Abstract
BACKGROUND:
Opioid-related adverse events are a serious problem in hospitalized patients. Little is known about patients who are likely to experience opioid-induced respiratory depression events on the general care floor and may benefit from improved monitoring and early intervention. The trial objective was to derive and validate a risk prediction tool for respiratory depression in patients receiving opioids, as detected by continuous pulse oximetry and capnography monitoring.
METHODS:
PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) was a prospective, observational trial of blinded continuous capnography and oximetry conducted at 16 sites in the United States, Europe, and Asia. Vital signs were intermittently monitored per standard of care. A total of 1335 patients receiving parenteral opioids and continuously monitored on the general care floor were included in the analysis. A respiratory depression episode was defined as respiratory rate ≤5 breaths/min (bpm), oxygen saturation ≤85%, or end-tidal carbon dioxide ≤15 or ≥60 mm Hg for ≥3 minutes; apnea episode lasting >30 seconds; or any respiratory opioid-related adverse event. A risk prediction tool was derived using a multivariable logistic regression model of 46 a priori defined risk factors with stepwise selection and was internally validated by bootstrapping.
RESULTS:
One or more respiratory depression episodes were detected in 614 (46%) of 1335 general care floor patients (43% male; mean age, 58 ± 14 years) continuously monitored for a median of 24 hours (interquartile range [IQR], 17–26). A multivariable respiratory depression prediction model with area under the curve of 0.740 was developed using 5 independent variables: age ≥60 (in decades), sex, opioid naivety, sleep disorders, and chronic heart failure. The PRODIGY risk prediction tool showed significant separation between patients with and without respiratory depression (P < .001) and an odds ratio of 6.07 (95% confidence interval [CI], 4.44–8.30; P < .001) between the high- and low-risk groups. Compared to patients without respiratory depression episodes, mean hospital length of stay was 3 days longer in patients with ≥1 respiratory depression episode (10.5 ± 10.8 vs 7.7 ± 7.8 days; P < .0001) identified using continuous oximetry and capnography monitoring.
CONCLUSIONS:
A PRODIGY risk prediction model, derived from continuous oximetry and capnography, accurately predicts respiratory depression episodes in patients receiving opioids on the general care floor. Implementation of the PRODIGY score to determine the need for continuous monitoring may be a first step to reduce the incidence and consequences of respiratory compromise in patients receiving opioids on the general care floor.
See Articles, p 1007, p 1025, p 1032
KEY POINTS.
Question: Can we use continuous capnography and oximetry monitoring data to derive a risk prediction tool to predict the risk of respiratory depression episodes in patients receiving parenteral opioid therapy on surgical and medical general care floors?
Findings: The PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) trial found a 46% incidence of respiratory depression and developed a novel respiratory depression risk prediction tool, including 5 easy-to-assess variables: age ≥60 years by decade, sex, opioid naivety, sleep disorders, and chronic heart failure.
Meaning: This risk prediction tool, based on continuous, blinded oximetry and capnography data, accurately predicts respiratory depression in patients receiving parenteral opioids on the general care floor.
The general care floor is a low-acuity inpatient environment. However, nearly half of all in-hospital cardiorespiratory events occur on the general care floor, often with catastrophic outcomes.1,2 A national registry identified 44,551 acute respiratory events in US hospitals, with an associated in-hospital mortality of nearly 40%.2 Early recognition of respiratory compromise through continuous respiratory monitoring on the general care floor has been advocated to reduce morbidity and mortality.3,4
Opioid-induced respiratory depression (OIRD) is one cause of respiratory compromise on the general care floor, in part, because opioid administration remains common practice.5 OIRD is traditionally defined using surrogate measures, such as hypoventilation with or without oxygen desaturation, and is often a diagnosis of exclusion.6 Its reported incidence between 0.3% and 21% is likely an underestimation of the true incidence.7,8 Opioid-related adverse events, including OIRD, are associated with increased length of stay (mean 5 additional days), readmission (15.8% vs 9.4% in patients without events), and cost (mean increase $10,000).9 The earliest warning of respiratory failure due to OIRD may be subtle changes in vital signs 6–8 hours before critical cardiac and respiratory decompensation ensues.10 Current general care floor monitoring often misses these early patterns or infers incorrect patterns, which are key to preventing catastrophic events. One investigation revealed that intermittent “spot check” monitoring every 4–6 hours missed >90% episodes of prolonged hypoxemia on the general care floor.7 Similarly, in a closed claims analysis of the American Society of Anesthesiologists (ASA), a majority of the >357 OIRD events evolved rapidly and almost all could have been prevented with adequate monitoring and timely responses.11 It remains difficult to predict which general care floor patients are likely to decompensate when receiving analgesic opioids.12
The PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) trial investigated the incidence and risk factors associated with respiratory depression episodes in hospitalized patients receiving parenteral opioids and monitored by continuous capnography and oximetry. A respiratory depression risk prediction model was derived and validated, and the PRODIGY risk prediction tool was created.
METHODS
Trial Design, Setting, and Participants
PRODIGY was a prospective trial conducted at 16 clinical sites in the United States, Europe, and Asia (Supplemental Digital Content, Table 1, http://links.lww.com/AA/D71), performed between April 2017 and May 2018 (ClinicalTrials.gov: NCT02811302; registered Study Chair: F.J.O.; registration date: June 2016). The trial was registered before patient enrollment and conducted in compliance with the Declaration of Helsinki and applicable laws and regulations of participating countries, including institutional review board (IRB) or Research Ethics Committee approval. Eligible patients included adults (≥18, 20, and 21 years in United States/Europe, Japan, and Singapore, respectively) able to provide written informed consent, expected to receive parenteral opioid therapy on the general care floor, and able to wear continuous monitoring equipment. Exclusion criteria were previously described (Supplemental Digital Content, Table 2, http://links.lww.com/AA/D71).1 Patients receiving opioids for nonsurgical pain were eligible for enrollment in the United States; all patients enrolled outside the United States were postsurgical. All adverse events were documented during continuous monitoring and at 30-day follow-up. Respiratory depression episodes were classified as respiratory opioid-related adverse events only if standard of care monitoring indicated the patient was symptomatic of respiratory depression and intervention was required.
Procedures
Enrolled patients were monitored in adherence to each sites’ practice (vital sign checks every 4–8 hours), as well as continuous capnography and pulse oximetry monitoring, collected with a Capnostream 20p or 35 portable bedside monitor (Medtronic, Boulder, CO). Continuous monitoring readings, alarms, and data were blinded to health care providers. Monitoring began after arrival on the general care floor and initiation of opioid therapy. Supplemental oxygen was allowed according to each sites’ clinical practice. A 30-day follow-up phone call was conducted. Clinical sites were limited to maximum 20% of total enrollment.
Trial Objectives
The primary objective was to derive and validate a risk prediction tool for respiratory depression in patients receiving opioids, as detected by continuous pulse oximetry and capnography monitoring (primary end point). A respiratory depression episode was defined as any of the following: respiratory rate ≤5 breaths/min (bpm) for ≥3 minutes, oxygen saturation (Spo2) ≤85% for ≥3 minutes, end-tidal carbon dioxide (Etco2) ≤15 or ≥60 mm Hg for ≥3 minutes, apnea episode lasting >30 seconds, or any respiratory opioid-related adverse event (Supplemental Digital Content, Table 3, http://links.lww.com/AA/D71). The ≥3-minute threshold was used to minimize technical artifacts and transient threshold deviations. An independent clinical event committee reviewed Etco2, Spo2, respiratory rate, and heart rate tracings and decided on the presence of a respiratory episode and excluded artifacts on a case-by-case basis using a predefined priority list (Supplemental Digital Content, Table 4, http://links.lww.com/AA/D71). To evaluate respiratory depression relatedness to opioids, the committee had access to time records of opioid use on the general care floor but was blinded to all other patient medical and clinical history.
A priori defined secondary objectives included (1) comparing patients with and without ≥1 respiratory depression episode, with end points including adverse event incidence and health care resource utilization and (2) calculating the predictive value of Etco2, respiratory rate, Spo2, and the Integrated Pulmonary Index (IPI) algorithm (determined by integrating Etco2, respiratory rate, Spo2, and heart rate signals into a single index using fuzzy logic)13 on the occurrence of respiratory depression for patients with ≥1 adjudicated respiratory depression episode, using sensitivity and specificity measures. Primary and secondary objectives used a modified full analysis set, including only enrolled patients who started continuous monitoring and received parenteral opioid therapy, because some patients were enrolled based on “planned” opioid administration but did not receive opioids. Patients with only respiratory opioid-related adverse events occurring outside of continuous monitoring were excluded from the modified full analysis set.
Sample Size Calculations
A total of 1650 patients were calculated as adequate, based on expected model derivation with 12 predictors, and a combined 12% reported incidence of respiratory rate ≤8 bpm, Spo2 ≤85%, or Etco2 ≥60 mm Hg, each for ≥3 minutes.1,7,14 Enrollment was monitored and ended with 1495 patients due to high prevalence (44%) of adjudicated respiratory depression episodes; this was sufficient to power statistical analysis.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics, including mean, standard deviation, median, minimum, maximum, and interquartile range (IQR) for continuous variables, and counts and percentages for categorical variables. To compare patients with and without respiratory depression episodes, categorical variables were analyzed with the χ2 test or the Fisher exact test, and continuous variable parameter comparisons were performed using t test or Wilcoxon test, as appropriate. Statistical tests used a 2-sided significance level of .05. All data analysis used SAS v9.4 (SAS Institute Inc, Cary, NC). This manuscript adheres to the applicable STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.
Risk Prediction Derivation
Medical and clinical information were collected at patient enrollment or during continuous monitoring through medical chart review; adverse events, protocol deviations, and health care resource utilization information were collected during 30-day follow-up. Due to low prevalence of some risk factors and the unexpected high respiratory depression occurrence, the risk prediction tool was derived using all patients, instead of the derivation–validation split defined in the published trial protocol.1
Forty-six a priori defined variables collected during the trial were assessed, including 12 variables previously described as respiratory depression risk factors (Supplemental Digital Content, Table 5, http://links.lww.com/AA/D71).3,15–19 Opioid naivety was defined as no history of opioid use as documented in available health records. Known or suspected sleep-disordered breathing included medical history of obstructive sleep apnea, the use of continuous positive airway pressure (CPAP), or confirmation of the Snoring, Tiredness, Observed apnea, blood Pressure (STOP) questions in the Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference, and Gender (STOP-BANG) questionnaire. Variable collinearity was tested by Spearman rho, where a correlation coefficient >0.25 was used to identify covariables, and clinical judgment used to determine covariable exclusion.20 Covariables with prevalence <0.5% or >90% were also excluded, resulting in 31 variables (Supplemental Digital Content, Table 5, http://links.lww.com/AA/D71).
Bivariable odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated for all 31 variables. The multivariable logistic regression model used stepwise selection with entry 0.25, stay criteria 0.15, and respiratory depression as the dependent variable. The model included all 2 × 2 interactions, with geography and effective length of monitoring (in quartiles, calculated as the total length of capnography monitoring excluding temporary gaps in monitoring) as random effects due to a correlation between monitoring length and respiratory depression occurrence. The main accuracy measure was the C-statistic equal to the area under the curve (AUC), derived from the mixed model. The final model was assessed using the calibration plot (predicted probability in deciles). Model prediction performance was assessed using R2, Somers D, γ, τ-a, and Brier score. Internal model validation was assessed by bootstrapping with stepwise variable selection, estimating the optimism (500 random samples with replacement from the original dataset), as recommended in the literature.21–23 The adjusted AUC was the difference between the AUC and optimism. Five-fold cross-validation with 10 replicates, for a total of 50 iterations, was performed to confirm bootstrapping results. Cross-validation was also performed using each trial site as a test set to confirm model performance across different hospital settings.24 The cross-validation performances were reported as the average AUC along with bias as the difference between the apparent and the test.
Risk Score Development
The PRODIGY risk score was calculated by multiplying each β coefficient by 10, rounding to the nearest integer, and adding all integers. The resulting continuous distribution of risk scores was stratified into 3 categories according to the risk level (tertiles), as planned before trial onset. The risk tool was assessed for accuracy using sensitivity and specificity, and respiratory depression incidence by risk levels was determined.
Missing Data
Because the clinical event committee adjudicated the respiratory depression episodes, there were no missing respiratory depression data. No imputation was used because missing covariable data were sufficiently low (5.2%), given that a 10% cutoff is typical for large samples and no established cutoff defining an acceptable percentage of missing data exists.25
RESULTS
Patient Characteristics and Prevalence of Respiratory Depression
Figure.
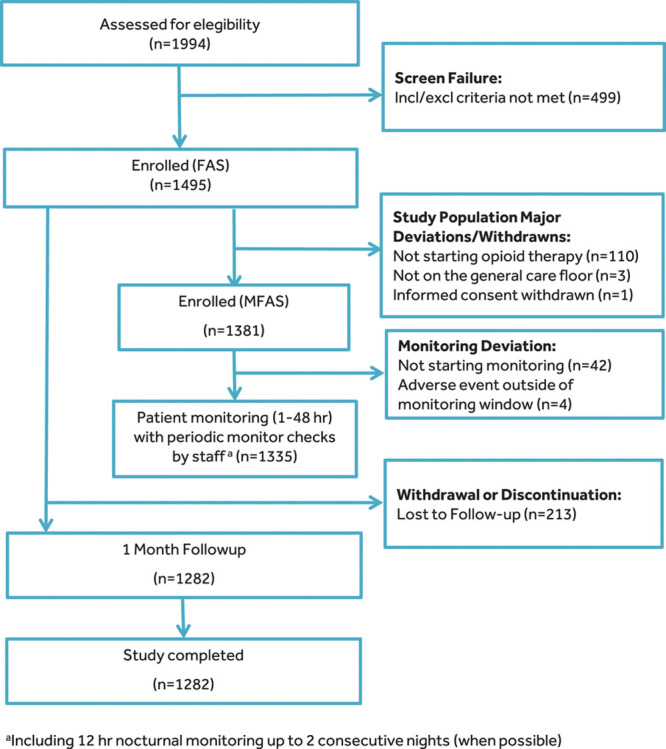
STROBE diagram detailing patient disposition throughout the trial, including patients in the FAS and the MFAS. aIncluding 12-h nocturnal monitoring up to 2 consecutive nights (when possible). FAS indicates full analysis set; Incl/Excl, Inclusion/Exclusion; MFAS, modified full analysis set; STROBE, STrengthening the Reporting of OBservational studies in Epidemiology.
In total, 1495 patients were enrolled, with 114 patients excluded due to major protocol deviations; 1282 patients completed 30-day follow-up (Figure). The median effective monitoring time was 24 hours (IQR, 17–26) for 1335 monitored patients (Figure; Supplemental Digital Content, Figure 1, http://links.lww.com/AA/D71). Median morphine milligram equivalents (MMEs) on the general care floor were 15 MMEs (IQR, 3–52) and 20 MMEs (IQR, 5–60) for patients with ≥1 or without respiratory depression episodes, respectively. The clinical event committee adjudicated 5768 potential respiratory depression episodes in 1347 patients, including some patients who did not receive parenteral opioids (Supplemental Digital Content, Figure 2, http://links.lww.com/AA/D71). Of all enrolled patients, 655 (44% of the full analysis set) were adjudicated as having at least 1 respiratory depression episode during the monitoring period, including 614 patients who received opioids and started continuous monitoring (46% of the modified full analysis set). Baseline characteristics varied by location, with US sites enrolling younger patients with a higher body mass index (BMI, ≥35 kg/m2), larger neck circumference, higher ASA physical status, and higher STOP-BANG score, compared to patients enrolled in Europe or Asia (Table 1). Of the 1185 patients who had ≥1 potential respiratory depression episode, 731 patients (62%) received supplemental oxygen during monitoring. A significantly higher percentage of patients enrolled in Asia were opioid naive (Table 1).
Table 1.
Patient Demographics and Baseline Characteristics
| Clinical Characteristics | United States (n = 769) | Europe (n = 254) | Asia (n = 312) | Total (n = 1335) | Significance (P)a |
|---|---|---|---|---|---|
| Age (y) | |||||
| Mean ± SD | 54.9 ± 14.7 | 60.2 ± 12.5 | 62.7 ± 12.4 | 57.7 ± 14.2 | <.001 |
| Median (IQR) | 56.0 (44.0–65.0) | 61.5 (53.0–69.0) | 65.0 (57.0–70.0) | 59.0 (49.0–68.0) | |
| Age (y) classes | |||||
| <60 | 252/769 (32.8) | 42/254 (16.5) | 35/312 (11.2) | 329/1335 (24.6) | <.001 |
| ≥60 to <70 | 184/769 (23.9) | 86/254 (33.9) | 122/312 (39.1) | 392/1335 (29.4) | |
| ≥70 to <80 | 103/769 (13.4) | 46/254 (18.1) | 75/312 (24.0) | 224/1335 (16.8) | |
| ≥80 | 24/769 (3.1) | 12/254 (4.7) | 13/312 (4.2) | 49/1335 (3.7) | |
| Sex (male) | 287/769 (37.3) | 126/254 (49.6) | 157/312 (50.3) | 570/1335 (42.7) | <.001 |
| BMI (kg/m2) | |||||
| Mean ± SD | 32.0 ± 9.2 | 27.8 ± 6.5 | 25.0 ± 4.5 | 29.6 ± 8.4 | <.001 |
| Median (IQR) | 30.1 (25.5–36.8) | 26.7 (23.3–30.7) | 24.5 (21.9–27.6) | 27.8 (23.7–33.3) | |
| BMI (kg/m2) | |||||
| <20 | 31/769 (4.0) | 10/252 (4.0) | 36/312 (11.5) | 77/1333 (5.8) | <.001 |
| ≥20 to <25 | 143/769 (18.6) | 89/252 (35.3) | 138/312 (44.2) | 370/1333 (27.7) | |
| ≥25 to <30 | 208/769 (27.0) | 83/252 (32.9) | 95/312 (30.4) | 386/1333 (29.0) | |
| ≥30 to <35 | 146/769 (19.0) | 44/252 (17.5) | 35/312 (11.2) | 225/1333 (16.9) | |
| ≥35 | 241/769 (31.3) | 26/252 (10.3) | 8/312 (2.6) | 275/1333 (20.6) | |
| Race/ethnicity | |||||
| American Indian or Alaska Native | 3/769 (0.4) | 0/254 (0) | 1/312 (0.3) | 4/1335 (0.3) | <.001 |
| Asian | 6/769 (0.8) | 2/254 (0.8) | 309/312 (99.0) | 317/1335 (23.7) | |
| Black or African American | 157/769 (20.4) | 1/254 (0.4) | 0/312 (0) | 158/1335 (11.8) | |
| Hispanic or Latino | 9/769 (1.2) | 0/254 (0) | 0/312 (0) | 9/1335 (0.7) | |
| Native Hawaiian or Other Pacific | 1/769 (0.1) | 0/254 (0) | 0/312 (0) | 1/1335 (0.1) | |
| White | 586/769 (76.2) | 148/254 (58.3) | 2/312 (0.6) | 736/1335 (55.1) | |
| Other | 7/769 (0.9) | 21/254 (8.3) | 0/312 (0) | 28/1335 (2.1) | |
| Current smoker | 120/769 (15.6) | 49/254 (19.3) | 30/312 (9.6) | 199/1335 (14.9) | .004 |
| Neck circumference (≥17 in M; ≥16 in F) | 351/766 (45.8) | 51/205 (24.9) | 17/312 (5.4) | 419/1283 (32.7) | <.001 |
| ASA physical status | |||||
| I | 4/744 (0.5) | 35/254 (13.8) | 75/312 (24.0) | 114/1310 (8.7) | <.001 |
| II | 266/744 (35.8) | 152/254 (59.8) | 206/312 (66.0) | 624/1310 (47.6) | |
| III | 448/744 (60.2) | 66/254 (26.0) | 31/312 (9.9) | 545/1310 (41.6) | |
| IV | 26/744 (3.5) | 1/254 (0.4) | 0/312 (0.0) | 27/1310 (2.1) | |
| Surgery demographics | |||||
| Surgical patient | 693/769 (90.1) | 254/254 (100.0) | 312/312 (100.0) | 1259/1335 (94.3) | <.001 |
| High-risk surgeryb | 39/769 (5.1) | 25/254 (9.8) | 18/312 (5.8) | 82/1335 (6.1) | .022 |
| Open surgery | 50/769 (6.5) | 21/254 (8.3) | 1/312 (0.3) | 72/1335 (5.4) | <.001 |
| Length of surgery (h) | |||||
| <2 | 290/769 (37.7) | 53/254 (20.9) | 87/312 (27.9) | 430/1335 (32.2) | <.001 |
| ≥2 to <4 | 311/769 (40.4) | 125/254 (49.2) | 119/312 (38.1) | 555/1335 (41.6) | |
| ≥4 | 168/769 (21.8) | 76/254 (29.9) | 106/312 (34.0) | 350/1335 (26.2) | |
| Opioid demographics | |||||
| Opioid epidural and IV route | 32/769 (4.2) | 8/254 (3.1) | 93/312 (29.8) | 133/1335 (10.0) | <.001 |
| Opioid Naivec | 551/769 (71.7) | 224/254 (88.2) | 301/312 (96.5) | (80.6) 1076/1335 | <.001 |
| Multiple opioids or concurrent CNS/sedating medication | 722/769 (93.9) | 220/254 (86.6) | 306/312 (98.1) | (93.5) 1248/1335 | <.001 |
| No. of distinct opioids | |||||
| 1 opioid | 69/769 (9.0) | 52/254 (20.5) | 8/312 (2.6) | 129/1335 (9.7) | <.001 |
| Opioid number >1 to <4 | 451/769 (58.6) | 152/254 (59.8) | 277/312 (88.8) | 880/1335 (65.9) | <.001 |
| Opioid number ≥4 | 249/769 (32.4) | 50/254 (19.7) | 27/312 (8.7) | 326/1335 (24.4) | |
| Cardiac disorders | |||||
| Aortic aneurysm | 8/769 (1.0) | 2/254 (0.8) | 0/312 (0) | 10/1335 (0.7) | .200 |
| Aortic valve disease | 12/769 (1.6) | 4/254 (1.6) | 2/312 (0.6) | 18/1335 (1.3) | .487 |
| CHF | 26/768 (3.4) | 7/254 (2.8) | 2/312 (0.6) | 35/1334 (2.6) | .023 |
| Coronary artery disease | 55/767 (7.2) | 10/254 (3.9) | 16/312 (5.1) | 81/1333 (6.1) | .126 |
| Hypertension | 366/769 (47.6) | 96/254 (37.8) | 150/312 (48.1) | 612/1335 (45.8) | .017 |
| Mitral valve disease | 13/769 (1.7) | 6/254 (2.4) | 1/312 (0.3) | 20/1335 (1.5) | .110 |
| Myocardial infarction | 21/768 (2.7) | 9/254 (3.5) | 4/312 (1.3) | 34/1334 (2.5) | .209 |
| Orthostatic hypotension | 3/769 (0.4) | 1/254 (0.4) | 0/312 (0) | 4/1335 (0.3) | .644 |
| Pulmonary hypertension | 8/769 (1.0) | 1/254 (0.4) | 0/312 (0) | 9/1335 (0.7) | .124 |
| Hepatobiliary disorders | |||||
| Liver failure | 2/769 (0.3) | 2/254 (0.8) | 6/312 (1.9) | 10/1335 (0.7) | .011 |
| Immune disorders | |||||
| Sarcoidosis | 3/769 (0.4) | 0/254 (0) | 0/312 (0) | 3/1335 (0.2) | .767 |
| Infections | |||||
| Multiple organ dysfunction syndrome | 2/769 (0.3) | 0/254 (0) | 0/312 (0) | 2/1335 (0.1) | 1.000 |
| Sepsis | 15/769 (2.0) | 2/254 (0.8) | 0/312 (0) | 17/1335 (1.3) | .014 |
| Metabolism and nutrition disorders | |||||
| Diabetes—type I | 18/769 (2.3) | 1/254 (0.4) | 0/312 (0) | 19/1335 (1.4) | .002 |
| Diabetes—type II | 124/769 (16.1) | 37/254 (14.6) | 53/312 (17) | 214/1335 (16.0) | .733 |
| Musculoskeletal and connective tissue disorders | |||||
| Muscular dystrophy | 2/769 (0.3) | 0/254 (0) | 0/312 (0) | 2/1335 (0.1) | 1.000 |
| Amyotrophic lateral sclerosis | 0/769 (0) | 0/254 (0) | 0/312 (0) | 0/1335 (0) | 1.000 |
| Renal and urinary disorders | |||||
| Kidney failure | 29/769 (3.8) | 14/254 (5.5) | 11/312 (3.5) | 54/1335 (4.0) | .412 |
| Respiratory, thoracic, and mediastinal disorders | |||||
| Acute bronchitis | 13/769 (1.7) | 0/254 (0) | 0/312 (0) | 13/1335 (1.0) | .006 |
| Asthma | 121/769 (15.7) | 18/254 (7.1) | 23/312 (7.4) | 162/1335 (12.1) | <.001 |
| Chronic bronchitis | 9/769 (1.2) | 3/254 (1.2) | 1/312 (0.3) | 13/1335 (1.0) | .402 |
| Chronic obstructive pulmonary disease | 60/769 (7.8) | 12/254 (4.7) | 7/312 (2.2) | 79/1335 (5.9) | .001 |
| Chronic restrictive lung disease | 3/769 (0.4) | 0/254 (0) | 0/312 (0) | 3/1335 (0.2) | .767 |
| Cystic fibrosis | 0/769 (0) | 0/254 (0) | 0/312 (0) | 0/1335 (0) | .767 |
| Emphysema | 5/769 (0.7) | 0/254 (0) | 0/312 (0) | 5/1335 (0.4) | .234 |
| Pneumonia | 15/769 (2.0) | 6/254 (2.4) | 9/312 (2.9) | 30/1335 (2.2) | .638 |
| Pulmonary fibrosis | 2/769 (0.3) | 0/254 (0) | 0/312 (0) | 2/1335 (0.1) | 1.000 |
| Sleep disordersd | 127/760 (16.7) | 13/195 (6.7) | 7/312 (2.2) | 147/1267 (11.6) | <.001 |
| Vascular disorders | |||||
| Cerebral aneurysm | 8/769 (1.0) | 0/254 (0) | 0/312 (0) | 8/1335 (0.6) | .066 |
| Peripheral vascular disease | 18/769 (2.3) | 8/254 (3.1) | 2/312 (0.6) | 28/1335 (2.1) | .090 |
| Stroke | 14/768 (1.8) | 5/254 (2.0) | 5/312 (1.6) | 24/1334 (1.8) | .946 |
| Transient ischemic attack | 11/768 (1.4) | 4/254 (1.6) | 2/312 (0.6) | 17/1334 (1.3) | .561 |
| STOP-BANG score class | |||||
| Low risk (0–2) | 343/756 (45.4) | 102/192 (53.1) | 180/312 (57.5) | 625/1260 (49.6) | <.001 |
| Intermediate risk (3–4) | 265/756 (35.1) | 60/192 (31.3) | 114/312 (36.5) | 439/1260 (34.8) | |
| High risk (5–8) | 148/756 (19.6) | 30/192 (15.6) | 18/312 (5.8) | 196/1260 (15.6) |
Medical patients were enrolled at US sites only, and medical history was collected from chart review.
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CHF, chronic heart failure; CNS, central nervous system; CPAP, continuous positive airway pressure; ESC/ESA, European Society of Cardiology/European Society of Anaesthesiology; F, female; IQR, interquartile range; IV, intravenous; M, male; SD, standard deviation; STOP, Snoring, Tiredness, Observed apnea, blood Pressure; STOP-BANG, Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference, and Gender.
aP values are not adjusted for multiple comparisons.
bHigh-risk surgery was defined using the revised ESC/ESA guidelines on noncardiac surgery.
cOpioid naive was defined as no use of any opioids in patient medication history.
dSleep disorders included medical history of obstructive sleep apnea, use of CPAP, or confirmation of the STOP questions in the STOP-BANG questionnaire.
Univariable Predictors of Respiratory Depression
Significant univariable respiratory depression predictors included age ≥70 to <80 or ≥80 years, male sex, BMI ≥20 to <25 or ≥35 kg/m2, opioid naivety, administration of ≥1 to <4 opioids, chronic heart failure, coronary artery disease, hypertension, type II diabetes, kidney failure, and asthma (Table 2).
Table 2.
Univariable Predictors of Respiratory Depression
| Clinical Characteristics | Patients With ≥1 Respiratory Depression Episode (n = 614), n/N (%) | Patients Without Respiratory Depression (n = 721), n/N (%) | OR (95% CI) | Significance (P) |
|---|---|---|---|---|
| Age (y) | ||||
| <60 | 73/614 (11.9) | 256/721 (35.5) | … | … |
| ≥60 to <70 | 213/614 (34.7) | 179/721 (24.8) | 2.47 (1.91–3.19) | .585 |
| ≥70 to <80 | 149/614 (24.3) | 75/721 (10.4) | 4.12 (2.99–5.68) | <.001 |
| ≥80 | 34/614 (5.5) | 15/721 (2.1) | 4.70 (2.51–8.81) | .014 |
| Sex (male) | 327/614 (53.3) | 243/721 (33.7) | 2.24 (1.80–2.80) | <.001 |
| BMI (kg/m2) | ||||
| <20 | 34/612 (5.6) | 43/721 (6.0) | … | … |
| ≥20 to <25 | 188/612 (30.7) | 182/721 (25.2) | 1.31 (0.80–2.14) | .029 |
| ≥25 to <30 | 186/612 (30.4) | 200/721 (27.7) | 1.18 (0.72–1.92) | .236 |
| ≥30 to <35 | 112/612 (18.3) | 113/721 (15.7) | 1.25 (0.75–2.11) | .130 |
| ≥35 | 92/612 (15.0) | 183/721 (25.4) | 0.64 (0.38–1.06) | <.001 |
| Current smoker | 90/614 (14.7) | 109/721 (15.1) | 0.96 (0.71–1.31) | .814 |
| Sleep disorders | 75/575 (13.0) | 72/692 (10.4) | 1.29 (0.92–1.82) | .145 |
| Cardiac disorders | ||||
| Aortic aneurysm | 8/614 (1.3) | 2/721 (0.3) | 4.75 (1.00–22.43) | .049 |
| Aortic valve disease | 12/614 (2.0) | 6/721 (0.8) | 2.38 (0.89–6.37) | .085 |
| CHF | 24/613 (3.9) | 11/721 (1.5) | 2.63 (1.28–5.41) | .009 |
| Coronary artery disease | 47/612 (7.7) | 34/721 (4.7) | 1.68 (1.07–2.65) | .025 |
| Hypertension | 321/614 (52.3) | 291/721 (40.4) | 1.62 (1.30–2.01) | <.001 |
| Mitral valve disease | 9/614 (1.5) | 11/721 (1.5) | 0.96 (0.40–2.33) | .929 |
| Myocardial infarction | 20/613 (3.3) | 14/721 (1.9) | 1.70 (0.85–3.40) | .132 |
| Pulmonary hypertension | 5/614 (0.8) | 4/721 (0.6) | 1.47 (0.39–5.51) | .565 |
| Hepatobiliary disorders | ||||
| Liver failure | 6/614 (1.0) | 4/721 (0.6) | 1.77 (0.50–6.30) | .379 |
| Infections | ||||
| Sepsis | 9/614 (1.5) | 8/721 (1.1) | 1.33 (0.51–3.46) | .562 |
| Metabolism and nutrition disorders | ||||
| Diabetes—type I | 9/614 (1.5) | 10/721 (1.4) | 1.06 (0.43–2.62) | .903 |
| Diabetes—type II | 112/614 (18.2) | 102/721 (14.1) | 1.35 (1.01–1.81) | .043 |
| Renal and urinary disorders | ||||
| Kidney failure | 33/614 (5.4) | 21/721 (2.9) | 1.89 (1.08–3.31) | .025 |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Acute bronchitis | 4/614 (0.7) | 9/721 (1.2) | 0.52 (0.16–1.69) | .277 |
| Asthma | 55/614 (9.0) | 107/721 (14.8) | 0.56 (0.40–0.80) | .001 |
| Chronic bronchitis | 7/614 (1.1) | 6/721 (0.8) | 1.38 (0.46–4.12) | .568 |
| Chronic obstructive pulmonary disease | 43/614 (7.0) | 36/721 (5.0) | 1.43 (0.91–2.26) | .122 |
| Vascular disorders | ||||
| Cerebral aneurysm | 5/614 (0.8) | 3/721 (0.4) | 1.96 (0.47–8.26) | .356 |
| Peripheral vascular disease | 16/614 (2.6) | 12/721 (1.7) | 1.58 (0.74–3.37) | .235 |
| Stroke | 13/613 (2.1) | 11/721 (1.5) | 1.40 (0.62–3.14) | .417 |
| Transient ischemic attack | 10/613 (1.6) | 7/721 (1.0) | 1.69 (0.64–4.47) | .290 |
| Surgery information | ||||
| High-risk surgery | 41/614 (6.7) | 41/721 (5.7) | 1.19 (0.76–1.86) | .453 |
| Open surgery | 34/614 (5.5) | 38/721 (5.3) | 1.05 (0.65–1.70) | .829 |
| Length of surgery (h) | ||||
| <2 | 184/614 (30.0) | 246/721 (34.1) | … | … |
| ≥2 to <4 | 264/614 (43.0) | 291/721 (40.4) | 1.21 (0.94–1.56) | .374 |
| ≥4 | 166/614 (27.0) | 184/721 (25.5) | 1.21 (0.91–1.60) | .467 |
| No. of distinct opioids | ||||
| Opioid naive | 520/614 (84.7) | 556/721 (77.1) | 1.64 (1.24–2.17) | <.001 |
| One opioid | 55/614 (9.0) | 74/721 (10.3) | … | … |
| Opioid number ≥1 to <4 | 431/614 (70.2) | 449/721 (62.3) | 1.29 (0.89–1.88) | .009 |
| Opioid number ≥4 | 128/614 (20.8) | 198/721 (27.5) | 0.87 (0.58–1.32) | .071 |
Abbreviations: BMI, body mass index; CHF, chronic heart failure; CI, confidence interval; OR, odds ratio.
Respiratory Depression Risk Assessment Tool
The multivariable model for respiratory depression prediction was developed using 1266 patients (69 missing data) and included 5 independent variables: age ≥60 to <70 years (OR, 2.24 [95% CI, 1.69–2.99]; P < .0001), ≥70 to <80 years (OR, 3.43 [95% CI, 2.41–4.89]; P < .0001), and ≥80 years (OR, 4.78 [95% CI, 2.33–9.80]; P < .0001); male sex (OR, 2.13 [95% CI, 1.65–2.74]; P < .0001); opioid naivety (OR, 1.34 [95% CI, 0.97–1.85]; P = .078); sleep disorders (known or suspected sleep disorders, including obstructive sleep apnea; OR, 1.61 [95% CI, 1.10–2.38]; P = .018); and chronic heart failure (OR, 2.12 [95% CI, 0.95–4.72]; P = .067) (Table 3). This multivariable model had an AUC of 0.76 (95% CI, 0.73–0.79) and a Hosmer–Lemeshow goodness-of-fit statistic test with P = .831 (Supplemental Digital Content, Figure 3, http://links.lww.com/AA/D71). The adjusted AUC was 0.7406, considering the low optimism (0.020). Bootstrapping selected the final model variables, including age, male sex, opioid naivety, sleep disorders, and chronic heart failure in 89%, 89%, 24%, 31%, and 37% of 500 replicates, respectively (Supplemental Digital Content, Table 6, http://links.lww.com/AA/D71). Complementary statistics comparing the fitted model with the null model included Brier score 0.19 (range for perfect model to null model, 0–0.25), scaled Brier score 22% (range, 0–100), R2 0.22 (range, 0–1), and adjusted R2 0.29 (range, 0–1).24 Rank correlation indexes measuring the ordinal association between 2 variables included Somers D 0.52, γ 0.53, and τ-a 0.26 (all on a −1 to 1 scale).24 Five-fold cross-validation of the multivariable model resulted in an average AUC of 0.78 with bias equal to −0.02; similar results were observed using cross-validation by trial site, where the average AUC was 0.83 and bias equaled −0.06.
Table 3.
Multivariable Model Prediction of Respiratory Depression, PRODIGY Scoring System, and Utilization
| Multivariable Model Predictors | Points if Clinical Characteristic = “Yes” | ||||
|---|---|---|---|---|---|
| Clinical Characteristic | Estimate | Standard Error | OR (95% CI) | Pr > t | |
| Age (y) | |||||
| <60 | Reference | … | … | … | 0 |
| ≥60 to <70 | 0.8077 | 0.1458 | 2.243 (1.685–2.985) | <0.0001 | 8 |
| ≥70 to <80 | 1.2323 | 0.1805 | 3.429 (2.407–4.886) | <0.0001 | 12 |
| ≥80 | 1.5647 | 0.3657 | 4.781 (2.333–9.798) | <0.0001 | 16 |
| Sex (M) | 0.7550 | 0.1284 | 2.128 (1.654–2.737) | <0.0001 | 8 |
| Opioid naive | 0.2912 | 0.1652 | 1.338 (0.968–1.850) | 0.0782 | 3 |
| Sleep disorders | 0.4755 | 0.1998 | 1.609 (1.087–2.381) | 0.0175 | 5 |
| Chronic heart failure | 0.7494 | 0.4085 | 2.116 (0.949–4.715) | 0.0668 | 7 |
| “Sum = PRODIGY Score” | |||||
| PRODIGY Score Distribution | |||||
| Low Risk | Intermediate Risk | High Risk | P | ||
| PRODIGY score | <8 points | ≥8 and <15 points | ≥15 points | ||
| % Pts with respiratory depression in risk category (n Pts in risk category with respiratory depression/n Pts in risk category) | 24% (83/351) | 42% (192/457) | 65% (299/458) | <.0001 | |
| Sensitivity (95% CI) | … | 0.86 (0.82–0.88) | 0.52 (0.48–0.56) | ||
| Specificity (95% CI) | … | 0.39 (0.35–0.42) | 0.77 (0.74–0.80) | ||
| PPV (95% CI) | … | 0.54 (0.50–0.57) | 0.65 (0.61–0.70) | ||
| NPV (95% CI) | … | 0.76 (0.72–0.81) | 0.66 (0.63–0.69) | ||
| OR (95% CI; P) | ORIL =2.34 (1.72–3.19; P < .001) ORHL = 6.07 (4.44–8.30; P < .001) |
ORHI =2.6 (1.99–3.39; P < .001) | |||
Abbreviations: CI, confidence interval; M, male; NPV, negative predictive value; OR, odds ratio; ORHI, odds ratio, high- versus intermediate-risk groups; ORHL, odds ratio, high- versus low-risk groups; ORIL, odds ratio, intermediate- versus low-risk groups; PPV, positive predictive value; PRODIGY, PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY; Pts, patients.
The PRODIGY score equaled the sum of points for each predictor, and score distribution was divided into low-, intermediate-, and high-risk tertiles (Table 3). The average predicted probability for a PRODIGY score <8, ≥8 to <15, and ≥15 was 0.18–0.39, 0.33–0.65, and 0.50–0.89, respectively. Within the PRODIGY score high-risk group, 65% of patients had ≥1 respiratory depression episode, compared to 42% and 24% of patients in the intermediate- and low-risk groups (Table 3). Patients with or without respiratory depression had significant separation in PRODIGY scores (P < .001) (Supplemental Digital Content, Figure 4, http://links.lww.com/AA/D71). High- and intermediate-risk scores had positive and negative predictive values equal to 0.65 and 0.54 and 0.66 and 0.76, respectively. The high- and low-risk groups had OR of 6.07 (95% CI, 4.44–8.30; P < .001) (Table 3), with high sensitivity and specificity for the intermediate- (≥8 points) and high-risk (≥15 points) groups, respectively.
Predictive Value of Continuous Monitoring Parameters
The most common capnography and pulse oximetry alarms were for apnea, low respiratory rate, and low Etco2, with adjudicated respiratory depression episodes occurring in 596, 155, and 141 patients, respectively (Table 4). No high Etco2 cases were observed. IPI <5 (1–10 scale with lower numbers indicating worse status) and apnea had the highest sensitivity (97.16; 95% CI, 95.84–98.16 and 95.01; 95% CI, 93.35–96.35, respectively), and low Spo2 with low Etco2 had the highest specificity (98.75; 95% CI, 98.36–99.06) in detecting respiratory depression episodes (Table 4).
Table 4.
Predictive Value of Etco2, Respiratory Rate, Spo2, Apnea, and IPI in Predicting Respiratory Opioid-Related Adverse Events
| Parameter | Pts With Device Episodes, n/N (%) | Pts With Respiratory Depressiona and Device Episodes, n/N (%) | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive Predictive Value % (95% CI) | Negative Predictive Value % (95% CI) |
|---|---|---|---|---|---|---|
| High Etco2 | 0/1185 (0.00) | 0/614b (0.00) | ... | ... | ... | ... |
| Low Etco2 | 748/1185 (63.12) | 141/614 (22.96) | 18.73 (16.20–21.46) | 57.07 (55.55–58.58) | 8.47 (7.28–9.80) | 76.79 (75.26–78.27) |
| Low respiratory rate | 685/1185 (57.81) | 154/614 (25.08) | 22.13 (19.43–25.02) | 68.06 (66.61–69.47) | 12.82 (11.18–14.61) | 80.46 (79.11–81.76) |
| Low Spo2 | 155/1185 (13.08) | 48/614 (7.82) | 6.13 (4.64–7.92) | 94.00 (93.24–94.70) | 17.82 (13.68–22.60) | 82.51 (81.40–83.59) |
| Apnea | 1150/1185 (97.05) | 596/614 (97.07) | 95.01 (93.35–96.35) | 13.76 (12.72–14.84) | 18.95 (17.80–20.14) | 92.85 (90.51–94.75) |
| IPI | ||||||
| IPI <3 | 1169/1185 (98.65) | 602/614 (98.05) | 96.25 (94.78–97.41) | 6.65 (5.91–7.45) | 17.95 (16.87–19.08) | 89.32 (85.33–92.53) |
| IPI <4 | 1169/1185 (98.65) | 602/614 (98.05) | 96.48 (95.04–97.60) | 6.60 (5.86–7.40) | 17.98 (16.90–19.11) | 89.84 (85.88–92.99) |
| IPI <5 | 1179/1185 (99.49) | 604/614 (98.37) | 97.16 (95.84–98.16) | 0.84 (0.59–1.17) | 17.22 (16.18–18.29) | 58.33 (44.88–70.93) |
| Low Etco2 and low respiratory rate | 615/1185 (51.90) | 112/614 (18.24) | 14.30 (12.06–16.79) | 72.27 (70.88–73.63) | 9.87 (8.29–11.64) | 79.89 (78.58–81.17) |
| Any alarm (excluding IPI) | 1183/1185 (99.83) | 613/614 (99.84) | 99.55 (98.84–99.88) | 0.58 (0.37–0.86) | 17.53 (16.48–18.61) | 85.71 (67.33–95.97) |
| High Etco2 and low respiratory rate | 0/1185 (0.00) | 0/614 (0.00) | ... | ... | ... | ... |
| High Etco2 and low Spo2 | 0/1185 (0.00) | 0/614 (0.00) | ... | ... | ... | ... |
| Low Etco2 and low Spo2 | 61/1185 (5.15) | 20/614 (3.26) | 2.50 (1.57–3.76) | 98.75 (98.36–99.06) | 29.73 (19.66–41.48) | 82.67 (81.59–83.72) |
Of the 1185 patients with device episodes, 731 patients received supplemental oxygen during monitoring, with 203 receiving supplemental oxygen at baseline.
Abbreviations: bpm, breaths/min; CI, confidence interval; Etco2, end-tidal carbon dioxide; IPI, Integrated Pulmonary Index; Pts, patients; Spo2, oxygen saturation.
aRespiratory depression is defined as respiratory rate ≤5 bpm, Spo2 ≤85%, or Etco2 ≤15 or ≥60 mm Hg for ≥3 minutes; apnea episode lasting >30 seconds; or any respiratory opioid-related adverse event.
bPatients with potential opioid-related adverse events only were excluded due to the lack of device monitoring.
Adverse Events and Health Care Resource Utilization
A total of 313 patients experienced ≥1 adverse event, including 22 opioid-related adverse events in 18 patients (Supplemental Digital Content, Table 7, http://links.lww.com/AA/D71). Ten opioid-related adverse events were detected by capnography and pulse oximetry monitoring; 11 occurred in the absence of continuous monitoring and were excluded during model derivation (Supplemental Digital Content, Table 8, http://links.lww.com/AA/D71). Of patients with opioid-related adverse events, 9, 6, and 3 were high-, intermediate-, and low-risk on the PRODIGY score, respectively. Patients with ≥1 respiratory depression episode (N = 655) were more likely to experience an adverse event that required action, with a relative risk of 1.36 (95% CI, 1.01–1.78; P = .040) for adverse events requiring prolonged hospitalization and 2.46 (95% CI, 1.73–3.50; P < .001) for adverse events requiring rescue, including rapid response team activation, versus patients without respiratory depression (Supplemental Digital Content, Table 9, http://links.lww.com/AA/D71). Of 46 adverse events with rescue action, 30 occurred in patients with ≥1 respiratory depression episode. Readmission (≤30 days) did not differ significantly between patients with or without respiratory depression. Mean hospital length of stay was 3 days longer in patients with ≥1 respiratory depression episode (10.5 ± 10.8 vs 7.7 ± 7.8 days; P < .0001; Supplemental Digital Content, Table 10, http://links.lww.com/AA/D71).
DISCUSSION
Despite its commonality on the general care floor, predicting respiratory depression remains challenging.12 Multiple prediction scores have been developed using postoperative pulmonary complications.26,27 The PRODIGY trial is novel because it derived a respiratory depression risk prediction tool using continuously monitored oximetry and capnography data, which were prospectively collected and evaluated using independent expert waveform verification.
The PRODIGY risk prediction tool performed similarly when validated by bootstrapping and by cross-validation (adjusted AUC, 0.74 and 0.76, respectively), confirming that the model performs well overall and across 16 individual trial sites. Prediction models for postoperative pulmonary complications have reported adjusted AUCs between 0.72 and 0.82,15,28–30 with one model reaching 0.88.26 The PRODIGY risk score compares well and is distinct from other prediction models, which are derived using intermittent vital signs or continuous pulse oximetry alone. The PRODIGY score identified 74% of patients with respiratory depression, with significant separation among low-, intermediate-, and high-risk groups. The 5 PRODIGY score variables are simple to assess, making the risk prediction tool easy to implement. Increasing age >60 years conferred the highest risk for respiratory depression. Age is a feature of other respiratory depression risk prediction models,26,27,31 and age-related changes in respiratory physiology and altered opioid pharmacokinetics could contribute to this observation.32
Postoperative hypoxemia (low Spo2) has been reported in 21% of postsurgical patients.7 While the PRODIGY trial saw a higher overall incidence of respiratory depression (46%), this could be attributed to the use of multiparameter continuous monitoring (Etco2, Spo2, respiratory rate, and heart rate). A majority of our patients used supplemental oxygen, translating into fewer (8%) hypoxemia episodes and more capnography-detected apneic and hypoventilation episodes.33 As expected, the combination of hypoventilation and hypoxemia was most specific for respiratory depression. In contrast to continuous oximetry and capnography monitoring, intermittent oximetry alone substantially underestimates respiratory depression incidence, missing >90% of hypoxemia episodes lasting >1 hour.7,33,34 PRODIGY observed no instances of high Etco2, which manifests during slowly developing respiratory depression. In cases of rapid cessation of breathing, as is typical in patients with sleep-disordered breathing, Etco2 levels tend to be low.35 This suggests that most OIRD developed rapidly without time for Etco2 accumulation and is consistent with evidence that patients can develop respiratory arrest shortly after a nursing visit.11
Improved OIRD detection on the general care floor has important clinical implications, as shown by a reduction of rapid response calls when using unblinded continuous capnography.36 In this trial, rescue, including rapid response team activation, and hospitalization prolongation due to an adverse event were 2.5 and 1.4 times higher, respectively, for patients with respiratory depression. Consistent with previous reports, patients with ≥1 respiratory depression episode experienced a 3-day longer hospital stay,9 equating to an economic burden of ≈$2000/d of inpatient hospitalization and potential costs due to complications and escalation of care.37,38
Overall, the number of opioid-induced respiratory complications requiring rescue was low. Ten patients required rescue treatment during continuous monitoring, and 11 patients needed rescue outside of continuous monitoring. This frequency of rescue treatment is consistent with recent studies using postoperative naloxone administration to define OIRD18,39,40 and supports the hypothesis that OIRD requiring rescue action occurs in ≈1 of 1000 general care floor patients.18,39 Combined, the potential severity of respiratory complications (death, anoxic brain injury) and the lack of OIRD detection by current monitoring practices highlight the need for risk prediction and adequate monitoring. Implementation of the PRODIGY score to determine the need for continuous monitoring may be a first step to reduce the consequences of respiratory compromise.
One strength of the PRODIGY trial design was inclusion of blinded, continuously monitored oximetry and capnography data and respiratory depression episode adjudication using an independent clinical event committee, allowing us to observe the true burden of respiratory depression. Furthermore, we included surgical and medical patients transferred to a range of general care floors in Europe, the United States, and Asia, making our findings generalizable. Although it remains to be seen if universal continuous monitoring will lead to a survival benefit for general care floor patients, our data suggest that screening with the easy-to-use PRODIGY score and the use of continuous monitoring at least in patients with high respiratory depression risk may prevent harm to the individual patient. For example, a 72-year-old male patient with chronic heart failure who is opioid naive is at a >2-fold risk of respiratory depression (PRODIGY score = 30; high risk) compared to a 67-year-old female patient with diabetes and chronic kidney disease who has previously received opioids (PRODIGY score = 8; intermediate risk) after a similar surgical procedure, when both receive opioids on the general care floor. Along with judicious use of opioids, this high-risk patient may benefit from continuous capnography and oximetry monitoring.
This work has some limitations. To evaluate OIRD episodes, the clinical event committee required access to opioid usage data, which could have influenced episode adjudication. Thus, opioid naivety, but not opioid doses and routes, was included in the model. Second, although only one-fifth of all adjudicated respiratory depression episodes were denoted as having ≥1 artifact, capnography data were subject to artifact and patient compliance. Finally, the trial was internally validated but lacks external validation on an independent cohort, primarily due to the nonavailability of a robust, continuously monitored patient dataset from the general care floor. Future studies are needed to validate the risk prediction tool.
Parenteral opioid use alone or in combination with sedatives is strongly associated with cardiopulmonary arrests on the general care floor.38 While judicious use of these interventions is important, continuous monitoring for patients receiving opioids is also deficient. A high incidence of respiratory depression episodes was detected using continuous monitoring and these may contribute to significant morbidity as seen by the higher incidence of rapid response activation and prolonged hospitalization. In the absence of interventional trials testing continuous monitoring and inpatient mortality, results from PRODIGY provide a tool for respiratory depression risk assessment and suggest that continuous monitoring with capnography and oximetry of patients identified as high risk for respiratory depression may improve their safety when parenteral opioid analgesia cannot be avoided.
CONTRIBUTORS
PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) Group Collaborators: in addition to the listed authors, the PRODIGY Group includes: Dr Marianne Tanios, Dr Eva Rivas, Dr Miluska Mejia, Kavita Elliott, and Assad Ali (Ashish Khanna, Cleveland Clinic); Dr Juan Fiorda-Diaz, Dr Ruben Carrasco-Moyano, Dr Ana Mavarez-Martinez, Dr Alicia Gonzalez- Zacarias, Cory Roeth, January Kim, and Alan Esparza-Gutierrez (Sergio Bergese, The Ohio State University Wexner Medical Center); Dr Carleara Weiss and Chiahui Chen (Carla Jungquist, University at Buffalo); Dr Arata Taniguchi, Yuko Mihara, and Makiko Ariyoshi (Hiroshi Morimatsu, Okayama University); Dr Ichiro Kondo, Dr Kentaro Yamakawa, Dr Yoshifumi Suga, Dr Kohei Ikeda, Dr Koji Takano, and Dr Yuuki Kuwabara (Shoichi Uezono, Jikei University); Dr Nicole Carignan, Dr Joyce Rankin, Katherine Egan, and Lakeisha Waters (Simon Lee, Emory University); Dr Ming Ann Sim, Dr Lyn Li Lean, Dr Qi En Lydia Liew, and Dr Lawrence Siu-Chun Law (Lian Kah Ti, National University Health System Singapore); James Gosnell and Salina Shrestha (Richard Urman, Brigham and Women’s Hospital); Chisom Okponyia and Mohammed H. Al-Musawi (Robert McIntyre, University of Colorado, Anschutz Medical Campus); Dr María José Parra Gonzalez (Carlos Tornero, Hospital Clínico Universitario de Valencia); Dr Claudia Neumann, Vera Guttenthaler, Olja Männer, Dr Achilles Delis, Dr Anja Winkler, Bahareh Marchand, and Frauke Schmal (Maria Wittmann, University Hospital Bonn); Dr Fuad Aleskerov, Dr Mohammedumer Nagori, Dr Muhammad Shafi, Gloria McPhee, Cynthia Newman, and Elizabeth Lopez (Dennis Auckley, MetroHealth Medical Center); Dr Sabrina Ma Har (Morgan Le Guen, Hôpital Foch); Dr Moumen Asbahi (Roy Soto, Beaumont Health); Kim Nordstrom McCaw (Frank Schramm, Providence Regional Medical Center); and Dr Maurice Theunissen and Dr Valerie Smit- Fun (Wolfgang Buhre, Maastricht University Medical Center).
ACKNOWLEDGMENTS
We thank the investigators, the staff, and the participants of the PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) trial for their valuable contributions. We also thank Christine Greening and Melanie Crystal (Medtronic, Boulder, CO) for clinical trial management and Katherine E. Liu, PhD (Medtronic, Minneapolis, MN) for medical writing support.
DISCLOSURES
Name: Ashish K. Khanna, MD.
Contribution: This author helped design the trial, acquire and interpret the data, and draft and revise the manuscript.
Conflicts of Interest: A. K. Khanna reports consulting fees from Medtronic, Edwards Lifesciences, and Philips North America.
Name: Sergio D. Bergese, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Carla R. Jungquist, NP, PhD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: C. R. Jungquist reports participation in the Medtronic Nurse Advisory Group.
Name: Hiroshi Morimatsu, MD, PhD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Shoichi Uezono, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Simon Lee, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Lian Kah Ti, MBBS, MMed.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Richard D. Urman, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: R. D. Urman reports a grant and personal fees from Merck and from 3M and Posimir.
Name: Robert McIntyre Jr, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Carlos Tornero, MD, PhD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Albert Dahan, MD, PhD.
Contribution: This author helped analyze and interpret the data, and revise the manuscript.
Conflicts of Interest: A. Dahan reports grants and personal fees for MSD Nederland BV, Grunenthal, and Medasense.
Name: Leif Saager, Dr Med.
Contribution: This author helped analyze and interpret the data, and revise the manuscript.
Conflicts of Interest: L. Saager reports a grant from Merck and personal fees from The 37 Company.
Name: Toby N. Weingarten, MD.
Contribution: This author helped analyze and interpret the data, and revise the manuscript.
Conflicts of Interest: T. N. Weingarten reports a grant from Merck and nonfinancial support from Respiratory Motion.
Name: Maria Wittmann, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Dennis Auckley, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Luca Brazzi, MD, PhD.
Contribution: This author helped analyze and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Morgan Le Guen, MD, PhD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Roy Soto, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Frank Schramm, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Sabry Ayad, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Roop Kaw, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Paola Di Stefano, MSc.
Contribution: This author helped design the trial, analyze the data, and revise the manuscript.
Conflicts of Interest: P. Di Stefano reports employment with Medtronic.
Name: Daniel I. Sessler, MD.
Contribution: This author helped interpret the data and revise the manuscript.
Conflicts of Interest: None.
Name: Alberto Uribe, MD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Vanessa Moll, MD, PhD.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: V. Moll reports personal fees for writing contributions to Merck Manuals.
Name: Susan J. Dempsey, MN.
Contribution: This author helped acquire and interpret the data, and revise the manuscript.
Conflicts of Interest: S. J. Dempsey reports participation in the Medtronic Nurse Advisory Group.
Name: Wolfgang Buhre, MD.
Contribution: This author helped design the trial, acquire and interpret the data, and draft and revise the manuscript.
Conflicts of Interest: W. Buhre reports grants from the European Union and Interreg Consortium, and personal fees from European Society of Anaesthesiology studies (PHOENICS and TETHYS) supported by B Braun Medical and Fresenius Medical Care, and from Medtronic.
Name: Frank J. Overdyk, MD.
Contribution: This author helped design the trial, interpret the data, and draft and revise the manuscript.
Conflicts of Interest: None.
This manuscript was handled by: David Hillman, MD.
Supplementary Material
FOOTNOTES
GLOSSARY
- ASA =
- American Society of Anesthesiologists
- AUC =
- area under the curve
- BMI =
- body mass index
- bpm =
- breaths/min
- CPAP =
- continuous positive airway pressure
- Etco2 =
- end-tidal carbon dioxide
- FAS =
- full analysis set
- IPI =
- Integrated Pulmonary Index
- IQR =
- interquartile range
- IRB =
- nstitutional review board
- MFAS =
- modified full analysis set
- MME =
- morphine milligram equivalent
- OIRD =
- opioid-induced respiratory depression
- OR =
- odds ratio
- PRODIGY =
- PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY
- Spo2 =
- oxygen saturation
- STOP =
- Snoring, Tiredness, Observed apnea, blood Pressure
- STROBE =
- STrengthening the Reporting of OBservational studies in Epidemiology
- STOP-BANG =
- Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference, and Gender
Published ahead of print 16 April 2020.
Funding: This work was supported by Medtronic. All authors have completed the International Committee of Medical Journal Editors (ICMJE) Form for Disclosure of Potential Conflicts of Interest. All authors except P.D.S. report financial support to the Investigator or Investigator’s Institution to fund the Medtronic-sponsored trial, as well as medical writing and editorial support by a medical writer employed by Medtronic.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
A full list of contributors can be found at the end of the article.
A steering committee comprised the authors of this manuscript, and some Medtronic personnel were involved in the design and conduct of the trial, and collection, management, and analysis of the data. The authors were responsible for data interpretation and preparation, review, and approval of the manuscript. Medtronic provided medical writing support, under the direction of the authors, to coordinate author reviews and assist in editing and journal styling. The corresponding author had final responsibility for the decision to submit for publication.
Clinical Trial Registry: ClinicalTrials.gov, ID: NCT02811302.
Contributor Information
Dr Marianne Tanios, (Ashish Khanna, Cleveland Clinic).
Dr Eva Rivas, (Ashish Khanna, Cleveland Clinic).
Dr Miluska Mejia, (Ashish Khanna, Cleveland Clinic).
Kavita Elliott, (Ashish Khanna, Cleveland Clinic).
Assad Ali, (Ashish Khanna, Cleveland Clinic).
Dr Juan Fiorda-Diaz, (Sergio Bergese, The Ohio State University Wexner Medical Center).
Dr Ruben Carrasco-Moyano, (Sergio Bergese, The Ohio State University Wexner Medical Center).
Dr Ana Mavarez-Martinez, (Sergio Bergese, The Ohio State University Wexner Medical Center).
Dr Alicia Gonzalez-Zacarias, (Sergio Bergese, The Ohio State University Wexner Medical Center).
Cory Roeth, (Sergio Bergese, The Ohio State University Wexner Medical Center).
January Kim, (Sergio Bergese, The Ohio State University Wexner Medical Center).
Alan Esparza-Gutierrez, (Sergio Bergese, The Ohio State University Wexner Medical Center).
Dr Carleara Weiss, (Carla Jungquist, University at Buffalo).
Chiahui Chen, (Carla Jungquist, University at Buffalo).
Dr Arata Taniguchi, (Hiroshi Morimatsu, Okayama University).
Yuko Mihara, (Hiroshi Morimatsu, Okayama University).
Makiko Ariyoshi, (Hiroshi Morimatsu, Okayama University).
Dr Ichiro Kondo, (Shoichi Uezono, Jikei University).
Dr Kentaro Yamakawa, (Shoichi Uezono, Jikei University).
Dr Yoshifumi Suga, (Shoichi Uezono, Jikei University).
Dr Kohei Ikeda, (Shoichi Uezono, Jikei University).
Dr Koji Takano, (Shoichi Uezono, Jikei University).
Dr Yuuki Kuwabara, (Shoichi Uezono, Jikei University).
Dr Nicole Carignan, (Simon Lee, Emory University).
Dr Joyce Rankin, (Simon Lee, Emory University).
Katherine Egan, (Simon Lee, Emory University).
Lakeisha Waters, (Simon Lee, Emory University).
Dr Ming Ann Sim, (Lian Kah Ti, National University Health System Singapore).
Dr Lyn Li Lean, (Lian Kah Ti, National University Health System Singapore).
Dr Qi En Lydia Liew, (Lian Kah Ti, National University Health System Singapore).
Dr Lawrence Siu-Chun Law, (Lian Kah Ti, National University Health System Singapore).
James Gosnell, (Richard Urman, Brigham and Women’s Hospital).
Salina Shrestha, (Richard Urman, Brigham and Women’s Hospital).
Chisom Okponyia, (Robert McIntyre, University of Colorado, Anschutz Medical Campus).
Mohammed H. Al-Musawi, (Robert McIntyre, University of Colorado, Anschutz Medical Campus)
Dr María José Parra Gonzalez, (Carlos Tornero, Hospital Clínico Universitario de Valencia).
Dr Claudia Neumann, (Maria Wittmann, University Hospital Bonn).
Vera Guttenthaler, (Maria Wittmann, University Hospital Bonn).
Olja Männer, (Maria Wittmann, University Hospital Bonn).
Dr Achilles Delis, (Maria Wittmann, University Hospital Bonn).
Dr Anja Winkler, (Maria Wittmann, University Hospital Bonn).
Bahareh Marchand, (Maria Wittmann, University Hospital Bonn).
Frauke Schmal, (Maria Wittmann, University Hospital Bonn).
Dr Fuad Aleskerov, (Dennis Auckley, MetroHealth Medical Center).
Dr Mohammedumer Nagori, (Dennis Auckley, MetroHealth Medical Center).
Dr Muhammad Shafi, (Dennis Auckley, MetroHealth Medical Center).
Gloria McPhee, (Dennis Auckley, MetroHealth Medical Center).
Cynthia Newman, (Dennis Auckley, MetroHealth Medical Center).
Elizabeth Lopez, (Dennis Auckley, MetroHealth Medical Center).
Dr Sabrina Ma Har, (Morgan Le Guen, Hôpital Foch).
Dr Moumen Asbahi, (Roy Soto, Beaumont Health).
Kim Nordstrom McCaw, (Frank Schramm, Providence Regional Medical Center).
Dr Maurice Theunissen, (Wolfgang Buhre, Maastricht University Medical Center).
Dr Valerie Smit-Fun, (Wolfgang Buhre, Maastricht University Medical Center).
Collaborators: Dr Marianne Tanios, Dr Eva Rivas, Dr Miluska Mejia, Kavita Elliott, Assad Ali, Dr Juan Fiorda-Diaz, Dr Ruben Carrasco-Moyano, Dr Ana Mavarez-Martinez, Dr Alicia Gonzalez-Zacarias, Cory Roeth, January Kim, Alan Esparza-Gutierrez, Dr Carleara Weiss, Chiahui Chen, Dr Arata Taniguchi, Yuko Mihara, Makiko Ariyoshi, Dr Ichiro Kondo, Dr Kentaro Yamakawa, Dr Yoshifumi Suga, Dr Kohei Ikeda, Dr Koji Takano, Dr Yuuki Kuwabara, Dr Nicole Carignan, Dr Joyce Rankin, Katherine Egan, Lakeisha Waters, Dr Ming Ann Sim, Dr Lyn Li Lean, Dr Qi En Lydia Liew, Dr Lawrence Siu-Chun Law, James Gosnell, Salina Shrestha, Chisom Okponyia, Mohammed H. Al-Musawi, Dr María José Parra Gonzalez, Dr Claudia Neumann, Vera Guttenthaler, Olja Männer, Dr Achilles Delis, Dr Anja Winkler, Bahareh Marchand, Frauke Schmal, Dr Fuad Aleskerov, Dr Mohammedumer Nagori, Dr Muhammad Shafi, Gloria McPhee, Cynthia Newman, Elizabeth Lopez, Dr Sabrina Ma Har, Dr Moumen Asbahi, Kim Nordstrom McCaw, Dr Maurice Theunissen, and Dr Valerie Smit-Fun
REFERENCES
- 1.Khanna AK, Overdyk FJ, Greening C, Di Stefano P, Buhre WF. Respiratory depression in low acuity hospital settings-seeking answers from the PRODIGY trial. J Crit Care. 2018;47:80–87. [DOI] [PubMed] [Google Scholar]
- 2.Andersen LW, Berg KM, Chase M, Cocchi MN, Massaro J, Donnino MW; American Heart Association’s Get With The Guidelines(®)-Resuscitation Investigators. Acute respiratory compromise on inpatient wards in the United States: incidence, outcomes, and factors associated with in-hospital mortality. Resuscitation. 2016;105:123–129. [DOI] [PubMed] [Google Scholar]
- 3.Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Manag Nurs. 2011;12:118–145.e10. [DOI] [PubMed] [Google Scholar]
- 4.DeVita MA, Smith GB, Adam SK, et al. “Identifying the hospitalised patient in crisis”–a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81:375–382. [DOI] [PubMed] [Google Scholar]
- 5.Izrailtyan I, Qiu J, Overdyk FJ, Erslon M, Gan TJ. Risk factors for cardiopulmonary and respiratory arrest in medical and surgical hospital patients on opioid analgesics and sedatives. PLoS One. 2018;13:e0194553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg. 2015;121:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–223. [DOI] [PubMed] [Google Scholar]
- 9.Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27:62–70. [DOI] [PubMed] [Google Scholar]
- 10.Vetro J, Natarajan DK, Mercer I, et al. Antecedents to cardiac arrests in a hospital equipped with a medical emergency team. Crit Care Resusc. 2011;13:162–166. [PubMed] [Google Scholar]
- 11.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122:659–665. [DOI] [PubMed] [Google Scholar]
- 12.Belcher AW, Khanna AK, Leung S, et al. Long-acting patient-controlled opioids are not associated with more postoperative hypoxemia than short-acting patient-controlled opioids after noncardiac surgery: a cohort analysis. Anesth Analg. 2016;123:1471–1479. [DOI] [PubMed] [Google Scholar]
- 13.Ronen M, Weissbrod R, Overdyk FJ, Ajizian S. Smart respiratory monitoring: clinical development and validation of the IPI™ (Integrated Pulmonary Index) algorithm. J Clin Monit Comput. 2017;31:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarter T, Shaik Z, Scarfo K, Thompson LJ. Capnography monitoring enhances safety of postoperative patient-controlled analgesia. Am Health Drug Benefits. 2008;1:28–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Canet J, Sabaté S, Mazo V, et al. ; PERISCOPE Group. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: a prospective, observational study. Eur J Anaesthesiol. 2015;32:458–470. [DOI] [PubMed] [Google Scholar]
- 16.Felhofer K. Developing a respiratory depression scorecard for capnography monitoring. Inov Pharm. 2013;4:(3):Article 128. [Google Scholar]
- 17.Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33:383–391. [DOI] [PubMed] [Google Scholar]
- 18.Weingarten TN, Herasevich V, McGlinch MC, et al. Predictors of delayed postoperative respiratory depression assessed from naloxone administration. Anesth Analg. 2015;121:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Joint Commission. Safe use of opioids in hospitals. Sentin Event Alert. 2012;49:1–5. [PubMed] [Google Scholar]
- 20.Yoo W, Mayberry R, Bae S, Singh K, Peter He Q, Lillard JW., Jr. A study of effects of multicollinearity in the multivariable analysis. Int J Appl Sci Technol. 2014;4:9–19. [PMC free article] [PubMed] [Google Scholar]
- 21.Miao Y, Stijacic Cenzer I, Kirby KA, Boscardin WJ. Estimating Harrell’s Optimism on Predictive Indices Using Bootstrap Samples. 2013San Francisco, CA: SAS Global Forum; [Google Scholar]
- 22.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC, Steyerberg EW. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat Methods Med Res. 2017;26:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001;25:464–469. [PubMed] [Google Scholar]
- 26.Canet J, Gallart L, Gomar C, et al. ; ARISCAT Group. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. [DOI] [PubMed] [Google Scholar]
- 27.Arozullah AM, Khuri SF, Henderson WG, Daley J; Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–857. [DOI] [PubMed] [Google Scholar]
- 28.Neto AS, da Costa LGV, Hemmes SNT, et al. ; LAS VEGAS. The LAS VEGAS risk score for prediction of postoperative pulmonary complications: an observational study. Eur J Anaesthesiol. 2018;35:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minkowitz HS, Scranton R, Gruschkus SK, Nipper-Johnson K, Menditto L, Dandappanavar A. Development and validation of a risk score to identify patients at high risk for opioid-related adverse drug events. J Manag Care Spec Pharm. 2014;20:948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brueckmann B, Villa-Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology. 2013;118:1276–1285. [DOI] [PubMed] [Google Scholar]
- 31.Gupta K, Prasad A, Nagappa M, Wong J, Abrahamyan L, Chung FF. Risk factors for opioid-induced respiratory depression and failure to rescue: a review. Curr Opin Anaesthesiol. 2018;31:110–119. [DOI] [PubMed] [Google Scholar]
- 32.Naples JG, Gellad WF, Hanlon JT. The role of opioid analgesics in geriatric pain management. Clin Geriatr Med. 2016;32:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam T, Nagappa M, Wong J, Singh M, Wong D, Chung F. Continuous pulse oximetry and capnography monitoring for postoperative respiratory depression and adverse events: a systematic review and meta-analysis. Anesth Analg. 2017;125:2019–2029. [DOI] [PubMed] [Google Scholar]
- 34.Weinger MB, Lee LA. No patient shall be harmed by opioid-induced respiratory depression. Anesthesia Patient Safety Foundation. 2011;26:21–40. [Google Scholar]
- 35.Olofsen E, Boom M, Nieuwenhuijs D, et al. Modeling the non-steady state respiratory effects of remifentanil in awake and propofol-sedated healthy volunteers. Anesthesiology. 2010;112:1382–1395. [DOI] [PubMed] [Google Scholar]
- 36.Stites M, Surprise J, McNiel J, Northrop D, De Ruyter M. Continuous capnography reduces the incidence of opioid-induced respiratory rescue by hospital rapid resuscitation ream. J Patient Saf. 2017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Ellison A. Average cost per inpatient day across 50 states. Becker’s Hospital CFO Report 2016. Available at: https://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html.Accessed November 27, 2018.
- 38.Overdyk FJ, Dowling O, Marino J, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11:e0150214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deljou A, Hedrick SJ, Portner ER, et al. Pattern of perioperative gabapentinoid use and risk for postoperative naloxone administration. Br J Anaesth. 2018;120:798–806. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld DM, Betcher JA, Shah RA, et al. Findings of a naloxone database and its utilization to improve safety and education in a tertiary care medical center. Pain Pract. 2016;16:327–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


