Abstract
The steep rise in food allergy (FA) has evoked environmental factors involved in disease pathogenesis, including the gut microbiota, diet and their metabolites. Early introduction of solid foods synchronizes with the “weaning reaction”, a time during which the microbiota imprints durable oral tolerance. Recent work has shown that children with FA manifest an early onset dysbiosis with the loss of Clostridiales species, which promotes the differentiation of ROR-γt+ regulatory T cells to suppress FA. This process can be reversed in preclinical mouse models by targeted bacteriotherapy. Here we review the dominant tolerance mechanisms enforced by the microbiota to suppress FA and discuss therapeutic intervention strategies that act to recapitulate the early life window of opportunity in stemming the FA epidemic.
Keywords: Bacteroidales, Clostridiales, FOXP3, MyD88, Regulatory T Cells, RORC, RORγt, Subdoligranulum variable, Oral Immunotherapy, Weaning reaction, fecal microbiota transplantation
Introduction
Food Allergy (FA) denotes a state of failure of oral tolerance to one or more foods that is associated with the emergence of a pathogenic T helper type 2 (Th2) allergic immune response directed against food antigens (Tordesillas et al., 2017a; Gowthaman et al., 2019). FA encompasses a number of heterogenous disorders segregated by anatomy and mechanisms involved in disease pathogenesis (Sicherer and Sampson, 2018). By far the most common FA disorder, and the focus of this review, involves IgE mediated allergic reactions to food. Other less common non-IgE mediated FA disorders include eosinophilic esophagitis, eosinophilic enteropathy and allergic enterocolitis, which can also co-exist with IgE mediated FA (Sicherer and Sampson, 2018). The incidence and prevalence of FA have sharply risen in the past decades affecting 8% of children and between 4 – 10 % of adults (Gupta et al., 2011; Gupta et al., 2019). Although genetic factors, including barrier and immune regulatory defects and variations in genes involved in the Th2 response, have been implicated in the susceptibility to FA, environmental factors appear to play a dominant role in accounting for the recent increase in the prevalence of FA. The nature of these factors, and in particular the critical role played by the gut microbiota in disease pathogenesis, has been the subject of intense scrutiny (Bunyavanich and Berin, 2019; Rachid and Chatila, 2016; Stephen-Victor and Chatila, 2019).
The hygiene hypothesis provided an initial framework to explain the alarming rise in allergic disorders, originally postulating the importance of early life exposure to microbial infections in decreasing the risk of developing allergic disorders (Strachan, 1989). This hypothesis has been modified to incorporate the influences mediated by commensal microbiota and of those factors that alter microbial colonization including diet, the environment in which a child is raised, antibiotic use and the mode of birth delivery (Riedler et al., 2001; Tamburini et al., 2016; von Mutius et al., 2000; Wold, 1998). Epidemiological studies in humans and experimental investigations in mice have outlined a critical role for the microbiota in regulating susceptibility to allergies. More specifically, healthy early life microbial colonization has been ascribed an instrumental role in establishing protection to allergies, including FA, whereas dysbiosis may engender susceptibility to FA (Abdel-Gadir et al., 2019; Azad et al., 2015; Braun-Fahrlander et al., 2002; Bunyavanich et al., 2016; Cahenzli et al., 2013; Fujimura et al., 2016; Rachid and Chatila, 2016).
In this review, we address the role of early life interactions of between the immune system and the gut microbiota, diet and their respective metabolites in regulating oral tolerance to food and the consequences of disrupting these interactions in engendering susceptibility to FA.
Dietary influence on oral tolerance and gut maturation
The neonatal immune system is exposed to a panoply of antigens that are derived both from the diet and the microbiota colonizing the mucosal surfaces. Although, certain commensals such as Segmented Filamentous Bacteria can induce inflammatory response in the gut, collectively commensals establish a state of mucosal tolerance that is mediated by several mechanisms, key amongst which are Treg cells and IgA (Macpherson et al., 2018; Tanoue et al., 2016; Wang et al., 2015). Emerging evidence supports a critical “neonatal window of opportunity” that is timed to establish mucosal homeostasis after birth (Gensollen et al., 2016; Hornef and Torow, 2020). The neonatal mucosal immune system is distinguished from that of adults in that it is more permissive in inducing long term tolerance. For instance, human foetal T cells are transcriptionally and functionally different from adult T cells and are primed to an immune-tolerant phenotype (Mold et al., 2010). Furthermore, a subset of thymic Treg cells that are generated in the perinatal period are functionally distinct from adult Treg cells. Notably this perinatal Treg cell subset can stably persist in the adult mice to impart tolerance from autoimmune diseases (Yang et al., 2015). Importantly, neonatal tolerance permissiveness does not compromise their effector innate immune responses. For example, neonatal sepsis and necrotizing enterocolitis (NEC) are life threatening diseases that are characterized by overwhelming inflammation causing significant morbidity (Neu and Walker, 2011; Shane et al., 2017). Thus, the neonatal immune system is differentially adapted than the adult immune system and is privileged in mounting a long term tolerogenic responses. Accordingly, early immune education such as that mediated by diet and commensals is critical for providing durable immune tolerance that persists in adulthood.
Microbial colonization occurs in the immediate post-natal period. Recent studies found no evidence for prenatal microbial colonization in the placenta, suggesting that earlier such reports may have been due to contamination (Aagaard et al., 2014; de Goffau et al., 2019; Leiby et al., 2018). Early life microbial colonization is characterized by dynamic changes in microbial diversity, which reaches equilibrium after the first few years of life and maintains relative stability thereafter absent intervening insults (Koenig et al., 2011; Spor et al., 2011). Exposure to the commensals triggers a vigorous immune response to the colonizing bacteria termed the “weaning reaction” (Al Nabhani et al., 2019). Notably in mice, the abundance of Clostridiales species surges around the age of weaning and induces RORγt+ Treg cells, which confer protection against gut pathologies such as colitis and allergic inflammation in adulthood (Al Nabhani et al., 2019). In addition, to the microbiota, dietary antigens may under certain conditions also induce the differentiation of antigen-specific T cells into RORγt+ Treg cells, and hence the weaning reaction may allow for the co-imprinting of food antigen-specific RORγt+ Treg cells (Kim et al., 2016). This immune reaction defines a critically timed neonatal window of opportunity that is essential to the tolerogenic imprinting of the immune system by appropriate commensals and foods. Reciprocally, interruption of this time window by events such as by treatment with antibiotics or inappropriate microbial exposure (dysbiosis) early in life may lead to a pathological imprinting of the immune system that may elicit irreversible and potentially deleterious consequences to the host later in life.
The weaning reaction.
Postnatal microbial colonization is shaped by the maternal microbiota, and by dietary and immunological components of the breast milk (Ganal-Vonarburg et al., 2020; Gomez de Aguero et al., 2016; Le Doare et al., 2018) (Figure 1). The developing infant gut microbiome undergoes three distinct phases of microbiome progression that is characterized by a developmental phase (months 3–14), a transitional phase (months 15–30), and a stable phase (months 31–46) (Stewart et al., 2018).The microbiota of the new-born is characterized by a bloom of Bifidobacteria and Lactobacillus species, which are adapted to process oligo- and polysaccharides present in the milk (Sela et al., 2008; Stewart et al., 2018). The transition from milk to solid food intake is associated with a switch in the gut microbiome in favour of blooming Clostridiales and Bacteroidales species, suggestive of a weaning reaction similar to that described in mice. The weaning reaction is characterized by an intense but transient pro-inflammatory wave of IFN-γ and TNF-α expressing gut T cells. Additionally, the expanding microbiota also induces an ROR-γt+ Treg cell population that persists through adulthood. Notably, maternal factors specifically breast milk and maternal IgA establish a pivotal early homeostatic set point which governs the transmission of ROR-γt+ Treg cell frequenceis in the gut from one generation to the other (Ramanan et al., 2020). Furthermore, ligands for the epidermal growth factor receptor (EGFR), present in breast milk regulate the weaning reaction (Al Nabhani et al., 2019), as the physiological post-natal decline in milk EGFR ligands was associated with the evolution of the weaning response. Interestingly, colonization of GF mice before (2 weeks) and not after (4 weeks) weaning recapitulates the proinflammatory wave of the weaning reaction, thus identifying a critically timed event mediated by the microbiota. Importantly, suppressing the proinflammatory wave of either TNF-α or IFN-γ had no bearing on the outcome of later life gut pathologies. In contrast, failure to imprint a tolerogenic response to the weaning microbiota was associated with heightened susceptibility to colitis and allergy later in life that was dependent on the microbiota induced ROR-γt+ Treg cells (Al Nabhani et al., 2019). These results suggest that early induction of ROR-γt+ Treg cells in the gut may potentially serve to foster long-term infectious tolerance into adulthood that may impact responses to both the commensal microbiota and foods. Conversely, onset of dysbiosis later in life that may result in the suppression of ROR-γt+ Treg cells to foment the development of food allergies (Figure 1).
Figure 1. Oral immune tolerance is shaped by early life dietary and microbial determinants.

In the immediate postnatal period, the infant gut microbiota is organized under the influence of Immunoglobulin A and other immunological components present in the mother’s milk. Maternal EGFR-L restrains the expansion of the infant’s gut microbiota and inhibits Goblet-Cell-Associated-Passages (GAPs). The physiological drop in EGFR-L around the time of weaning (transition to a solid food diet) activates a critically timed window during which the gut microbiota expands and changes composition with the blooming Clostridales species. The concurrent opening of GAPs in the small intestine and colon facilitates the entry of luminal antigens to induce the formation of ROR-γt+ Treg cells that are imprinted to both bacteria and foods. This imprinting persists into adulthood, while its disruption due to dysbiosis or other insults may result in increased susceptibility to FA. Recapitulation of the weaning reaction by means of microbial therapy can induce the ROR-γt+ Treg cells and curtail the development of gut pathologies.
Consistent with these results, studies by Abdel-Gadir et al aimed at assessing changes in the faecal bacterial composition of FA infants in the first years of life demonstrated that patients with FA manifested an early onset gut dysbiosis that was characterized by the loss of Subdoligranulum variabile around the age of weaning that persisted into early childhood (Abdel-Gadir et al., 2019). Additionally, Clostridiales species (clusters I, IV, XI and XIVa), showed significant differences in specific age groups. The relevance of these changes was emphasized by the demonstration that treatment of FA-prone Il4raF709 mice with S. variabile or with a consortium of Clostridiales species reflective of those affected by the dysbiosis abrogated the induction of FA in these mice. This protection was mediated by the induction of gut RORγt+ Treg cells, which were increased by microbial therapy. The importance of RORγt+ Treg cells in regulating FA was demonstrated by the failure of the microbial therapy to protect against disease in Il4raF709 mice with Treg cell-specific deletion of Rorc, encoding ROR-γt (Abdel-Gadir et al., 2019).
The goblet cell intake during the weaning reaction and after.
The gut lamina propria consists of different types of antigen presenting cells (APCs) including classical and non-classical dendritic cells (DCs), monoycte-derived DCs and resident macrophages which play essential redundant roles in inducing tolerance ((Bogunovic et al., 2009; Esterhazy et al., 2016; Persson et al., 2013; Schlitzer et al., 2013; Schulz et al., 2009; Varol et al., 2009). Although, tolerogenic responses can be induced in the peyer’s patch and mucosal lymphoid tissues, gut draining lymph nodes are critical sites for the induction of oral tolerance (Spahn et al., 2001; Spahn et al., 2002). Furthermore, the small intestinal gut-draining lymph nodes are highly compartmentalized with the proximal sites preferentially inducing a tolerogenic response and the distal gut-draining lymph nodes inducing a pro-inflammatory response (Esterhazy et al., 2019). Additionally, tolerance to luminal antigens can also be induced in the small intestine (SI) and the distal colon (Kim et al., 2016; Veenbergen et al., 2016).
Goblet cells, whose role in mucus production contributes to epithelial barrier functions, play a key role in supporting tolerance acquisition to luminal antigens mediated by LP-APCs. The transient formation of goblet-cell-associated passages (GAPs) in the SI and colon act as a portal for the transfer of luminal antigens to CD103+ dendritic cells (DCs) in the lamina propria to induce Treg cell differentiation (McDole et al., 2012). Interestingly the formation of colonic GAPs is initiated by the drop in EGF-R ligands, thus coinciding with the weaning reaction to augment Treg cell generation and imprint tolerance to gut bacteria. However, after weaning, enhanced sensing of the microbiota by the goblet cells results in the closure of the colonic GAPs by mechanisms dependent on goblet-cell intrinsic MyD88 signalling (Knoop et al., 2017; Kulkarni et al., 2020). Furthermore, a recent report demonstrated that GAPs early in life facilitated the imprinting of tolerogenic properties in LP-DCs and macrophages. Notably, abrogation of GAPs incapacitated the induction of ROR-γt+ Treg cells in the small intestine and colon, impairing the generation of tolerance to dietary antigens (Kulkarni et al., 2020). Thus, the transient formation of colonic GAPs, which is tightly monitored by EGF-R ligands and the microbiota, constitutes a critical window in the tolerogenic imprinting of the immune system (Fig. 1).
Early life dietary interventions in FA.
The emergence of transient time window early in life that is key to establishing oral tolerance is supported by the results of trials of dietary intervention in infants at high risk for FA, such as those with moderate to severe eczema. As FA is particularly common in early childhood, initial European and American clinical practice guidelines recommended delayed introduction of allergenic foods in the diet of infants at risk for FA as a mean to decrease the development of FA (2000; Anvari et al., 2017; Host et al., 1999). However, several FA such as those to peanut and tree nuts are typically persistent, while others including milk allergy that used to subside early in childhood now persist for much longer periods and frequently fail to regress (Bock et al., 2001; Savage et al., 2016; Skripak et al., 2007). Furthermore, the recommendation to avoid common food allergens in infants and young children with high risk for FA failed to curtail the FA epidemic, leading to the withdrawal of the clinical practice guidelines on food avoidance early in life (Greer et al., 2008). A critical breakthrough in that regard came with observations that early introduction of allergenic food was associated with a decreased risk for developing FA. A number of population-based studies supported the view that early introduction was associated with a reduced incidence in the development of FA to peanut, egg and milk later in life (Du Toit et al., 2008; Katz et al., 2010; Koplin et al., 2010). Building on these observations, the first double-blind, randomized controlled study of early peanut introduction (LEAP, Learning Early About Peanut) demonstrated that introduction of peanut between the ages of 4 and 11 months in high risk atopic infants (i.e. infants with either egg allergy and/or moderate to severe eczema) was associated with a dramatic reduction in the development of peanut allergy at age 60 months. This landmark study provided compelling evidence for clinical practice and supported a paradigm shift for the early introduction of allergenic foods as a critical modality to prevent FA (Du Toit et al., 2015).
Although the LEAP study critically advanced our understanding of FA, several aspects pertinent to early intervention strategies remain to be clarified. For example, does early introduction of peanut provide tolerance to other allergic foods such as tree nuts? Follow-up studies so far suggest that this is not the case (du Toit et al., 2018). Individual food supplementation thus appears to protect against the specific allergen but does not seem to impact the underlying loss of oral tolerance intrinsic to FA, suggesting that that the underlying dysbiosis may not be resolved by oral immunotherapy with specific foods. This is a critical issue as FA subjects are very frequently allergic to several foods (Gupta et al., 2018; Sicherer et al., 2010), and early exposure to multiple allergens may prove difficult to implement. Another open question revolves around the persistence over time of the protection provided by early food introduction. The Persistence of Oral Tolerance to Peanut (LEAP-ON) study suggested that most subjects placed on early peanut supplementation as part of the LEAP study remained tolerant to peanut one year after discontinuing peanut ingestion. It remains unclear however whether such tolerance persists long-term and into adulthood (Du Toit et al., 2016). Additionally, it remains to be elucidated how early introduction of large quantities of a particular food such as peanut may influence the dynamics of the gut microbiota, relevant to the regulation of FA responses. A better understanding of these issues may emerge from studies analysing the outcome of early dietary interventions in FA in the context of the weaning reaction and the nature of the immune responses to dietary antigens generated during this narrow window.
Dietary and microbial metabolites regulating food allergy.
The gut microbiota is largely composed of symbiotic commensals with a few species functioning as pathobionts (Round and Mazmanian, 2009). While commensals generally promote tolerogenic immune responses, their localization and abundance are tightly regulated by the host. Aberrant translocation of commensal bacteria from the gut to other organs or misdirected immune responses to commensals can precipitate an inflammatory response that mirrors that induced by pathogenic microbes (Hand et al., 2012). The risk of dysregulated commensal translocation is mitigated by their segregation from the mucosa by a layer of mucus which nevertheless allows bidirectional movement of metabolic intermediates produced by the host and the microbes (Postler and Ghosh, 2017). Select microbiota-produced metabolites that critically promote oral tolerance and barrier integrity to suppress pathogenic responses to ingested foods, including short chain fatty acids, tryptophan metabolites and bile-acid metabolites (Fig. 2).
Figure 2. Microbial metabolites regulates gut immune tolerance and barrier integrity.
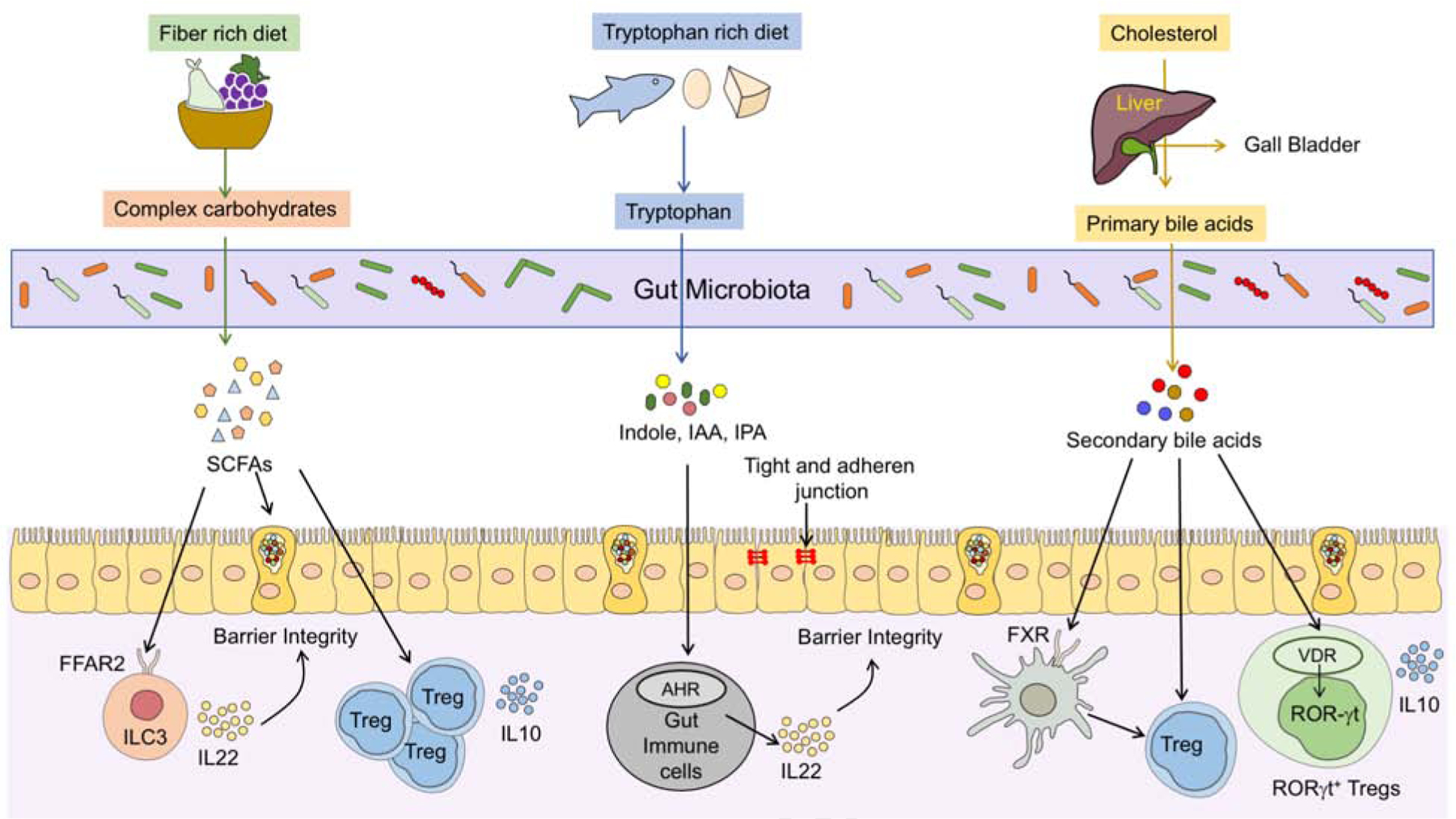
Diet and host-derived metabolites generated by bacterial processing regulate mucosal immune tolerance and promote epithelial barrier integrity. SCFAs, derived by gut microbial fermentation of complex carbohydrates, induce the differentiation of gut Treg cells and augment their production of IL10. SCFAs also enforce epithelial barrier integrity by inducing mucus secretion from goblet cells and IL-22 production by ILC3, the latter by a FFAR2-dependent mechanism. Catabolism of dietary proteins results in the release of tryptophan and its catabolism into metabolites such as indole, indole propionic acid (IPA) and indolealdehyde (IAld). These metabolites decrease intestinal permeability by augmenting the epithelial cell tight and adherens junctions by signalling through the pregnane X receptor (PXR). Additionally, indole derivates activate the aryl hydrocarbon receptor (AHR) in immune cells to induce the barrier protecting cytokine IL22. Primary bile acids produced in the liver are further modified by the gut microbiota to secondary bile acids, which act to induce the differentiation of ROR-γt Treg cell by signalling through the vitamin-D receptor to promote mucosal immune tolerance.
SCFAs are produced in the gut by the microbial fermentation of complex and non-digestible carbohydrates such as dietary fibres. Notably, dietary polysaccharides can affect host microbiome interaction by directly shaping the structure and composition of our gut microbes (Porter and Martens, 2017) The degradation of complex carbohydrates is mediated by glycoside hydrolases and polysaccharide lyases, two types of carbohydrate-active enzymes (CAZymes) (Garron and Henrissat, 2019). The mammalian system is majorly dependent on the colonic microbiota for the degradation of complex carbohydrates as the human genome has a minimal repertoire of CAZymes that is dedicated to the degradation of dietary carbohydrates (approximately 17 enzymes) (Cantarel et al., 2012; El Kaoutari et al., 2013). In comparison to the human genome, Bacteroidetes thetaiotaomicron alone comprises of more than 170 polysaccharide degrading enzymes (Cantarel et al., 2012; Xu et al., 2003). The most abundant SCFAs produced by the gut microbial fermentation are acetate, butyrate, and propionate. B. thetaiotaomicron which produces copious amounts of acetate was shown to enhances the differentiation of goblet cells and mucus secretion (Wrzosek et al., 2013). Indeed, stimulation of epithelial cells with SCFAs that contain acetate induces the expression of mucin and mucus production by differentially altering the ratio of prostaglandins 1 and 2 (Willemsen et al., 2003). The production of mucus by epithelial cells is critically involved in maintaining barrier integrity and in preventing leakage of food and bacterial antigens from the lumen. Moreover, mucus not only barricades exogenous antigen from the gut epithelial lining, but it also contains immunomodulatory proteins such as MUC2, which imprints tolerogenic features in DCs, by stimulating the production of IL-10 and TGF-β1 production (Shan et al., 2013). Furthermore, the metabolite sensing receptor free fatty acid receptor 2 (Ffar2) which recognizes different SCFAs with varying affinity plays a critical role in promoting epithelial barrier integrity. Ffar2 and their agonism in innate lymphoid cells 3 (ILC3) promotes their expansion and function in the colon. Deletion of Ffar2 in ILC3s was associated with a decrease in ILC3-derived interleukin-22 (IL-22) leading to impaired gut epithelial function characterized by altered mucus-associated proteins and antimicrobial peptides and increased susceptibility to colonic injury and bacterial infection (Chun et al., 2019). In addition to the role of SCFAs in mediating barrier integrity, SCFAs are also implicated in promoting Treg cell responses in the gut, a key cell type associated in imparting tolerance to mucosal antigens in the gut.
Gut Treg cell differentiation is shaped by the microbiota and their metabolites. Treg cell frequencies are particularly high in the lamina propria, especially in the colon, and they positively corelate with the microbial mass in the GI tract (Atarashi et al., 2011). Colonization of germ-free (GF) mice with commensals promotes the de novo differentiation of induced (i)Treg cells in the gut (Atarashi et al., 2013; Atarashi et al., 2011). This induction is enhanced by feeding the mice with a high-fibre diet (Furusawa et al., 2013). SCFAs released by the breakdown of dietary fibre are involved in the induction of colonic Treg cells by increasing the histone 3 acetylation at the Foxp3 promoter (Arpaia et al., 2013; Furusawa et al., 2013). Investigation of the role of SCFAs in regulating FA responses has yielded controversial results thus far. One study found that supplementation of extensively hydrolysed casein formula along with Lactobacillus rhamnosus GG promoted tolerance to cow’s milk allergy partly by expanding butyrate producing bacterial strains (Berni Canani et al., 2016). Similarly in a mouse model of FA, oral immunotherapy was augmented by using non-digestible oligosaccharides (Vonk et al., 2017). Furthermore, global deletion in mice of Ffar2, encoding the free fatty acid receptor 2 (also known as G protein-coupled receptor-43) and Gpr109a, which recognizes different SCFAs with varying affinity, rendered mice susceptible to FA (Tan et al., 2016). However, in the Il4raF709 mouse model of FA, in which Treg cells play a critical role in mediating tolerance to FA, SCFAs failed to rescue allergic responses. Importantly, although SCFAs promote induced Treg (iTreg) cell differentiation and stability, they failed to induce ROR-γt+ Treg cells, a subset of Treg cells induced by the microbiota which regulates Type 2 immunity including FA and Type 17 responses such as in colitis (Abdel-Gadir et al., 2019; Ohnmacht et al., 2015; Sefik et al., 2015). A possible resolution to these conflicting data would invoke the action of different metabolites at distinct stages of Treg cell differentiation. In such a scenario, SCFAs may act to retain and expand the nascent Treg cell pool in the gut, with additional microbial sensing by Treg cell-intrinsic Myd88 acting to imprint a ROR-γt+ signature.
A growing body of evidence suggests that microbiota-derived tryptophan metabolites play an important role in gut homeostasis and the gut/brain axis. Tryptophan is an essential amino acid that the host cells cannot synthesize and as such needs to be exogenously supplemented in dietary intake. Tryptophan is present in all plants and animals. Three major pathways are operative in the gut to degrade tryptophan to either serotonin, kynurenine or indole (Agus et al., 2018). Indole derivatives are either directly generated or secondarily metabolized by the gut microbiota to act as ligands for Aryl hydrocarbon receptor (AHR) and xenobiotic sensors (Stockinger et al., 2014). In vitro and in vivo studies in mice have established a role for indoles in regulating epithelial barrier integrity (Bansal et al., 2010; Shimada et al., 2013). Indole administration in GF mice induces the expression of Cldn7, Ocln, Tjp1, Ctnnb1 and Cdh1 which promote adherens junctions to prevent leakage of luminal contents (Shimada et al., 2013). In addition, indole propionic acid (IPA) was also found to enhance epithelial integrity in mice by serving as a ligand for the xenobiotic sensor pregnane X receptor (PXR) (Venkatesh et al., 2014). In accordance, PXR deficient mice were associated with a leaky gut (Venkatesh et al., 2014). The role of the microbiota in regulating epithelial junctions by IPA was emphasized in a recent study that employed Clostridiales sporogens lacking the ability to synthesize IPA (C. sporogens fldc). GF mice colonized with C. sporogens fldc but not the wild-type C. sporogens displayed compromised barrier integrity (Dodd et al., 2017). Furthermore, indole derivates engage with AHR to induce IL-22 a key cytokine that regulates barrier functions at the mucosal surfaces (Lamas et al., 2016).
Emerging evidences point to an association between FA and tryptophan metabolites. Supplementation of D-tryptophan produced from probiotic bacterial strains of Bifidobacterium and Lactobacillus was shown to suppress allergic inflammation in the lungs by increasing gut microbial diversity and by boosting gut Treg cells (Kepert et al., 2017). By employing untargeted metabolomic profiling, Crestani et al uncovered a disease-specific metabolomic signature in FA by comparing FA children with healthy control and asthmatic subjects. Furthermore, the presence of a positive history of food-induced anaphylaxis was associated with increased levels of serum indole propionate, while the presence of multiple FA was characterized by decreased serum levels of kynurenine and serotonin (Crestani et al., 2020). Another study suggested that tolerance acquisition in FA patients was associated with increased serum kynurenine/tryptophan ratio, an index of tryptophan metabolism (Buyuktiryaki et al., 2016). Interestingly, studies in mice have implicated an important role for indole propionate in regulating gut barrier functions (Venkatesh et al., 2014). The increased in circulating indole propionate in FA subjects with a history of anaphylaxis may point to it being a putative protective mechanism, although this remains to be validated. An example of how a microbially-derived metabolite may modulate allergic diseases was previously provided by studies showing that 12,13-diHOME increases the risk of asthma by impeding the development of immune tolerance (Fujimura et al., 2016). Hence, the precise documentation of the fecal and circulating metabolic profiles in FA subjects, and investigating candidate metabolites in relevant mouse models may be critical in defining the precise functions of these metabolites in FA.
Primary bile acids are synthesized from the catabolism of cholesterol in the liver. They are conjugated to either glycine or taurine and secreted into the intestinal lumen where they are further modified by the gut microbiota to form a multitude of bioactive secondary bile acid species. Two main classes of receptors are involved in mediating bile acid recognition: The G protein coupled receptors and the nuclear receptors.
While the role of bile acids in regulating cardiovascular diseases, hepatic diseases and inflammatory bowel disease (IBD) is well documented (Brown and Hazen, 2018; Wahlstrom et al., 2016), their function in regulating allergic diseases is currently unknown. Our metabolomic studies in FA children revealed that FA is associated with an altered profile of secondary bile acids in comparison to asthma. Although a putative mechanism of action of secondary bile acids in regulating FA remains to be elucidated, recent studies have implicated a key role for secondary bile acids in regulating mucosal tolerance in the gut by inducing the differentiation of Treg cells. (Campbell et al., 2020; Hang et al., 2019; Song et al., 2020). Distinct derivatives of secondary bile acids are implicated in regulating specific facets of T cell responses. While 3-oxoLAC directly binds to retinoid-related orphan receptor and inhibits the differentiation of Th17 responses, IsoalloLCA promotes the differentiation of Treg cells in a conserved non-coding sequence 3 (CNS3) dependent manner (Hang et al., 2019). Additionally, secondary bile acids can induce the differentiation of ROR-γt+ Treg cells by directly engaging with the vitamin D receptor expressed in Treg cells. Moreover, engineering bacterial consortia to express IsoDCA resulted in the induction of colonic ROR-γt+ Tregs in mice (Campbell et al., 2020). Furthermore, the secondary bile acid 3β-hyrdoxydeoxycholic (IsoDCA) acid augments the generation of Treg cells by suppressing the immunostimulatory properties of DCs (Campbell et al., 2020). Whether the reduced ROR-γt+ Treg cells observed in FA subjects is a consequence of deficient production by the microbiota of key secondary bile acid metabolites implicated in ROR-γt+ Treg cell differentiation remains to be established. In the studies by Song et. al, it was noted that the induction of ROR-γt+ Treg cells by bile acid metabolites was restricted to the colon, reflected in its critical function in regulating colonic inflammation in mice (Song et al., 2020). However, the pathology in IgE-mediated FA is majorly associated with the small intestine where antigenic priming and tolerance to food antigens are governed. Hence delineating the regional effects of metabolites regulating ROR-γt+ Treg cells in the gut, specifically within the small intestinal lamina propria, will be critical in uncovering the mechanisms of tolerance induction to food antigens and provide avenues to target them.
Sphingolipids are a large class of complex lipids involved in several biological functions ranging from structural role in the cellular membrane to key signalling molecules (Hannun and Obeid, 2018). The metabolism of sphingolipids revolves around the generation of ceramides, central molecules that serve as precursors for a multitude of complex sphingolipids, such as cerebrosides and glycosphingolipids. Recent application of untargeted metabolomics have identified a marked sphingolipid dysregulation associated with the presence of FA, with FA children showing significantly lower serum levels of multiple ceramides and sphingolipids as compared to children with asthma and not-atopic controls (Crestani et al., 2020). Lee-Sarwar et al also found lower sphingolipid levels in the stools of FA subjects (Lee-Sarwar et al., 2018). Sphingolipids are both produced endogenously and are also derived from selected gut bacteria, in particular Bacteroidetes. Invariant natural killer (iNK) T can recognize lipid moieties, including sphingolipids, and have been postulated to play a role in the intercommunication between the host and the gut microbiota and in the establishment of oral tolerance (Dowds et al., 2015). Studies conducted in mouse models of colonic inflammation suggested that early-life exposure to inhibitory sphingolipids provided by commensal Bacteroides species, is a critical step in preventing harmful expansion of iNKT cells in the gut and in protecting against intestinal inflammation (An et al., 2014; Olszak et al., 2012). Nevertheless, Crestani et al. did not observe a difference in the frequency or activation status of circulating iNKT cells between children with and without FA, in spite of the marked differences in their sphingolipid profiles (Crestani et al., 2020). While iNKT cells may have a role in regulating intestinal inflammation, alternative mechanisms mediating the effects of sphingolipids, including those of microbial origin, on oral tolerance in FA, remain to be elucidated.
Therapeutic Interventions in FA.
The current standard of care for FA patients consists of strict avoidance of the allergenic food and the use of rescue medications such as antihistamines and epinephrine in the event of allergic reactions stemming from accidental food exposures. In the last decade several clinical trials of food-specific oral and epicutaneous immunotherapy (OIT and EPIT, respectively) have been undertaken (Fleischer et al., 2019; Jones et al., 2017b; Vickery et al., 2018). The first peanut-protein based product for OIT has recently been approved by the Food and Drug Administration for the mitigation of allergic reactions, including anaphylaxis, in children with peanut allergy. The rational for OIT and EPIT is to build a state of unresponsiveness to food allergens, as defined by an increased threshold required to elicit allergic symptoms during an oral challenge with the culprit food. In the case of OIT, unresponsiveness is achieved by administering food allergens to FA subjects regularly, starting at doses below the threshold for inducing a systemic reaction and then progressively escalating to predefined maintenance doses that are thought to prevent or attenuate systemic allergic reactions elicited by comparable amounts of food protein (Kim and Burks, 2020). In the case of EPIT, continuous release of microgram amounts of food allergen protein from a patch applied on the skin was shown to increase unresponsiveness to oral food allergens ingested in the context of food challenges performed before and after patch treatment (Jones et al., 2017a).
Regular exposure to food antigens by oral immunotherapy upregulate the function of allergen-specific Treg cells, which play an instrumental role in mediating active unresponsiveness (Abdel-Gadir et al., 2018a; Abdel-Gadir et al., 2018b; Syed et al., 2014). EPIT has also been described to induce allergen-specific Treg cells that suppress FA through a TGF-β1 dependent inhibition of mast cells. (Tordesillas et al., 2017b). Additionally, immunotherapy is associated with the suppression of allergen-specific IgE antibodies and a concomitant induction of allergen-specific blocking IgG4 antibodies to suppress mast cell and basophil functions (Burton et al., 2014; James et al., 2011; Kulis et al., 2018; Tsai et al., 2020), induction of effector CD4 T cells anergy (Rolland and O’Hehir, 1998) and deletion of antigen-specific effector Th2 cells (Wambre et al., 2014).
A recent large double blind, randomized controlled study of OIT in 496 peanut allergic children who developed allergic symptoms upon ingestion of <100mg peanut protein at the beginning of the study, showed that 67% of the children were able to tolerate 600mg or more peanut protein at the exit food challenge upon active treatment with peanut OIT. Moderate and severe allergic reactions, including anaphylaxis, were more frequent in participants receiving active treatment as compared to the placebo group (Vickery et al., 2018). In contrast to OIT, EPIT is associated with less frequent and severe adverse reactions, though at the expenses of decreased efficacy in inducing unresponsiveness. Continuous exposure to food allergens appears to be critical to the efficacy of OIT and EPIT, as both interventions fail to induce sustained, long-term immune tolerance in the majority of patients. This is evidenced by the decrease in the amount of food protein able to elicit allergic reactions once therapy is discontinued. In the case of peanut allergy, up to 85% of patients on OIT revert back to reacting to smaller amounts of peanut by 6 months after cessation of therapy (Syed et al., 2014). Trials of intermittent, rather than regular food ingestion have revealed a dramatic reduction in the rate of sustained unresponsiveness from 50% to 15% (Vickery et al., 2014). OIT carries the risk of significant side effects, including allergic reactions (with anaphylaxis reported in up to 10% of patient) and eosinophilic esophagitis, resulting in the withdrawal of 10–20% of treated subjects (Chu et al., 2019). Thus, while immunotherapy for FA is promising, there remains the need for more effective and/or safe therapies, either as stand-alone or as adjuncts to OIT/EPIT.
Early life dysbiosis has been hypothesized to be a critical factor in the development of FA. Initial reports characterizing the gut microbiota of FA and heathy infants found evidence of dysbiosis in FA subjects, an observation that was reproduced by several other groups (Abdel-Gadir et al., 2019; Azad et al., 2015; Bunyavanich et al., 2016). These observations were correlative and since early life gut microbiota colonization is influenced by diet, environment and genetics the role of dysbiosis in the pathogenesis of FA remained speculative. Evidence for a mechanistic role of dysbiosis in the pathogenesis of FA was provided by studies in a mouse model of FA. Fecal microbiota transplantation (FMT) from sensitized FA-prone Il4raF709 mice to FA-resistant GF WT mice was able to reproduce the hallmarks of FA, including the development of antigen-specific IgE responses and anaphylaxis upon oral challenge (Noval Rivas et al., 2013). In contrast, FA-resistant wild-type mice harbour a gut microbiota signature which has the capacity to supress diseases when transferred into GF FA-susceptible Il4raF709 mice (Abdel-Gadir et al., 2019). Importantly, these studies suggested a candidate role for FMT as a treatment approach for FA.
The above promising results involving FMT between mice were corroborated by more recent studies involving human to mouse FMT. By employing mice models of FA, Feehley et al and Abdel-Gadir et al demonstrated that healthy individuals are endowed with a gut flora that has the capacity to supress FA reactions in FA-prone mice (Abdel-Gadir et al., 2019; Feehley et al., 2019). In contrast, FMT from FA infants failed to mitigate FA responses in the same mouse models. Of note, the studies by Abdel-Gadir et al demonstrated that FMT from healthy but not from FA infants resulted in a significant induction of ROR-γt+ Treg cells in the gut of recipient mice, suggesting that the gut flora of FA children was ineffective in its capacity to induce ROR-γt+ Treg cells. A similar observation has been made in other gut inflammatory conditions (inflammatory bowel diseases, IBD), wherein collectively the gut flora of IBD subjects was shown to have an impaired capacity to induce ROR-γt+ Treg cells and consequently to fail to curtail Th2 and Th17 responses (Britton et al., 2019). Taken together, the compelling evidence of FMT experiments in mice in their ability to modulate oral tolerance has led to its pilot application in humans, with a phase I trial of FMT in peanut allergic adults currently under way (Albuhairi and Rachid, 2020; Stephen-Victor and Chatila, 2019). Considerations in the choice of FMT donors include ascertainment of their non-atopic background and strict and supervised exclusion of suspect food allergens from their diet.
Although FMT-based approaches are currently the treatment of choice for patients with recurrent and refractory C. difficile infection (van Nood et al., 2013), the therapeutic application of FMT in FA poses a different set of considerations and challenges. C. difficile infection occurs in the context of a gut environment that is depleted of microbiota as a result of antibiotic therapy (an empty niche), which can then be reversed by FMT (Theriot et al., 2016). In contrast, the dysbiosis present in FA is more subtle, involving changes in a select number of taxa (Stephen-Victor and Chatila, 2019) and leads to hypothesize whether the creation of an empty niche with antibiotic treatment may be necessary to make available and potentiate its effects in FA subjects. In that regard, the recent promising results reported in randomized trials for ulcerative colitis subjects are relevant in that the latter also involves immune dysregulation influenced by the microbiota (Cammarota and Ianiro, 2019; Imdad et al., 2018). Side effects of FMT need to be considered, including the risk for potential life threatening infections by multi-drug-resistant organisms that can be transmitted by FMT (DeFilipp et al., 2019). There is also a potential for increased gut bacterial translocation with FMT therapy which may be responsible for unwanted infectious complications and/or inflammatory responses.
Additionally, it is unclear how FMT therapy may impact pathobiont responses. This is particularly pertinent as pathobionts promote tolerogenic responses under healthy conditions, while they may revert to being pathogenic in susceptible hosts. Studies in mice for example demonstrate that pathobionts such as Heliobacteria hepaticus promote the differentiation of ROR-γt+ Treg cells in the colon to curtail the development of colitis, while the same bacteria induce a detrimental response in IL-10 deficient mice that aggravates colitis by enhancing Th17 responses (Xu et al., 2018). Altogether, FMT-based therapy has a strong potential to benefit FA subjects and to reset the underlying dysbiosis. Its implementation would require further investigations to establish its efficacy especially in younger patients, all the while exercising rigorous screening for multi-drug-resistant bacteria and other potential pathogens.
The identification of individual immunomodulatory bacteria from human subjects that are able to suppress FA in preclinical mouse models provides a rationale for designing targeted bacteriotherapy, either with individual bacteria or with small bacterial consortia, as a novel therapeutic strategy for FA. A critical attribute of early life dysbiosis in FA is the loss of immunomodulatory Clostridiales bacterial species. Administration of a defined consortium of Clostridiales species, related to taxa directly impacted by dysbiosis in FA infants, supressed FA in GF-Il4raF709 mice and in specific pathogen free (SPF) Il4raF709 mice. The ability to supress FA is not however limited to Clostridiales species, as immunomodulatory bacterial consortia belonging to the order of Bacteroidales were also found to protect from FA responses in mice. Notably, bacteriotherapy induced the differentiation of ROR-γt Treg cells via a Treg cell-intrinsic Myd88 signalling pathway. The induction of ROR-γt Treg cells is associated with a reciprocal contraction of Th2-skewed and GATA3 expressing Treg cells, which function to promote diseases pathogenesis in FA (Noval Rivas et al., 2015) (Fig. 3). The essential role played by ROR-γt Treg cells in suppressing FA was demonstrated by Treg cell-specific deletion of ROR-γt which completely abrogated the protection imparted by bacteriotherapy. Aside from ROR-γt+ Treg cell induction, commensals of the order Clostridiales have been implicated in suppressing FA by promoting epithelial barrier integrity. In a mouse model of peanut allergy, a consortium of Clostridiales was shown to prevent the systemic leakage of peanut proteins from the gut by inducing IL-22 secretion from ILC3 (Stefka et al., 2014).
Figure 3. Microbial regulation of oral immune tolerance to food and its breakdown in FA.
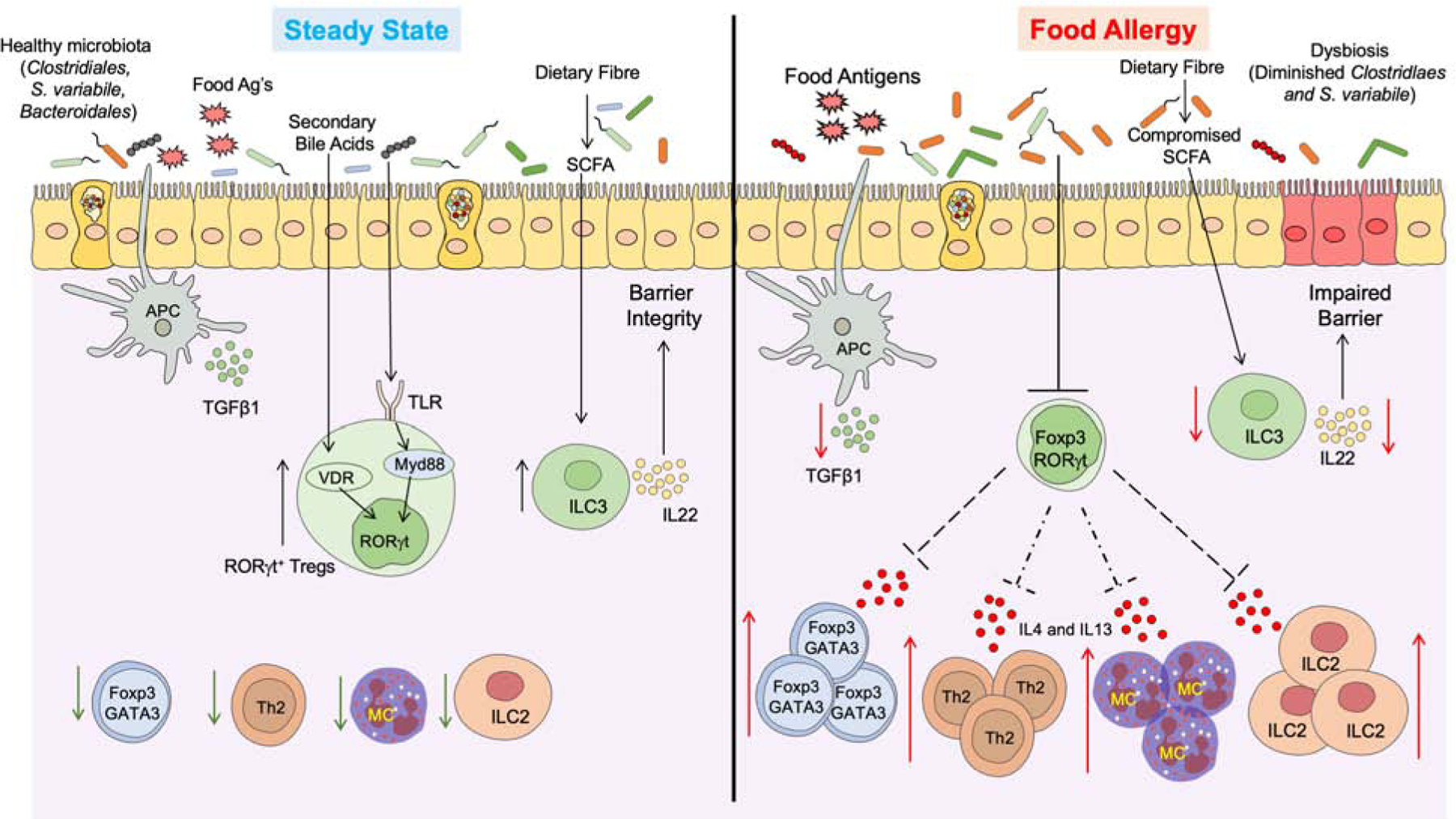
(Left) Under homeostatic conditions antigen presenting cells (classical CD103+ dendritic cells) sample the gut lumen and promote the formation of nascent dietary antigen-specific iTreg cells. Commensals, including Clostridiales and Bacteroidales species, deliver further signals via MyD88 in nascent iTreg cells to drive the expression of ROR-γt. Additionally differentiation signals include secondary bile acid metabolites, which promote the differentiation of ROR-γt by activating vitamin-D receptor in iTreg cells. ROR-γt+ Treg cells may regulate tolerance to dietary antigens by inhibiting antigen-specific helper Th 2 (Th2) cell responses, impeding the formation of pathogenic Th2 cell-like reprogramming of Treg cells, suppression of mast cells cell and innate lymphoid cell (ILC)-2 activation. SCFAs produced by the degradation of complex carbohydrates activates ILC3-dependent secretion of IL22 to promote barrier functions. (Right) The underlying dysbiosis in FA compromises barrier integrity and impairs the differentiation of ROR-γt+ iTreg cells, while augmenting the expansion of Treg cells with a Th2 cell-like phenotype that express GATA3 and IL-4. These pathogenic Treg cells license the gut tissue immune response by Th2 cells, Mast cells, and ILC2 to promote disease pathogenesis.
Specific FA-protective bacteria belonging to the Clostridiales species that are impacted by dysbiosis have been recently identified. By analysing the fecal microbiota composition of healthy and FA infants between the ages of 1 to 30 months, Abdel-Gadir et al found that S. variabile was supressed in the FA group. Interestingly, the loss of S. variabile in FA infants occurred around the time when a solid food diet is consolidated (12 months of age), which coincides with early-life immune imprinting (Abdel-Gadir et al., 2019). Monotherapy with S. variabile resulted in ROR-γt+ Treg cells induction and mitigated FA in mice, suggesting that the loss of FA-protective bacterial strains, such as S. variabile, due to dysbiosis may act as a disease switch in the break-down of oral tolerance in FA infants. In a separate study in which fecal material from healthy and cow milk allergic infants was transferred to GF mice, Feehley et al identified Anaerostipes caccae as the most abundant bacteria taxa present in GF mice that had received FMT from healthy subjects (Feehley et al., 2019). Colonization of GF mice with A. caccae was able to mitigate the allergic response to milk. However, it remains to be elucidated whether A. caccae can supress FA in the context of a conventional microbiota and the mechanism underlying its immunomodulatory properties.
Together, the above studies provide a proof of concept for the use of bacteriotherapy in the treatment of FA. However, several aspects involving the application of microbial therapy in human FA subjects are still unknown and will require further investigations, including its efficacy, safety and the optimal timing of treatment. Also the potential of using microbial therapy as an adjunct therapy with OIT to imprint long-term tolerance needs to be tested. The observation that heat-killed bacteria failed to rescue FA in murine models suggests a critical role for metabolites secreted by live microorganisms in mediating the immunomodulatory functions of bacteriotherapy. In this regard, the precise identity and mixture of metabolites potentially involved in this process, including secondary bile acids, SCFAs and Toll-like receptor and AHR ligands, will require additional and in-depth investigations. Overall, compelling evidence has emerged that bacterial-based approaches may be useful in restoring oral tolerance in FA individuals and may represent a new class of precision therapies that can aid in curbing the FA epidemic (Fig. 4).
Figure 4: Current and projected treatment modalities for FA.
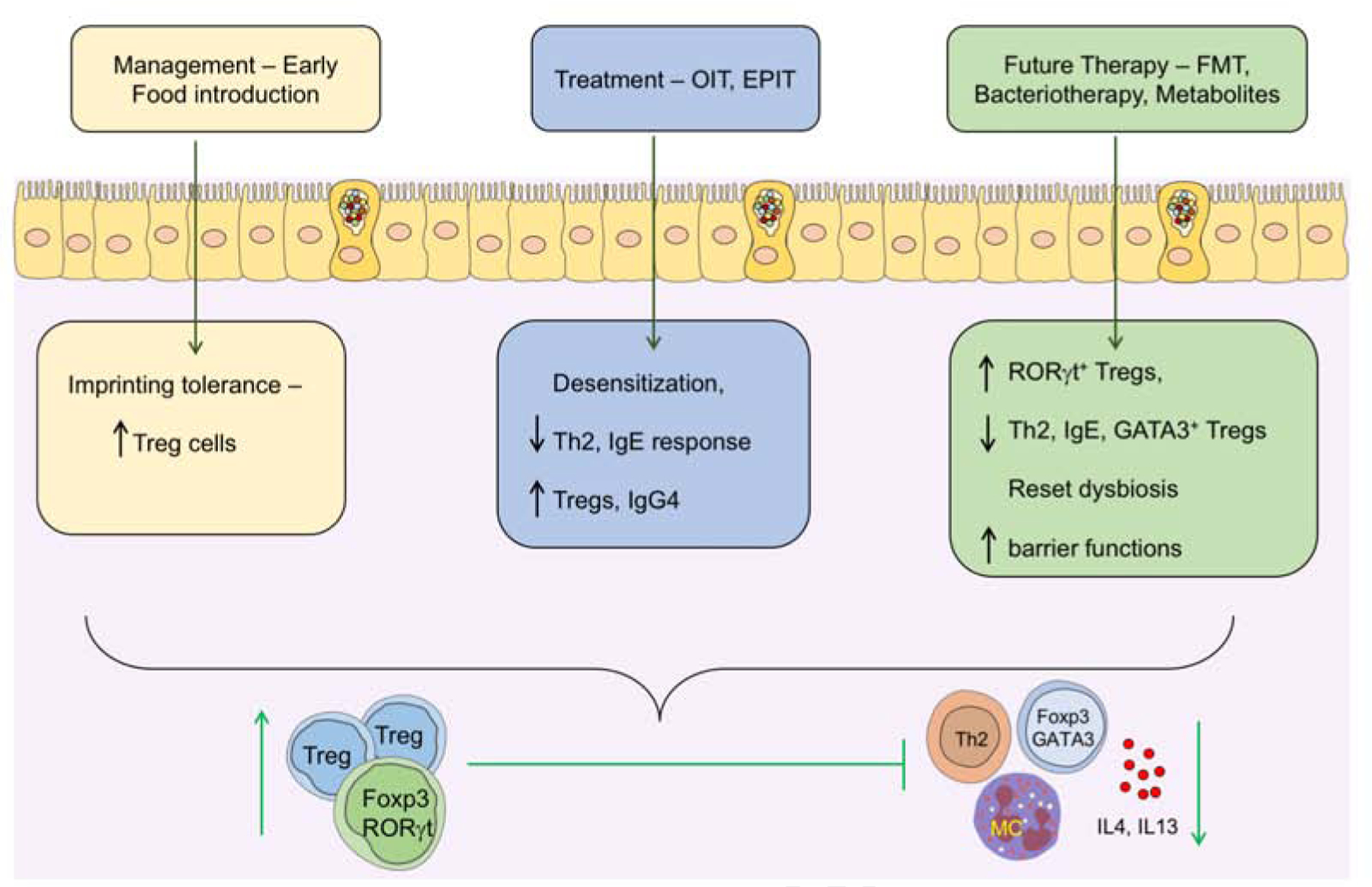
Introduction of common food allergens early in life leads to the development of tolerance possibly by coinciding with the weaning reaction and imprinting durable allergen-specific iTregs cells. In FA subjects who fail to imprint early life tolerance, treatment with either OIT or EPIT results in a state of desensitization that may evolve in some patients into sustained unresponsiveness or oral tolerance to the suspect food. Immunotherapy may act by several mechanisms including the induction of allergen-specific iTregs cells and the inhibition of mast cells and basophils by concomitantly augmenting allergen-specific blocking IgG4 antibodies while suppressing allergen-specific IgE responses. Immunotherapy may also promote the deletion of Th2 cells, and the reversal of pathogenic Th2 cell-like reprogramming of Treg cells. Because immunotherapy fails to induce sustained oral tolerance in many patients, alternate therapies targeting the gut microbiota and their metabolites are currently being explored, including FMT, rationally designed bacterial consortia and precision-based individual bacterial strains and microbiota-derived metabolites. These therapies have the potential to recapitulate early life imprinting by inducing ROR-γt+ Treg cells and, in the case of microbial therapies, to reset the underlying dysbiosis.
Concluding Remarks
Recent studies have detailed an essential role for the microbiota and their metabolites in imprinting oral tolerance to food and identified a critical time window early in life essential for this imprinting to take place. Disruption of this imprinting process by insults that impinge on the weaning reaction, whether due to dysbiosis-promoting events or delayed introduction of solid food, may engender heightened susceptibility to FA. Critically, this disruption may also take place later in life through insults that disrupt the consolidation and maintenance of tolerogenic imprinting, possibly through new onset dysbiosis. Thus an essential research goal is to elucidate the precise molecular mechanisms underlying the permissive state of infectious tolerance present early in life and means of reproducing them to re-establish oral tolerance at an older age. Related to that goal is a better understanding of the environmental insults that disrupt oral tolerance at any age and means of readdressing them. Particularly relevant is the capacity to manipulate the microbiota by dietary interventions to selectively expand and sustain tolerogenic commensals. Also of high therapeutic interest is the capacity to employ bioactive microbial metabolites in lieu of live bacteria to reprogram the gut immune system in favor of oral tolerance. Marshalling these approaches to recapitulate the early life window of tolerance at any age may prove decisive to stem the ongoing epidemic of FA.
Acknowledgements
This work was supported by the National Institutes of Health grants no. R01 AI126915 and R21 AI132843 to T.A.C, and by the Bunning Food Allergy Fund the Jasmine and Paul Mashikian Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
T.A.C. is an inventor on published US patent application, 15/801,811, that covers methods and compositions for the prevention and treatment of FA using microbial treatments. T.A.C. and E.S.-V. have pending patent applications related to the use of probiotics in enforcing oral tolerance in FA (62/758,161, and, 62/823,866). T.A.C. is founders of and has equity in Consortia Tx.
References
- (2000). American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 106, 346–349. [PubMed] [Google Scholar]
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, and Versalovic J (2014). The placenta harbors a unique microbiome. Sci Transl Med 6, 237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Gadir A, Massoud AH, and Chatila TA (2018a). Antigen-specific Treg cells in immunological tolerance: implications for allergic diseases. F1000Res 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Gadir A, Schneider L, Casini A, Charbonnier LM, Little SV, Harrington T, Umetsu DT, Rachid R, and Chatila TA (2018b). Oral immunotherapy with omalizumab reverses the Th2 cell-like programme of regulatory T cells and restores their function. Clin Exp Allergy 48, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Gadir A, Stephen-Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H, Wang L, Li N, Crestani E, Spielman S, et al. (2019). Microbiota therapy acts via a regulatory T cell MyD88/RORgammat pathway to suppress food allergy. Nat Med 25, 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agus A, Planchais J, and Sokol H (2018). Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 23, 716–724. [DOI] [PubMed] [Google Scholar]
- Al Nabhani Z, Dulauroy S, Marques R, Cousu C, Al Bounny S, Dejardin F, Sparwasser T, Berard M, Cerf-Bensussan N, and Eberl G (2019). A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 50, 1276–1288 e1275. [DOI] [PubMed] [Google Scholar]
- Albuhairi S, and Rachid R (2020). Novel Therapies for Treatment of Food Allergy. Immunol Allergy Clin North Am 40, 175–186. [DOI] [PubMed] [Google Scholar]
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, and Kasper DL (2014). Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvari S, Chokshi NY, Kamili QU, and Davis CM (2017). Evolution of Guidelines on Peanut Allergy and Peanut Introduction in Infants: A Review. JAMA Pediatr 171, 77–82. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, and Rudensky AY (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. (2013). Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, Mandhane PJ, Turvey SE, Subbarao P, Becker AB, et al. (2015). Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy 45, 632–643. [DOI] [PubMed] [Google Scholar]
- Bansal T, Alaniz RC, Wood TK, and Jayaraman A (2010). The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A 107, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, Calignano A, Khan AA, Gilbert JA, and Nagler CR (2016). Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J 10, 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock SA, Munoz-Furlong A, and Sampson HA (2001). Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol 107, 191–193. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. (2009). Origin of the lamina propria dendritic cell network. Immunity 31, 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, et al. (2002). Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 347, 869–877. [DOI] [PubMed] [Google Scholar]
- Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, Li Z, Mortha A, Merad M, Das A, et al. (2019). Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORgammat(+) Regulatory T Cells and Exacerbate Colitis in Mice. Immunity 50, 212–224 e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, and Hazen SL (2018). Microbial modulation of cardiovascular disease. Nat Rev Microbiol 16, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunyavanich S, and Berin MC (2019). Food allergy and the microbiome: Current understandings and future directions. J Allergy Clin Immunol 144, 1468–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunyavanich S, Shen N, Grishin A, Wood R, Burks W, Dawson P, Jones SM, Leung DYM, Sampson H, Sicherer S, and Clemente JC (2016). Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol 138, 1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, Koleoglou KJ, Chatila TA, Schneider LC, Rachid R, et al. (2014). Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol 134, 1310–1317 e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyuktiryaki B, Sahiner UM, Girgin G, Birben E, Soyer OU, Cavkaytar O, Cetin C, Arik Yilmaz E, Yavuz ST, Kalayci O, et al. (2016). Low indoleamine 2,3-dioxygenase activity in persistent food allergy in children. Allergy 71, 258–266. [DOI] [PubMed] [Google Scholar]
- Cahenzli J, Koller Y, Wyss M, Geuking MB, and McCoy KD (2013). Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G, and Ianiro G (2019). FMT for ulcerative colitis: closer to the turning point. Nat Rev Gastroenterol Hepatol 16, 266–268. [DOI] [PubMed] [Google Scholar]
- Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, Jin WB, Guo CJ, Violante S, et al. (2020). Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel BL, Lombard V, and Henrissat B (2012). Complex carbohydrate utilization by the healthy human microbiome. PLoS One 7, e28742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, Brozek JL, and Schunemann HJ (2019). Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet 393, 2222–2232. [DOI] [PubMed] [Google Scholar]
- Chun E, Lavoie S, Fonseca-Pereira D, Bae S, Michaud M, Hoveyda HR, Fraser GL, Gallini Comeau CA, Glickman JN, Fuller MH, et al. (2019). Metabolite-Sensing Receptor Ffar2 Regulates Colonic Group 3 Innate Lymphoid Cells and Gut Immunity. Immunity 51, 871–884 e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani E, Harb H, Charbonnier LM, Leirer J, Motsinger-Reif A, Rachid R, Phipatanakul W, Kaddurah-Daouk R, and Chatila TA (2020). Untargeted metabolomic profiling identifies disease-specific signatures in food allergy and asthma. J Allergy Clin Immunol 145, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, Parkhill J, Charnock-Jones DS, and Smith GCS (2019). Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, and Hohmann EL (2019). Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med 381, 2043–2050. [DOI] [PubMed] [Google Scholar]
- Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, and Sonnenburg JL (2017). A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551, 648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowds CM, Blumberg RS, and Zeissig S (2015). Control of intestinal homeostasis through crosstalk between natural killer T cells and the intestinal microbiota. Clin Immunol 159, 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, Fox AT, Turcanu V, Amir T, Zadik-Mnuhin G, et al. (2008). Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol 122, 984–991. [DOI] [PubMed] [Google Scholar]
- Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, et al. (2015). Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 372, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, Bahnson HT, Fisher HR, Feeney M, Radulovic S, Basting M, et al. (2018). Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J Allergy Clin Immunol 141, 1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, Brough HA, Santos AF, Harris KM, Radulovic S, et al. (2016). Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N Engl J Med 374, 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kaoutari A, Armougom F, Gordon JI, Raoult D, and Henrissat B (2013). The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 11, 497–504. [DOI] [PubMed] [Google Scholar]
- Esterhazy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, and Mucida D (2019). Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569, 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterhazy D, Loschko J, London M, Jove V, Oliveira TY, and Mucida D (2016). Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat Immunol 17, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehley T, Plunkett CH, Bao R, Choi Hong SM, Culleen E, Belda-Ferre P, Campbell E, Aitoro R, Nocerino R, Paparo L, et al. (2019). Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med 25, 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer DM, Greenhawt M, Sussman G, Bégin P, Nowak-Wegrzyn A, Petroni D, Beyer K, Brown-Whitehorn T, Hebert J, Hourihane JO, et al. (2019). Effect of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Ingestion Among Children With Peanut Allergy: The PEPITES Randomized Clinical Trial. JAMA 321, 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, et al. (2016). Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 22, 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. [DOI] [PubMed] [Google Scholar]
- Ganal-Vonarburg SC, Hornef MW, and Macpherson AJ (2020). Microbial-host molecular exchange and its functional consequences in early mammalian life. Science 368, 604–607. [DOI] [PubMed] [Google Scholar]
- Garron ML, and Henrissat B (2019). The continuing expansion of CAZymes and their families. Curr Opin Chem Biol 53, 82–87. [DOI] [PubMed] [Google Scholar]
- Gensollen T, Iyer SS, Kasper DL, and Blumberg RS (2016). How colonization by microbiota in early life shapes the immune system. Science 352, 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. (2016). The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302. [DOI] [PubMed] [Google Scholar]
- Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, Joseph J, Gertie JA, Xu L, Collet MA, et al. (2019). Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer FR, Sicherer SH, Burks AW, American Academy of Pediatrics Committee on, N., American Academy of Pediatrics Section on, A., and Immunology (2008). Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 121, 183–191. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, and Holl JL (2011). The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 128, e9–17. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, and Nadeau KC (2018). The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, Schleimer RP, and Nadeau KC (2019). Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw Open 2, e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, Maynard CL, Elson CO 3rd, and Belkaid Y (2012). Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science 337, 1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, et al. (2019). Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, and Obeid LM (2018). Sphingolipids and their metabolism in physiology and disease. Nature reviews. Molecular cell biology 19, 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornef MW, and Torow N (2020). ‘Layered immunity’ and the ‘neonatal window of opportunity’ - timed succession of non-redundant phases to establish mucosal host-microbial homeostasis after birth. Immunology 159, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Host A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, Bresson JL, Hernell O, Lafeber H, Michaelsen KF, et al. (1999). Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child 81, 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A, Nicholson MR, Tanner-Smith EE, Zackular JP, Gomez-Duarte OG, Beaulieu DB, and Acra S (2018). Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev 11, CD012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, Jacobson MR, Kimber I, Till SJ, and Durham SR (2011). Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol 127, 509-516.e501-505. [DOI] [PubMed] [Google Scholar]
- Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, Henning AK, Berin MC, Chiang D, Vickery BP, et al. (2017a). Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol 139, 1242–1252 e1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, Henning AK, Berin MC, Chiang D, Vickery BP, et al. (2017b). Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol 139, 1242–1252.e1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, and Leshno M (2010). Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol 126, 77–82 e71. [DOI] [PubMed] [Google Scholar]
- Kepert I, Fonseca J, Muller C, Milger K, Hochwind K, Kostric M, Fedoseeva M, Ohnmacht C, Dehmel S, Nathan P, et al. (2017). D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J Allergy Clin Immunol 139, 1525–1535. [DOI] [PubMed] [Google Scholar]
- Kim EH, and Burks AW (2020). Food allergy immunotherapy: OIT and EPIT. Allergy. [DOI] [PubMed] [Google Scholar]
- Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, and Surh CD (2016). Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351, 858–863. [DOI] [PubMed] [Google Scholar]
- Knoop KA, Gustafsson JK, McDonald KG, Kulkarni DH, Coughlin PE, McCrate S, Kim D, Hsieh CS, Hogan SP, Elson CO, et al. (2017). Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, and Ley RE (2011). Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108 Suppl 1, 4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, Tey D, Slaa M, Thiele L, Miles L, et al. (2010). Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol 126, 807–813. [DOI] [PubMed] [Google Scholar]
- Kulis MD, Patil SU, Wambre E, and Vickery BP (2018). Immune mechanisms of oral immunotherapy. J Allergy Clin Immunol 141, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni DH, Gustafsson JK, Knoop KA, McDonald KG, Bidani SS, Davis JE, Floyd AN, Hogan SP, Hsieh CS, and Newberry RD (2020). Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol 13, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, et al. (2016). CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22, 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doare K, Holder B, Bassett A, and Pannaraj PS (2018). Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front Immunol 9, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Sarwar K, Kelly RS, Lasky-Su J, Moody DB, Mola AR, Cheng TY, Comstock LE, Zeiger RS, O’Connor GT, Sandel MT, et al. (2018). Intestinal microbial-derived sphingolipids are inversely associated with childhood food allergy. J Allergy Clin Immunol 142, 335–338 e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby JS, McCormick K, Sherrill-Mix S, Clarke EL, Kessler LR, Taylor LJ, Hofstaedter CE, Roche AM, Mattei LM, Bittinger K, et al. (2018). Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Yilmaz B, Limenitakis JP, and Ganal-Vonarburg SC (2018). IgA Function in Relation to the Intestinal Microbiota. Annu Rev Immunol 36, 359–381. [DOI] [PubMed] [Google Scholar]
- McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, and Miller MJ (2012). Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, and McCune JM (2010). Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 330, 1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J, and Walker WA (2011). Necrotizing enterocolitis. N Engl J Med 364, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, and Chatila TA (2015). Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 42, 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, Chehoud C, Kuczynski J, DeSantis T, Warrington J, et al. (2013). A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol 131, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, et al. (2015). MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349, 989–993. [DOI] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, and Blumberg RS (2012). Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, and Agace WW (2013). IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38, 958–969. [DOI] [PubMed] [Google Scholar]
- Porter NT, and Martens EC (2017). The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu Rev Microbiol 71, 349–369. [DOI] [PubMed] [Google Scholar]
- Postler TS, and Ghosh S (2017). Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab 26, 110–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachid R, and Chatila TA (2016). The role of the gut microbiota in food allergy. Curr Opin Pediatr 28, 748–753. [DOI] [PubMed] [Google Scholar]
- Ramanan D, Sefik E, Galvan-Pena S, Wu M, Yang L, Yang Z, Kostic A, Golovkina TV, Kasper DL, Mathis D, and Benoist C (2020). An Immunologic Mode of Multigenerational Transmission Governs a Gut Treg Setpoint. Cell 181, 1276–1290 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E, and Team AS (2001). Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 358, 1129–1133. [DOI] [PubMed] [Google Scholar]
- Rolland J, and O’Hehir R (1998). Immunotherapy of allergy: anergy, deletion, and immune deviation. Curr Opin Immunol 10, 640–645. [DOI] [PubMed] [Google Scholar]
- Round JL, and Mazmanian SK (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J, Sicherer S, and Wood R (2016). The Natural History of Food Allergy. J Allergy Clin Immunol Pract 4, 196–203; quiz 204. [DOI] [PubMed] [Google Scholar]
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, et al. (2013). IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, and Pabst O (2009). Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 206, 3101–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, et al. (2015). MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349, 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, et al. (2008). The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A 105, 18964–18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, et al. (2013). Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane AL, Sanchez PJ, and Stoll BJ (2017). Neonatal sepsis. Lancet 390, 1770–1780. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, and Takeda K (2013). Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One 8, e80604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicherer SH, Munoz-Furlong A, Godbold JH, and Sampson HA (2010). US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol 125, 1322–1326. [DOI] [PubMed] [Google Scholar]
- Sicherer SH, and Sampson HA (2018). Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 141, 41–58. [DOI] [PubMed] [Google Scholar]
- Skripak JM, Matsui EC, Mudd K, and Wood RA (2007). The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol 120, 1172–1177. [DOI] [PubMed] [Google Scholar]
- Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C, and Kasper DL (2020). Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature 577, 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, Koni PA, Ruddle NH, Flavell RA, Rennert PD, and Weiner HL (2001). Induction of oral tolerance to cellular immune responses in the absence of Peyer’s patches. Eur J Immunol 31, 1278–1287. [DOI] [PubMed] [Google Scholar]
- Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, and Kucharzik T (2002). Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur J Immunol 32, 1109–1113. [DOI] [PubMed] [Google Scholar]
- Spor A, Koren O, and Ley R (2011). Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9, 279–290. [DOI] [PubMed] [Google Scholar]
- Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo GY, Cao S, Theriault BR, et al. (2014). Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A 111, 13145–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen-Victor E, and Chatila TA (2019). Regulation of oral immune tolerance by the microbiome in food allergy. Curr Opin Immunol 60, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GA, et al. (2018). Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Di Meglio P, Gialitakis M, and Duarte JH (2014). The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 32, 403–432. [DOI] [PubMed] [Google Scholar]
- Strachan DP (1989). Hay fever, hygiene, and household size. BMJ 299, 1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O’Riordan G, et al. (2014). Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 133, 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini S, Shen N, Wu HC, and Clemente JC (2016). The microbiome in early life: implications for health outcomes. Nat Med 22, 713–722. [DOI] [PubMed] [Google Scholar]
- Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, and Mackay CR (2016). Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell reports 15, 2809–2824. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Atarashi K, and Honda K (2016). Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 16, 295–309. [DOI] [PubMed] [Google Scholar]
- Theriot CM, Bowman AA, and Young VB (2016). Antibiotic-Induced Alterations of the Gut Microbiota Alter Secondary Bile Acid Production and Allow for Clostridium difficile Spore Germination and Outgrowth in the Large Intestine. mSphere 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordesillas L, Berin MC, and Sampson HA (2017a). Immunology of Food Allergy. Immunity 47, 32–50. [DOI] [PubMed] [Google Scholar]
- Tordesillas L, Mondoulet L, Blazquez AB, Benhamou PH, Sampson HA, and Berin MC (2017b). Epicutaneous immunotherapy induces gastrointestinal LAP(+) regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol 139, 189–201 e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M, Mukai K, Chinthrajah RS, Nadeau KC, and Galli SJ (2020). Sustained successful peanut oral immunotherapy associated with low basophil activation and peanut-specific IgE. J Allergy Clin Immunol 145, 885–896.e886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. (2013). Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368, 407–415. [DOI] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, and Jung S (2009). Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31, 502–512. [DOI] [PubMed] [Google Scholar]
- Veenbergen S, van Berkel LA, du Pre MF, He J, Karrich JJ, Costes LM, Luk F, Simons-Oosterhuis Y, Raatgeep HC, Cerovic V, et al. (2016). Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103(+) dendritic cells. Mucosal Immunol 9, 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al. (2014). Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41, 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, Burk C, Hiegel A, Carlisle S, Christie L, et al. (2014). Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol 133, 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, Hourihane JO, Jones SM, Shreffler WG, Marcantonio A, Zawadzki R, et al. (2018). AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med 379, 1991–2001. [DOI] [PubMed] [Google Scholar]
- von Mutius E, Braun-Fahrlander C, Schierl R, Riedler J, Ehlermann S, Maisch S, Waser M, and Nowak D (2000). Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy 30, 1230–1234. [DOI] [PubMed] [Google Scholar]
- Vonk MM, Diks MAP, Wagenaar L, Smit JJ, Pieters RHH, Garssen J, van Esch B, and Knippels LMJ (2017). Improved Efficacy of Oral Immunotherapy Using Non-Digestible Oligosaccharides in a Murine Cow’s Milk Allergy Model: A Potential Role for Foxp3+ Regulatory T Cells. Front Immunol 8, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom A, Sayin SI, Marschall HU, and Backhed F (2016). Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab 24, 41–50. [DOI] [PubMed] [Google Scholar]
- Wambre E, DeLong JH, James EA, Torres-Chinn N, Pfützner W, Möbs C, Durham SR, Till SJ, Robinson D, and Kwok WW (2014). Specific immunotherapy modifies allergen-specific CD4(+) T-cell responses in an epitope-dependent manner. J Allergy Clin Immunol 133, 872–879.e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Charbonnier LM, Noval Rivas M, Georgiev P, Li N, Gerber G, Bry L, and Chatila TA (2015). MyD88 Adaptor-Dependent Microbial Sensing by Regulatory T Cells Promotes Mucosal Tolerance and Enforces Commensalism. Immunity 43, 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen LE, Koetsier MA, van Deventer SJ, and van Tol EA (2003). Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 52, 1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold AE (1998). The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy 53, 20–25. [DOI] [PubMed] [Google Scholar]
- Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, et al. (2013). Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, and Gordon JI (2003). A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299, 2074–2076. [DOI] [PubMed] [Google Scholar]
- Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, Galan C, Belkaid Y, Bonneau R, and Littman DR (2018). c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Fujikado N, Kolodin D, Benoist C, and Mathis D (2015). Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]


