVID-19 disease, and these are tabulated in Table 1.
Fig. 9.
Schematic diagram of the role of human monoclonal antibodies to block SARS-CoV-2
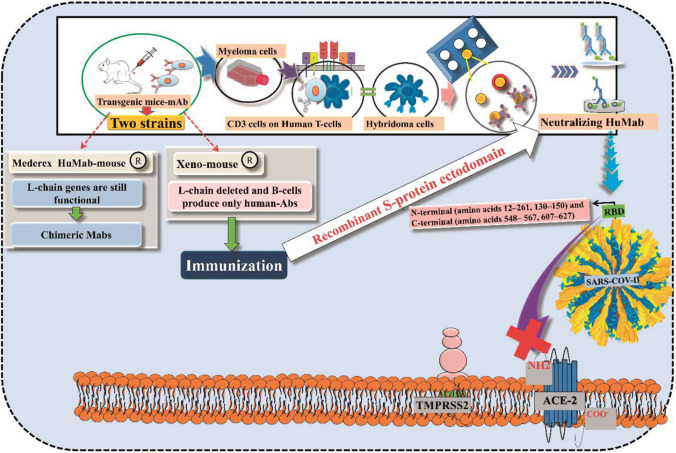
The human monoclonal antibodies (hmAbs) are developed by using several strategies against SARS-CoV-2 including preparation of hybridomas (using a transgenic mouse), phage display technologies and the immortalization of convalescent B cells. At present, the hmAbs production has been carried out with two strains of transgenic mice, i.e., Medarex HuMab-Mouse and Xeno-mouse [54]. The difference between these two strains of mice is that mouse L chain genes are still functional in the Medarex HuMAb-Mouse; thus, these mice can produce chimeric mAbs. The Xeno-Mouse from Amgen has all the mouse L chain genes deleted, and B cells produce only human Abs. One of these mice monoclonal antibodies [chimeric (Medarex HuMAb) (or) hybridomas (Xeno-mouse)] was immunized to produce neutralizing antibodies and further evaluated. The developed neutralizing antibodies bind to a specific portion to the RBD (N-terminal (amino acids 12–261, 130–150), and C-terminal of the RBD (amino acids 548–567, 607–627)) to prevent fusion of SARS-CoV-2 with the target cells.
COVID-19 convalescent plasma
Due to emergency of COVID-19 patients, recently, FDA has recommended to healthcare providers and investigators the use of convalescent plasma collected from individuals who have recovered from COVID-19 that may contain antibodies to SARS-CoV-2. However, till now, this therapy has not yet been shown to be effective and safe against this disease. Thus, it is very essential to investigate the safety and effectiveness of COVID-19 convalescent plasma in clinical trials. For this therapy, FDA has recommended some guidelines as follows [55]: (1) the pathways for use of investigational COVID-19 convalescent plasma; (2) patient eligibility; (3) collection of COVID-19 convalescent plasma, including donor eligibility and donor qualifications; (4) labeling, and (5) record keeping.
The pharmacological safety data including dose, drug–drug interactions and toxicities of selected potential drug candidates are provided in Table 2.
Table 2.
Pharmacological safety data of selected potential drug candidates [11, 12, 14, 34, 38, 39, 43–45, 57–59, 64, 69, 70, 89]
| Drug | Dose | Drug–drug interaction | Toxicity |
|---|---|---|---|
| Chloroquine phosphate (Aralen) [11, 12, 14, 43, 89] | 500 mg by mouth every 12–24 h × 5–10 days | CYP2D6 and CYP3A4 substrate |
Mild: Abdominal cramps and gastrointestinal intolerance Serious: Cardiovascular effects with QTc prolongation, hematologic effects, central nervous system effects, hypoglycemia, retinal toxicity and neuropsychiatric effect |
| Hydroxychloroquine sulfate (Plaquenil) [11, 12, 44, 89] | 400 mg by mouth daily × 5 days OR 400 mg by mouth every 12 h × 1 day, then 200 mg by mouth every 12 h × 4 days OR 200 mg by mouth 3 times/day for 10 days | CYP2D6, CYP3A4, CYP3A5 and CYP2C8 substrate |
Mild: Gastrointestinal disorders Serious: Blood and lymphatic system disorders, Cardiac disorders (cardiomyopathy, QT interval prolongation, and ventricular arrhythmias), Ear and labyrinth disorders, Eye disorders (Irreversible retinopathy with retinal pigmentation changes), maculopathies (macular degeneration), decreased dark adaptation |
| Favipiravir [60, 61, 89] | 1600 mg doses on the first day and two doses of 600 mg for the next 13 days | CYP2C8 and aldehyde oxidase inhibitor | Mild: Diarrhea, Serious: Hyperuricemia, elevated transaminases, reduction in neutrophil count, teratogenicity |
| Lopinavir/ritonavir (Kaletra) [45, 57, 64, 89] | 400 mg/100 mg by mouth every 12 h for up to 14 days OR lopinavir/ritonavir 400-mg/100-mg per 5-mL oral solution | P-gp substrate; CYP3A4 inhibitor and substrate; CYP2D6 substrate; CYP1A2, CYP2B6, CYP2C9, CYP2C19 Inducer; UGT1A1 inducer |
Mild: Gastrointestinal disorders Serious: Hyperlipidemia, cardiac conduction disorders, hepatotoxicity, pancreatitis |
| Losartan [34] |
Initial dose: 50 mg orally once a day Maximum dose: 100 mg orally once a day |
Interacts with drugs that may increase the level of potassium in the blood such as ACE inhibitors including benazepril/lisinopril, birth control pills containing drospirenone |
Mild: Dizziness, chest pain, hypoglycemia Serious: Hyperkalemia, hypotension, and orthostatic hypotension |
| Remdesivir [14, 57–59, 89] | 200 mg × 1, 100 mg every 24 h IV infusion | Not a significant inducer/inhibitor of CYP enzymes | Reversible elevated transaminases, kidney injury, liver toxicity |
| Tocilizumab (Actemra) [69, 70, 89] | 400 mg IV or 8 mg/kg × 1–2 doses. If required, then second dose after 8–12 h of first dose | IL-6 reduces mRNA expression for several CYP450 isoenzymes, including CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 |
Mild: Upper respiratory tract infections (chances of tuberculosis), headache, nasopharyngitis, hypertension Serious: Gastrointestinal perforations, hematologic effects, hepatotoxicity, hypersensitivity reactions |
| Umifenovir (Arbidol) [38, 39, 89] | 200 mg every 8 h by mouth 7–14 days | Metabolized by CYP3A4, flavin-containing monooxygenase (FMO) family, and UDP-glucuronosyltransferase (UGT) family |
Mild: Gastrointestinal upset Serious: Allergic reaction, elevated transaminases |
Vaccines
Due to the worldwide outbreak of the COVID-19, the general public are keenly watching the progress of development of COVID-19 vaccines [56]. Dr. Anthony S. Fauci, Director of National Institute of Allergy and Infectious Diseases (NIAID), said that “finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority.” But development of a new vaccine against a new disease is not an easy task. Various research institutes and industries are giving their full efforts to develop a vaccine against this pandemic disease with its earliest. Thankfully, the progress is rapid due to various reasons such as: (1) sharing the efforts to sequence the genetic material of SARS-CoV-2 by China throughout the world, (2) coronaviruses were already on the radar of health science researchers, (3) the knowledge from SARS and MERS caused by corona viruses and (4) also learnings from the vaccines against SARS and MERS which were stopped or postponed when those outbreaks were controlled may still be used to defeat COVID-19. The progress of most promising vaccines against the pandemic disease COVID-19 being made by various industries and research institutes is tabulated in Table 3. Several miscellaneous vaccines under investigation for COVID-19 are listed under Box 2.
Table 3.
The progress in development of vaccines by different companies and institutes throughout the world [16–20, 90, 91]
| Vaccine under trial | Name of the company | Platform | Discussion | Current status |
|---|---|---|---|---|
| mRNA-1273 [16] | Moderna | RNA | This is a novel lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine that encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2. The protein S complex is necessary for membrane fusion and infection of host cells. Thus, the stabilized prefusion coronavirus spike protein responsible for Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) can be used as a vaccine antigen to produce robust neutralizing antibody responses. The vaccine is packed with mRNA. The genetic material originates from DNA and makes proteins. Moderna carries its vaccine with mRNA that encodes the correct coronavirus proteins, which are injected into the body. The immune cells present in the lymph nodes can process this mRNA and start making the protein in the right way for new immune cells to identify it and mark it for damage | Phase-III clinical trial (NCT04470427) |
| Ad5-nCoV [17] | CanSino Bio and Institute of Biotechnology of the Academy of Military Medical Sciences | Non-Replicating Viral Vector | In China, Ad5-nCoV is the first novel coronavirus vaccine for COVID-19. This is a genetically engineered vaccine candidate with the replication-defective adenovirus type 5 as the vector to express SARS-CoV-2 spike protein. Preclinical animal studies of this vaccine candidate showed that it can persuade a strong immune response in animal models. Preclinical animal safety studies also exhibited a good safety profile | A Phase-III trial in Saudi Arabia is currently underway |
| AZD1222 (previously called ChAdOx1) [18] | Jenner Institute-University of Oxford | Non-Replicating Viral Vector | This vaccine candidate was developed by Jenner Institute based on a chimp adenovirus vector. For the clinical trials, recruiting 510 healthy volunteers, aged between 18 and 55 years, in a single-blinded, randomized, placebo-controlled, multi-center study to determine efficacy, safety, and immunogenicity of this vaccine | Phase-II/III clinical trial |
| LV-SMENP-DC [90] | Shenzhen Geno-Immune Medical Institute | Lentiviral | A synthetic minigene has been engineered based on conserved domains of the viral structural proteins and a polyprotein protease. The entry of SARS-CoV-2 is intermediated through attachment of the S protein to the ACE2 receptor and the viral replication depends on molecular mechanisms of all of these viral proteins. In this vaccine, a competent lentiviral vector system (NHP/TYF) was used to convey COVID-19 minigenes to express viral proteins and immune modulatory genes to modify dendritic cells (DCs) and to activate T cells | Phase-I/II clinical trial (NCT04276896) |
| Bacillus Calmette–Guérin (BCG) Vaccine [19] | Research group, Netherlands | Live Attenuated Virus | The Research group will recruit 1000 healthcare workers in eight Dutch hospitals who will either receive the BCG vaccine, or a placebo | Phase-III clinical trial |
| BCG Vaccine [20] | Murdoch Children’s Research Institute | Live Attenuated Virus | This is one of the oldest vaccines available in the market. It is made from live, attenuated bovine tuberculosis bacillus, Mycobacterium bovis which induces an adaptive immune response against tuberculosis bacterium. Due to availability in the market, they utilize this vaccine directly in Phase-III clinical trials. This is an open-label, two-group, Phase-III randomized controlled trial in up to 4170 healthcare workers across Australia | Phase-III clinical trial (NCT04327206) |
| Oral bacTRL-Spike [91] | Symvivo Corporation | Oral vaccine for COVID-19 | bacTRL-Spike-1 will be the first-in-human study of bacTRL-Spike, and the first-in-human use of orally delivered bacTRL. It is a genetically modified, probiotic-based oral vaccine for COVID-19. The study design is a phase 1, randomized, observer-blind, placebo-controlled trial in 84 healthy adults [63 receiving active vaccine in bacterial medium and 21 receiving placebo (bacterial medium only)] | Phase-I clinical trial (NCT04334980) |
Box 2.
|
Live attenuated vaccines (AJVaccines, Altimmune, Arcturus Therapeutics and Duke-NUS Medical School, Baylor College of Medicine, BIOCAD, Biological Research Institute, Codagenix) Molecular clamp vaccine (CSL and The University of Queensland) Synthetic mRNA that stimulates the immune system to produce antibodies (CureVac) Two Ii-Key peptide vaccine candidates (EpiVax) Ii-Key peptide vaccine (ExpreS2ion Biotechnologies, Generex Biotechnology) Ankara virus-like particles (MVA-VLP) (Geovax and Bravovax) COVID-19 S-Trimer (GlaxoSmithKline) Adenovirus-based vector vaccine (Greffex) gp96-based vaccine (Heat Biologics) INO-4800; currently in preclinical stage (Inovio Pharmaceuticals) Plant-based vaccine (Institute Pasteur, Themis Bioscience, University of Pittsburgh, Johnson & Johnson, Medicago) Modified avian coronavirus vaccine (MIGAL Galilee Research Institute) BNT162 (Merck, Novavax, Peter Doherty Institute for Infection and Immunity, Pfizer and BioNTech) A chimera that combines DNA from the SARS-CoV-2 with a harmless virus that can stimulate the immune system (Sanofi) Formalin-inactivated and alum-adjuvanted candidate vaccine (Sinovac) Gene-encoded antibody vaccine (Sorrento Therapeutics, Inc. and SmartPharm Therapeutics Inc.) DNA-based vaccine (Takis Biotech) Vaccine candidate based on Tonix’s horsepox vaccine, TNX-1800 (Tonix Pharmaceuticals and Southern Research) CoronaVac (Sinovac; Phase-III), Adjuvant recombinant vaccine candidate (Anhui Zhifei Longcom Biopharmaceutical, Institute of Microbiology of the Chinese Academy of Sciences; Phase-II), ZyCoV-D (Zydus Cadila; Phase-II), Covaxin (Bharat Biotech, National Institute of Virology; Phase-II), BBIBP-CorV (Beijing Institute of Biological Products, China National Pharmaceutical Group (Sinopharm); Phase-I/II), GX-19 (Genexine; Phase-I/II), Sputnik V (Gamaleya Research Institute, Acellena Contract Drug Research and Development; Phase-I/II), Self-amplifying RNA vaccine (Imperial College London; Phase-I/II), LUNAR-COV19 (Arcturus Therapeutics and Duke-NUS Medical School; Phase-I/II), mRNA-based vaccine (CureVac; Phase-I/II), SCB-2019 (GlaxoSmithKline, Sanofi, Clover Biopharmaceuticals, Dynavax and Xiamen Innovax; Phase-I), COVAX-19 (Vaxine Pty Ltd.; Phase-I), NVX-CoV2373 (Novavax; Phase-I), V590 (Merck, IAVI; Phase-I), GRAd-COV2 (ReiThera, Leukocare, Univercells, Phase-I) |
Under parenthesis companies and research institutes are mentioned
In silico modeling applied to search the future drug candidates
To combat the COVID-19 pandemic, researchers must fight with the time to save as many lives as possible. To save the time and speed up the drug discovery process, in silico modeling provides one the best possible options. Till now, in most of the cases, researchers are trying the computational repurposing theory of existing approved drugs (synthetic as well as from natural origin) for SARS-CoV-2 employing docking, homology modeling and molecular dynamics (MD) studies to identify probable magic drugs for COVID-19. Huang et al. [21] computationally designed a short protein fragment or peptide which may block the coronaviruses’ ability to enter human cells by binding to the viral protein which is one of the first kinds of peptide treatments routing for experimental efforts.
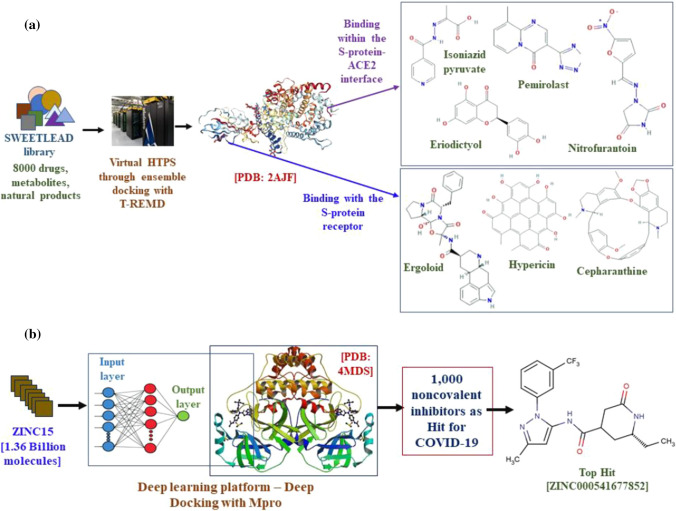
Smith and Smith [22] analyzed 8000 small drug molecules and natural products (SWEETLEAD library database) employing restrained temperature replica-exchange MD simulations combining virtual screening through the ensemble docking to identify the effective drug for COVID-19 which might stop the virus by two ways: (a) disrupting S protein and ACE2 receptor interface stability; or (b) by troubling the capability of the S protein to recognize the ACE2 receptor. The simulation was performed in the IBM’s supercomputer SUMMIT which is recognized as the world’s most powerful computer with 200 petaflops speed. The authors reported the predicted binding affinities of all studied molecules for isolated SARS-CoV-2 S protein and the S protein human ACE2 receptor interface, followed by proposed 77 small molecules by docking studies (47 ligands employing interface docking and 30 ligands identified by the isolated S protein docking). Among the identified 77 molecules, the authors finally reported top 7 regulatory approved small molecules [Pemirolast (Zinc ID: 5783214), Isoniazid pyruvate (Zinc ID: 4974291), Eriodictyol (Zinc ID: 58117) and Nitrofurantoin (Zinc ID: 3875368) binding within the S protein-ACE2 interface; Ergoloid (Zinc ID: 3995616), Cepharanthine (Zinc ID: 30726863), and Hypericin (Zinc ID: 3780340) binding with the S protein receptor] which may serve as possible drug candidates for COVID-19 (Fig. 10a).
Fig. 10.
Schematic workflows performed by Smith and Smith [22] (a) and by Ton et al. [23] (b)
Ton et al. [23] identified 1000 noncovalent inhibitors for SARS-CoV-2 main protease (Mpro) using 1.36 billion compounds from the ZINC15 library employing deep docking platform using Glide SP module which utilizes QSAR models trained on docking scores. The 4MDS protein with 1.6 Å resolution bound to a noncovalent inhibitor was used for the docking study, and among the identified 1000 noncovalent inhibitors, ZINC000541677852 is the top hit drug candidate (Fig. 10b).
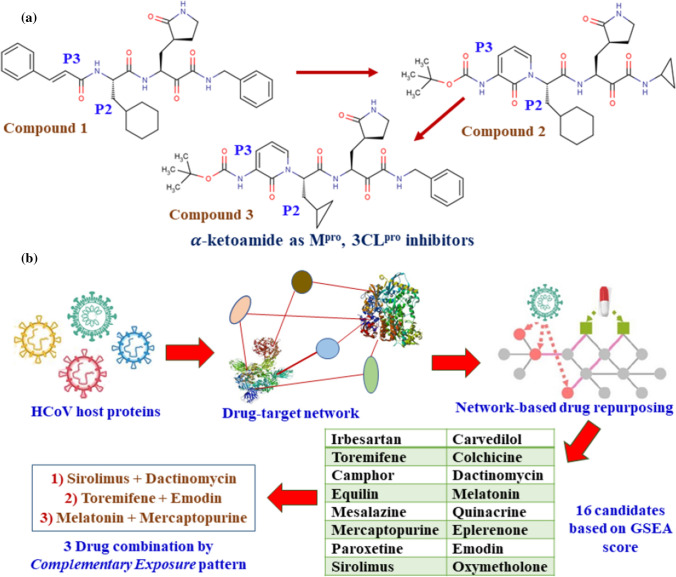
α-Ketoamide inhibitors are proposed as new drug candidates by designing, synthesis, followed by a docking study on the main protease (Mpro, 3CLpro) which has a crucial role in processing the polyproteins that are translated from the viral RNA [24]. Three different PDB structures were employed for the study; they are 6Y2E, 6Y2F and 6Y2G. The authors modified previously designed inhibitor (1) (earlier used for beta-, alpha coronaviruses and 3C proteases of enteroviruses) by incorporating the P3-P2 amide bond into a pyridone ring to enhance the half-life of the newly designed (2) compound in plasma followed by replacing the hydrophobic cinnamoyl moiety with less hydrophobic Boc group (Fig. 11a). Although the inhibitory concentration decreased from 0.18 ± 0.02 µM (1) to 2.39 ± 0.63 µM (2), but molecule 2 reported three times higher plasma binding and 19 times better plasma solubility compared to molecule 1. Further, to improve the antiviral activity against betacoronaviruses of clade b, the authors replaced the P2 cyclohexyl moiety of molecule 1 by the smaller cyclopropyl fragment to produce compound 3 which showed IC50 value of 0.67 ± 0.18 μM for the purified recombinant SARS-CoV-2 Mpro.
Fig. 11.
Schematic workflows performed by Zhang et al. [24] (a) and by Zhou et al. [25] (b)
Zhou et al. [25] reported an integrative antiviral drug repurposing analysis employing pharmacology-based network medicine platform by a two-step process: (a) quantifying the relationship between the human coronaviruses (HCoV) and host interactome and (b) identifying the drug targets in the human protein–protein interaction network. As per phylogenetic analyses, SARS-CoV-2 has the highest nucleotide sequence identity (79.7%), followed by the envelope and nucleocapsid protein sequence identities of 96% and 89.6%, respectively, with SARS-CoV. Employing the network proximity analyses between drug targets and HCoV-associated proteins followed by gene set enrichment analysis (GSEA), it was possible to identify 16 probable anti-HCoV drugs (e.g., melatonin, mercaptopurine, and sirolimus, etc.). Later, the drugs were validated through HCoV-induced transcriptomics data in human cell lines and enrichment analyses of drug-gene signatures. The authors also reported three effective drug combinations among the probable hits identified through ‘complimentary exposure’ pattern (Fig. 11b).
Grifoni et al. [26] employed the Immune Epitope Database and Analysis Resource (IEDB) to characterize the sequence similarity between SARS-CoV and SARS-CoV-2 through homology modeling. The epitope prediction identified a priori potential B and T cell epitopes for SARS-CoV-2 which are the promising targets for immune recognition, followed by the discovery of diagnostics and future vaccines.
Within a short period of time, a huge number of in silico results were deposited in the preprint servers from all over the world and at the same time a few are already published. As in most of the cases, multiple in silico drug designing and virtual screening tools were employed for repurposing theory of approved existing drugs [94], thus, major information is gathered in Table 4 to avoid similar discussion several times [95–119].
Table 4.
| Implied in silico methods and tools | Database | PDB ID | Pharmacological target and mechanism of action of study | Number and name of leads/hits |
|---|---|---|---|---|
| Classical MD (NAMD and the CHARMM36 protein force field) | Peptides | 2AJF | Peptide inhibitors are formed by two sequential self-supporting α-helices extracted from the protease domain of ACE2 which provide a stable binding and are extremely specific to the SARS-CoV-2 receptor-binding domains | 3 (Inhibitor 2, Inhibitor 3 and Inhibitor 4) [95] |
| Docking followed by VS (AutoDdock Vina, SMINA) | SuperDRUG2 (3639 approved drugs) | 6LU7, 5R82, 6YB7 | Main protease (Mpro) was utilized in MD and VS of approved drugs followed by ranking of drug candidates based on predicted binding energy | 2 (Saquinavir and Beclabuvir) [96] |
| VS through docking (PLANTS) followed by MD (AMBER, MOE2019) | Selleckchem and TargetMol (3118 FDA-approved drug) | 6LU7, 4MDS | VS of two databases on two targets (Mpro in complex with peptidomimetic inhibitor and SARS-CoV 3C-Like proteinase 3CLpro) | 4 (Indinavir, lopinavir, atazanavir, cobicistat) [97] |
| VS employing docking study (AutoDock 4.2) | ZINC (1615 FDA-approved drug) | 6LU7 | Repurposing of FDA-approved drugs as inhibitors for Mpro protein of SARS-CoV-2 | 1 (Simeprevir) [98] |
| Hierarchical VS through docking (GLIDE), MD (AMBER), Binding Free Energy Calculations (MM-PBSA-WSAS) | DrugBank (Approved, investigational, experimental drugs) | 6LU7 | Repurposing of DrugBank molecules as inhibitors for Mpro protein of SARS-CoV-2 | 7 (Carfilzomib, Eravacycline, Valrubicin, Lopinavir, Elbasvir, Streptomycin, PubChem 23727975) [99] |
| Docking (Molegro Virtual Docker) | Terpenoids (9) | 6LU7 | Terpenoids of plant origin were checked as inhibitors of Mpro of SARS-CoV-2 | 1 (Ginkgolide A) [100] |
| Docking (AutoDock Vina) | DrugBank 5.1.5 | 6Y84, 6Y2F, 6Y2G, 6W63 | Drug repurposing against SARS-CoV-2 Mpro or 3CLpro | 29 (Remdesivir, Asunaprevir, Ciluprevir, Simeprevir, Danoprevir, Glecaprevir, etc.) [101] |
| Docking (DOCK6), MD (SOMD, GROMACS) | ZINC (1615 FDA-approved drug) | 6LU7 | Combination of docking and MD simulations of FDA-approved drugs to identify inhibitors that could potentially bind to the active site of SARS-CoV-2 Mpro | 11 (Dronedarone, Aliskiren, cobicistat, Isavuconazonium, Capreomycin, Tessalon, Pradaxa, Saquinavir, Ceftolozane, Naloxegol Carfilzomib) [102] |
| Docking (Autodock 4.2) | Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) | 5E6J, 1UJ1, 6CAD | Screening of Chinese medicinal herbs that are frequently used in treating viral respiratory infections through docking and network pharmacology analysis targeting 3CLpro, papain-like protease (PLpro) and spike protein | 13 (betulinic acid, coumaroyltyramine, tanshinone IIa cryptotanshinone, sugiol, desmethoxyreserpine, dihomo-γ-linolenic acid, lignan, dihydrotanshinone, kaempferol, quercetin, moupinamide, N-cis-feruloyltyramine) [103] |
| Homology Modeling (ICM 3.7.3), Docking (ICM) | ZINC (2924), Natural products (1066), Anti-viral drugs (78) | 2K87,2FKV,2W2G, 3E9S, 2K87, 2AW0, 2AHM, 3EE7,1Z8A 5NFY,5NUR, 6JYT, 5NFY, 2H85, 3R24, 1YO4, 3SCI, 1SSK, 2CJR, 5X29 | 19 SARS-CoV-2 targets and 1 human target were prepared by homology modeling. Along with these targets and human ACE2 target, selected databases were screened for repurposing of drug candidates and targets | 42 PLpro inhibitors, 53 3CLpro inhibitors, 40 RdRp inhibitors [104] |
| Molecule Transformer-Drug Target Interaction (MT-DTI), Docking (AutoDock Vina) | Drug Target Common (DTC) database, BindingDB database, FDA-approved antiviral drug | 3CLpro, RdRp, 2′-O-ribose methyltransferase, 3′-to-5′ exonuclease, endoRNAse | Pre-trained deep learning-based drug–target interaction model named MT-DTI was used to identify commercially available antiviral for SARS-CoV-2 | 6 (Atazanavir, remdesivir, efavirenz, ritonavir, dolutegravir, lopinavir) [105] |
| Homology modeling (Blastp, Modeller 9.12), Docking (Maestro, Schrodinger), MD (DESMOND) | DrugBank (2300 approved drug), Super Natural II (3,00,000) | 2HSX | Modeled non-structural protein NSP1 employing homology modeling, docking and followed by MD simulation-based VS to identify new drug candidates through drugs repurposing theory | 32 (Remdesivir, Edoxudine, Esculin, Acarbose, Shogaol, Glycyrrhizic acid, Gingeronone) [106] |
| Homology modeling (SWISS-MODEL), Docking (AutoDock Vina), MD (GROMACS) | LOPAC | 2AJF, 6VSB | High-throughput VS was employed to investigate FDA-approved drugs against both ACE2 host cell receptor and the S protein to identify drug candidate through repurposing theory | 5 inhibitors of ACE2 receptor (TNP, Eptifibatide acetate, GNF5, RS504393, GR 127935 hydrochloride hydrate); 5 inhibitors of viral S protein (KT185, KT203 GSK1838705A, BMS195614, RS504393) [107] |
| Docking (AutoDock Vina), VS (MTiOpenScreen) | Drugs-lib (7173) | 2DUC | Molecular modeling of the 3CLpro of the SARS-CoV-2 followed by VS performed for already approved drug | 16 (Diosmin, Hesperidin, MK-3207, Venetoclax, Dihydroergocristine, Bolazine, R428, Ditercalinium, Etoposide, Ledipasvir UK-432097, Teniposide, Irinotecan, Velpatasvir, Eluxadoline, Lumacaftor) [108] |
| Docking (AutoDock Vina), VS (MTiOpenScreen), MD (AMBER 16) | Approved drugs (28) | 6LU7, 1UK4, 6M0J, 6NUR | Searching of antagonists which will inhibit the Mpro of the SARS-CoV-2 virus, modulate the ACE2 receptors and reduce viral replication by inhibiting NSP12 RNA Polymerase | 4 (Simeprevir, baricitinib, paritaprevir, remdesivir) [109] |
| Docking, MD (SYBYL-X 1.1) | Essential oils in garlic including organosulfur compounds (18) | 6LU7 | Inhibitory effect of essential oils from garlic is established and found interactions with the amino acids of the human ACE2 protein and the Mpro of SARS-CoV-2 | 2 (Allyl disulfide, Allyl trisulfide) [110] |
| Docking (AutoDock Vina) | Artemisinin and derived compounds (12) | 6LZG | Explained how HCQ interferes in the prevention of Lys353 in human ACE2 from interacting with the corresponding binding hotspot exist on the Spike protein through docking. This was followed by screening of artemisinin derived compounds to show better docking score (two mode of interactions with Lys31 and Lys353 binding hotspots of the Spike protein) of them compare to HCQ | 3 (Artesunate, Artenimol Artemisinin) [111] |
| MD (CHARMM36) | CLQ, HCQ | 6VSB | Structural and molecular modeling reported that CLQ and HCQ bind to sialic acids and gangliosides with high affinity (S protein uses sialic acids linked to host cell surface gangliosides binding domain at the tip of the N-terminal domain (amino acids 111-158) of the SARS- CoV2 for entry to host along with ACE2) | 2 (CLQ-OH[preferably], CLQ) [112] |
| Docking (PatchDock, FireDock) | HCQ, Curcumin (2) | 6Y84, 2GHV | Molecular modeling indicated that combination of HCQ with curcumin disrupt the stability of SARS-CoV-2 receptor proteins effectively by binding with main protease and S1 RBD. The combination therapy has higher efficacy than single dose of HCQ | Combination therapy of HCQ and Curcumin [113] |
| Docking (GLIDE- Schrodinger), Binding free energy calculations (MM-GBSA) | SELLEKCHEM, DrugBank, Repurposing hub | 6LU7, 6M03 | Mpro target was utilized in molecular docking and VS of approved drugs followed by binding energy calculation to identify potential drug candidate | 6 (Pepstatin A, Leupeptin Hemisulfate, Nelfinavir, Birinapant, Lypression, Octeotide) [114] |
| 2D-QSAR, Docking (Autodock tool 1.5.6) followed by VS | Binding DB (69 molecules as 3CLpro for QSAR model), ZINC15 database and Chemical Abstracts Service (CAS) reported as COVID-19 antiviral candidates for VS | 6LU7 | MLR based 2D-QSAR model was developed using 3CLpro inhibitor followed by docking study was also explored. Further, inhibitory activity of a total of 50,437 compounds was computed. Top 100 compounds were reported with inhibitory activity in mM. | 100 lead compounds from ZINC15 and CAS database [115] |
| Pharmacophore (vROCS (OpenEye)) and Docking study followed by VS (AutoDock 4.2) | Four pharmacophores (OEW, remdesivir, hydroxychloroquine and N3) and 50,000 natural compounds from ZINC database for VS | 6LU7 and 6Y7M | Pharmacophore and docking based virtual screening performed employing natural compounds of ZINC database targeting SARS-CoV-2M protease | 11 ligands as (ZINC1845382, ZINC1875405, ZINC2092396, ZINC2104424, ZINC44018332, ZINC2101723, ZINC2094526, ZINC2094304, ZINC2104482, ZINC3984030, and ZINC1531664) [116] |
| Sequence analysis and homology modeling followed by VS employed docking (AutoDock VINA) and later using machine learning technique CNN (BindScope) | MCULE database (44,704,142) | 6VSB | 44 million compounds were screened to find potential inhibitor able to inhibit the surface glycoprotein responsible for virus entry and binding | 3 molecules (benzylfuran-2(5H)-one); ((2,5-difluorophenyl)thio)-2,2-difluoroacetic acid) and (2-methylfuran-3-yl)methanesulfonyl fluoride) [117] |
| Similarity searching and Docking (Autodock Vina, version 1.1.2 and MOE v.2019) and ADMET profiling (SwissADME) | FooDB (22,880), Dark Chemical Matter (DCM) (139,329), ZINC (top 10 hit from literature), Actives (11) | 6LU7, 5N5O | Consensus VS of DCM and Food Chemicals as potential inhibitors of SARS-CoV-2 MPro | 105 hits of which, three are commercially available [118] |
| Docking (GLIDE-Schrodinger), MM-GBSA, ADMET profiling (pkCSM and ProTox-II), MD (DESMOND- Schrodinger) | MolPort database (10,305) | 6LU7, 6Y2E, 6Y2F, 6Y2G, 6M03, 6Y84, 5RF8, 5RG0, 5R8T | Protein reliability analysis among 9 Mpro proteins followed by VS through docking, MM-GBSA calculation, ADMET profiling and MD to find natural Mpro inhibitors for SAS-COV-2 which is already commercially available for further testing | 3 hits (two natural compounds MolPort-005-944-636 and MolPort-005-945-924, as well as a Noricaritin (MolPort-039-052-338) derived from Epimedium brevicornu Maxim) [119] |
An interesting aspect is that most of the studies are based on repurposing theory of existing approved drug employing docking and MD supported VS. Majorly the authors relied on approved antiviral drugs along with DrugBank database, Zinc database, Natural compounds’ databases along with one of the most discussed molecules of recent time, HCQ. Thus, the basic ideas of implication of in silico tools and repurposing of approved drugs are same, but the only difference is selection of the target protein in different studies. Without any doubt, the crystal structure of COVID-19 main protease (Mpro) in complex with an inhibitor N3 (PDB: 6LU7) is the most accessed protein for the drug discovery. But based on the target type, the choice of proteins can be different. Thus, to assist researchers, we have classified the target proteins into 14 types covering 110 PDB crystal ID from PDB (https://www.rcsb.org/) available until April 12, 2020, and enlisted in Table 5.
Table 5.
Type of target protein to identify the efficient drug molecule of SARS-CoV-2
| Type of target protein | PDB crystal ID |
|---|---|
| Structure of the 2019-nCoV HR2 Domain | 6LVN |
| Nsp9 RNA-binding protein of SARS-CoV-2 | 6W4B, 6W9Q |
| N-terminal RNA-binding domain of the SARS-CoV-2 nucleocapsid phosphoprotein | 6YI3, 6M3M, 6VYO |
| SARS-CoV-2 main protease (Mpro/3CLpro) | 5R84, 5R83, 5R7Y, 5R80, 5R82, 5R81, 5R8T, 5R7Z, 5REA, 5REC, 5REB, 5REE, 5RED, 5REG, 5REF, 5RE9, 5RE8, 5RE5, 5RE4, 5RE7, 5RE6, 5RFB, 5RFA, 5RFD, 5RFC, 5RFF, 5RFE, 5RFH, 5RFG, 5REY, 5REX, 5RF9, 5REZ, 5RF2, 5REP, 5RF1, 5RES, 5RF4, 5RER, 5RF3, 5REU, 5RF6, 5RET, 5RF5, 5REW, 5REV, 5RF7, 5REI, 5REH, 5REK, 5REJ, 5REM, 5REL, 5REO, 5RF0, 5REN, 5RFZ, 5RFY, 5RFR, 5RFQ, 5RFT, 5RFS, 5RFV, 5RFU, 5RFX, 5RFW, 5RFJ, 5RFI, 5RFL, 5RFK, 5RFN, 5RFM, 5RFP, 5RFO, 5RG0, 6Y2E, 6Y84, 6W63, 6YB7, 5RF8, 6Y2G, 6Y2F, 6LU7 |
| ADP ribose phosphatase of NSP3 from SARS-CoV-2 | 6VXS, 6W02. 6W6Y |
| papain-like protease (PLpro) of SARS-CoV-2 | 6W9C |
| SARS-CoV-2 RNA-dependent RNA polymerase | 7BTF, 6M71 |
| post fusion core of 2019-nCoV S2 subunit | 6LXT |
| NSP16–NSP10 Complex from SARS-CoV-2 | 6W4H, 6W61, 6W75 |
| NSP15 Endoribonuclease from SARS-CoV-2 | 6VWW, 6W01 |
| SARS-CoV-2 receptor-binding domain in complex with human antibody CR3022 | 6W41 |
| Spike glycoprotein with a single receptor-binding domain (RBD) [plus bound to ACE2] | 6VSB, 6VXX, 6M0J, 6VYB, 6LZG |
| Chimeric receptor-binding domain complexed with its receptor human ACE2 | 6VW1 |
| RBD/ACE2-B0AT1 complex | 6M17 |
Is interspecies modeling the future of COVID-19 drug discovery? An unexplored concept under in silico tools
Multiple incidents illustrated the outbreak of corona virus in animals, especially in pets [120, 121]. Animals from dogs, cats, tigers, lions to minks are already tested positive and showed mild to severe symptoms of corona virus, followed by death of few instances. Although these events are scattered and not enough to study in the middle of human crisis, we cannot ignore the fact of human to animal transmission. If human to animal transmission is true, then there is a possibility of mutations, insertions and deletions in the genome sequence of this deadly virus in these animals in future with probabilities of zoonotic transfer of a stronger form of present SARS-CoV-2 virus from them to human in the near future. Thus, these small incidents need to be checked very carefully to avoid any future transmission.
The above-mentioned facts help to build an interspecies analysis by Kar and Leszczynski [122] for animals to human transmission or vice versa to aid in the drug discovery process of COVID-19 in upcoming days. The in silico interspecies-quantitative structure–activity relationship (i-QSAR) modeling correlates and then extrapolates the response (activity/toxicity) data from animal source to human which will be helpful for drug discovery. Due to genome sequence similarity of SARS-CoV-2 with the pangolins and bat, experimental data of drug candidates to pangolins/bat along with structural and physicochemical properties of drugs can be correlated with human response endpoint which is the first step of modeling. In the next step, extrapolation of animal data to human data can lead to no human testing through developed i-QSAR model. Once the acceptable predictive i-QSAR model is ready, there might be no need of future animal testing. As bat and pangolins are endangered species, thus future introspection can be performed in dogs and cats due to their better accessibility as well as considering them possible carriers of SARS-CoV-2 from the recent incidents.
Conclusion
Researchers from all over the world are trying to find the medicines which will help to stop the transmission of the virulent SARS-CoV-2 virus, mitigate the symptoms of the infected patients and help to lower the death toll throughout the world. Unfortunately, till now there is no silver gunshot which can solve this pandemic COVID-19. We have reported here a comprehensive and updated discussion on drug or drug candidates under investigation with their probable targets and mechanisms of action which might not be clear for many cases earlier, along with the effort of ongoing vaccine trials, monoclonal antibodies therapy and convalescent plasma treatment. We have presented here the ongoing computational efforts related to in silico tools to explore the probable drug candidates against COVID-19 along with the up-to-date target protein information. The information provided from in silico approaches may work as a magic bullet for the medicinal chemists to accelerate the drug discovery and development process. Overall, this review provides a strong intellectual foundation to support progress of ongoing research related to thorough knowledge of drugs or investigational drugs, vaccines and in silico approaches which can be helpful for the development of new drug candidates for the treatment of COVID-19.
Acknowledgements
PKO thanks the UGC, New Delhi, for financial assistance in the form of a fellowship (F./PDFSS-2015-17-WES-11996). SK and JL are thankful to the National Science Foundation (NSF/CREST HRD-1547754 and NSF/RISE HRD-1547836) for financial support. JGK thanks the Ministry of Chemicals & Fertilizers, Department of Pharmaceuticals, Government of India, and the National Institute of Pharmaceutical Education and Research Kolkata (NIPER-Kolkata) for providing financial assistance in the form of a fellowship. KR thanks Science and Engineering Research Board (SERB), New Delhi, for financial assistance under the MATRICS scheme (File Number MTR/2019/000008).
Compliance with ethical standards
Conflict of interest
There are no conflicts to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Probir Kumar Ojha and Supratik Kar have contributed equally to this work.
Contributor Information
Kunal Roy, Email: kunalroy_in@yahoo.com, Email: kunal.roy@jadavpuruniversity.in.
Jerzy Leszczynski, Email: jerzy@icnanotox.org.
References
- 1.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Coronavirus pandemic data. https://www.worldometers.info/coronavirus/. Accessed 11 May 2020
- 4.Zeng ZQ, Chen DH, Tan WP, et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur J Clin Microbiol Infect Dis. 2018;37:363–369. doi: 10.1007/s10096-017-3144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Zai J, Wang X, Li Y. Potential of large “first generation” human-to-human transmission of 2019-nCoV. J Med Virol. 2020;92:448–454. doi: 10.1002/jmv.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14:e1007236. doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Zhou Q, Liet Y, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung TS, Liu DX. Human coronavirus: host–pathogen interaction. Annu Rev Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 11.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J AntiMicrob Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautret P, Lagierac J-C, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Cao R, Zhanget L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19. ClinicalTrials.gov website: https://clinicaltrials.gov/ct2/show/NCT04470427. Accessed 13 Aug 2020
- 17.Phase I Clinical Trial of a COVID-19 Vaccine in 18–60 Healthy Adults (CTCOVID-19). ClinicalTrials.gov website: https://clinicaltrials.gov/ct2/show/NCT04313127. Accessed 11 May 2020
- 18.A Study of a Candidate COVID-19 Vaccine (COV001). ClinicalTrials.gov website: https://www.clinicaltrials.gov/ct2/show/NCT04324606. Accessed 11 May 2020
- 19.de Vrieze J (2020) Can a century-old TB vaccine steel the immune system against the new coronavirus? Science. https://www.sciencemag.org/news/2020/03/can-century-old-tb-vaccine-steel-immune-system-against-new-coronavirus. Accessed 11 May 2020
- 20.BCG Vaccination to Protect Healthcare Workers Against COVID-19 (BRACE). ClinicalTrials.gov website: https://clinicaltrials.gov/ct2/show/NCT04327206. Accessed 11 May 2020
- 21.Huang X, Pearce R, Zhang Y (2020) Computational design of peptides to block binding of the SARS-CoV-2 spike protein to human ACE2. Preprint at https://www.biorxiv.org/content/10.1101/2020.03.28.013607v1.full.pdf [DOI] [PMC free article] [PubMed]
- 22.Smith M, Smith JC (2020) Repurposing therapeutics for covid-19: Supercomputer-based docking to the sars-cov-2 viral spike protein and viral spike protein-human ace2 interface. Preprint at 10.26434/chemrxiv.11871402.v3
- 23.Ton AT, Gentile F, Hsing M, Ban F, Cherkasov A. Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3 billion compounds. Mol Inform. 2020;39:2000028. doi: 10.1002/minf.202000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Lin D, Sun X, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell discov. 2020;6:1–18. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–680. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang LS, Wang YR, Ye DW, Liu QQ. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int J Antimicrob Agents. 2020;55:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P, Wang X. COVID-19: a new challenge for human beings. Cell Mol Immunol. 2020;17:555–557. doi: 10.1038/s41423-020-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46:579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du L, Yang Y, Zhou Y, Lu L, Li F, Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin Ther Targets. 2017;21:131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novel coronavirus structure reveals targets for vaccines and treatments (2020) https://www.nih.gov/news-events/nih-research-matters/novel-coronavirus-structure-reveals-targets-vaccines-treatments. Accessed 11 May 2020
- 34.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glowacka I, Bertram S, Herzog P, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y. Compensation of ACE2 function for possible clinical management of 2019-nCoV-induced acute lung injury. Virol Sin. 2020;35:256–258. doi: 10.1007/s12250-020-00205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 38.The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection (ELACOI). ClinicalTrials.gov website: https://clinicaltrials.gov/ct2/show/NCT04252885. Accessed 11 May 2020
- 39.Kadam RU, Wilson IA. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci. 2017;114:206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64:e00399-20. doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 44.Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5:e00293. doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature. 2020;578:347–348. doi: 10.1038/d41586-020-00444-3. [DOI] [PubMed] [Google Scholar]
- 46.Mifsud EJ, Hayden FG, Hurt AC. Antivirals targeting the polymerase complex of influenza viruses. Antivir Res. 2019;169:104545. doi: 10.1016/j.antiviral.2019.104545. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Wang XJ. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genomics. 2020;47:119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopinavir; Ritonavir (All Populations Monograph). Elsevier (2020) https://www.elsevier.com/__data/assets/pdf_file/0010/990730/Lopinavir,-Ritonavir-Drug-Monograph_3.17.2020.pdf. Accessed 11 May 2020
- 49.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagstaff KM, Rawlinson SM, Hearps AC, Jans DA. An AlphaScreen(R)-based assay for high-throughput screening for specific inhibitors of nuclear import. J Biomol Screen. 2011;16:192–200. doi: 10.1177/1087057110390360. [DOI] [PubMed] [Google Scholar]
- 51.Yu X, Zhang S, Jiang L, et al. Structural basis for the neutralization of MERS-CoV by a human monoclonal antibody MERS-27. Sci Rep. 2015;5:13133. doi: 10.1038/srep13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Widjaja I, Wang C, van Haperen R, et al. Towards a solution to MERS: protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein. Emerg Microbes Infect. 2019;8:516–530. doi: 10.1080/22221751.2019.1597644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian X, Li C, Huang A, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott CT. Mice with a human touch. Nat Biotechnol. 2007;25:1075–1077. doi: 10.1038/nbt1007-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Recommendations for Investigational COVID-19 Convalescent Plasma, US FDA (2020) https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds?utm_campaign=What%27sNew2020-03-24&utm_medium=email&utm_source=Eloqua. Accessed 11 May 2020
- 56.Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo D. Old weapon for new enemy: drug repurposing for treatment of newly emerging viral diseases. Virol Sin. 2020;35:253–255. doi: 10.1007/s12250-020-00204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Expanded Access Remdesivir (RDV; GS-5734™). ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/results?cond=Coronavirus&term=&type=&rslt=&age_v=&gndr=&intr=remdesivir&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 11 May 2020
- 60.‘Favilavir’: First approved drug to possibly treat coronavirus. The Science Times. https://www.sciencetimes.com/articles/25053/20200317/favilavir-first-approve-drug-treat-coronavirus.htm. Accessed 11 May 2020
- 61.Favipiravir combined with tocilizumab in the treatment of Corona Virus Disease 2019. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04310228?cond=Coronavirus&intr=Tocilizumab&draw=2&rank=1. Accessed 11 May 2020
- 62.Arabi YM, Shalhoub S, Mandourah Y, et al. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin Infect Dis. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu W, Morse JS, Lalonde T, Xu S. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Surviving sepsis campaign rapid guidelines of the management of critically ill adults with coronavirus disease 2019. ESICM. https://www.esicm.org/ssc-covid19-guidelines/. Accessed 11 May 2020]
- 65.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38:379–391. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 66.Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Int Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Witt BJ, Garrison EA, Champion HC, Kadowitz PJ. L-163,491 is a partial angiotensin AT1 receptor agonist in the hindquarters vascular bed of the cat. Eur J Pharmacol. 2020;404:213–219. doi: 10.1016/s0014-2999(00)00612-9. [DOI] [PubMed] [Google Scholar]
- 68.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roche initiates Phase III clinical trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 pneumonia. Roche. https://www.roche.com/media/releases/med-cor-2020-03-19.htm. Accessed 11 May 2020
- 71.Kiniksa Announces Early Evidence of Treatment Response with Mavrilimumab in 6 Patients with Severe COVID-19 Pneumonia and Hyperinflammation. PipelineReview. https://pipelinereview.com/index.php/2020040174191/Antibodies/Kiniksa-Announces-Early-Evidence-of-Treatment-Response-with-Mavrilimumab-in-6-Patients-with-Severe-COVID-19-Pneumonia-and-Hyperinflammation.html. Accessed 11 May 2020
- 72.Craven J COVID-19 therapeutics tracker. Regulatory Focus. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-therapeutics-tracker. Accessed 11 May 2020
- 73.Fitzhugh M Humanigen preps lenzilumab for potential battle with deadly COVID-19 symptom. Bioworld. https://www.bioworld.com/articles/433890-humanigen-preps-lenzilumab-for-potential-battle-with-deadly-covid-19-symptom. Accessed 11 May 2020
- 74.FDA Approves Emergency IND Use of Humanigen’s Lenzilumab For Compassionate Use In COVID-19 Patients. ACCESSWIRE. https://www.accesswire.com/583610/FDA-Approves-Emergency-IND-Use-of-Humanigens-Lenzilumab-For-Compassionate-Use-In-COVID-19-Patients. Accessed 11 May 2020
- 75.Novant Health Initiates Phase 2 COVID-19 Trial with CytoDyn’s Leronlimab. CytoDyn. https://content.equisolve.net/_7bdf444ef2b4daaec17d4324fda3a32a/cytodyn/news/2020-04-07_Novant_Health_Initiates_Phase_2_COVID_19_Trial_411.pdf. Accessed 11 May 2020
- 76.Roivant pushing gimsilumab testing for ARDS in COVID-19 patients. ThePharmaLetter. https://www.thepharmaletter.com/article/roivant-pushing-gimsilumab-testing-for-ards-in-covid-19-patients. Accessed 11 May 2020
- 77.First patient outside U.S. treated in global Kevzara® (sarilumab) clinical trial program for patients with severe COVID-19. https://www.sanofi.com/-/media/Project/One-Sanofi-Web/Websites/Global/Sanofi-COM/Home/media-room/press-releases/2020/2020-03-30-07-00-00-2008040-en.pdf. Accessed 11 May 2020
- 78.RELIEF THERAPEUTICS and NeuroRx, Inc. File FDA IND for Aviptadil to Treat COVID-19-induced Respiratory Distress. P&T Community. https://www.ptcommunity.com/wire/relief-therapeutics-and-neurorx-inc-file-fda-ind-aviptadil-treat-covid-19-induced-respiratory. Accessed 11 May 2020
- 79.EUSA Launches Trial of Siltuximab for COVID-19 Complications. FDA NEWS. https://www.fdanews.com/articles/196378-eusa-launches-trial-of-siltuximab-for-covid-19-complications. Accessed 11 May 2020
- 80.Immunoregulatory Therapy for 2019-nCoV. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04268537. Accessed 11 May 2020
- 81.Alexion to start Soliris in COVID-19 Phase II trial in next few days. GlobalData. https://www.globaldata.com/alexion-to-start-soliris-in-covid-19-phase-ii-trial-in-next-few-days-says-globaldata/. Accessed 11 May 2020
- 82.Bevacizumab in Severe or Critical Patients With COVID-19 Pneumonia (BEST-CP). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04275414. Accessed 11 May 2020
- 83.CD24Fc as a Non-antiviral Immunomodulator in COVID-19 Treatment (SAC-COVID). ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT04317040. Accessed 11 May 2020
- 84.Colchicine Counteracting Inflammation in COVID-19 Pneumonia (ColCOVID-19). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04322565. Accessed 11 May 2020
- 85.Covid-19 drug research: second phase of clinical trial of new drug candidate, SNG001 For SARS-CoV-2 coronavirus initiated. Thailand Medical News. https://www.thailandmedical.news/news/covid-19-drug-research-second-phase-of-clinical-trial-of-new-drug-candidate,-sng001-for-sars-cov-2-coronavirus-initiated. Accessed 11 May 2020
- 86.BIDMC launches clinical trial to assess common anti-clotting medication for treatment of COVID-19-related respiratory failure. BIDMC. https://www.bidmc.org/about-bidmc/news/2020/04/covid-19-anti-clotting-medication. Accessed 11 May 2020
- 87.Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. World Health Organization. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 11 May 2020
- 88.Use of Ascorbic Acid in Patients with COVID 19. ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT04323514. Accessed 11 May 2020
- 89.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 90.Immunity and safety of Covid-19 synthetic minigene vaccine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04276896. Accessed 11 May 2020
- 91.Evaluating the safety, tolerability and immunogenicity of bacTRL-spike vaccine for prevention of COVID-19. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04334980. Accessed 11 May 2020
- 92.COVID-19 Treatment and Vaccine Tracker. Milken Institute (2020). https://milkeninstitute.org/sites/default/files/2020-03/Covid19%20Tracker%20032020v3-posting.pdf?fbclid=IwAR2rX6EhbvBMcGf3eqnQNimiz1u7KPJVM3aeXN1HmgmCX3QlfbVTrcV3cQQ. Accessed 11 May 2020
- 93.Craven, J. COVID-19 vaccine tracker. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker. Accessed 15 August 2020
- 94.Roy K. In silico drug design: repurposing techniques and methodologies. New York: Academic Press; 2019. [Google Scholar]
- 95.Han Y, Kral P (2020) Computational design of ACE2-based short peptide inhibitors of SARS-CoV2. ChemRxiv. Preprint at 10.26434/chemrxiv.12061734.v1
- 96.Talluri S (2020) Virtual screening based prediction of potential drugs for COVID-19. Preprints at 10.20944/preprints202002.0418.v2
- 97.Contini A (2020) Virtual screening of an FDA approved drugs database on two COVID-19 coronavirus proteins. Preprint at 10.26434/chemrxiv.11847381
- 98.Hosseini FS, Amanlou M (2020) Simeprevir, potential candidate to repurpose for coronavirus infection: virtual screening and molecular docking study. Preprints at 10.20944/preprints202002.0438.v1 [DOI] [PMC free article] [PubMed]
- 99.Wang J (2020) Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. Preprint at 10.26434/chemrxiv.11875446.v1 [DOI] [PMC free article] [PubMed]
- 100.Shaghaghi N (2020) Molecular docking study of novel COVID-19 protease with low risk terpenoides compounds of plants. Preprint at 10.26434/chemrxiv.11935722.v1
- 101.Chakraborti S, Bheemireddy Sneha, Srinivasan, N (2020) Repurposing drugs against main protease of SARS-CoV-2: mechanism based insights supported by available laboratory and clinical data. ChemRxiv. Preprint. 10.26434/chemrxiv.12057846.v2 [DOI] [PubMed]
- 102.Mendoza-Martinez C, Rodriguez-Lezama A (2020) Identification of potential inhibitors of SARS-CoV2 main protease via a rapid in-silico drug repurposing approach. Preprint at 10.26434/chemrxiv.12085083.v1
- 103.Zhang DH, Wu KL, Zhang X, Deng SQ, Peng B. In silico screening of Chinese herbalmedicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. 2020;18:152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beck BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV2) through a drug-target interaction deep learning model. Comput Struct Biotechnol. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharma A, Tiwari V, Sowdhamini R (2020) Computational search for potential COVID-19 drugs from FDA-approved drugs and small molecules of natural origin identifies several anti-virals and plant products. Preprint at 10.26434/chemrxiv.12091356.v1 [DOI] [PMC free article] [PubMed]
- 107.Choudhary S, Malik YS, Tomar S (2020) Identification of SARS-CoV2 cell entry inhibitors by drug repurposing using in silico structure-based virtual screening approach. Preprint at 10.26434/chemrxiv.12005988.v1 [DOI] [PMC free article] [PubMed]
- 108.Chen YW, Yiu CPB, Wong KY. Prediction of the SARS-CoV2 (2019-nCoV) 3C-like protease (3CLpro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oliveira LD, Davi M, Oliveira TD, Mota K (2020) Comparative computational study of SARS-CoV2 receptors antagonists from already approved drugs. Preprint at 10.26434/chemrxiv.12044538.v2
- 110.Thuy BTP, My TTA, Hai NTT, et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sehailia M, Chemat S (2020) In-silico studies of antimalarial-agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31-binding hotspots of SARS-CoV2 spike protein than hydroxychloroquine: potential repurposing of artenimol for COVID-19. Preprint at 10.26434/chemrxiv.12098652.v1 [DOI] [PMC free article] [PubMed]
- 112.Fantini J, Scala CD, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV2 infection. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srivastava A, Singh D (2020) Destabilizing the structural integrity of SARS-CoV2 receptor proteins by curcumin along with hydroxychloroquine: an insilco approach for a combination therapy. Preprint at 10.26434/chemrxiv.12090438.v1
- 114.Mittal L, Kumari A, Srivastava M, Singh M, Asthana S (2020) Identification of potential molecules against COVID-19 main protease through structure-guided virtual screening approach. Preprint at 10.26434/chemrxiv.12086565.v2 [DOI] [PMC free article] [PubMed]
- 115.Kumar V, Roy K. Development of a simple, interpretable and easily transferable QSAR model for quick screening antiviral databases in search of novel 3Clike protease (3CLpro) enzyme inhibitors against SARS-CoV diseases. SAR QSAR Environ Res. 2020;31:511–526. doi: 10.1080/1062936X.2020.1776388. [DOI] [PubMed] [Google Scholar]
- 116.Andrade BS, Ghosh P, Barh D, Tiwari S, Silva RJS, Soares WRDA, Melo TS, Freitas AS, González-Grande P, Palmeira LS, Alcantara LCJ, Giovanetti M, Góes-Neto A, Azevedo VADC. Computational screening for potential drug candidates against the SARS-CoV-2 main protease [version 1; awaiting peer review] F1000Research. 2020;9:514. doi: 10.12688/f1000research.23829.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Onawole AT, Sulaiman KO, Kolapo TU, Akinde FO, Adegoke RO. COVID-19: CADD to the rescue. Virus Res. 2020;285:198022. doi: 10.1016/j.virusres.2020.198022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santibáñez-Morán MG, López-López Edgar, Prieto-Martínez FD, Sánchez-Cruz Norberto, Medina-Franco JL (2020) Consensus virtual screening of dark chemical matter and food chemicals uncover potential inhibitors of SARS-CoV-2 main protease. Preprint at 10.26434/chemrxiv.12420860.v1 [DOI] [PMC free article] [PubMed]
- 119.Kapusta K, Kar S, Collins JT, Franklin LM, Kolodziejczyk W, Leszczynski J, Hill GA. Protein reliability analysis and virtual screening of natural inhibitors for SARS-CoV-2 main protease (Mpro) through docking, molecular mechanic & dynamic, and ADMET Profiling. J Biomol: Struct Dyn; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grimm D. Should pets be tested for coronavirus? Science. 2020 doi: 10.1126/science.abc0029. [DOI] [Google Scholar]
- 121.Shi J, Wen Z, Zhong G, et al. Susceptibility of ferrets, cats, dogs, and different domestic animals to SARS coronavirus-2. Science. 2020;368:1016. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kar S, Leszczynski J. From animal to human–interspecies analysis provides novel way of ascertaining and fighting COVID-19. The Innovation. 2020 doi: 10.1016/j.xinn.2020.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]