Abstract
2Naphthalene sulfonate (2NS) is an intermediate compound used in textile industries. Being nonbiodegradable, the concerns regarding its biotoxicity have risen. In the present investigation the toxic effects of 2NS were analyzed with the help of Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR), which was used to monitor changes in the vibrational modes of functional groups within the biomolecules. After calculating LD 50, one half of LD 50 i.e. 0.33 mg/15 g b.w. was intraperitoneally administrated and the brain tissue was collected for investigation after 96 h of exposure. The spectra observed revealed the significant differences in absorbance and areas between control and treated groups reflecting the change in proteins, lipids and nucleic acid due to toxicity induced by 2NS. In addition, protein secondary structure analysis was focused in this study, which reveals alterations in α helix and β sheet structure after 2NS intoxication. Histopathology of brain was also studied, which reveals changes in the histology of brain in group treated with 2NS. In conclusion, the study highlighted the application of ATR-FTIR and histopathology for toxicity assessment.
Keywords: 2Naphthalene sulfonate (2NS), ATR-FTIR, histopathology, LD 50, Channa punctatus
Introduction
The aquatic bodies serve as a sink for number of contaminants with varying hazardous potentials [1]. Run off from contaminated land surface or industrial effluents are likely avenues by which most water bodies receive contaminants. Source of these contaminants are known to cause direct toxic effects on aquatic species [2]. Synthetic compounds mostly involved in industrial or agricultural plants, unless particularly eliminated by wastewater treatment plants, may remain in effluent and get discharged into the water bodies as trace contaminants. Aquatic bodies carrying these trace pollutants may become direct source of drinking water, which can prove to be fatal for humans [3]. Thus, concern regarding the safety of environment has gained importance.
In industrial sector, textile industries majorly contribute to water pollution. Wastewater effluents from the textile and other dye-stuff industries contain significant amounts of synthetic dyes in addition to harmful chemicals involving intermediates involved in the synthesis of dyes. Dyes persist in natural water bodies for quite a long time and render the water unfit for its intended use, at the same time the aquatic organisms also remain in contact with them for a very long time. Thus, high chances for accumulation of these compounds in aquatic organisms are there. Organisms may uptake these xenobiotics via dermal or dietary routes of exposure. 2Naphthalene sulfonate (2NS) is an intermediate involved in the production of dyes, surfactants and dispersants. It is also used in concrete finishing, tanning of hides and in the production of pharmaceuticals and agrochemicals [4–6]. Due to its sulfonated nature maximum stability is imparted to the benzene ring making the compound highly resistant to biodegradation. Thus, the concern regarding its toxicity has risen.
Due to high specific oxygen consumption rate and high lipid content, brain is considered to be highly susceptible to oxidative stress. To study the effects of water-borne contaminants, fishes are most reliable experimental model. Being the first recipient they can metabolize, concentrate and store those contaminants in their bodies as higher vertebrates. Channa punctatus, being widely distributed and available throughout the year, is considered as an excellent model species. Thus, for the present inquisition brain tissue of C. punctatus was studied.
Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) is a reliable biospectroscopy technique, which generates a spectrum indicating biological constituents existing in cells or tissues, using the ability to absorb and vibrate at specific frequency of the electromagnetic spectrum with the possibilities of monitoring cellular alterations as well as tracking these changes over time [7]. Attenuated total reflection is the most accomplished of all spectroscopic sampling techniques as it demands very compact sampling preparation and can be used on any kind of samples possessing any morphology, while maintaining the structural integrity of the sample. It helps in identifying the structural variation of biomolecules including proteins, lipids and nucleic acids [8]. Actually, peculiar frequencies, intensities and bandwidths in an IR spectrum led to the characterization of the functional groups of biomolecules, providing insight into the conformational and structural alterations in biological constituents. Along with ATR-FTIR, histopathological analysis also gives critical data to estimate alterations occurred in tissues after external manifestations and has recently achieved an important place in morphotoxicology [9]. Thus, present study was performed in order to assess the toxic potential of 2NS on brain tissue of C. punctatus with the help of modern technique i.e. ATR-FTIR along with histopathology highlighting its application to distinguish and determine sublethal effects of potential toxins in the surroundings using sentinel animals.
Materials and Methods
Test chemical
2NS was purchased from Himedia Research laboratory, Mumbai, India (CAS No. 120–18-3)
Experimental design: The freshwater fish C.punctatus commonly called as snakehead fish weighing15 ± 2 g were procured from local outlets and acclimatized in 200 l capacity glass aquarium for 15 to 20 days under laboratory conditions. The water of aquarium was changed daily in order to avoid the accumulation of waste material and to reduce its ammonia content, and fishes were fed with synthetic diet. The mean values of physicochemical parameters of water were determined, which comes out to be: temperature 26.7°C, pH values 7.2, dissolved oxygen 5.63, electrical conductivity 630 μs/cm and total dissolved solids (TDS) 307 mg/L. Ethical approval for this study was not required as C. punctatus is a food fish.
After LD 50 determination, which comes out to be 0.66 mg/15 g b.w. [10], 0.33 mg/15 g b.w. (one half of LD50) was selected for further experiment. Two groups were taken in which one was control and other was treated and each group containing 10 fishes. Fishes maintained in tap water were considered as control group, whereas fishes in which 2NS was intraperitoneally injected in the midline of the pelvic fins in a constant volume of distilled water were considered as treated group. The experiment was performed in triplicates. After 96 h fishes from both groups were sacrificed without giving anesthesia and the brain tissue collected. Out of the collected material one part was lyophilized for 24 h and finally analyzed for revealing alterations in bio-constituents with the help of ATR-FTIR and other part was used for histopathology.
Sample preparation for ATR-FTIR study
Brain tissues of control and exposed fishes were washed in phosphate buffer saline. After lyophilization of 24 h the samples were grounded to powder with the help of pestle and mortar and analysis was performed using a Cary 630 FTIR Spectrometer in the spectral range of 4000 to 400 cm−1 using an FTIR imaging system coded for 64 scans at 16 cm−1 resolution taking total 30 s as measurement time. The diamond ATR sensor was cleaned with ethyl alcohol or acetone before each sample measurements. Each sample analysis was performed in triplicates.
Sample preparation for histopathology
Brain tissues isolated from both groups of fish were rinsed and cleaned in Physiological saline solution (0.75% NaCl). Fixation was carried out in 10% formalin buffer solution for 48 h and was processed through graded series of alcohols. Further, the samples were cleared in xylene and finally embedded in paraffin wax before being sectioned at 5 μm. Specimens were further stained with eosin and hematoxylin. Finally, the prepared sections were observed and photographed using light microscope.
Statistical analysis
The data were analyzed using t-test (SPSS 11).
Results
ATR-FTIR
The present investigation shows the effect of 2NS on the biomolecules of brain tissue of C. punctatus using ATR-FTIR technique. The spectra were normalized with the help of ORIGIN 8.0. Alterations observed in peak areas of infrared bands were assessed to obtain structural and molecular information about the particular sample. Table 1 presents the general band assignment, whereas Table 2 presents significant alterations observed in different peak areas of specified bio-constituents present in brain tissue after 2NS intoxication as compared to control. Figure 1 presents the spectrum of control and 2NS intoxicated brain tissues in spectral range of 4000–400 cm−1. The spectrum is complex and possesses number of bands originating from different functional groups. Therefore, the spectra were further analyzed by forming three different spectrums ranging from 800 to 1800 cm−1, 3100 to 3600 cm−1 and 2800 to 3050 cm−1. Figure 2 presents the representative ATR-FTIR spectra of the control and 2NS exposed brain tissues of C. punctatus in the 3600–3100 cm−1 region. The absorptions in this particular region are mainly dominated by the amide A of proteins arising from N–H and O–H stretching modes of proteins and intermolecular H bonding. The weak band observed at ∼3081 cm−1 represents N–H stretching of proteins of amide B. Intensity of absorbing bands arising at 3293 and 3081 cm−1 are generally attributed to amide A and B bands arising from N–H stretching of proteins. The alterations observed in the area of these bands show the toxic effect of 2NS on amide A and B band. Figure 3 presents the representative ATR-FTIR spectra of the control and 2NS administered brain tissues of C. punctatus in the 3050–2800 cm−1 region. This region is mainly attributed to lipids. The broad band observed at ∼2955 cm−1was assigned to asymmetric stretching vibration of methyl group. This band mainly monitors the lipid in the biological system. Lipids play important role in membrane fluidity. Exposure and diffusion of membrane constituents is governed by lipids. The change in methylene stretching bands of the lipid hydrocarbon chain helps in analyzing lipid fluidity. In the present analysis, the area of CH2 asymmetric stretching vibration (2926 cm−1) decreases from 9.8 to 5.61due to 2NS intoxication. This reduction reveals the alterations in constitution of acyl chains. Additionally, the decrease in area of CH2 stretching band positioned at 2854 cm−1 was found. The value decreases from 4.12 to 1.81, suggesting the decrease in content of CH2 groups in the intoxicated brain tissues. shows the representative FTIR spectra of the control and 2NS exposed brain tissues of C. punctatus in the 1800–800 cm−1 region. The band observed at 1451 cm−1 is because of bending vibration of CH2 present in proteins as well as lipids. The decrease observed in the area of this band was 14.17%. The bands observed at 1230 and 1081 cm−1 are due to asymmetric and symmetric modes of phosphodiester group present in nucleic acids, respectively. The alteration in area observed in these bands reveals the toxic potential of 2NS.
Table 1.
General band assignments of the FTIR spectra
| Lipids | Wavenumber (cm−1) | |
|---|---|---|
| 1468, 1461 | CH3-asymmetric bending: mainly lipids | |
| 1736 | Carbonyl C=O stretch: lipids | |
| 3011 | Olefinic HC=CH stretch: lipids | |
| 2959, 2958 | CH3-asymmetric stretch: mainly lipids | |
| 2851, 2855 | CH2-asymmetric stretch: mainly lipids | |
| Nucleic acids | 1237 | (PO2 asymmetric stretch: mainly nucleic acids |
| 1170 | CO–O–C asymmetric stretching: phospholipids | |
| 1080 | PO2-symmetric stretching: mainly nucleic acids | |
| 961–964 | C–N+–C stretch: nucleic acids | |
| Proteins | 1653, 1654 | Amide I: C=O stretching of proteins |
| 1550, 1543 | Amide II: N–H Bending/C–N stretching of proteins | |
| 3302 | Amide A: mainly N–H stretching of proteins |
Table 2.
Alterations in different peak areas in brain after treatment with 2NS
| Wavenumber(cm−1) | Control | Exposed | |
|---|---|---|---|
| Lipids | 1451 | 3.81 ± 0.02 | 3.27* ± 0.04 |
| 2926 | 9.88 ± 0.15 | 5.61* ± 0.37 | |
| 2854 | 4.12 ± 0.13 | 1.81* ± 0.24 | |
| Proteins | 1640 | 12.89 ± 0.67 | 19.15* ± 0.33 |
| 1543 | 6.63 ± 0.33 | 10.19* ± 0.48 | |
| 3293 | 27.35 ± 0.85 | 37.48* ± 0.67 | |
| 3081 | 6.93 ± 0.86 | 4.93* ± 0.56 | |
| Nucleic acids | 1230 | 5.81 ± 0.87 | 3.50* ± 0.27 |
| 1080 | 14.97 ± 0.14 | 12.08* ± 0.85 | |
| 961 | 1.58 ± 0.07 | 1.93* ± 0.05 |
*Difference between control and exposed groups.
The areas of each peak were calculated with ORIGIN 8.0 SOFTWARE. The values are the mean along with standard deviation for each group (n = 3). Comparisons were carried out by Students t test. The degree of significance was P ≤ 0.05.
Figure 1.
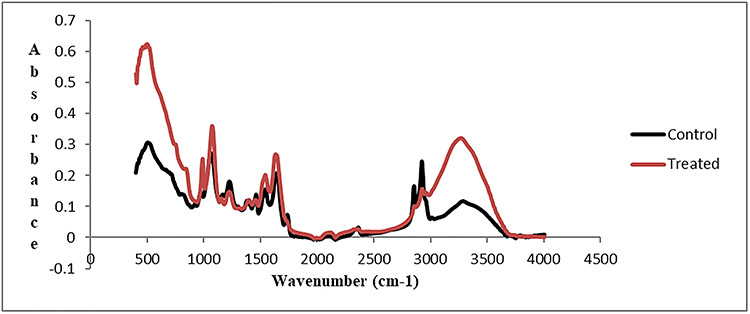
The representative FTIR spectrum of the brain tissue of C. punctatus in 4000–400 cm−1 region.
Figure 2.

The representative FTIR spectra of the control and 2NS exposed brain tissues of C. punctatus in the 3600–3050 cm−1 region.
Figure 3.
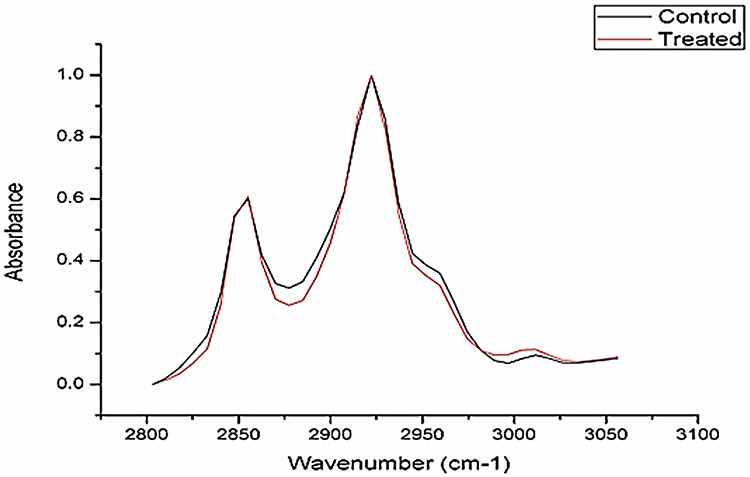
The representative FTIR spectra of the control and 2NS exposed brain tissues of C. punctatus in the 2800–3100 cm−1 region.
Figure 4.

The representative FTIR spectra of the control and 2NS exposed brain tissues of C. punctatus in the 800–1800 cm−1 region.
Further, Table 3 provides the detailed analysis of the bands present in the range of 1700 to 1600 cm−1 that also attributes to amide A vibrational mode, which further indicate sensitivity to conformational changes in the secondary structure. The band observed at 1654 cm−1 is mainly assigned to α helical structure. This amide A band is formed by involving C=O bond, N–H and C–N bending and alteration observed in this band reveals the effect of toxic material on protein confirmation. The significant increase was observed in the area of band observed at 1654 cm−1 reveals the alteration observed in amide A-band confirmation. Table 3 also presents the peak at 1625 cm−1, which attributes to β sheet. The peak arising at 1662 and 1640 cm−1 indicates β turns and random coils and the alteration observed in all these band areas from control indicates the toxic effect of 2NS on protein secondary structure.
Table 3.
The mean values of peak areas of main protein secondary structures for control as well as 2NS intoxicated brain tissue
| Structure | Peak centers (cm−1) | Control | Exposed |
|---|---|---|---|
| α-helix | 1654 | 9.19 ± 0.01 | 9.70* ± 0.10 |
| β-sheet | 1625 | 8.55 ± 0.01 | 9.70* ± 0.10 |
| Turns and bends | 1662 | 4.54 ± 0.05 | 4.40 ± 0.17 |
| Random coil | 1640 | 12.89 ± 0.67 | 19.15* ± 0.33 |
*Difference between control and exposed groups.
The areas of each peak were calculated with ORIGIN 8.0 SOFTWARE. The values are the mean for each group (n = 3). Comparisons were carried out by Students t test. The degree of significance was P ≤ 0.05.
Histopathology
The present study shows the histopathological changes observed in brain tissue after intraperitoneal administration of 2NS. Brain histopathology of control fish revealed normal histological architect without any indication of deformities however the treated fish after 96 h showed areas of necrosis and vacuolization, indicating neurotoxic effect of 2NS (Fig. 5A and B).
Figure 5.
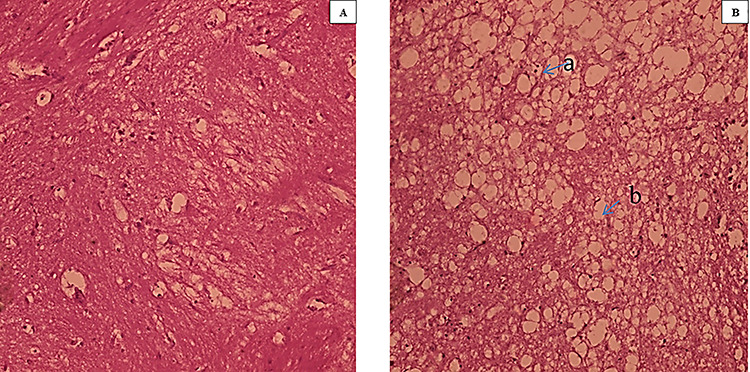
(A and B): Histopathology slides of brain tissue (×100) A: Control B: Treated (a: necrosis, b: vacuolization).
Discussion
Analysis of alterations in biochemical constituents was performed by ATR-FTIR in 96 h exposed groups. Fourier transform infrared (FTIR) spectroscopy is being extensively used to study structural as well as concentration changes in biochemical constituents. The intensity of the absorption bands in the FTIR spectrum is directly related to the concentration of the molecules. Obinaju et al. [16] illustrated the potential application of ATR-FTIR spectroscopy to study, signature and understand the changes occurring in subcellular components of the cell as a result of exposure to potential mutagens. Chemically induced changes to biological molecules, particularly genetic molecules such as DNA are known to predispose organisms to pathologic disease conditions such as cancers [17]. Thus, variations in nucleic acid should be considered as major risk. In the present study, alteration observed at wavenumber 3302 cm−1, showing 37.03% significant increase (P ≤ 0.05), implies variation in the strength of protein and amide hydrogen bonding. The significant increase at 1654 and 1543 cm−1 was observed, which comes out to be 48.56% and 53.69%, respectively. These sharp bands observed at 1654 and ~1543 cm−1 correspond to amide I and amide II vibration of structural protein, respectively. The amide I absorption is mainly associated with the C=O stretching vibration of the protein amide. The amide II absorption arises from amide N–H bending vibration (60%) coupled to C-N stretching vibration (40%) mode of the polypeptide and protein backbone [11]. Both are sensitive and often used to determine protein secondary structure. This prominent alteration in protein region leads to determination of small peaks present in 1600−1700 cm−1 region, which is attributed to proteins structures. The peak areas of exposed groups were compared with the untreated (control) group. The results obtained for brain tissue show alteration in the band at 1654 cm−1, which was assigned to alpha-helix structure; the bands at 1625, 1632 and 1684 cm−1 were assigned to beta-sheet structure; the band around 1647 cm−1 was assigned to random coil and the bands at 1662 and 1669 cm−1 arose from beta turn [18, 19]. The bands at 1468 cm−1 arise mainly from the CH2 asymmetric bending and COO– symmetric stretching modes, respectively [11].
Along with proteins, damage to lipids was also assessed in the present study. For lipids, 1468, 2959, 2855 cm−1 wavenumbers were studied. Lipids play a key role in the membrane fluidity. By affecting the conformation of membrane proteins, they govern exposure and diffusion of membrane components. The changes in lipid fluidity can be detected by analyzing the methylene stretching bands of the lipid hydrocarbon chains. The peak area of the CH2 asymmetric stretching vibration (2959 cm−1) was found to be decreased from 9.88 to 5.61 showing 43.21% significant reduction. Ma et al. [20] found that the anatase nTiO2-treated mouse brain undergoes severe oxidative stress and produce reactive oxygen species (ROS) due to its biological activities under light. This confirms that the observed changes in biochemical constituents in the brain tissues of C. punctatus could be due to the overproduction of ROS. This leads to the breakdown of balance of the oxidative/antioxidative system in the brain, resulting in the lipid peroxidation, which leads to the alterations in the structure of biomolecules.
Alterations in nucleic acids were also assessed in the present study. The significant reduction in mean values of peak areas at frequency 1088 can be associated with alteration in nucleic acids structure. Significant alteration was also observed at 961 cm−1 frequency which indicates alteration in C-N + -C stretch present in nucleic acids thus showing brain being prone to DNA damage after the exposure of 2NS. The ability to detect slight changes in the IR spectra of samples at wavenumbers representative of biomolecules, e.g., symmetric (1088) and asymmetric (1234) PO-2 bands is associated with nucleic acids [21,22]. The area at 1234 cm−1 band was found to be significantly reduced.
In order to speculate the effect of changes in the structure of major biomolecules on the integrity of tissue, histopathological evaluation of brain was also considered. Alterations correlated with the histopathology of brain due to different pollutants are scarce. Thus, the present analysis was undertaken to fill the lacuna in this regard. The present study showed normal histology indicating no deformities in case of control group (Fig. 5). However, in those administered with 2NS, areas of necrosis and vacuolization in cerebrum of brain were observed indicating the neurotoxic nature of 2NS. Sarma et al. [23] observed massive degeneration in cerebrum of Labeo rohita after exposure of endosulfan. Similarly, severe necrosis in brain tissue was observed after exposure of hexachlorocyclohexane in L. rohita [24]. The study clearly indicated that alterations in the main biochemical constituents might lead to the histopathological changes in brain tissue.
Conclusion
In conclusion, the present investigation indicates significant alterations in the major biochemical constituents such as lipids, nucleic acids and proteins after 2NS intoxication, which may lead to structural and molecular changes in the brain tissue ultimately leading to histopathological effects. The study highlighted the use of ATR-FTIR as a novel tool for toxicity assessment.
Conflicts of Interest
None declared.
Acknowledgement
Authors are sincerely thankful to Council of Scientific and Industrial Research-University Grants Commission (CSIR-UGC) for awarding fellowship to S.M. and also University Grants Commission Special Assistance Programme (UGC SAP, New Delhi) for providing infrastructural facilities.
References
- 1. Kelly SA, Giulio RTD. Developmental toxicity of estrogenic alkylphenols in killifish (Fundulus heteroclitus). Environ Toxicol Chem 2000;19:2564–70. [Google Scholar]
- 2. Bayen S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: a review. Environ Int 2012;48:84–101. [DOI] [PubMed] [Google Scholar]
- 3. Murray KE, Thomas SM, Bodour AA. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ Pollut 2010;158:3462–71. [DOI] [PubMed] [Google Scholar]
- 4. Storm T, Reemtsma T, Jekel J. Use of volatile amines as ion-pairing agents for the high-performance liquid chromatographic tandem mass spectrometric determination of aromatic sulfonates in industrial wastewater. J Chromatogr A 1999;854:175–85. [DOI] [PubMed] [Google Scholar]
- 5. Suter MJF, Riediker S, Giger W. Selective determination of aromatic sulfonates in landfill leachates and groundwater using microbore liquid chromatography coupled with mass spectrometry. Anal Chem 1999;71:897–904. [DOI] [PubMed] [Google Scholar]
- 6. Riediker S, Suter MJF, Giger W. Benzene- and naphthalenesulfonates in leachates and plumes of landfills. Water Res 2000;34:2069–79. [Google Scholar]
- 7. Trevisan J, Angelov PP, Patel II et al. Syrian hamster embryo (SHE) assay (pH 6.7) coupled with infrared spectroscopy and chemometrics towards toxicological assessment. Analyst 2010;135:3266–72. [DOI] [PubMed] [Google Scholar]
- 8. Taillandier E, Liquier J, Taboury JA. Infrared spectral studies on DNA conformations In: Clarke RJH, Hester RE (eds). Advances in Infrared and Raman Spectroscopy, 12. New York: Wiley Heyden, 1985, 65–114. [Google Scholar]
- 9. Lamastra L, Suciu NA, Trevisan M. Sewage sludge for sustainable agriculture: contaminants’ contents and potential use as fertilizer. Chem Biol Technol Agric 2018;5:10. [Google Scholar]
- 10. Mehra S, Chadha P. Bioaccumulation and toxicity of 2 naphthalene sulfonate: an intermediate compound used in textile industries. Toxicol Res 2020;9:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dogan A, Siyakus G, Severcan F. FTIR spectroscopic characterization of irradiated hazelnut. Food Chem 2007;100:1106–14. [Google Scholar]
- 12. Severcan F, Toyran N, Kaptan N et al. Fourier transform infrared study of the effect of diabetes on rat liver and heart tissues in the C–H region. Talanta 2000;53:55–9. [DOI] [PubMed] [Google Scholar]
- 13. Cakmak G, Togan I, Severcan F. 17b-Estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: a comparative study with nonylphenol. Aquat Toxicol 2006;77:53–63. [DOI] [PubMed] [Google Scholar]
- 14. Toyran N, Turan B, Severcan F. Selenium alters the lipid content and protein profile of rat heart: an FTIR microspectroscopic study. Arch Biochem Biophys 2007;458:184–93. [DOI] [PubMed] [Google Scholar]
- 15. Benedetti E. Bramanti E., Papineschi F., et al. Determination of the relative amounts of nucleic acids and proteins in leukemic and normal lymphocytes by means of Fourier transform infrared microscopy. Appl Spectrosc 199751,792–797. [Google Scholar]
- 16. Obinaju BE, Graf C, Halsall C et al. Linking biochemical perturbations in tissues of the African catfish to the presence of polycyclic aromatic hydrocarbons in Ovia River, Niger Delta region. Environ Pollut 2015b;201:42–9. [DOI] [PubMed] [Google Scholar]
- 17. Malins DC, Anderson KM, Stegeman JJ et al. Biomarkers signal contaminant effects on the organs of English sole (Parophrys vetulus) from Puget sound. EHP 2006;114:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dogan A, Siyakus G, Severcan F. FTIR spectroscopic characterization of irradiated hazelnut. Food Chem 2007;100:1106–14. [Google Scholar]
- 19. Wolkers WF, Looper SA, McKiernan AE et al. Membrane and protein properties of freeze-dried mouse platelets. J Membr Biol 2002;19:201–10. [DOI] [PubMed] [Google Scholar]
- 20. Toyran N, Zorlu F, Donmez G et al. Chronic hypoperfusion alters the content and structure of proteins and lipids of rat brain homogenates: a Fourier transform infrared spectroscopy study. Eur Biophys J 2004;33:549–54. [DOI] [PubMed] [Google Scholar]
- 21. Ma L, Liu J, Li N et al. Biomaterials 2010;31:99–105. [DOI] [PubMed] [Google Scholar]
- 22. Lasch P, Pacifico A, Diem M. Spatially resolved IR microspectroscopy of single cells. Biopolymers 2002;67:335–8. [DOI] [PubMed] [Google Scholar]
- 23. Sarma K, Pal A, Sahu N et al. Biochemical and histological changes in the brain tissue of spotted murrel, Channa punctatus (Bloch), exposed to endosulfan. Fish Physiol Biochem 2010;597–603. [DOI] [PubMed] [Google Scholar]
- 24. Das BK, Mukherjee SC. A histological study of carp (Labeo rohita) exposed to hexachlorocyclohexane. Vet Arh 2000;70:169–80. [Google Scholar]


