Abstract
Rag GTPases play a crucial role in mTORC1 activation by promoting its recruitment to the lysosomal surface in a nutrient-dependent manner. A study now identifies a family of lysosomal G-protein couple receptors as modulators of Rag GTPases localization and activation, adding one more component to the fast growing mTOR regulatory network.
The protein kinase complex mTORC1 plays a critical role integrating signals from different pathways to regulate proliferation, cell growth, and anabolic processes1. In response to amino acid stimulation, mTORC1 is recruited to the lysosomal surface, where it is activated by the small GTPase Rheb2. The amino acid–dependent translocation of mTOR requires Rag GTPases (Rags) and Ragulator, a pentameric protein complex that anchors the Rags to lysosomes3. The Rag proteins function as heterodimers in which the active complex consists of GTP-bound RagA or B complexed with GDP-bound RagC or D. Amino acids trigger the GTP loading of RagA/B proteins, thus promoting binding to raptor and recruitment of the mTORC1 complex to lysosomes4. Meanwhile, the activity of Rheb is modulated by growth factors, suggesting that different stimuli (e.g. growth factors and amino acids) must cooperate to activate mTORC1. In line with these findings, growing evidence reveals that mTORC1 activity may be modulated by a wide variety of stimuli including energy, glucose, cholesterol and several stressors, suggesting the potential existence of additional, yet-uncharacterized mTORC1 regulators. In this issue of Nature Cell Biology, Gan et al. identify the lysosome-localized G-protein receptor-like protein GPR137B as a component of the mTORC1 regulatory network5 (Figure 1).
Figure 1.
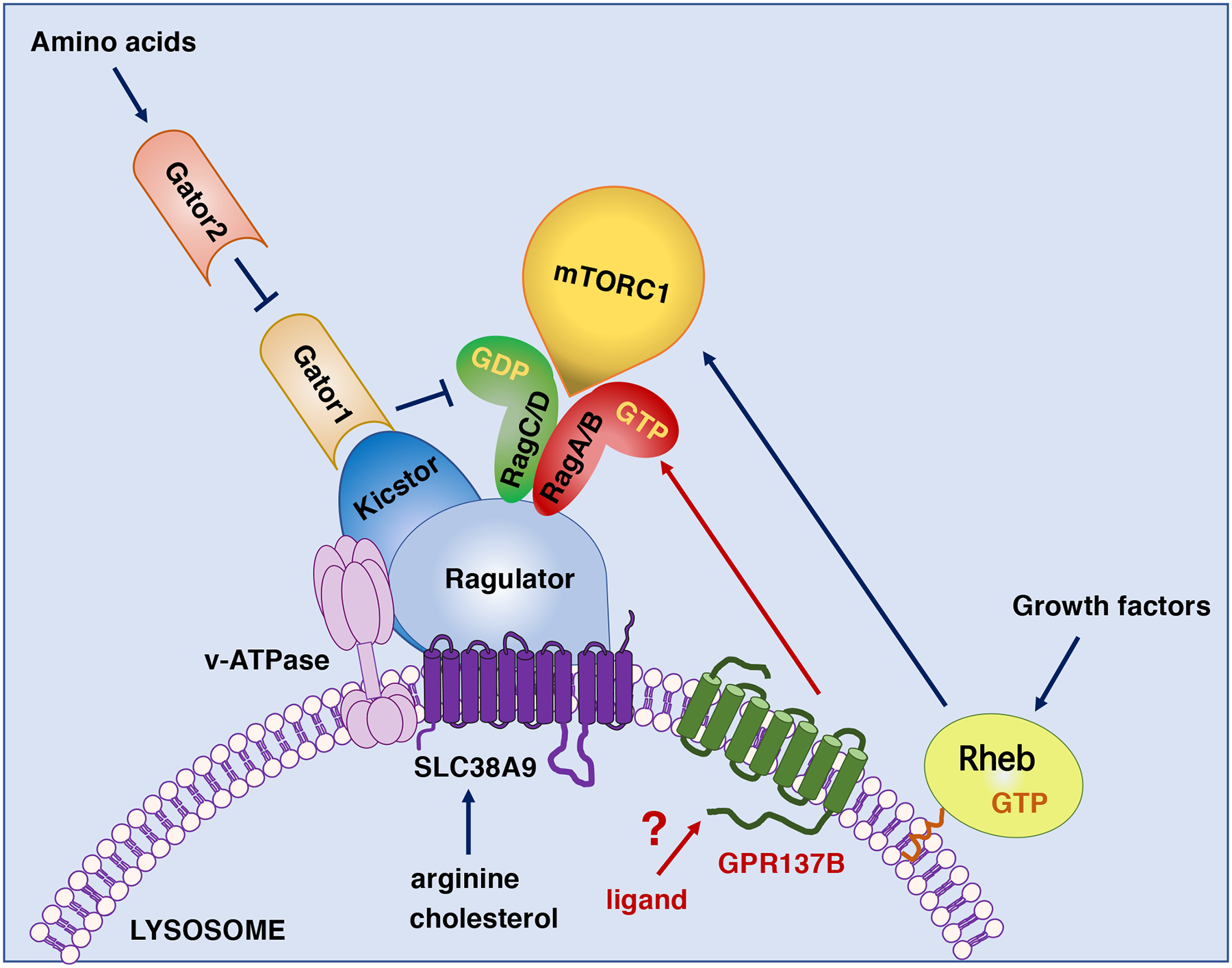
Schematic showing regulation of mTORC1 in mammalian cells. Activation of mTOR at the lysosomal surface requires Rag-mediated recruitment of the mTORC1 complex and Rheb, which allosterically unlocks the mTOR kinase activity. Multiple nutrient signals, including amino acids, cholesterol and glucose converge to regulate Rag activation, whereas Rheb activity is stimulated by growth factors. Rags sense amino acid levels inside the lysosome through SLC38A9, as well as cytosolic amino acids via the Gator1/Gator2 complex. Ragulator and Kicstor mediate recruitment of Rags and Gator to the lysosomes, respectively. GPR137B modulates mTORC1 activity by increasing Rag lysosomal localization and activation.
To identify regulators of mTORC1 activity and localization, Gan et al. took an unbiased approach and performed a microscopy-based genome-wide human siRNA screen. Over 21,000 pools of siRNA, each one consisting of four siRNAs targeting individual genes, were initially tested in the non-transformed human fibroblasts cell line Hs68. More than 400 pools resulted in impaired mTORC1 activity, as assessed by reduced phosphorylation of the mTORC1 substrate rp60. To focus on those proteins that specifically regulate mTORC1 distribution, the authors monitored mTORC1 colocalization with the lysosomal marker Lamp2 in cells starved and treated with cycloheximide, a means to rule out proteins that regulate amino acid uptake from outside the cell. This approach reduced the list to only 15 genes, with RagC as the top hit, in agreement with the well-known role of Rags in mTORC1 recruitment to lysosomes5.
Gan et al. focused on GPR137B, a lysosomal protein of unknown function with homology to G-protein coupled receptors (GPCRs). Three transmembrane proteins have been shown to regulate mTORC16, the proton pump v-ATPase, the arginine transporter SLC38A9, and the cholesterol transporter NPC1, thus making GPR137B a particularly attractive candidate. The authors showed that GPR137B depletion resulted in reduced mTORC1 lysosomal localization, decreased S6K and 4EBP1 phosphorylation, and increased autophagy. Conversely, GPR137B over-expression led to enhanced mTORC1 translocation to lysosomes and increased S6K and 4EBP1 activity, even in the absence of nutrients. It is important to note that GPR137B over-expression did not affect mTORC1 activity in serum-starved cells, suggesting that GPR137B is more relevant to amino acid-dependent mTORC1 regulation. This possibility was confirmed by experiments showing that the co-immunoprecipitation of GPR137B with mTOR, raptor, and Rags was not detected in Rag-deficient cells. Furthermore, the interaction of GPR137B and raptor was amino acid sensitive, whereas binding between GPR137B and Rags was not. In addition, Gan et al. found that increased recruitment of mTORC1 to lysosomes following GPR137B over-expression did not occur in cells depleted of Rags, whereas GPR137B depletion did not affect mTORC1 regulation in cells expressing constitutive active Rags or cells depleted of the RagA GAP GATOR1. Together, these results suggested that GPR137B likely modulates mTORC1 activity through regulating Rags.
Further analysis revealed that GPR137B regulates Rag activity in two ways. First, GPR137B increased Rag concentration on lysosomal membranes. As such, depletion of GPR137B reduced the amount of RagA and RagC present on the lysosomal surface, whereas GPR137B over-expression increased RagA translocation. Second, GPR137B promoted RagA GTP loading. This was evidenced by the reduced amino acid-induced interaction of RagA with raptor and mTOR observed in GPR137B-depleted cells. In addition, Gan et al. showed that GPR137B over-expression increased binding of RagA to raptor and mTOR in both control and starved conditions, indicating that GPR137B can increase RagA GTP-loading even in the absence of amino acids. The mechanism by which GPR137B modulate Rag activity will require further investigation. Interestingly, additional data revealed that over-expression of GPR137B had no effect when LAMPTOR was depleted, indicating that GPR137B likely binds and regulate Rags in a Ragulator-dependent manner.
A recent study identified a nutrient-regulated affinity switch that facilitates mTORC1-Rag interactions, while destabilizing binding of Rags to Ragulator7. These observations were confirmed by Gan el al. in the present study. In agreement with the idea that active Rags display reduced affinity for Ragulator, the authors found increased RagA and RagC dissociation from lysosomes following amino acid stimulation. Likewise, GDP-loaded Rags showed reduced dissociation as assessed by FRAP. Importantly, GPR137B over-expression in starved cells increased Rag exchange, while GPR137B depletion reduced the exchange. This is consistent with the interpretation that GPR137B promotes RagA GTP loading. The results from both studies agree with a model in which GTP loading into RagA stimulates mTORC1 recruitment to lysosomes, while at the same time promoting dissociation of the mTORC1-Rag complex. However, the interpretation of the physiological consequence of this dissociation slightly differs between these two studies. Lawrence et al. suggests a model whereby mTORC1 is likely more active in close proximity to lysosomes, given that recent evidence indicates that Rheb must physically interact with mTORC1 to relieve an auto-inhibitory intramolecular interaction2. In this regard, the dissociation of the mTORC1-Rag complex may constitute a mechanism to prevent mTORC1 hyperactivation. In contrast, the findings from Gan el al. suggest that the mTORC1-Rag complex may remain active in the cytosol, thus facilitating mTORC1 interaction with cytosolic substrates. In this case one of the main functions of GPR137B may be to facilitate the dissociation of the active mTORC1-Rag complex into the cytosol.
Gan et al. also confirmed the role of GPR137B in Rag regulation in vivo. Similar to what has been observed in Rag knockouts8, depletion of GPR137B in zebrafish resulted in the accumulation of enlarged lysosomes in microglial cells. Rags are also known to be critical for facilitating mTORC1-dependent regulation of the transcription factor TFEB9. Depletion or inactivation of Rags leads to constitutive TFEB nuclear accumulation and activation8,9. Consistently, the expression of several TFEB targets appeared upregulated in GPR137B knockout zebrafish.
Gan et al. showed that in addition to GPR137B, two other members of the family, GPR137 and GPR137C, localized to lysosomes and increased mTOR translocation upon over-expression, suggesting that they may also function as mTOR-Rags modulators. Indeed, depletion of either GPR137B or GPR137 in HAP-1 cells results in phenotypes consistent with reduced Rag activity, including enlarged lysosomes, enhanced autophagy and reduced RagA lysosomal accumulation5. These results are of particular interest considering the recent implication of GPR137B and GPR137 in different types of cancer. GPR137 plays a pro-oncogenic role in ovarian cancer by promoting proliferation and metastasis10, while GPR137 depletion inhibits growth of medulloblastoma, hepatoma, prostate, colon, pancreatic, and urinary bladder cancer cells11–14. Given the well-characterized role of mTOR in cancer progression, it will be critical to assess whether the contribution of GPR137 to cancer is linked to alterations in mTOR activity and regulation. It is also important to keep in mind that GPCRs are usually effective drug targets. Several GPCRs have been targeted for the treatment of a multitude of conditions including obesity, hypertension, allergies, psychotic disorders and pain, among others15. Therefore, targeting GPR137B is an attractive possibility for therapeutic intervention in cancer.
Another essential consideration for future study is the identification of those cellular cues that may modulate the interaction between GPR137B and Rags. GPR137B is an orphan GPCR, meaning that its endogenous ligands remain unknown. Recent evidence suggests that some orphan receptors are constitutively activated, and this might be the case for GPR137B, as well. However, another intriguing possibility is that GPR137B is activated in response to specific stimuli, such as ions or amino acids. If this is the case, GPR137B may play a critical role communicating changing conditions inside the lysosomal lumen to the rest of the cell, a question to be explored by future studies.
Footnotes
Competing Interest
The author declares no competing interest.
References
- 1.Saxton RA & Sabatini DM Cell 168, 960–976 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H et al. Nature 552, 368–373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sancak Y et al. Cell 141, 290–303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sancak Y et al. Science 320, 1496–501 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gan, et al. Nat. Cell Biol This issue (2019). [Google Scholar]
- 6.Lawrence RE & Zoncu R Nat. Cell Biol 21, 133–142 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Lawrence RE et al. Nat. Cell Biol 20, 1052–1063 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen K, Sidik H & Talbot WS Cell Rep. 14, 547–559 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martina JA & Puertollano RJ Cell Biol. 200, 475–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang LQ et al. Tumori. 104, 330–337 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Wang C et al. Biotechnol. Appl. Biochem 62, 868–73 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Shao X et al. Cell Biol. Int 39, 418–26 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Cui X et al. Biotechnol. Appl. Biochem 62, 861–867 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Du Y et al. Biotechnol. Appl. Biochem 62, 855–60 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Franco R, Martínez-Pinilla E, Navarro G & Zamarbide M Prog. Neurobiol 150, 21–38 (2017). [DOI] [PubMed] [Google Scholar]


