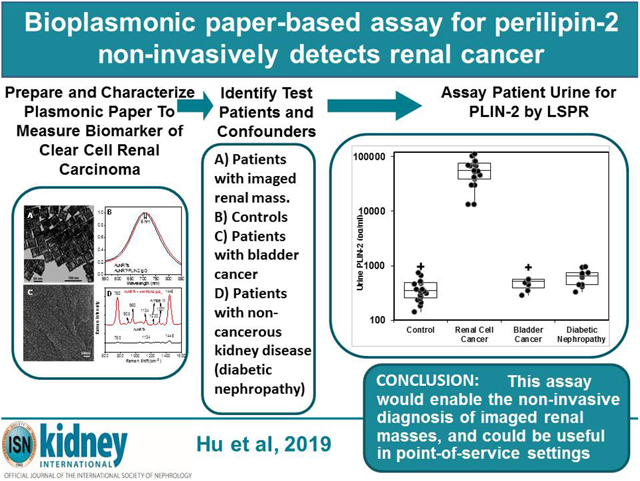
Abstract
Renal cell carcinoma (RCC) has poor survival prognosis because it is asymptomatic at an early, more curative stage. Recently, urine perilipin-2 (PLIN-2) was demonstrated to be a sensitive and specific biomarker for the noninvasive, early detection of RCC and an indispensable indicator to distinguish cancer from a benign renal mass. However, current Western blot or ELISA PLIN-2 assays are complicated, expensive, time-consuming or insensitive, making them unsuitable for routine analysis in clinical settings. Here we developed a plasmonic biosensor based on the high refractive index sensitivity of gold nanorattles for the rapid detection of PLIN-2 in patient urine. The paper-based plasmonic assay is highly sensitive and has a dynamic range of 50 pg/ml to 5 μg/ml PLIN-2. The assay is not compromised by variations in urine pH or high concentrations of interfering proteins such as albumin and hemoglobin, making it an excellent candidate for routine clinical applications. The urine PLIN-2 assay readily distinguished patients with pathologically proven clear cell carcinomas of various size, stage and grade (55.9 [39.5, 75.8] ng/ml, median [1st and 3rd quartile]) from age-matched controls (0.3 [0.3, 0.5] ng/ml), patients with bladder cancer (0.5 [0.4, 0.6] ng/ml) and patients with diabetic nephropathy (0.6 [0.4, 0.7] ng/ml). Urine PLIN-2 concentrations were roughly proportional to tumor size (Pearson coefficient 0.59). Thus, this cost-effective and label-free method represents a novel approach to conduct a non-invasive population screen or rapid differential diagnosis of imaged renal masses, significantly facilitating the early detection and diagnosis of RCC.
Keywords: Cancer Diagnostics, Renal Cancer, Bioplasmonic Assay, Cancer Biomarkers
Graphical Abstract
Introduction
Kidney cancer accounts for 3% to 4% of the newly found cases of malignant tumors in adults.1 Unfortunately, renal cell carcinoma (RCC) is generally asymptomatic at early stages, and is thereby frequently advanced when a diagnosis is made. Most renal masses are incidentally discovered by imaging modalities such as computed tomography and magnetic resonance imaging, but imaging cannot reliably distinguish between cancerous and benign tumors.2,3 The conventional approach to a suspected renal mass is partial or radical nephrectomy. The problem is that 20% of imaged renal masses are not cancer, and patients incur the loss of normal kidney tissue,2,3 reducing renal function and accelerates progression of underlying chronic kidney diseases.3,4
Recently, we reported that urine perilipin-2 (PLIN-2) and aquaporin-1 (AQP1) are sensitive and specific biomarkers for early noninvasive detection of clear cell or papillary subtypes of RCC (together accounting for 85–90% of malignant RCC).5,6 These biomarkers were not increased in patients with common non-cancerous kidney diseases (i.e. diabetic nephropathy, glomerulonephritis, urinary tract infection5), or other urinary tract cancers (bladder or prostate6), thus demonstrating specificity. Results suggested that AQP1 and PLIN-2 have potential utility for differential diagnosis of imaged renal masses.5,6 However, the Western blot procedure for PLIN-2 is complicated, expensive, is semi-quantitative and time consuming, which is not suitable for analysis of urine samples in clinical lab-settings. It is also challenging to develop an enzyme-linked immunosorbent assay (ELISA) for PLIN-2, particularly for screening of patient urine samples, due to insufficient sensitivity and effects of interindividual variability in urine pH, A convenient and cost-effective assay is needed for fast and quantitative testing of urine PLIN-2. This could significantly facilitate differential diagnosis of imaged renal masses to avoid unnecessary renal excision surgery and its consequences.
Plasmonic biosensors, based on reflective index sensitivity of localized surface plasmon resonance (LSPR), are highly sensitive, cost-effective and label-free biodiagnostic platforms. LSPR of noble metal nanostructures involving the coherent oscillation of dielectrically confined conduction electrons is a sensitive methodology.7–9 Hollow plasmonic nanostructures such as gold nanoshells, nanocages and nanorattles exhibit significantly higher refractive index sensitivity compared to their solid counterparts, making them excellent candidates for plasmonic biosensors.10 Recently, we have demonstrated that gold nanorattles (AuNRTs) exhibit much higher refractive index sensitivity compared to gold nanorods with similar LSPR wavelength.11
Plasmonic paper, common filter paper adsorbed with biofunctionalized nanostructures, is a highly attractive biodiagnostic platform for point-of-care and resource-limited settings.12–16 Our previous work has provided proof-of-concept for bioplasmonic paper as a platform technology for the detection and quantification of AQP1, another biomarker for RCC diagnosis.17
Herein, we report a facile approach for rapid and highly sensitive detection of PLIN-2 in clinical urine samples. Common filter paper uniformly absorbed with commercial monoclonal antibody-conjugated AuNRTs is employed as a label-free plasmonic biosensor. This assay is significantly more sensitive than Western blotting, and provides the requisite sensitivity for quantifying clinical urine PLIN-2 concentrations for RCC. Moreover, this technology represents a highly attractive diagnostic platform for rapid detection and quantification of target bioanalytes in pre-clinical and clinical settings.
Results
A schematic illustration summarizing the building of the bioplasmonic paper to detect PLIN-2 using gold nanorattles (AuNRTs) and a monoclonal antibody is depicted in Figure 1. The technology is versatile with the assay specificity dependent on the specificity of the antibody and the sensitivity of the assay is amplified by the nanoparticle. The paper base allows easy handling and processing.
Figure 1.
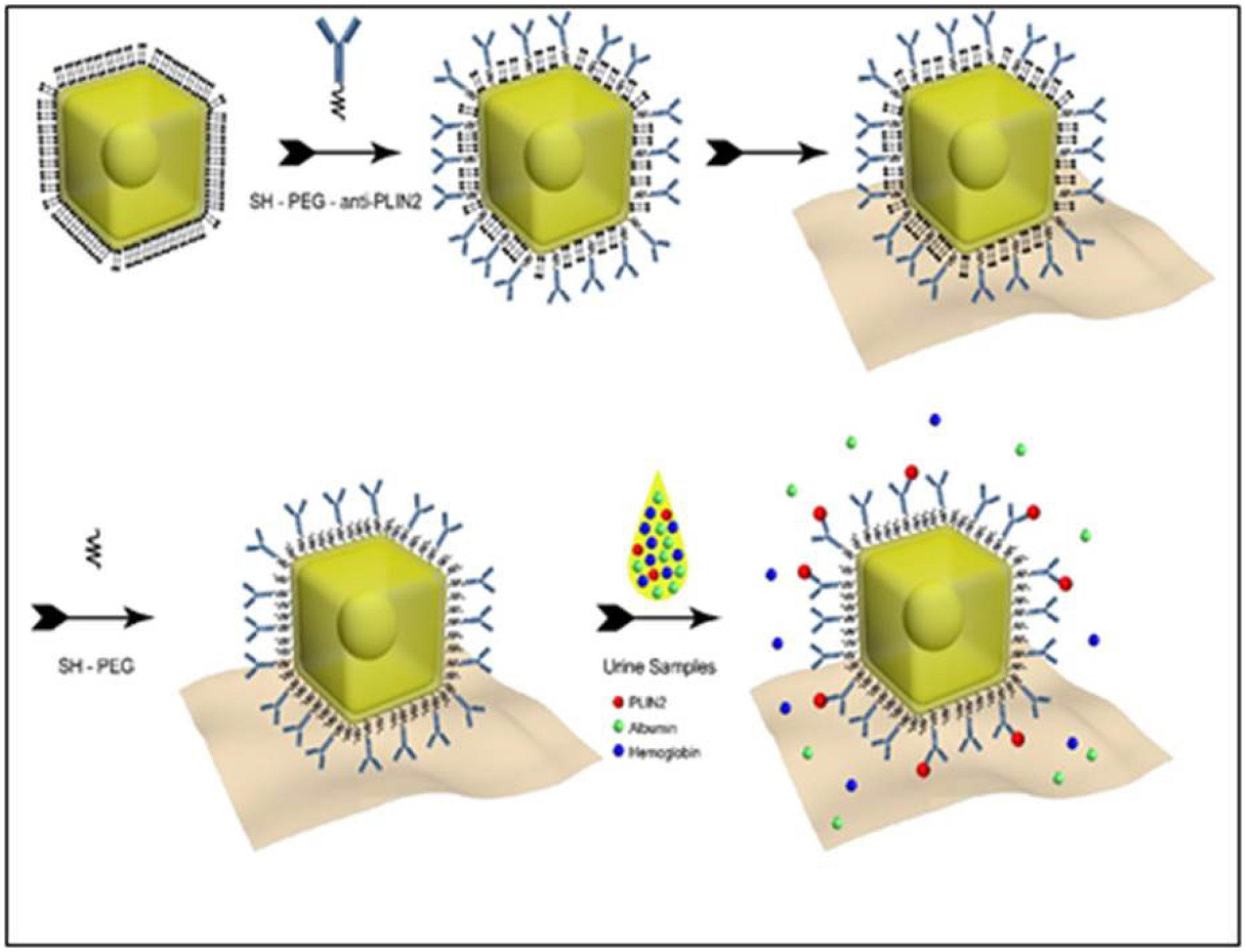
Schematic illustration of plasmonic paper manufacture. Gold nanorattles (AuNRTs) coated with cetyltrimethylammonium chloride (CTAC) were functionalized with monoclonal antibody by means of a bifunctional polyethylene glycol (PEG). The functionalized AuNRTs were then coated on filter paper and non-specific binding sites neutralized by further PEGylation. The paper-bound functionalized AuNRTs then bind specific analyte; in this case, perilipin-2 (PLIN-2) and cause a red shift in the localized surface plasmon resonance (LSPR) for the AuNRTs.
Measurement of PLIN-2 in human urine
The LSPR wavelength shift of the bioplasmonic paper upon exposure to kidney cancer patient urine samples was found to be significantly higher compared to that of healthy volunteers (example Figure 2A). Results were also compared to PLIN-2 concentrations previously determined by Western blot analysis.5,6 Urine PLIN-2 concentrations from the 20 patients with RCC were 43 [29, 68] ng/ml (median [1st and 3rd quartile]), and were significantly higher (P<0.001, Kruskal-Wallis) than those of 20 controls at 0.3 [0.3, 0.5] ng/ml, those of 8 patients with bladder cancer at 0.5[0.4, 0.6] ng/ml or 10 patients with diabetic nephropathy at 0.6[0.4, 0.7] ng/ml (Figure 2B). There was no overlap in PLIN-2 concentration ranges between RCC and all other groups. Equally important; the urine PLIN-2 concentrations were related to tumor size (Figure 2C) with a Spearman correlation coefficient of 0.59 (95% confidence interval 0.16 to 0.81, P<0.009). A Spearman plot of the urine PLIN-2 concentrations of the patients with RCC measured by the plasmonic papers and previously by Western blotting (Figure 2D) indicates an excellent correlation (R2 0.94, P<0.001). However, it should be noted that the Western blot analysis appears to tail off at low PLIN-2 concentrations.
Figure 2. A).
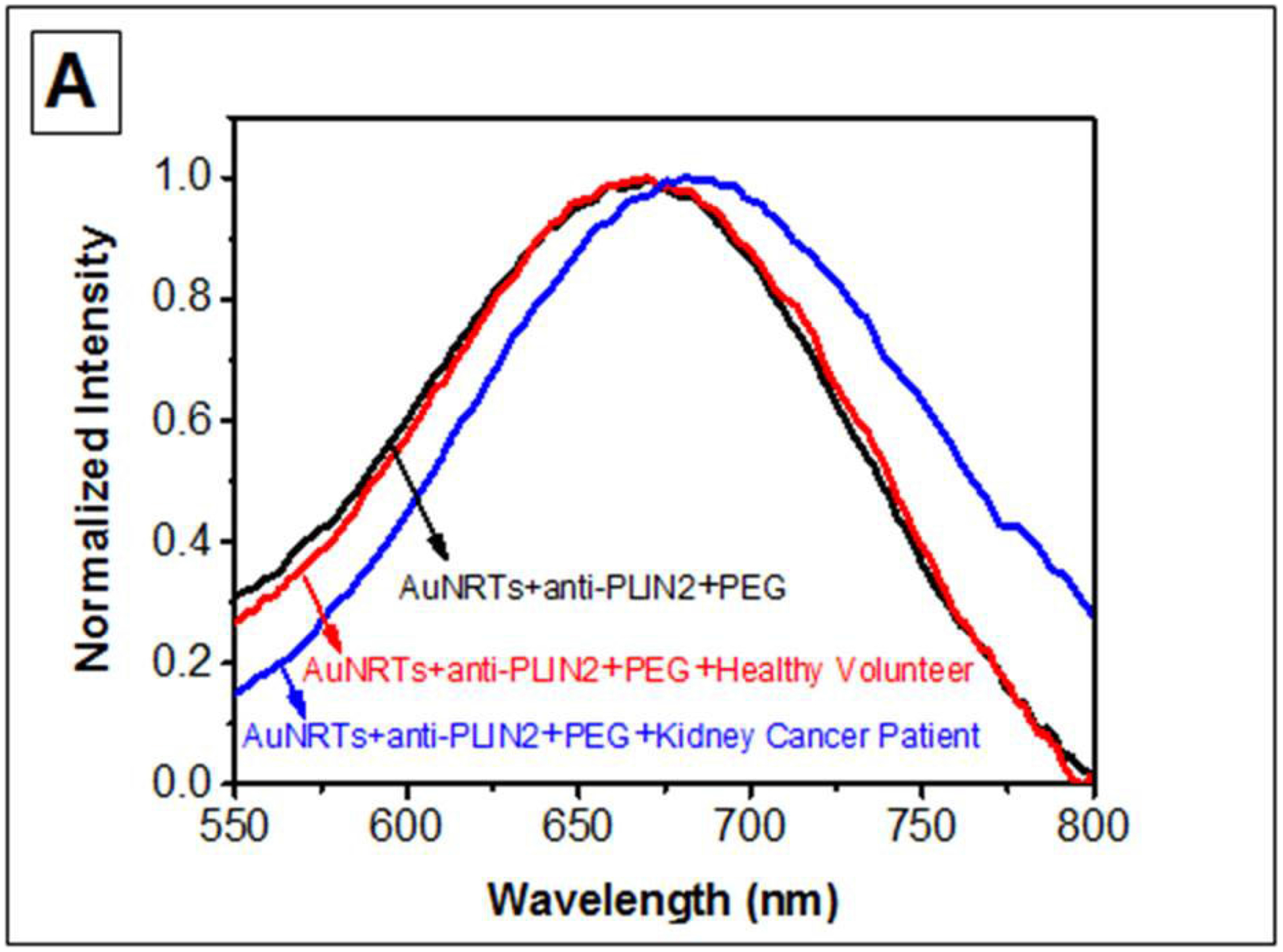
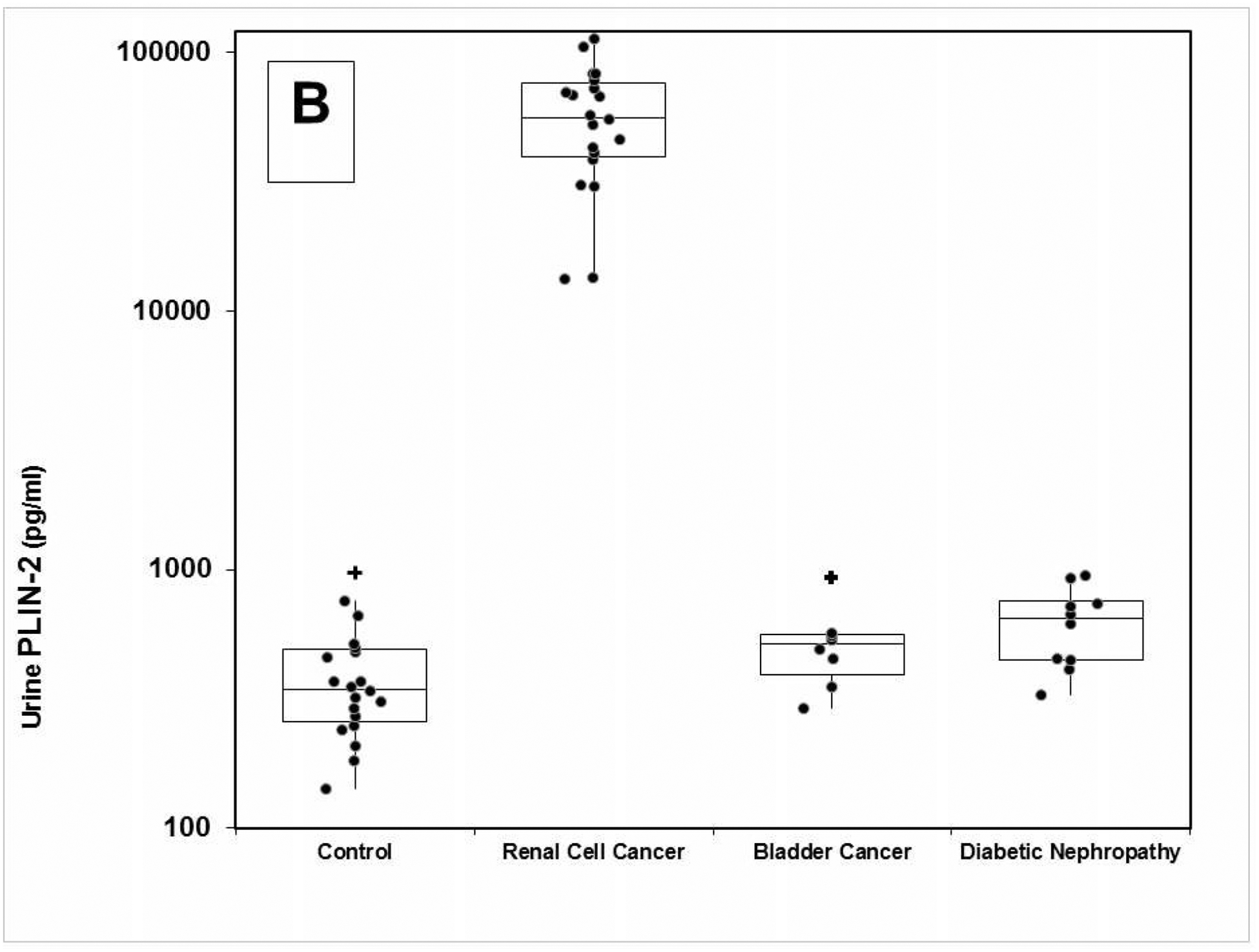
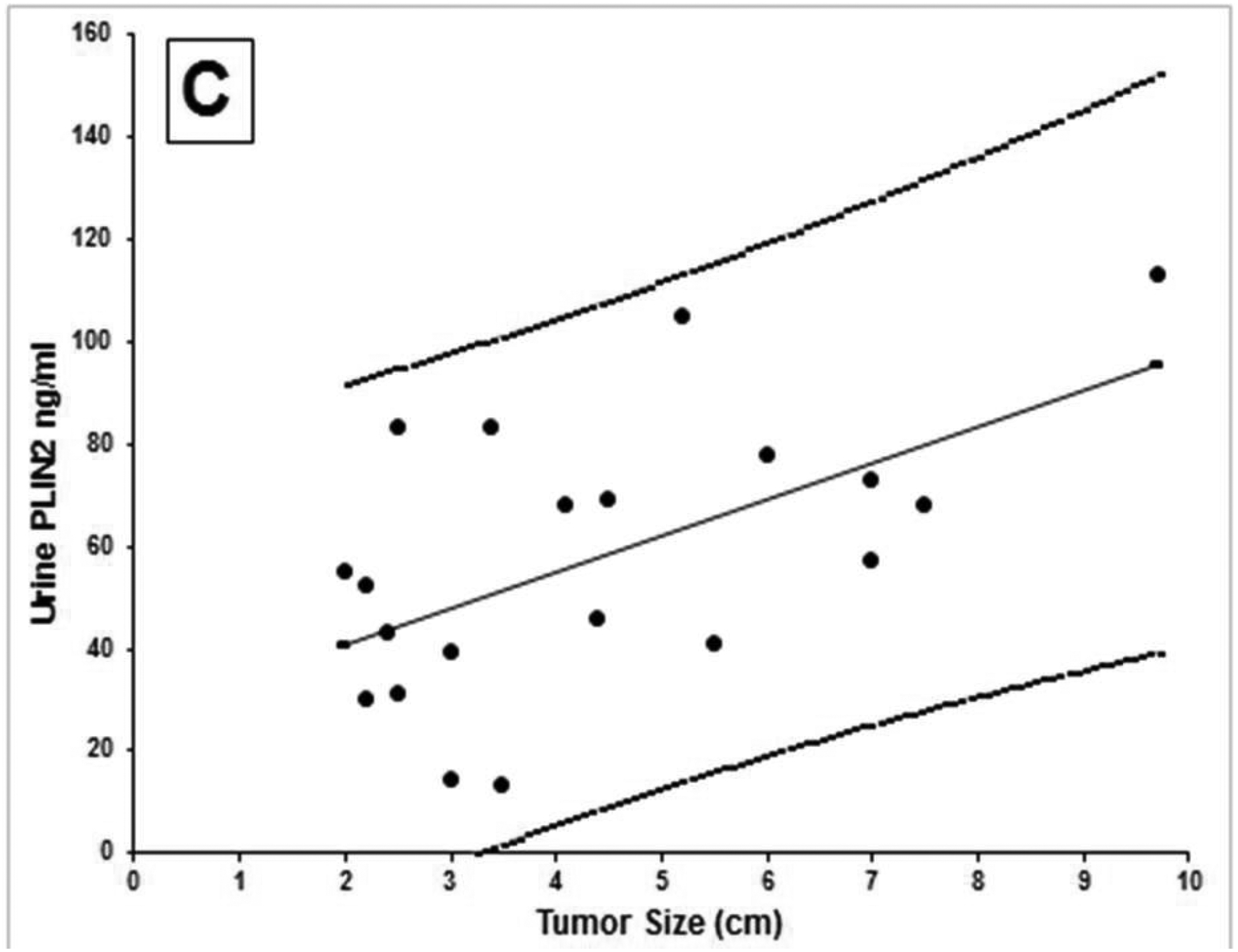
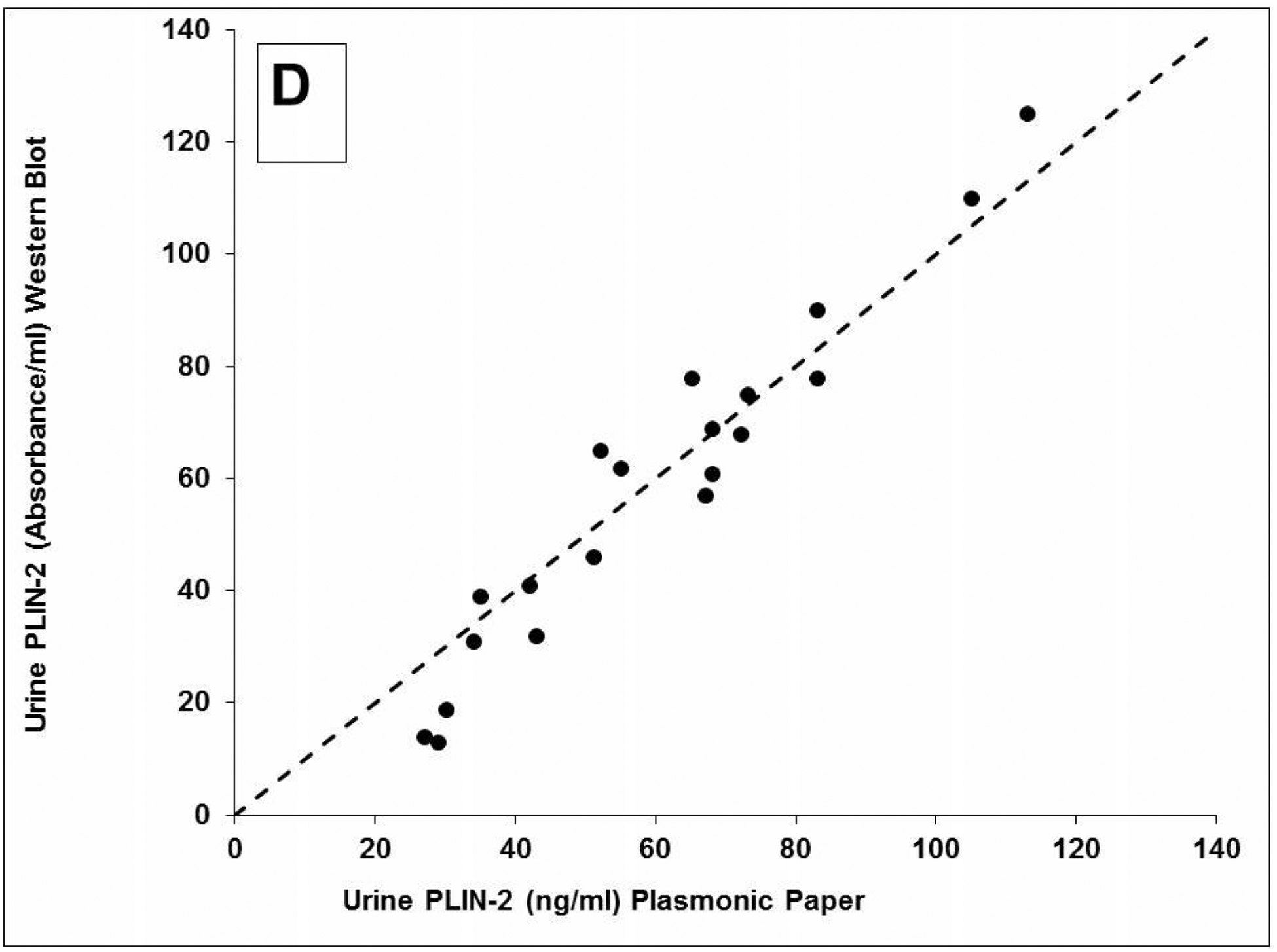
A) Representative localized surface plasmon resonance spectra of anti-PLIN-2 functionalized AuNRTs (black), functionalized AuNRTs incubated with urine from a healthy volunteer (red), and functionalized AuNRTs incubated with urine from a patient with reanl clear cell carcinoma (blue). B) Box and whisker plot of the urine PLIN-2 concentrations of 20 control patients, 20 patients with renal cell cancer, 8 patients with bladder cancer and 10 patients with diabetic nephropathy. The median with whiskers and boxes depicting the interquartile range is shown. The concentration of each patient is depicted as a solid dot or a plus sign. The plus signs are outliers more than 1.5 times but less than 3.0 times the interquartile range. Patients with renal cell cancer have significantly higher urine PLIN-2 concentrations than that of other cohorts (P<0.001 by Kruskal-Wallis). C) Urine PLIN-2 concentration of patients with renal cell cancer as a function of tumor size. The Spearman correlation coefficient is 0.59 (P=0.009). Regression line (solid). 95% prediction interval (dashed lines). D) Urine PLIN-2 concentration determined by plasmonic paper vs relative absorbance by Western blot. Dashed line of identity. Spearman correlation 0.94 (P<0.001).
Discussion
Plasmonic paper serves as a simple and versatile platform for implementing plasmonic biosensors. Due to the high sensitivity of the assay, and lack of potential interference by albuminuria or hematuria, urine PLIN-2 concentrations in normal individuals could be accurately measured and there was a clear difference in urine PLIN-2 concentrations between normal individuals, patients with bladder cancer, patients with diabetic nephropathy and patients with a pathologically proven clear cell carcinoma. A limitation of the study is the small number of patients in this proof-of-concept analysis. A larger multi-center blinded study incorporating patients with a wide variety of non-cancerous kidney diseases, more patients with cancerous urologic diseases and many more control individuals would be necessary to validate this assay. The assay enables fast, sensitive, cost-effective and non-invasive diagnosis of kidney cancer, especially in at risk groups. For example, the Veterans Health Administration now targets kidney cancer as a specific condition based on the exposure of soldiers, their families and base personnel to contaminated drinking water.18 There is also a significantly increased incidence of RCC among autoworkers exposed to metal working fluids.19 There is no current cost effective high throughput means of identifying individuals with RCC. Our assay could be of importance in identifying those with RCC in military bases or autoworker cohorts or other populations. Additionally, this assay would enable the differential diagnosis of imaged renal masses, and could be useful in point-of-care settings such as hospital radiology departments and stand-alone imaging clinics. The need for differential diagnosis of imaged renal mass is compelling. Nationally, about 45,000 partial and radical nephrectomies were performed in the United States alone in 2015.3 It is estimated that 18% of these would have undergone surgery for removal of a benign mass (extrapolation of SEER 18 Data Base). A non-invasive biomarker assay to differentiate a benign mass from renal cell cancer could potentially have prevented unnecessary surgery in these cases. Future prospective studies are needed to validate this bioplasmonic paper based assay in population screening and to differentially diagnose imaged renal masses on a larger scale.
Methods
Synthesis of Bioplasmonic Paper
We employed a seed-mediated approach in combination with galvanic replacement reaction to synthesize AuNRTs.11. These AuNRTs were conjugated with a commercially available human PLIN-2-specific monoclonal antibody as the biorecognition element. The successful conjugation of the PLIN-2 antibody was evidenced by a 6 nm red shift in the LSPR wavelength of the AuNRT (Supplemental Figure 2B). Uniform adsorption of bioconjugated plasmonic nanostructures on the paper substrate, which ensures optical homogeneity of the plasmonic paper substrates, is of great importance in determining the limit and accuracy of detection. Scanning electron microscopy (SEM) images revealed that the AuNRTs were uniformly distributed on paper substrates without significant aggregation or patchiness (Supplemental Figure 2C). Furthermore, surface enhanced Raman scattering (SERS) spectra confirm successful conjugation of anti-PLIN-2 IgG2B to the AuNRTs (Supplemental Figure 2D). Characteristic Raman spectral bands at 905 cm−1, 960 cm−1, 1233 cm−1, and 1307 cm−1 (correspond to symmetric C-C-N stretching and CH3 rocking vibration and asymmetric C-C-N stretching of the IgG2B) confirm the successful biofunctionalization of the nanostructures with antibodies.
Measurement of PLIN-2 in Patient Urine
Urine from 20 patients with documented clear cell renal carcinomas, 8 patients with documented bladder cancer and 20 normal individuals were obtained after written informed consent from September 2013 through February 2014. Approval was acquired from the Washington University Institutional Review Board. The studies were registered with ClinicalTrials.gov NCT00851994 and NCT01538823. Urine from patients with diabetic nephropathy was originally obtained from the P30 (DK079333) Kidney Translational Research Core of the Washington University George M. O’Brien Center for Kidney Disease Research as part of a previous study.20 Characterization of the patient cohorts is presented in the supplemental information.
The concentration of PLIN-2 was measured in October-November 2016, again in November 2017 and in March 2019 in a blinded fashion by immersing the plasmonic paper substrates into the different calibration standards or patient urine (diluted 10-fold) for 2 hours, after which they were completely rinsed with water and dried under a stream of nitrogen. Six vis-NIR extinction spectra were obtained for each paper substrate using spectrophotometer coupled to an optical microscope before and after exposing to samples to assure uniformity of the readings. The average LSPR wavelength before incubation of the paper substrates was subtracted from the average wavelength after incubation (Figure 2A) and the PLIN-2 concentration calculated based upon the standard curve run within each assay.
A Translational Statement, details of the synthesis and characterization of the bioplasmonic papers, patient demographics and statistical methods used are presented in the Supplemental Information.
Supplementary Material
Acknowledgements
The authors would like to thank Clinical Coordinators Marilee Fisher, Cynthia Tang and Aaron Steinberg for consenting patients and biobanking patient urine samples and Ross Rosen (student Department of Energy, Environmental, and Chemical Engineering) for help with the patient PLIN-2 measurements. Urine from patients with diabetic nephropathy were originally obtained from the P30 (DK079333) Kidney Translational Research Core of the Washington University George M. O’Brien Center for Kidney Disease Research as part of a previous study.8
Disclosures
PLIN-2, also known as ADFP, is subject in part to patents US9091690, US9494590, EP2433137 and EP2863225 to EDK and JJM and patent US9410949B2 to EDK, JJM and SS. To date, there are no financial interests.
The work was supported by grants 3706 and 3952 from The Foundation for Barnes-Jewish Hospital and by National Institutes of Health grants CA141521 to JJM; DK100759 to SS and a grant from the National Natural Science Foundation of China 8130655 to RH. JJM, SS and EDK are among founding members of Auragent Bioscience, LLC. Auragent is a small business startup developing plasmonic amplification of fluorescent signals. To date, Auragent has no financial interest in LSPR-based assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. July 2016;66(4):271–289. [DOI] [PubMed] [Google Scholar]
- 2.Akdogan B, Gudeloglu A, Inci K, Gunay LM, Koni A, Ozen H. Prevalence and predictors of benign lesions in renal masses smaller than 7 cm presumed to be renal cell carcinoma. Clinical genitourinary cancer. June 2012;10(2):121–125. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DC, Vukina J, Smith AB, et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. The Journal of urology. January 2015;193(1):30–35. [DOI] [PubMed] [Google Scholar]
- 4.Thorstenson A, Bergman M, Scherman-Plogell AH, et al. Tumour characteristics and surgical treatment of renal cell carcinoma in Sweden 2005–2010: a population-based study from the national Swedish kidney cancer register. Scandinavian journal of urology. June 2014;48(3):231–238. [DOI] [PubMed] [Google Scholar]
- 5.Morrissey JJ, Mellnick VM, Luo J, et al. Evaluation of Urine Aquaporin-1 and Perilipin-2 Concentrations as Biomarkers to Screen for Renal Cell Carcinoma: A Prospective Cohort Study. JAMA oncology. May 2015;1(2):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrissey JJ, Mobley J, Figenshau RS, Vetter J, Bhayani S, Kharasch ED. Urine aquaporin 1 and perilipin 2 differentiate renal carcinomas from other imaged renal masses and bladder and prostate cancer. Mayo Clinic proceedings. January 2015;90(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li T, Vongehr S, Tang S, Dai Y, Huang X, Meng X. Scalable Synthesis of Ag Networks with Optimized Sub-monolayer Au-Pd Nanoparticle Covering for Highly Enhanced SERS Detection and Catalysis. Scientific reports. November 15 2016;6:37092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novo C, Funston AM, Mulvaney P. Direct observation of chemical reactions on single gold nanocrystals using surface plasmon spectroscopy. Nature nanotechnology. October 2008;3(10):598–602. [DOI] [PubMed] [Google Scholar]
- 9.Shao Q, Wu P, Gu P, Xu X, Zhang H, Cai C. Electrochemical and spectroscopic studies on the conformational structure of hemoglobin assembled on gold nanoparticles. The journal of physical chemistry. B July 7 2011;115(26):8627–8637. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Xia Y. Gold and silver nanoparticles: a class of chromophores with colors tunable in the range from 400 to 750 nm. The Analyst. June 2003;128(6):686–691. [DOI] [PubMed] [Google Scholar]
- 11.Luan J, Liu KK, Tadepalli S, et al. PEGylated Artificial Antibodies: Plasmonic Biosensors with Improved Selectivity. ACS applied materials & interfaces. September 14 2016;8(36):23509–23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas A, Brimer A, Slocik JM, Tian L, Naik RR, Singamaneni S. Multifunctional analytical platform on a paper strip: separation, preconcentration, and subattomolar detection. Analytical chemistry. April 16 2013;85(8):3977–3983. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q CyJ, Cao S, Kharasch ED, Singamaneni S, Morrissey JJ. Rapid point of care, paper-based plasmonic biosensor for Zika virus diagnosis. Advanced Biosystems. 2017;1:1700096. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Hankus ME, Tian L, Pellegrino PM, Singamaneni S. Highly sensitive surface enhanced Raman scattering substrates based on filter paper loaded with plasmonic nanostructures. Analytical chemistry. December 1 2011;83(23):8953–8958. [DOI] [PubMed] [Google Scholar]
- 15.Tadepalli S, Kuang Z, Jiang Q, et al. Peptide Functionalized Gold Nanorods for the Sensitive Detection of a Cardiac Biomarker Using Plasmonic Paper Devices. Scientific reports. November 10 2015;5:16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian L, Tadepalli S, Park SH, et al. Bioplasmonic calligraphy for multiplexed label-free biodetection. Biosensors & bioelectronics. September 15 2014;59:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian L, Morrissey JJ, Kattumenu R, Gandra N, Kharasch ED, Singamaneni S. Bioplasmonic paper as a platform for detection of kidney cancer biomarkers. Analytical chemistry. November 20 2012;84(22):9928–9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Veterans A. Diseases Associated With Exposure to Contaminants in the Water Supply at Camp Lejeune. Final rule. Federal register. January 13 2017;82(9):4173–4185. [PubMed] [Google Scholar]
- 19.Shrestha D, Liu S, Hammond SK, et al. Risk of renal cell carcinoma following exposure to metalworking fluids among autoworkers. Occupational and environmental medicine. October 2016;73(10):656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrissey JJ, Kharasch ED. The specificity of urinary aquaporin 1 and perilipin 2 to screen for renal cell carcinoma. The Journal of urology. May 2013;189(5):1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


