Abstract
For this child, at this particular moment, how much anesthesia should I give? Determining the drug requirements of a specific patient is a fundamental problem in medicine. Our current approach uses population-based pharmacological models to establish dosing. However, individual patients, and children in particular, may respond to drugs differently. In anesthesiology, we have the advantage that we can monitor our patients in real time and titrate drugs to the desired effect. Examples include blood pressure management or muscle relaxation. Although the brain is the primary site of action for sedative-hypnotic drugs, the brain is not routinely monitored during general anesthesia or sedation, a fact that would surprise many patients. One reason for this is that, until recently, physiologically principled approaches for anesthetic brain monitoring have not been articulated. In the past few years, our knowledge of anesthetic brain mechanisms has developed rapidly. We now know that anesthetic drug effects are clearly visible in the electroencephalogram (EEG) of adults and reflect underlying anesthetic pharmacology and brain mechanisms. Most recently, similar effects have been characterized in children. In this article, we describe how EEG monitoring could be used to guide anesthetic management in pediatric patients. We review previous evidence and present multiple case studies showing how drug-specific and dose-dependent EEG signatures seen in adults are visible in children and infants, including those with neurological disorders. We propose that the EEG can be used in the anesthetic care of children to enable anesthesiologists to better assess the drug requirements of individual patients in real time and improve patient safety and experience.
In the United States, an estimated 6 million children undergo anesthesia for medical procedures each year.1 Determining anesthetic drug requirements for children can pose unique challenges. Our current approach uses population-based pharmacological models to gauge dosing. Anesthetic drugs profoundly affect autonomic, respiratory, cardiovascular, and thermoregulatory systems, which are all monitored continuously throughout a procedure. To patients and others outside the field of anesthesiology, it is perhaps surprising that the brain is not routinely monitored during general anesthesia or sedation. One reason for this is that, until recently, physiologically principled approaches for anesthetic brain monitoring have not been articulated.
In the past few years, our knowledge of anesthetic brain mechanisms has developed rapidly. Anesthetic and sedative agents have been shown to induce profound changes in brain activity that can be observed on the electroencephalogram (EEG).2–5 EEG oscillations are the result of highly synchronized postsynaptic potentials stemming from shared rhythmic firing among a population of neurons (Figure 1A, B).6,7 Scalp electrodes of the EEG measure the oscillatory electrical potentials that emanate from the cerebral cortex (Figure 1C).6–8 This rhythmic activity is thought to mediate different aspects of brain function by facilitating coordination and information sharing within and between neural circuits.6–8 Anesthetic and sedative drugs induce highly pronounced, abnormal patterns of oscillations that are thought to disrupt communication within the brain.3,9–14 These drug-induced patterns of oscillations can easily be seen in the raw EEG signals and in the computed EEG spectrogram, as we will illustrate throughout this article. Importantly, specific oscillatory patterns have been found to reflect a patient’s current level of unconsciousness under anesthesia,3 which makes it possible to track the brain states of a patient in real time during an anesthetic.
Figure 1.
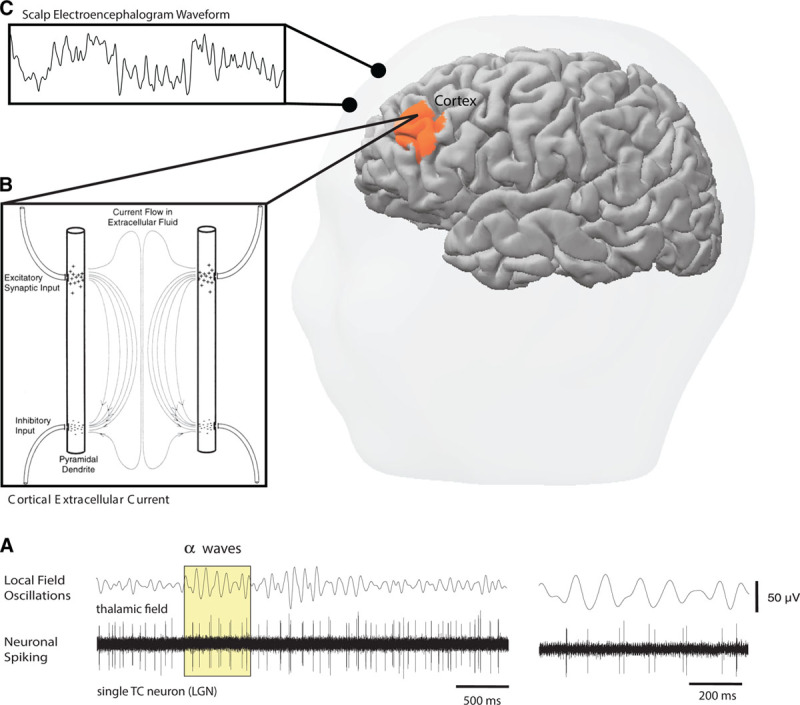
Schematic illustrating the origins of the EEG signal. In the central nervous system, populations of neurons firing in rhythmic patterns produce wave-like postsynaptic potentials of varying frequencies that are thought to help orchestrate both the local and global sharing of information (A). The anatomical geometry of cortical neurons allows for the production of potentials that are large enough to be measured at the scalp (B). Continuously measuring the scalp electrical potentials produced by the cerebral cortex yields the EEG waveform (C). EEG indicates electroencephalogram; LGN, lateral geniculate nucleus; TC, thalamocortical. The Figure is reproduced with permission from Purdon PL, Sampson A, Pavone KJ, Brown EN, “Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures,” Anesthesiology, 2015, 123, 4, 937–960.4
In this article, we will illustrate how EEG monitoring could be used to help manage anesthetic care in children. We do this using a combination of literature review, didactic tutorial, and case studies. The case data were recorded in pediatric patients receiving general anesthesia or sedation. The EEG was observational and not used for clinical management. As we will show, the anesthesia-induced EEG patterns seen in adults are also observed in children, implying that monitoring concepts developed for adult patients could be applied in children as well. We conclude by describing how the EEG changes throughout development, suggesting potential monitoring strategies that could be tailored specifically to infants (0–12 months).
Case 1: A 2-Year-Old Patient Undergoing Endoscopy and Colonoscopy
Overview.
To provide an example of an EEG recording in a clinical setting, we report a case in which a 2-year-old male patient underwent endoscopy and colonoscopy at Massachusetts General Hospital. Clinical vitals collected included blood pressure, heart rate, respiratory rate, and oxygen saturation. A 4-electrode frontal EEG was applied for observational purposes but was not used to guide anesthetic management. The patient received 25 mcg fentanyl and was induced with sevoflurane and nitrous oxide. He was maintained on a propofol infusion at a rate of 100 μg/kg/min, and the endoscopy procedure was well tolerated. However, shortly after the colonoscopy procedure was initiated, the patient began moving in response to a procedural stimulus, and a bolus of propofol was given along with a change in propofol rate to 250 μg/kg/min. The patient subsequently stopped moving, and the remainder of the procedure was uncomplicated.
The EEG Spectrogram.
What was the brain state leading up to the movement at the beginning of the colonoscopy procedure? And how did it compare to the brain state during the remainder of the colonoscopy after administration of the propofol bolus?
These questions can be readily answered by examining the computed EEG spectrogram and the unprocessed EEG waveforms recorded during this case, shown in Figure 2. The unprocessed EEG waveform and EEG spectrogram show patterns that vary in a dose-dependent manner that can track anesthesia-induced brain states.2–4,9,15–18 These stereotypical patterns can be viewed on many commercially available EEG monitors.
Figure 2.
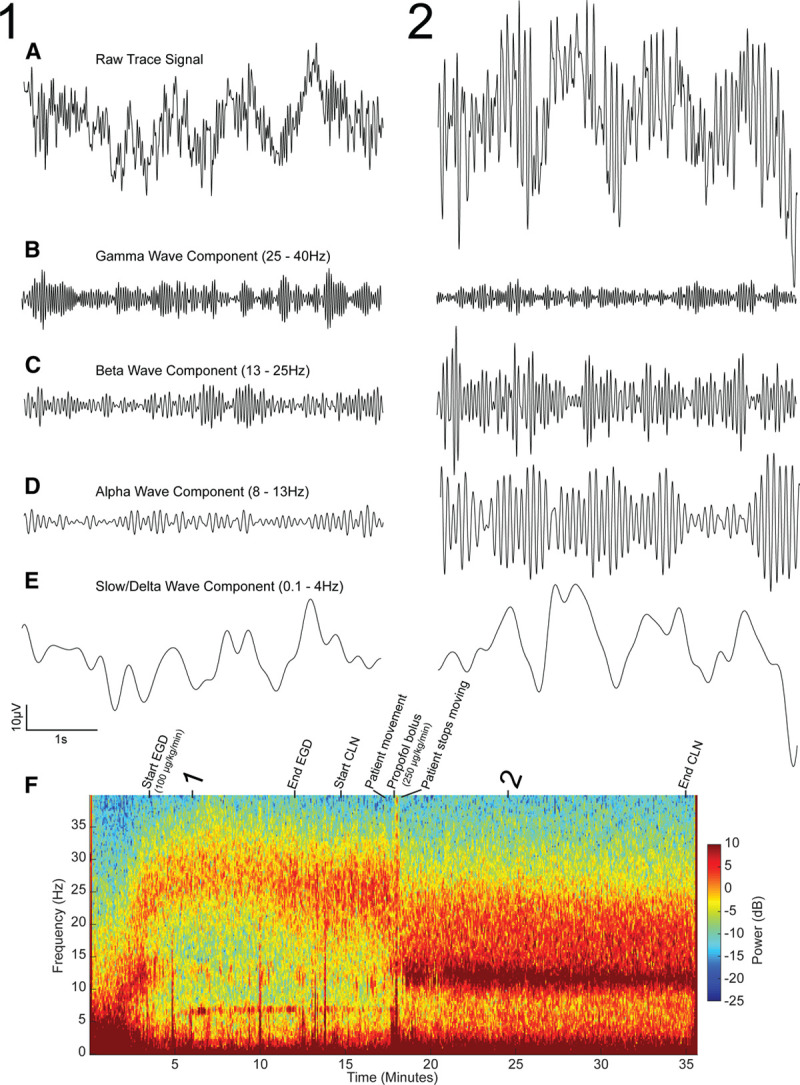
The data presented for the first case involving the 2-year-old patient receiving propofol anesthesia. Representative raw trace signals from timepoints 1 (sedated state) and 2 (fully unconscious state) (A). Bandpass filtered components of the EEG signal presented in A in the gamma (25–40 Hz), beta (13–25 Hz), alpha (8–13 Hz), and slow/delta (0.1–4 Hz) ranges, respectively (B–E). The spectrogram for the full case annotated with procedural events (F). The downward trend in peak frequency into the alpha range tracks the behavioral state of the patient throughout the procedure. CLN indicates colonoscopy; EEG, electroencephalogram; EGD, endoscopy.
The traditional approach for reading the EEG is to examine the unprocessed EEG waveform.4,5,19 This approach can be highly effective because the anesthesia-induced oscillations for the most part are easy to see in the EEG waveform, and because changes in brain state are visible instantaneously.4,5 However, 1 limitation of the unprocessed EEG waveform is that the precise frequency structure of anesthesia-induced brain oscillations can be difficult to discern. Precise visualization of this structure is important because the oscillations will vary over time as a function of changing anesthetic drug doses or patient arousal. To address these limitations, we can use the power spectrum and spectrogram to more precisely visualize the oscillatory structure in the EEG. The power spectrum is a function that indicates the size (power) of oscillations at each frequency. The spectrogram is a variant of the spectrum that displays the power at each frequency over time, making it possible to observe how the frequency structure changes over time. Spectrograms are therefore well suited for visualizing how the EEG changes over time, say during the course of an anesthetic.
The EEG spectrum and spectrogram are first computed by splitting or decomposing the unprocessed EEG waveform (Figure 2A) into its different frequency components.20,21 This decomposition is similar to how a prism splits white light into different frequencies or colors. Just as we use colors to describe light at different frequencies, we use specific nomenclature to describe EEG oscillations at different frequencies—slow (<1 Hz), delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–25 Hz), and gamma (25–40 Hz; Figure 2B–E). Like a painting with different shades of color, the EEG has a subtle structure that is only approximated by these conventionally defined frequency bands. Nonetheless, this nomenclature is a useful way of describing different oscillations in the EEG. The power is related to the amplitude of the EEG wave at a given frequency: power ∝ (amplitude).2 The power is plotted as a function of frequency on a decibel scale, defined as 10 log10 (power), and is represented by a color scale in the spectrogram (Figure 2F).20,21
In our case of the 2-year-old patient, a few prominent frequency bands with high power are visible on the patient’s spectrogram (Figure 2F). At first, we see a low-frequency, slow-delta oscillation, which transitions to high-frequency beta and gamma oscillations within the first 5 minutes (Figure 2F, timepoint 1). However, after approximately 17 minutes, this beta/gamma oscillation decreases in frequency into the beta and alpha frequency range and increases in power (Figure 2F, timepoint 2). At this point, we see 2 oscillations, 1 at an alpha/beta frequency, and 1 at a slow/delta frequency, which persist throughout the remainder of the case. Overall, this patient’s spectrogram illustrates how brain activity in the various frequency ranges can vary throughout a procedure. This is also illustrated in Figure 2B–E, in which the components of the EEG at timepoints 1 and 2 are filtered into different frequency bands. We note that the changes in the EEG spectrogram between timepoints 1 and 2 are easier to discern than the changes in the unprocessed EEG waveform (Figure 2A).
Dose Dependence of the EEG.
It has previously been established in adults that anesthesia-induced EEG changes are dose-dependent.3,9,22–28 Gibbs et al.2 first documented this phenomenon in the 1930s,2 and many subsequent studies have also reported a similar relationship.22–26,28 Altogether, these studies show that increasing the rate of anesthetic administration alters the frequency and amplitude of the oscillations visible on the EEG. Anesthetics that act primarily by potentiating gamma-aminobutyric acid (GABA), such as propofol and the ether derivatives sevoflurane, isoflurane, and desflurane, tend to produce a “slowing” in the EEG in which lower frequency oscillations are amplified and higher frequencies are reduced in power.4,18 When analyzed in a quantitative fashion using the spectrogram, we see that this “slowing” is structured, composed of 2 oscillations, one in the alpha/beta band and another in the slow/delta band.4,18 In the presented case, both the EEG spectrogram and EEG waveform reveal dose-dependent changes similar to those seen in adults.4 The patient was initially maintained on an infusion of propofol to achieve a sedative state for both the endoscopy and colonoscopy. The corresponding spectrogram (Figure 2F) shows higher frequency beta and gamma oscillations appearing shortly after induction that persist as propofol is infused at a rate of 100 µg/kg/min. At around 17 minutes into the procedure, however, shortly after a propofol bolus and an increased infusion rate of 250 μg/kg/min, peak power increases and shifts noticeably to lower frequencies. The filtered EEG components (Figure 2B–E) also illustrate the sharp increase in amplitude in the slow/delta and alpha/beta ranges at timepoint 2 compared to timepoint 1, representing an overall power increase in lower frequencies.
Behavioral States: Sedation, Unconsciousness, and Emergence.
The association between patient behavior and dose-dependent EEG changes has been well characterized in adult patients receiving propofol,3,29 and specific EEG signatures have been correlated with the behavioral states of sedation, unconsciousness, and emergence.3 When adult patients receive lower doses of propofol, inducing a state of sedation in which there remains some probability that the patient may respond to stimuli, the EEG shows increased beta and gamma oscillations (Figure 3A, B).3 In the presented case, an almost identical pattern is visible on the EEG before movement, with a prominent oscillation spanning the beta and gamma frequency ranges (Figure 2F).
Figure 3.
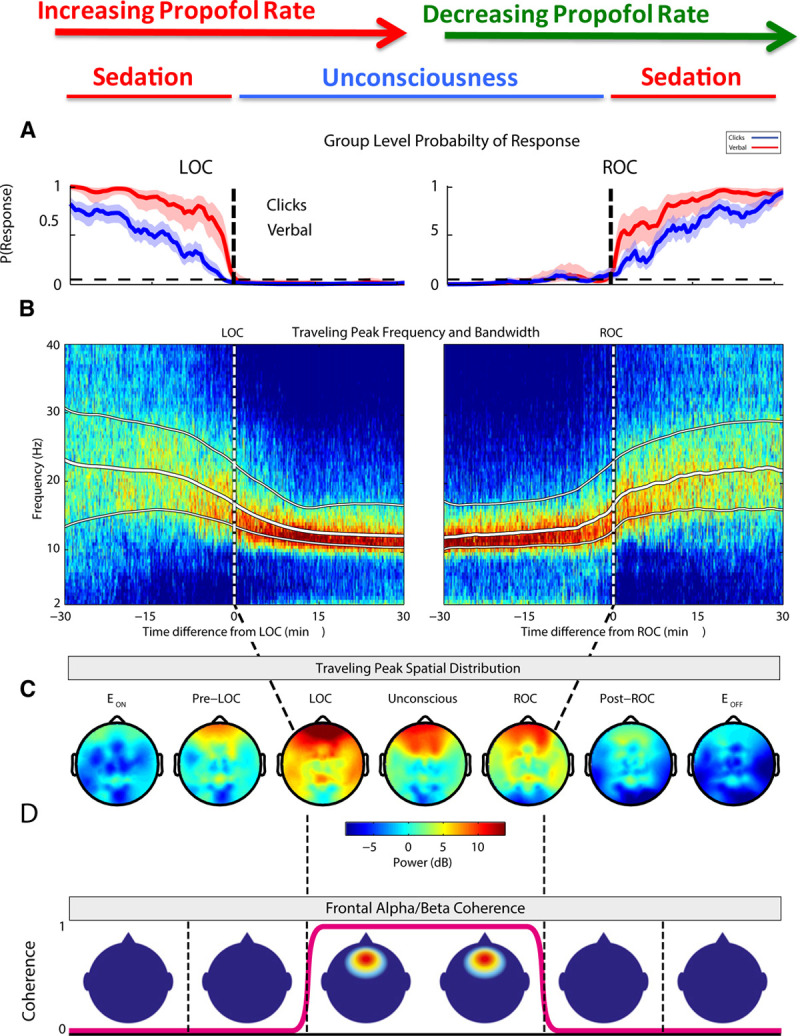
The time course of behavioral and EEG changes in adults in response to a varied infusion rate of propofol. Group-level auditory stimulus response-probability curves (A), baseline-normalized spectrograms plotted with white lines representing the median, 25th, and 75th percentiles of the traveling peak bandwidth (B), and spatial differences in median frequency power (C) and coherence (D), all aligned with LOC and ROC behavioral timepoints. EEG indicates electroencephalogram; LOC, loss of consciousness; ROC, return of consciousness. The Figure was adapted with permission from Purdon et al.3
In adult patients, when higher doses of propofol are administered to produce unconsciousness, prominent alpha and slow oscillations develop, coupled with the loss of beta and gamma oscillations (Figure 3A, B).3,4 Additionally, during the unconscious state, robust alpha oscillations are observed primarily in the frontal leads (Figure 3C) and express high coherence (Figure 3D), which is a measure of correlation between different signals from 2 electrodes at a given frequency. As consciousness begins to return and patients re-enter the sedated state, all of these changes are reversed and the alpha and slow/delta bands appear to “unzip” (Figure 3A, B).3,4 In the presented case, after anesthetic administration is increased in response to patient movement, a distinctive downward shift in frequencies can be seen on the spectrogram (Figure 2F). Prominent high-amplitude alpha oscillations appear, gamma power decreases, and slow/delta power increases, which all coincide with the patient becoming unresponsive to procedural stimuli. Taken as a whole, this case illustrates how EEG patterns associated with sedation and unconsciousness in adults can be readily observed in a 2-year-old patient.
Drug Signatures.
In adults, the anesthesia-induced EEG spectrum has a structure that corresponds to the class or mechanism of the drug being administered.4,15 The spectral signature representing a state of unconsciousness for adults receiving propofol anesthesia shows pronounced bands of slow-delta and alpha power.4 This same pattern is observed in the 2-year-old patient after 17 minutes, when the patient was deemed clinically to be in a state of general anesthesia maintained with propofol. The EEG signature of unconsciousness for sevoflurane, an anesthetic drug commonly used in pediatric practice, and the other ether derivatives, appears similar to that of propofol.4 Both propofol and the ether derivatives are thought to produce unconsciousness primarily through the enhancement of GABAergic inhibition15,27; this common molecular mechanism of action may explain the similarity in their EEG spectra.15,30 One notable difference between the EEG spectra of propofol and sevoflurane is that at higher sevoflurane concentrations, the peak alpha frequency can decrease below what is typically observed under propofol, and increased power may be observed in the theta (4–8 Hz) band.4 The mechanisms for these effects are not known, but could relate to sevoflurane’s actions at sites besides GABA receptors.
The remaining cases in the article feature propofol and sevoflurane as the primary anesthetic drugs. However, it is worth noting that anesthetic drugs appear to have EEG signatures that reflect their underlying drug class (eg, dexmedetomidine as an α-2 agonist, ketamine as an N-Methyl-d-aspartic acid [NMDA] antagonist).4
Age Dependence.
We saw how the form of the propofol-induced spectrogram in this 2-year-old patient was similar to that of an adult. In some ways, this is surprising, since we know that the brains of young children are still developing and are quite different from adults. Recent studies of the anesthesia-induced EEG in children show that, while the form of the oscillations is the same in adults and children greater than about 1 year of age, these oscillations are much larger in children, that is, the oscillations have higher power in children.31,32 As we see in Figure 4A, B, throughout development, overall spectral power increases from birth until about 5–8 years of age and then declines over the next 15 years until a plateau is reached in young adulthood.31,32 These changes in overall spectral power appear to parallel underlying neuronal development.34–36 We see also that the spectrograms of children >1 year of age receiving propofol (Figure 4C) and sevoflurane (Figure 4D) general anesthesia have the slow-delta and alpha band signature seen in adults.31,32,37,38 This age-dependent effect is also observed in the presented case, in which EEG power is increased at all frequencies compared to the typical adult spectrum, but with distinct peaks in the slow-delta and alpha frequencies that are the key indicators of the unconscious state in adults receiving propofol or sevoflurane.
Figure 4.
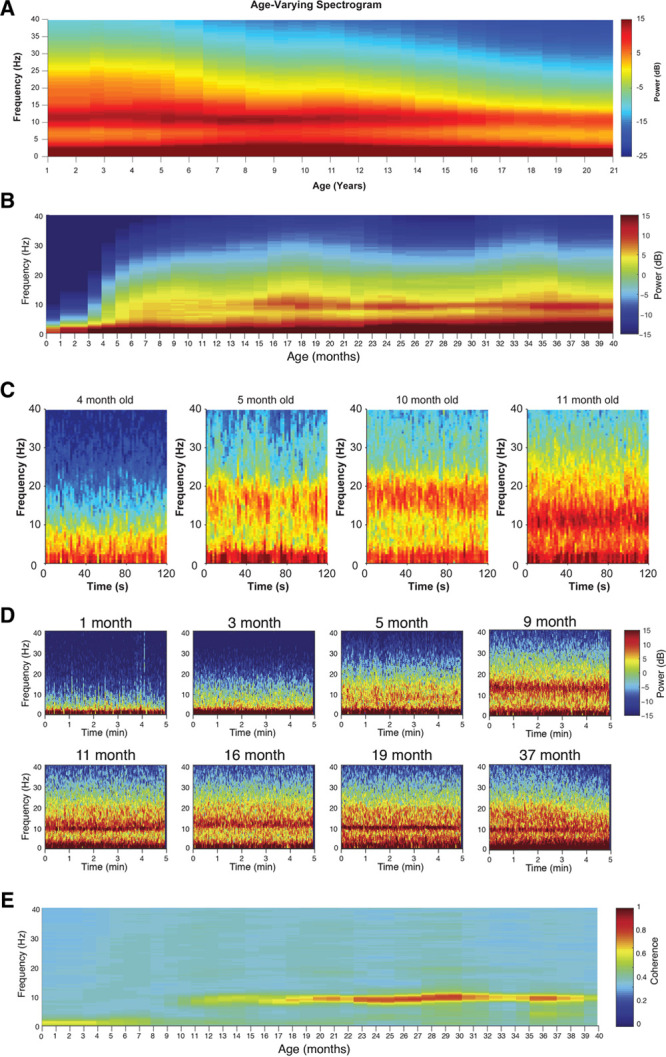
Combined spectrograms of propofol-induced frontal EEG activity for patients 1–21 y of age. Prominent alpha and slow oscillations are present across the entire age range in spite of shifts in overall power that are dependent on age (A). Combined spectrograms of sevoflurane-induced, frontal EEG activity for patients 0–40 mo of age (B). Four individual spectrograms of frontal EEG power from infants receiving propofol anesthesia across the first year of life (C). Individual spectrograms of frontal EEG power from infants receiving sevoflurane anesthesia across the first 3 y of life (D). High-frequency power is largely absent in 0- to 4-mo-old infants, but an alpha/beta oscillation emerges around 9 mo that travels downward in peak frequency until around 1 y of age (C, D). Combined coherograms of sevoflurane-induced, frontal EEG activity for patients 0–40 mo of age (E). Only recordings when patients were maintained at surgical levels of anesthesia are presented. EEG indicates electroencephalogram. Figure panels B, D, and E were adapted from the British Journal of Anaesthesia, 120, Cornelissen L, Kim SE, Lee JM, Brown EN, Purdon PL, Berde CB, “Electroencephalographic Markers of Brain Development During Sevoflurane Anaesthesia in Children up to 3 years old,” 1274–1286, 2018, with permission from Elsevier.33 Figure panels A and C were adapted from Lee JM, Akeju O, Terzakis K, et al, “A Prospective Study of Age-Dependent Changes in Propofol-Induced Electroencephalogram Oscillations in Children,” Anesthesiology, 2017, 127, 2, 293–306.32
Discussion.
Currently, the approach to administering anesthetic drugs is based on pharmacokinetic and pharmacodynamic (PK/PD) models to inform drug dose or infusion rate. These models often include covariates such as the patient’s age, weight, and sex. However, even when this information is used, predicted effect-site drug concentration can be an imprecise predictor of unconsciousness, with wide interindividual variability in the predicted effect-site concentration necessary to produce loss of consciousness.39 Furthermore, since PK/PD models vary significantly by age40,41 and children grow and develop at different rates, it is difficult to extrapolate these models to the pediatric population. Although there are several proposed models for the pediatric population, there is not a consensus for which of these models is the most reliable.42
In an attempt to convey direct information about the brain that would be relevant to anesthetic drug administration, processed EEG monitors were developed from the EEG to represent a patient’s level of consciousness as a single value “index” between 0 and 100.43–46 However, these monitors do not adjust for the underlying neural mechanisms of each anesthetic drug and are instead believed to operate under the assumption that all anesthetic drugs induce unconsciousness through EEG slowing with increasing doses, despite evidence showing this trend to be true for only certain drugs and not others.2–4,15,24 Processed EEG monitors also do not correct for the individual neurophysiology of each patient, which has been shown to vary greatly with age.32,33,47–49 Perhaps unsurprisingly, these indices have been ineffective in reducing the incidence of patient awareness during surgery in adults,50,51 with even greater variation in the pediatric population.52,53 Recent evidence has shown that processed EEG monitors perform particularly worse in the infant population,54,55 which is consistent with the knowledge that young infants show different EEG patterns under anesthesia from those observed in older children and adults.31–33,38 In particular, we have hypothesized that in many cases, appropriately anesthetized children would have inappropriately “high” index readings, incorrectly suggesting a “lighter” state of unconsciousness. We make this prediction based on the fact that most index-based processed EEG monitors use higher frequency beta and gamma power as an indicator of “lightening” anesthesia, yet power in these frequencies are elevated in children even when they are adequately anesthetized.31,32 Alternatively, a number of groups have recently advocated for using the unprocessed EEG and EEG spectrogram to manage the brain states of adult patients during sedation and general anesthesia.3,4,31,37 Given the broad similarities in the form of the EEG signal in children and adults, this same approach would provide a reasonable alternative to existing 0–100 processed EEG monitors that do not account for a child’s state of brain development.
Case 2: A 9-Month-Old Female Patient Undergoing a Ureteroureterostomy
Unique Features of the Anesthesia-Induced EEG in Infants.
The previous case illustrated how the EEG signatures of propofol-induced sedation and unconsciousness in a 2-year-old patient were similar in form to those of adults. These EEG patterns are consistently present in children >1 year of age.31–33,56 Do the same relationships hold for infants?
Infants show EEG patterns under anesthesia that are different from those observed in older children and adults.31–33,38 Infants receiving propofol (Figure 4C) and sevoflurane (Figure 4D) for general anesthesia present the signature slow power band, but there are notable differences in the higher frequency ranges and in coherence that occur within the first 12 months.31–33,38 While slow power seems to gradually increase from birth until 1 year of age, oscillations within the 5–20 Hz range are mostly absent until 4 months of age (Figure 4B–D).33,38,47 A wide band of beta oscillations appears around this time and becomes progressively more consolidated into the alpha band through the remainder of the first year of life (Figure 4B–D).33 Surprisingly, the alpha oscillations do not become coherent until approximately 1 year of age (Figure 4E).31,33 Despite such developmental differences, EEG monitoring strategies can still be applied to the infant population, so long as the age of the patient and the associated age-dependent EEG features are taken into account.
Overview.
To provide a clinical example of EEG recording from the infant population, we report a case in which a 9-month-old female patient underwent a ureteroureterostomy procedure at Boston Children’s Hospital (Figure 5). Clinical vitals collected during the procedure included heart rate, blood pressure, end-tidal CO2, end-tidal sevoflurane, end-tidal isoflurane, and oxygen saturation. A 33-electrode EEG cap was applied as part of a research study (approved by the Boston Children’s Hospital Institutional Review Board) but was again not used to guide anesthetic management.
Figure 5.
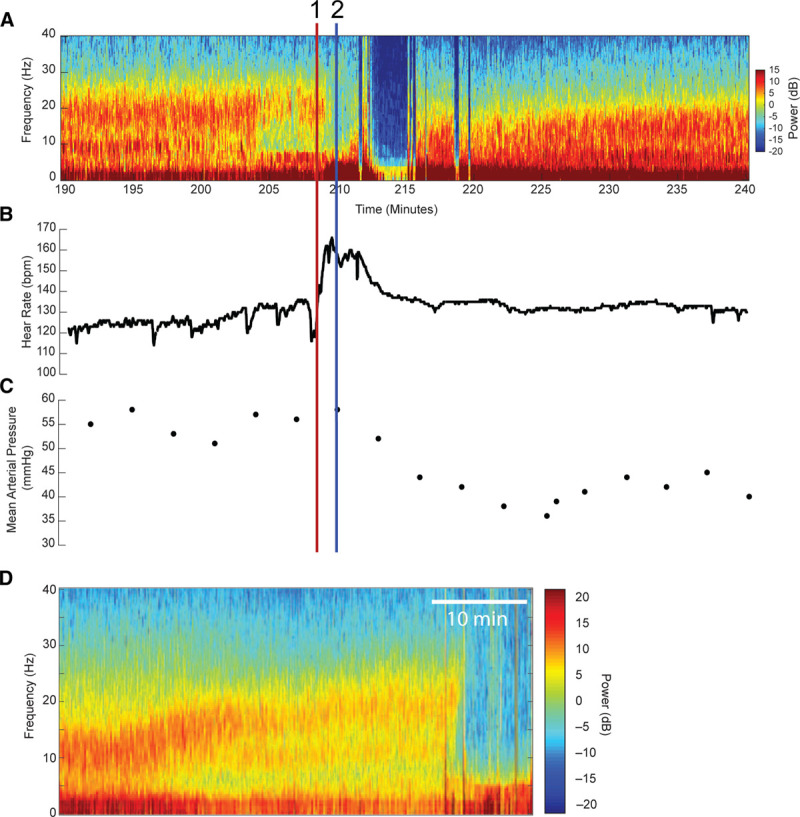
The data presented for the second case involving the 9-mo-old patient receiving inhaled anesthesia. The raw spectrogram for the period of the procedure involving multiple changes in the patient’s brain and behavioral states (A). Signatures of awakening, profound unconsciousness, and adequate unconsciousness are all present and easily identifiable despite the patient’s young age. Changes in the EEG spectrogram preceded changes in patient behavior, while changes in heart rate (B) and blood pressure (C) both followed the awakening event (timepoint 1, red line). The blue line (timepoint 2) represents the timepoint when the patient stops moving. D, An identical pattern of brain and behavioral activity has been previously reported by Cornelissen et al.33 EEG indicates electroencephalogram.
General anesthesia was induced using sevoflurane. Rocuronium was administered and the trachea was intubated. A caudal block was administered using bupivacaine 0.25%, 7.5 mL. General anesthesia was maintained with isoflurane and nitrous oxide. Fentanyl was administered 3 times during the procedure: at minutes 11 (10 µg), 124 (5 µg), and 274 (10 µg). Morphine was given at minutes 37 (0.2 mg) and 357 (0.3 mg). Approximately 208 minutes after surgery start, the patient started moving in response to surgical stimulus (Figure 5, timepoint 1 red line), with a subsequent increase in heart rate to 167 bpm (Figure 5B) and transient decrease in oxygen saturation to 80%. A propofol bolus of 40 mg was given, and the end-tidal concentration of isoflurane was increased from 0.7% to 1.2%. The patient subsequently stopped moving (Figure 5, timepoint 2 blue line), with a decrease in heart rate back to ~130 bpm and drop in mean arterial pressure to 35 mm Hg (Figure 5B, C). The remainder of the case was uneventful.
The EEG spectrogram for this case shows visible features corresponding to, and even anticipating, the key events. The EEG spectrogram (Figure 5A) shows a prominent slow/delta band as well as an expanded band of alpha and beta power (7–23 Hz) from minutes 190 to 204, and clinical observations made during this time indicate the patient was unconscious and unresponsive. After minute 204, the power within the 7–15 Hz range is attenuated, and the EEG spectrogram shows a pronounced beta oscillation spanning 15–23 Hz. Four minutes later, at minute 208, the patient moves (Figure 5, timepoint 1 red line). The patient is administered a bolus of propofol around this time and movement ceases, coincident with a sharp increase in slow/delta power around minute 210 (Figure 5, timepoint 2), followed by a period of burst suppression lasting ~6 minutes. This period of burst suppression includes long periods of isoelectricity, in which the EEG waveform is flat, reflecting the absence of cortical activity. These isoelectric periods appear as dark blue vertical bands in the spectrogram. A second brief episode of isoelectric EEG is also observed at around 218 minutes into the case. After this, the patient’s EEG shows a progressive return to the original pattern of slow/delta and broad alpha and beta oscillations, seen between minutes 190 and 204.
Discussion.
This case (Figure 5A) illustrates how the EEG could potentially be used to monitor the drug-specific and dose-dependent effects of anesthetic drugs in infants. As reported by Cornelissen et al,33 sevoflurane-induced unconsciousness in 9-month-old patients (Figure 4D) is associated with EEG power in the slow/delta and alpha and beta (10–20 Hz) frequency bands. We see this pattern in the case of this 9-month-old patient from minutes 198 to 204 (Figure 5A), suggesting that the patient was unconscious during this time. From minutes 204 to 208 and before patient movement, there is a noticeable shift seen on the EEG: beta oscillations begin to shift upward in frequency and become weaker, while power within the 7–15 Hz range disappears. These changes appear consistent with the previously discussed “unzipping” pattern of emergence from anesthesia in adults (Figure 3)3,4 and in children57 and suggest that the patient entered a “lighter” anesthetic state during this time. Given that the patient began to move as this state progressed, the spectrogram pattern between minutes 204 and 208 likely represents the infant equivalent of emergence seen in older patients.33 A recent study of the EEG signatures of emergence in children reports loss of alpha oscillations before gross body movement in children as young as 4 months. Figure 5D shows an example of this effect in the spectrogram from an 8-month-old patient emerging from sevoflurane in N2O/O2, showing the same “unzipping” pattern.57 In the present case, after the propofol bolus, there is a significant increase in slow power (minutes 209–212), which is a characteristic change seen in adults representing a deeper state of unconsciousness.4 The following 6 minutes of EEG recording shows a spectrogram that is blue at nearly all frequencies above approximately 1 Hz, indicating that the EEG signal has significantly diminished in size. This spectrogram corresponds to a state of burst suppression, an EEG pattern characterized by alternating periods of activity (“bursts”) and periods of electrical silence (“suppressions”), the latter of which may last seconds to 10s of seconds, or even longer.58,59 (In some literature, these suppression periods may also be referred to as “discontinuities.”)48 As the effects of the propofol bolus wear off, the patient’s spectrogram transitioned back to the slow/delta and expanded alpha and beta power band pattern seen when the patient was unconscious and not moving.
Both case 1 and case 2 illustrate how the EEG can be used to monitor brain states and anticipate events before they occur. In case 2, the EEG revealed signs of emergence ~5 minutes before gross body movement. In contrast, the heart rate, the more conventional means of anesthetic monitoring, did not change until movement was observed. Early recognition of EEG pattern changes suggestive of emergence from general anesthesia could have theoretically prevented the movement and anesthetic overcorrection seen in both case 1 and case 2. The overcorrection observed in case 2 was notable for a number of reasons. First, this overcorrection appears to have had a major systemic physiological side effect, as the infant’s mean arterial pressure subsequently dropped by 20 mm Hg. Recent literature suggests that significant changes in mean arterial pressure from baseline could result in poor postoperative outcomes.60 Second, it produced a striking episode of burst suppression and isoelectricity, which we will discuss in greater detail in the next case.
Case 3: A 19-Year-Old Autistic Patient Undergoing Sigmoidoscopy
Overview.
To provide another example of burst suppression, we next present a case in which a 19-year-old male patient with severe autism and chronic constipation complicated by rectal ulcer was scheduled for elective anal rectal motility studies and sigmoidoscopy at Massachusetts General Hospital. The patient was nonverbal and had been placed in a residential facility for behavioral issues including aggression. Preoperative medications included benztropine, valproate, escitalopram, olanzapine, mesalamine, and omeprazole. Although antiepileptic medication had been prescribed due to multifocal epileptiform discharges identified on EEG at age 8, the patient did not carry a formal diagnosis of epilepsy. The patient received oral ketamine 3.3 mg/kg and midazolam 0.2 mg/kg for premedication. Monitoring in the endoscopy suite included noninvasive blood pressure cuff, 5-lead electrocardiogram (ECG), pulse oximetry, and end-tidal CO2. A 4-lead frontal EEG was again recorded for observational purposes only and was not being monitored by the clinical team to manage the anesthetic.
The patient underwent anal rectal motility studies before propofol administration to better assess baseline rectal tone. During this time, approximately 6 minutes of EEG were recorded, showing broad beta-band power (Figure 6). Subsequently, the patient was induced with a propofol bolus of 70 mg and a propofol infusion of 350 µg/kg/min was initiated. After the induction period, the EEG spectrogram shows a combination of slow-delta oscillations and beta oscillations, gradually transitioning to slow-delta and alpha oscillations (Figure 6A, timepoint A).
Figure 6.
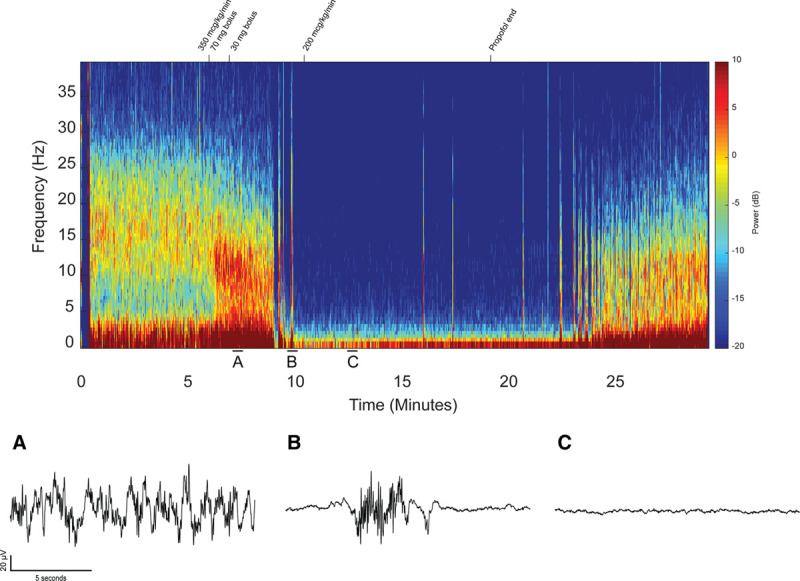
The full case raw spectrogram for the 19-y-old receiving primarily propofol anesthesia highlighted by a prolonged state of isoelectricity. The spectrogram is annotated with the changes in propofol infusion that occurred throughout the case. Representative raw trace signals from timepoints A (fully unconscious state), B (profoundly unconscious state of burst suppression), and C (profoundly unconscious state of isoelectricity) are presented.
Approximately 3 minutes after the start of the propofol infusion, there is a period of burst suppression lasting ~60 seconds on the EEG. The raw trace signal (Figure 6B) at timepoint B further illustrates the alternating burst and suppression epochs characteristic of burst suppression. At this time, the patient became apneic, and oxygen saturation transiently decreased below 90% for approximately 1 minute, reaching a minimum of 82% oxygen saturation. Subsequently, a very prolonged period of suppression is visible on the EEG spectrogram, lasting a total of 12 minutes, despite the fact that the propofol infusion rate had been reduced to 200 µg/kg/min shortly after minute 10. This prolonged suppression period, sometimes referred to as “isoelectric” in the EEG literature, appears as a flatline on the EEG waveform at timepoint C (Figure 6C). This isoelectric pattern persists throughout a period of time when the patient is hemodynamically stable and oxygen saturation was >99%, belying the fact that the patient was in a profoundly unconscious state equivalent to a deep state of coma. About 2 minutes after the propofol infusion was stopped, there is a second period of burst suppression lasting ~3 minutes. Just before the EEG electrodes were removed for transport, the EEG shows slow-delta and alpha oscillations characteristic of continued propofol-induced unconsciousness.
The patient failed to emerge from unconsciousness for over 2 hours. Throughout his delayed emergence, the patient had remained hemodynamically stable with spontaneous ventilation. However, he did not arouse to vocal or physical stimulation, and demonstrated 0/5 motor tone on neurological examination. He was ultimately discharged almost 3 hours after his endoscopic procedure had ended.
Discussion.
In this case, the patient carried a diagnosis of severe autism, which has been associated with a disruption of GABAergic signaling and a high rate of comorbid epilepsy and epileptiform EEG signs.61,62 Although autistic patients are thought to have higher anesthetic requirements, evidence from a recent study suggests that they may actually have lower anesthetic requirements: autistic patients are twice as likely to be in a state of burst suppression compared to neurotypically developing children at comparable anesthetic doses.56 Additionally, even in the absence of clinical epilepsy, the use of antiepileptics such as valproic acid has been associated with decreased anesthetic requirements.63 Thus, it is possible that autistic patients, by way of their underlying neuropathology, their use of medications to control autism-related comorbidities, or both, could have lower anesthetic requirements. The patient also received premedication with ketamine and midazolam. Ketamine’s antagonistic effect on NMDA receptors, at low doses, promotes cortical excitability due to selective inhibition of GABAergic interneurons, which is thought to produce gamma-band oscillations.4,15 Midazolam at sedative doses is associated with beta oscillations.4,15 We hypothesize that the beta and gamma oscillations we observed at the beginning of the case reflect the combined effect of ketamine and midazolam.64
As emphasized earlier, EEG burst suppression suggests a level of anesthesia-induced unconsciousness beyond what is required for sedation or general anesthesia. We hypothesize that this prolonged state of EEG suppression could have contributed to this patient’s delayed emergence, consistent with previously documented postoperative effects associated with intraoperative burst suppression.65,66 As in the previous case, overadministration of anesthetic drugs can have other consequences; in this case, it may have contributed to respiratory depression and a drop in oxygen saturation to 82%. Given this patient’s complex history, it would be difficult to predict the patient’s anesthetic requirements a priori. The apparent overadministration of anesthetics in this case was not appreciated by standard physiological monitors such as ECG, blood pressure, pulse oximetry, and respiratory rate at the time. On the other hand, it is clear from the EEG recorded during this case that the typical EEG patterns associated with propofol-induced unconsciousness were visible, including slow and alpha waves, early in the case, as well as burst suppression and isoelectricity later in the case. Therefore, the EEG could have been used clinically to identify these readily recognizable patterns and to manage the anesthetic dosing and choice of anesthetic medications accordingly. More generally, this case provides another example of how the EEG can be used to provide individualized real-time feedback on a patient’s brain state during general anesthesia.
CONCLUSIONS AND FUTURE DIRECTIONS
In the first years of life, the brain undergoes rapid developmental changes. Although many features of the anesthesia-induced EEG signal change during this time, the overall qualitative features of this signal, as well as the dose-dependent changes, mirror those seen in adults.4 Therefore, direct interpretation of the EEG and EEG spectrogram, as illustrated in this article, could provide a reasonable and more informative alternative for monitoring the brain during general anesthesia and sedation than standard vital signs monitoring alone. It would be feasible for anesthesiologists to learn to recognize age- and drug-specific EEG signatures, as described here and elsewhere, to aid in anesthetic management.3,4,31–33,38 Use of the EEG in this way could enable anesthesiologists to better assess the drug requirements of individual patients in real time and improve patient safety and experience. In addition, characterizing age-dependent changes in anesthesia-induced EEG patterns in children and infants could offer a unique window into the mechanisms of brain development.31–33
DISCLOSURES
Name: Steven P. Brandt, BA.
Contribution: This author helped conceive and write major sections of the manuscript and designed figures.
Conflicts of Interest: None.
Name: Elisa C. Walsh, MD.
Contribution: This author helped collect case data and write sections of the manuscript.
Conflicts of Interest: None.
Name: Laura Cornelissen, PhD.
Contribution: This author helped collect case data.
Conflicts of Interest: None.
Name: Johanna M. Lee, MD.
Contribution: This author helped collect case data and write sections of the manuscript.
Conflicts of Interest: None.
Name: Charles Berde, MD, PhD.
Contribution: This author helped collect case data.
Conflicts of Interest: None.
Name: Erik S. Shank, MD.
Contribution: This author helped collect case data.
Conflicts of Interest: None.
Name: Patrick L. Purdon, PhD.
Contribution: This author helped conceive and write major sections of the manuscript.
Conflicts of Interest: P. L. Purdon is an inventor on pending patents on brain monitoring using the electroencephalogram. One of these patents is under nonexclusive license by Massachusetts General Hospital to Masimo Corporation. P. L. Purdon receives institutionally distributed licensing royalties for this license. P. L. Purdon is also a co-founder of PASCALL Systems, Inc, a start-up company developing closed-loop physiological control for anesthesiology. P. L. Purdon has received speaker’s honoraria from Masimo Corporation.
This manuscript was handled by: Maxime Cannesson, MD, PhD.
FOOTNOTES
GLOSSARY
- CLN =
- colonoscopy
- ECG =
- electrocardiogram
- EEG =
- electroencephalogram
- EGD =
- endoscopy
- GABA =
- gamma-aminobutyric acid
- LGN =
- lateral geniculate nucleus
- LOC =
- loss of consciousness
- NMDA =
- N-Methyl-d-aspartic acid
- PK/PD =
- pharmacokinetic and pharmacodynamic
- ROC =
- return of consciousness
- TC =
- thalamocortical
Funding: None.
Conflicts of Interest: See Disclosures at the end of the article.
Reprints will not be available from the authors.
Listen to this Article of the Month podcast and more from OpenAnesthesia.org® by visiting http://journals.lww.com/anesthesia-analgesia/pages/default.aspx.
REFERENCES
- 1.DeFrances CJ, Cullen KA, Kozak LJ. National hospital discharge survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007;165:1–209. [PubMed] [Google Scholar]
- 2.Gibbs FA, Gibbs EL, Lennox WG. Effect on the electro-encephalogram of certain drugs which influence nervous activity. Arch Intern Med (Chic). 1937;60:154–166. [Google Scholar]
- 3.Purdon PL, Pierce ET, Mukamel EA, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–E1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical electroencephalography for anesthesiologists: part I: background and basic signatures. Anesthesiology. 2015;123:937–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett C, Voss LJ, Barnard JP, Sleigh JW. Practical use of the raw electroencephalogram waveform during general anesthesia: the art and science. Anesth Analg. 2009;109:539–550. [DOI] [PubMed] [Google Scholar]
- 6.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hämäläinen M, Hari R, Ilmoniemi R, Knuutila J, Lounasmaa OV. Magnetoencephalography: theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413. [Google Scholar]
- 8.Buzsáki G. Rhythms of the Brain. 2006New York, NY: Oxford University Press; [Google Scholar]
- 9.Cimenser A, Purdon PL, Pierce ET, et al. Tracking brain states under general anesthesia by using global coherence analysis. Proc Natl Acad Sci U S A. 2011;108:8832–8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supp GG, Siegel M, Hipp JF, Engel AK. Cortical hypersynchrony predicts breakdown of sensory processing during loss of consciousness. Curr Biol. 2011;21:1988–1993. [DOI] [PubMed] [Google Scholar]
- 12.Lewis LD, Weiner VS, Mukamel EA, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–E3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, Steyn-Ross ML, Steyn-Ross DA, Wilson MT, Sleigh JW. EEG slow-wave coherence changes in propofol-induced general anesthesia: experiment and theory. Front Syst Neurosci. 2014;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vizuete JA, Pillay S, Ropella KM, Hudetz AG. Graded defragmentation of cortical neuronal firing during recovery of consciousness in rats. Neuroscience. 2014;275:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akeju O, Pavone KJ, Westover MB, et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014;121:978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akeju O, Westover MB, Pavone KJ, et al. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology. 2014;121:990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prerau MJ, Brown RE, Bianchi MT, Ellenbogen JM, Purdon PL. Sleep neurophysiological dynamics through the lens of multitaper spectral analysis. Physiology (Bethesda). 2017;32:60–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babadi B, Brown EN. A review of multitaper spectral analysis. IEEE Trans Biomed Eng. 2014;61:1555–1564. [DOI] [PubMed] [Google Scholar]
- 21.Mitra P, Bokil H. Observed Brain Dynamics. 2007New York, NY: Oxford University Press; [Google Scholar]
- 22.Martin JT, Faulconer A, Jr, Bickford RG. Electroencephalography in anesthesiology. Anesthesiology. 1959;20:359–376. [DOI] [PubMed] [Google Scholar]
- 23.Faulconer A, Pender JW, Bickford RG. The influence of partial pressure of nitrous oxide on the depth of anesthesia and the electro-encephalogram in man. Anesthesiology. 1949;10:601–609. [DOI] [PubMed] [Google Scholar]
- 24.Kiersey DK, Bickford RG, Faulconer A., Jr. Electro-encephalographic patterns produced by thiopental sodium during surgical operations; description and classification. Br J Anaesth. 1951;23:141–152. [DOI] [PubMed] [Google Scholar]
- 25.Faulconer A., Jr. Correlation of concentrations of ether in arterial blood with electro-encephalographic patterns occurring during ether-oxygen and during nitrous oxide, oxygen and ether anesthesia of human surgical patients. Anesthesiology. 1952;13:361–369. [DOI] [PubMed] [Google Scholar]
- 26.Bart AJ, Homi J, Linde HW. Changes in power spectra of electroencephalograms during anesthesia with fluroxene, methoxyflurane and ethrane. Anesth Analg. 1971;50:53–63. [PubMed] [Google Scholar]
- 27.Gugino LD, Chabot RJ, Prichep LS, John ER, Formanek V, Aglio LS. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth. 2001;87:421–428. [DOI] [PubMed] [Google Scholar]
- 28.Tinker JH, Sharbrough FW, Michenfelder JD. Anterior shift of the dominant EEG rhytham during anesthesia in the Java monkey: correlation with anesthetic potency. Anesthesiology. 1977;46:252–259. [DOI] [PubMed] [Google Scholar]
- 29.Feshchenko VA, Veselis RA, Reinsel RA. Propofol-induced alpha rhythm. Neuropsychobiology. 2004;50:257–266. [DOI] [PubMed] [Google Scholar]
- 30.Ishizawa Y, Ahmed OJ, Patel SR, et al. Dynamics of propofol-induced loss of consciousness across primate neocortex. J Neurosci. 2016;36:7718–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akeju O, Pavone KJ, Thum JA, et al. Age-dependency of sevoflurane-induced electroencephalogram dynamics in children. Br J Anaesth. 2015;115(suppl 1):i66–i76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JM, Akeju O, Terzakis K, et al. A prospective study of age-dependent changes in propofol-induced electroencephalogram oscillations in children. Anesthesiology. 2017;127:293–306. [DOI] [PubMed] [Google Scholar]
- 33.Cornelissen L, Kim SE, Lee JM, Brown EN, Purdon PL, Berde CB. Electroencephalographic markers of brain development during sevoflurane anaesthesia in children up to 3 years old. Br J Anaesth. 2018;120:1274–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petanjek Z, Judaš M, Šimic G, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murata Y, Colonnese MT. Thalamic inhibitory circuits and network activity development. Brain Res. 2019;1706:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Shen D, Lin W. Resting-state functional MRI studies on infant brains: a decade of gap-filling efforts. Neuroimage. 2019;185:664–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purdon PL, Pavone KJ, Akeju O, et al. The ageing brain: age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth. 2015;115(suppl 1):i46–i57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornelissen L, Kim SE, Purdon PL, Brown EN, Berde CB. Age-dependent electroencephalogram (EEG) patterns during sevoflurane general anesthesia in infants. Elife. 2015;4:e06513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwakiri H, Nishihara N, Nagata O, Matsukawa T, Ozaki M, Sessler DI. Individual effect-site concentrations of propofol are similar at loss of consciousness and at awakening. Anesth Analg. 2005;100:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnider TW, Minto CF, Gambus PL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88:1170–1182. [DOI] [PubMed] [Google Scholar]
- 41.Schnider TW, Minto CF, Shafer SL, et al. The influence of age on propofol pharmacodynamics. Anesthesiology. 1999;90:1502–1516. [DOI] [PubMed] [Google Scholar]
- 42.Sepúlveda P, Cortínez LI, Sáez C, et al. Performance evaluation of paediatric propofol pharmacokinetic models in healthy young children. Br J Anaesth. 2011;107:593–600. [DOI] [PubMed] [Google Scholar]
- 43.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847. [DOI] [PubMed] [Google Scholar]
- 44.Schneider G, Gelb AW, Schmeller B, Tschakert R, Kochs E. Detection of awareness in surgical patients with EEG-based indices--bispectral index and patient state index. Br J Anaesth. 2003;91:329–335. [DOI] [PubMed] [Google Scholar]
- 45.Prichep LS, Gugino LD, John ER, et al. The patient state index as an indicator of the level of hypnosis under general anaesthesia. Br J Anaesth. 2004;92:393–399. [DOI] [PubMed] [Google Scholar]
- 46.Jäntti V, Alahuhta S. Spectral entropy–what has it to do with anaesthesia, and the EEG? Br J Anaesth. 2004;93:150–151. [DOI] [PubMed] [Google Scholar]
- 47.Sury MR, Worley A, Boyd SG. Age-related changes in EEG power spectra in infants during sevoflurane wash-out. Br J Anaesth. 2014;112:686–694. [DOI] [PubMed] [Google Scholar]
- 48.Cornelissen L, Bergin AM, Lobo K, Donado C, Soul JS, Berde CB. Electroencephalographic discontinuity during sevoflurane anesthesia in infants and children. Paediatr Anaesth. 2017;27:251–262. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi K, Shigemi K, Sawa T. Neonatal electroencephalography shows low sensitivity to anesthesia. Neurosci Lett. 2012;517:87–91. [DOI] [PubMed] [Google Scholar]
- 50.Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–1108. [DOI] [PubMed] [Google Scholar]
- 51.Avidan MS, Jacobsohn E, Glick D, et al. ; BAG-RECALL Research Group. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365:591–600. [DOI] [PubMed] [Google Scholar]
- 52.Samarkandi AH. The bispectral index system in pediatrics–is it related to the end-tidal concentration of inhalation anesthetics? Middle East J Anaesthesiol. 2006;18:769–778. [PubMed] [Google Scholar]
- 53.Tirel O, Wodey E, Harris R, Bansard JY, Ecoffey C, Senhadji L. Variation of bispectral index under TIVA with propofol in a paediatric population. Br J Anaesth. 2008;100:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sciusco A, Standing JF, Sheng Y, Raimondo P, Cinnella G, Dambrosio M. Effect of age on the performance of bispectral and entropy indices during sevoflurane pediatric anesthesia: a pharmacometric study. Paediatr Anaesth. 2017;27:399–408. [DOI] [PubMed] [Google Scholar]
- 55.McKeever S, Johnston L, Davidson AJ. Sevoflurane-induced changes in infants’ quantifiable electroencephalogram parameters. Paediatr Anaesth. 2014;24:766–773. [DOI] [PubMed] [Google Scholar]
- 56.Walsh EC, Lee JM, Terzakis K, et al. Age-dependent changes in the propofol-induced electroencephalogram in children with autism spectrum disorder. Front Syst Neurosci. 2018;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cornelissen L, Donado C, Lee JM, et al. Clinical signs and electroencephalographic patterns of emergence from sevoflurane anaesthesia in children: an observational study. Eur J Anaesthesiol. 2018;35:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A. 2012;109:3095–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis LD, Ching S, Weiner VS, et al. Local cortical dynamics of burst suppression in the anaesthetized brain. Brain. 2013;136:2727–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCann ME, Schouten AN, Dobija N, et al. Infantile postoperative encephalopathy: perioperative factors as a cause for concern. Pediatrics. 2014;133:e751–e757. [DOI] [PubMed] [Google Scholar]
- 61.Robertson CE, Ratai E-M, Kanwisher N. Reduced GABAergic action in the autistic brain. Curr Biol. 2016;26:80–85. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto T, Sasaki M, Sugai K, Hanaoka S, Fukumizu M, Kato T. Paroxysmal discharges on EEG in young autistic patients are frequent in frontal regions. J Med Invest. 2001;48:175–180. [PubMed] [Google Scholar]
- 63.Ouchi K, Sugiyama K. Required propofol dose for anesthesia and time to emerge are affected by the use of antiepileptics: prospective cohort study. BMC Anesthesiol. 2015;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forsyth A, McMillan R, Campbell D, et al. Comparison of local spectral modulation, and temporal correlation, of simultaneously recorded EEG/fMRI signals during ketamine and midazolam sedation. Psychopharmacology (Berl). 2018;235:3479–3493. [DOI] [PubMed] [Google Scholar]
- 65.Fritz BA, Kalarickal PL, Maybrier HR, et al. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg. 2016;122:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andresen JM, Girard TD, Pandharipande PP, Davidson MA, Ely EW, Watson PL. Burst suppression on processed electroencephalography as a predictor of postcoma delirium in mechanically ventilated ICU patients. Crit Care Med. 2014;42:2244–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]


