Abstract
Present study was planned to investigate the ameliorative effect of silver nanoparticles (AgNPs) on acetaminophen-induced nephrotoxicity. Our results demonstrate that therapy of AgNPs at three different doses (50, 100 and 150 μg/kg once only) prevented the acetaminophen (2 g/kg once only) induced acute renal toxicity. AgNPs treated animals also show less intensity in the histological alterations in kidneys and corroborating the results of analysis of serum urea and creatinine. In addition, AgNPs therapy prevented the acetaminophen-induced oxidative stress, which was confirmed by the alleviated lipid peroxidation, enhanced renal reduced glutathione content and restored enzymatic activities of superoxide dismutase, catalase and adenosine triphosphatase in kidney. Thus, our results demonstrate a possible protective potential of AgNPs on renal toxicity induced by acetaminophen. This study will definitely lead to the development of therapeutic drug against nephrotoxicity, after further clinical and preclinical studies.
Keywords: silver nanoparticles, nephrotoxicity, acetaminophen, oxidative stress, GSH
Graphical Abstract
Graphical Abstract.
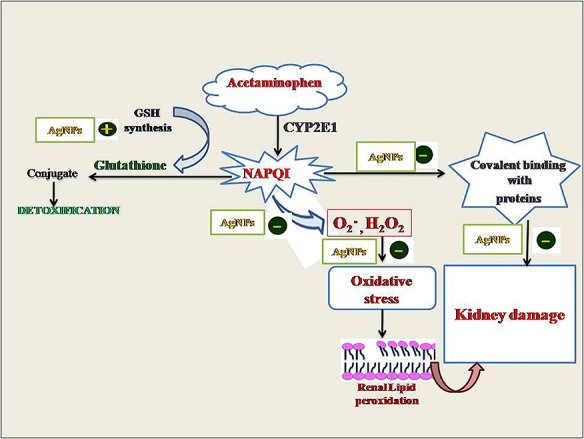
Introduction
Acetaminophen (APAP), commonly prescribed analgesic and antipyretic drug in clinical practice stands out as the most toxic drug among the drugs with potentially toxic effect [1]. It has been reported that APAP overdose potentially induce hepatorenal damage in experimental animals and humans [1–6] and in severe cases to death. Acute renal failure has been reported in ~1–2% of patients because of APAP overdose [7–10]. Toxicity of APAP in both hepatic and extrahepatic tissues is closely related to its metabolism [11]. At therapeutic dose, APAP in liver is conjugated with glucuronate and sulfate to generate water soluble, nontoxic compounds that are excreted in bile. A small amount of APAP is metabolized into highly reactive and toxic metabolite, N-acetyl-p-benzoquinone-imine (NAPQI) by the microsomal P-450 enzyme system. Intracellular reduced glutathione (GSH) conjugates with NAPQI, forming mercapturic acid conjugate, which is excreted via kidneys, hence plays a crucial role in detoxification of APAP [12]. However, at overdose of APAP, the amount of the active NAPQI exceeds the binding capacity of GSH, which results in the accumulation of NAPQI. Thus active NAPQI binds with the intracellular macromolecules that lead to tissue damage. Furthermore, consequent activation of lysosomal enzymes initiate tissue necrosis and finally organ dysfunction [10, 13]. Proximal tubules are the target of APAP toxicity because of their active absorptive and secretory activities [14, 15]. As APAP can induce life-threatening kidney lesions, the search for therapy for APAP-induced nephrotoxicity is of clear toxicological importance [16–19].
Researchers pay much more attention toward the scientific use of nanoparticles because of their amazing physicochemical properties. Metallic nanoparticles show promising applications in the field of medicine, biology and material science. Noble metal such as silver shows enormous potential for biomedical applications. It has been successfully used as novel diagnostic and therapeutic agents and to deliver pharmaceutics [20, 21]. Thus, the interests in the use of silver nanoparticles (AgNPs) are continuously increased. Literature has revealed the significant medicinal properties in AgNPs from ancient times like antimicrobial, antiangiogenic, antitumor, anti-inflammatory, hepatoprotective and antioxidant activities [6, 22–24]. However, various studies have demonstrated that AgNPs are able to cause important structural and functional alterations in different organs including kidneys [25, 26]. Thus AgNPs in both cell-based models and animal studies elucidate a diverse picture of biological impact, showing both therapeutic as well as toxic effects. Thus it is apparent that any toxic effect by AgNPs is extremely dependent on the mode of synthesis, size, shape and selection of dose of AgNPs [25]. Looking into a variety of biomedical applications of AgNPs, an attempt has been made for the evaluation of therapeutic potential of AgNPs in ameliorating APAP-induced nephrotoxicity. To the best of our knowledge, this is the first study to show the nephroprotective efficacy of pure AgNPs against APAP toxicity. We used 3–5 nm sized AgNPs in present study, which can be efficiently excreted through kidneys and the AgNPs does not accumulate inside the body. Moreover, unlike AgNPs synthesized by chemical reduction that requires the addition of dispersing agent to avoid the aggregation of nanoparticles, the AgNPs used in present investigation were synthesized by physical vapor deposition (PVD) and evenly dispersed in sterilized water, without the addition of dispersing agent to maintain 99.99% purity.
Materials and Methods
Animals and chemicals
Female albino rats of Wistar strain (160 ± 10 g b.w.) were used in this study. Animals were housed under standard husbandry conditions (25 ± 2°C temperature, 60–70% relative humidity and 12 h photoperiod). The rats were fed on standard pellet diet and water ad libitum. Animals were treated and cared in accordance with the guidelines recommended by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India (CPCSEA/501/01/a). Experimental protocols were approved by the Institutional Animal Ethical Committee of Jiwaji University, Gwalior.
Preparation of AgNPs
AgNPs (3–5 nm) were procured from manufacturer, Gold NanoTech, Inc., Taipei, Taiwan. Nanoparticles were synthesized by PVD, suspended in sterilized water, to maintain 99.99% purity of AgNPs and the unique technology was applied to allow our AgNPs to be evenly dispersed in sterilized water. Therefore, unlike nanosilver made with chemical reduction in which additional dispersing agent is required to avoid the aggregation of nanoparticles, the AgNPs used in present study are evenly suspended in ultrapure water without addition of dispersing agent. This further increases the purity of AgNPs.
Chemicals
APAP was procured from Smithkline Bee-cham, (Batch no. 0103). Silymarin and other chemicals were procured from Sigma–Aldrich Company, Ranbaxy, New Delhi and Himedia Laboratories Ltd Mumbai, India. All diagnostic kits were procured from E-Merck, India Pvt Ltd; auto analyzer (Micro Lab 200, Merck) was used for their measurements.
Preparation of doses and treatments
Preparation of doses was described in our previous study [6]. Briefly a suspension of APAP (2.0 g/5 ml/kg) was made in hot distilled water and administered orally by using oral gavage. Colloidal solution of AgNPs were prepared in distilled water and different doses of AgNPs (50, 100 and 150 mg/5 ml/kg p.o.) were administered to the animals orally. Silymarin (50 mg/5 ml/kg, p.o.) was prepared in 1% gum acacia and silymarin was given as positive control.
Experimental protocol
Animals were divided into seven groups of six animals each. Group I served as control, Group II was administered AgNPs at a dose of 150 mg/kg p.o. once only and served as AgNPs per se. Group III–VII were administered (APAP) at a dose of 2 g/kg p.o. once only. Group III served as experimental control (APAP per se). After 24 h of APAP administration, group IV–VI were treated with three different doses (50, 100 and 150 μg/kg p.o.) of AgNPs once only and animals of Group VII were administered silymarin at a dose of 50 mg/kg p.o. once only, as a standard drug. Animals of all groups were sacrificed after 24 h of the last treatment.
Collection of serum
Blood samples were collected from retro-orbital venous sinus [27]. Serum was harvested after keeping the blood at room temperature for 30 min followed by centrifugation at 2000 rpm for 15 min and stored at −20°C until analyzed.
Biochemical assay of kidney function tests
Serum was used for the estimation of markers of kidney function test like serum urea and creatinine that were measured using diagnostic kits and auto analyzer (Micro Lab 200, Merck).
Tissue biochemical assays
Kidney tissues were excised immediately after necropsy. Tissues were rinsed in ice cold normal saline and blotted to dry for tissue biochemical estimations. Tissues were homogenized with a Remi Motor Homogenizer (RQ-122) using glass tube and Teflon pistle. Tissues were homogenized in different ice cold buffers [150 mM KCL for lipid peroxidation (LPO), 1% sucrose for GSH, normal saline for superoxide dismutase (SOD) and catalase (CAT), hypotonic solution for adenosine triphosphatase (ATPase)]. Homogenates were immediately processed to determine LPO [28], reduced GSH [29], CAT [30], SOD [31] and ATPase [32].
Histopathological study
After necropsy kidney slices were fixed immediately in Bouin’s fixative and paraffin sections of 5 μm thickness were cut. Hematoxylin–eosin stained slides were observed under light microscope [33].
Statistical analysis
Results are presented as mean ± SEM of six animals used in each group. Data were subjected to statistical analysis through one way analysis of variance (ANOVA) considering significant at 5% level followed by student’s t-test considering P ≤ 0.05 [34]. Percent of protection was calculated by the following formula [6].
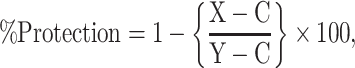 |
where, X = APAP + AgNPs, C = control, Y = APAP.
Result
Results of serum biochemical tests
A marked elevation in the kidney specific markers like serum urea and creatinine was observed after acute exposure of APAP when compared with control group (Table 1). AgNPs at all the three doses (50, 100 and 150 μg/kg) restored altered urea and creatinine level toward control in a dose dependent manner. Maximum recovery was observed at both the higher doses (100 and 150 μg/kg) in restoring the levels of urea and creatinine when analyzed statistically.
Table 1.
Protective potential of AgNPs on kidney function tests
| Treatments | Urea (mg/dl) | Creatinine (mg/dl) |
|---|---|---|
| Control | 18.0 ± 0.99 | 0.22 ± 0.01 |
| AgNPs per se | 20.0 ± 1.10 | 0.23 ± 0.01 |
| APAP per se | 39.0 ± 2.15b | 1.10 ± 0.06b |
| APAP + AgNPs 50 μg/kg % protection | 32.8 ± 1.81 (29%) | 0.83 ± 0.04* (31%) |
| APAP + AgNPs 100 μg/kg % protection | 28.0 ± 1.54* (52%) | 0.60 ± 0.03* (57%) |
| APAP + AgNPs 150 μg/kg % protection | 27.7 ± 1.53* (54%) | 0.60 ± 0.03* (57%) |
| APAP + S 50 mg/kg % protection | 23.8 ± 1.31* (72%) | 0.30 ± 0.01* (91%) |
| ANOVA | 26.901a | 107.52a |
Data are mean ± SEM; N = 6; S, silymarin.
aSignificant at 5% for ANOVA.
bAPAP vs control.
*APAP + therapy vs APAP at P ≤ 0.05.
Results of biochemical analysis of renal homogenate
Table 2 depicts significant elevation in renal lipid peroxidation and a remarkable fall in renal GSH after intoxication of APAP. Increased lipid peroxidation was expressed in terms of enhanced Thiobarbituric Acid Reactive Species (TBARS) in APAP exposed rats, which indicated renal peroxidative damage (P ≤ 0.05). AgNPs at all the three doses (50, 100 and 150 μg/kg) and silymarin at 50 mg/kg showed significant depletion in LPO and hence restored GSH when compared to APAP per se; however, maximum recovery was observed at 100 and 150 μg/kg doses (P ≤ 0.05), which was confirmed by the percent protection.
Table 2.
Protective efficacy of AgNPs on lipid peroxidation and reduced glutathione concentrations in renal homogenate
| Treatments | LPO (n moles TBARS/mg protein) | GSH (μmole/g) |
|---|---|---|
| Control | 0.32 ± 0.01 | 8.00 ± 0.44 |
| AgNPs per se | 0.30 ± 0.01 | 8.00 ± 0.44 |
| APAP per se | 2.18 ± 0.12b | 4.00 ± 0.22b |
| APAP + AgNPs 50 μg/kg % protection | 1.70 ± 0.09* (26%) | 6.00 ± 0.33* (50%) |
| APAP + AgNPs 100 μg/kg % protection | 1.20 ± 0.06* (53%) | 6.50 ± 0.35* (62%) |
| APAP + AgNPs 150 μg/kg % protection | 1.18 ± 0.06* (54%) | 6.60 ± 0.36* (65%) |
| APAP + S 50 mg/kg % protection | 0.68 ± 0.03* (81%) | 7.40 ± 0.40* (85%) |
| ANOVA | 122.04a | 16.687a |
Data are mean ± SEM4; N = 6.
aSignificant at 5% for ANOVA.
bAPAP vs control.
*APAP + therapy vs APAP at P ≤ 0.05.
Significant decline (P ≤ 0.05) was found in SOD, CAT and ATPase activity in kidney after APAP administration (Table 3). ANOVA showed significant recovery in the activities of SOD, CAT and ATPase by the therapy of AgNPs at all the three doses, but the most effective restoration was observed at 100 and 150 μg/kg doses. No adverse effects were found in the blood and tissue biochemical parameters AgNPs per se group, which confirmed the nontoxic effect of AgNPs.
Table 3.
Protective effect of AgNPs on activities of SOD and CAT and ATPase in kidney homogenate
| Treatments | SOD (U/mg protein) | CAT (U/mg protein) | ATPase (mg Pi/100 g/min) |
|---|---|---|---|
| Control | 69.0 ± 3.81 | 83.0 ± 4.58 | 1829 ± 101 |
| AgNPs per se | 71.0 ± 3.92 | 82.0 ± 4.53 | 1830 ± 101 |
| APAP per se | 36.0 ± 1.99b | 39.0 ± 2.15b | 1021 ± 56.4b |
| APAP + AgNPs 50 μg/kg % protection | 55.0 ± 3.04* (57%) | 60.0 ± 3.31* (48%) | 1230 ± 68.0* (26%) |
| APAP + AgNPs 100 μg/kg % protection | 60.5 ± 3.34* (74%) | 71.5 ± 3.95* (74%) | 1550 ± 85.6* (65%) |
| APAP + AgNPs 150 μg/kg % protection | 61.6 ± 3.40* (77%) | 71.8 ± 3.96* (74%) | 1560 ± 86.2* (67%) |
| APAP + S 50 mg/kg % protection | 66.0 ± 3.64* (91%) | 79.6 ± 4.40* (92%) | 1640 ± 90.6* (77%) |
| ANOVA | 14.868a | 19.061a | 14.776a |
Data are mean ± SEM; N = 6.
aSignificant at 5% for ANOVA.
bAPAP vs control.
*APAP + therapy vs APAP at P ≤ 0.05.
Histopathological examinations of kidney
Light microscope evaluation of kidneys in control group showed normal morphology of renal parenchyma with well-defined Bowman’s capsule and tubules (Fig. 1A). Kidney of rats after acute administration of APAP showed severe deterioration in cortical region and hypercellularity in glomeruli, diameters of the tubules were decreased. Apical nuclei were also seen in epithelial cells of tubules. Disrupted endothelial lining was also observed (Fig. 1B). With the treatment of AgNPs at 50 μg/kg dose, significant improvement in proximal and distal convoluted tubules was seen; however, tubular obstruction still persists (Fig. 1C). Better results were observed at the dose of 100 and 150 μg/kg treatment of AgNPs, improved structure of glomeruli was noted. The tubules and glomeruli were well organized, the nuclear organization in the epithelium of collecting tubules was normal. Clear and wide lumens were observed in the renal tubules (Fig. 1D and E). Therapy of silymarin showed remarkable improvement in histoarchitecture of kidney (Fig. 1F).
Figure 1.
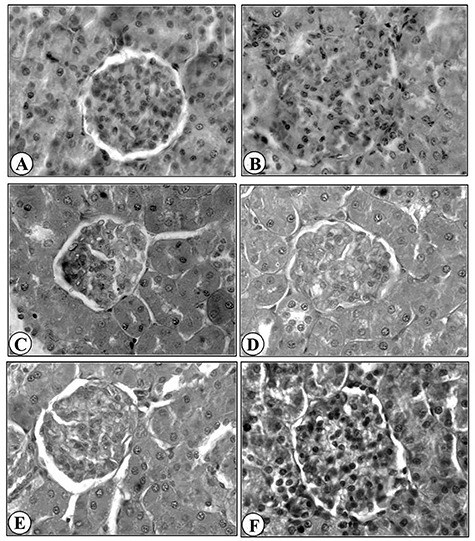
Histopathology of Kidney (×400); Kidney of control rat (A), Kidney of rat intoxicated with APAP 2 g/kg (B), Kidney of rat treated with AgNPs 50 mg/kg after APAP intoxication (C), Kidney of rat treated with AgNPs 100 mg/kg after APAP intoxication (D), Kidney of rat treated with AgNPs 150 mg/kg after APAP intoxication (E), Kidney of rat treated with Silymaran 50 mg/kg after APAP intoxication (F).
Discussion
Nephrotoxicity and hepatotoxicity are the potential problems associated with APAP intoxication. APAP is widely, used as analgesic and antipyretic drug in general medicine hence an assessment of its relative toxicity is important. Thus, present study was planned to investigate the ameliorative effect of AgNPs on APAP-induced nephrotoxicity in rats. Acute exposure of APAP to rats led to an altered kidney functions and antioxidant capacities. Therapy of AgNPs and silymarin significantly ameliorated the alteration of biochemical and antioxidant variables induced by APAP intoxication, suggesting their protective efficacy. AgNPs also improved the structure of kidney that was evaluated on the basis of histopathological findings.
Urea and creatinine are important indicators of renal damage in clinical findings [35, 36]. Thus serum urea and creatinine were evaluated to demonstrate kidney damage. It has been reported that the elevated levels of urea and creatinine in serum are the major diagnostic symptoms of kidney dysfunction because the rate of production exceeds the rate of clearance due to the defect in renal function [37]. Our results depicted that the levels of urea and creatinine were significantly enhanced by APAP intoxication to rats when compared with control group, demonstrating the deterioration of the renal function. Our results are in consistent with our previous study in which we noticed remarkable increase of urea and creatinine in the serum of APAP treated rats [5]. Our results are also in accordance with the findings of other researchers who reported that APAP-induced kidney damage is observed by enhanced serum urea and creatinine [4, 38, 39]. Therapy of AgNPs restored urea and creatinine near to normal that states the role of AgNPs in averting the kidney dysfunction.
APAP-induced nephrotoxicity may be due to the metabolic activation of APAP to the reactive metabolite, NAPQI [40, 41]. When large doses of APAP are ingested, more NAPQI is formed that results in more severe depletion in GSH, which leads to accumulation of NAPQI and gets covalently bound with macromolecules and cellular protein [42]. This process interrupt homeostasis and initiates tissue necrosis and ultimately to organ dysfunction. Concentration of intracellular GSH is therefore a key determinant of the extent of APAP-induced renal injury, hence researchers focus their interest on compounds that act as antioxidants and are capable of stimulating GSH synthesis. In present investigation renal GSH level was declined and activities of major renal antioxidant enzymes (SOD and CAT) were significantly inhibited due to APAP intoxication, which we have also observed in our previous study [5]. Our results also corroborate with the other investigations [13, 43, 44]. Therapy of AgNPs to APAP intoxicated rats significantly raised GSH level and activities of renal SOD and CAT toward normal. This increase in both the nonenzymatic and enzymatic antioxidants may play a considerable role in the mechanism of the nephroprotective effect of AgNPs.
Furthermore, it has been reported that LPO might be a contributory factor to the development of renal toxicity [45, 46]. Depletion of renal GSH allows lipid peroxidation, TBARS are a good indicator of the degree of lipid peroxidation [36, 43]. In the present investigation, we have also observed a significant elevation in the TBARS levels in the kidney tissue of rats intoxicated with APAP alone compared with the control. Probably the restoration of membrane damage by AgNPs is partially related to its ability to scavenge lipid peroxidation initiating agents. A significant decline in LPO was observed in AgNPs treated rats. The results obtained with AgNPs are comparable to those seen with silymarin.
ATPase is a mitochondrial lipid-dependent membrane-bound enzyme. Membrane fluidity is distorted by the alteration in membrane lipids, which in turn affect ATPase activity and as a result energy dependent cellular function [47, 48]. APAP intoxication provoked significant inhibition in ATPase activity in kidney, which might be due to dysfunctional changes in mitochondria and cell membrane permeability. AgNPs treatment prevented membrane lesion to a large extent with concomitant recovery in the activity ATPase by recovering cell membrane permeability.
Our biochemical investigations are also supported by histological observations. In the present investigation, histopathological examinations showed a clear evidence of nephrotoxicity following the acute intoxication of APAP. Acute tubular necrosis was the most relevant histopathological change. These results are in agreement with our previous investigation describing the renal histological alterations following the administration of APAP [5]. Therapy of AgNPs Showed recovery in the APAP-induced histological alterations kidney. The ameliorative effect of AgNPs on the nephrotoxicity induced by APAP possibly depends on its ability to mainly enhance the GSH synthesis, which lead to excretion of more NAPQI and activities of SOD, CAT and ATPase are increased. Hence AgNPs inhibit the generation of peroxide and superoxide radical thus, tissues are protected from damage.
Conclusion
Thus it is concluded that on the basis of biochemical results and histopathological findings, the present data confirmed that AgNPs might be a potential therapeutic agent against experimentally induced APAP nephrotoxicity via its antioxidant and free radical-scavenging properties. However, further investigations are needed to demonstrate the exact mechanism of AgNPs on APAP-induced nephrotoxicity.
Acknowledgment
Authors thank Jiwaji University, Gwalior, India for providing laboratory facilities and UGC, SAP-II for financial support (F.4-1/2006(BSR)/7-97/2007(BSR), 26 June 2012).
Conflict of interest statement
None declared.
References
- 1. Kaplowitz N. Acetaminophen hepatotoxicity: what do we know don’t we know, and what do we do next? Hepatology 2004;40:23–6. [DOI] [PubMed] [Google Scholar]
- 2. Eguia L, Materson BJ. Acetaminophen-related acute renal failure without fulminant liver failure. Pharmacotherapy 1997;17:363–70. [PubMed] [Google Scholar]
- 3. Ghosh J, Das J, Manna P et al. . Acetaminophen induced renal injury via oxidative stress and TNF-α production: therapeutic potential of arjunolic acid. Toxicology 2010;268:8–18. [DOI] [PubMed] [Google Scholar]
- 4. Karthivashan G, Kura AU, Arulselvan P et al. . The modulatory effect of Moringa oleifera leaf extract on endogenous antioxidant systems and inflammatory markers in an acetaminophen-induced nephrotoxic mice model. Peer J 2016;4:e2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reshi MS, Shrivastava S, Jaswal A et al. . Gold nanoparticles ameliorate acetaminophen induced hepato-renal injury in rats. Exp Toxicol Pathol 2017a;69:231–40. [DOI] [PubMed] [Google Scholar]
- 6. Reshi MS, Uthra C, Yadav D et al. . Silver nanoparticles protect acetaminophen induced acute hepatotoxicity: a biochemical and histopathological approach. Regul Toxicol Pharmacol 2017b;90:36–41. [DOI] [PubMed] [Google Scholar]
- 7. Bailey B, Amre OK, Gaudreault F. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med 2003;31:299–305. [DOI] [PubMed] [Google Scholar]
- 8. Bajt ML, Knight TR, Lemasters JJ et al. . Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci 2004;80:343–9. [DOI] [PubMed] [Google Scholar]
- 9. Mour G, Feinfeld DA, Caraccio T et al. . Acute renal dysfunction in acetaminophen poisoning. Ren Fail 2005;27:381–3. [PubMed] [Google Scholar]
- 10. Mazer M, Perrone J. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations and management. J Med Toxicol 2008;4:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu J, Cui H, Behr M et al. . In vivo mechanisms of tissue-selective drug toxicity: effects of liver-specific knockout of the NADPH-cytochrome P450 reductase gene on acetaminophen toxicity in kidney, lung and nasal mucosa. Mol Pharmacol 2005;67:623–30. [DOI] [PubMed] [Google Scholar]
- 12. Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis 1990;10:267–78. [DOI] [PubMed] [Google Scholar]
- 13. Ahmad ST, Arjumand W, Nafees S et al. . Hesperidin alleviates acetaminophen induced toxicity in wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol Lett 2012;208:149–61. [DOI] [PubMed] [Google Scholar]
- 14. Blakely P, McDonald BR. Acute renal failure due to acetaminophen ingestion: a case report and review of the literature. JAmSocNephrol 1995;6:48–53. [DOI] [PubMed] [Google Scholar]
- 15. Trumper L, Monasterolo LA, Ochoa E et al. . Tubular effects of acetaminophen in the isolated perfused rat kidney. ArchToxicol 1995;69:248–52. [DOI] [PubMed] [Google Scholar]
- 16. Abraham P. Vitamin C may be beneficial in the prevention of paracetamol-induced renal damage. Clin Exp Nephrol 2005;9:24–30. [DOI] [PubMed] [Google Scholar]
- 17. Isik B, Bayrak R, Akcay A et al. . Erdosteine against acetaminophen-induced renal toxicity. MolCell Biochem 2006;287:185–91. [DOI] [PubMed] [Google Scholar]
- 18. Abdel Zaher AO, Abdel Hady RH, Mahmoud MM et al. . The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology 2008;243:261–70. [DOI] [PubMed] [Google Scholar]
- 19. Abdel Zaher AO, Abdel Rahman MM, Hafez MM et al. . Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology 2007;234:124–34. [DOI] [PubMed] [Google Scholar]
- 20. Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol 2007;18:26–30. [DOI] [PubMed] [Google Scholar]
- 21. Li C. A targeted approach to cancer imaging and therapy. Nat Mater 2014;13:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sriram MI, Kanth SB, Kalishwaralal K et al. . Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int J Nanomedicine 2010;5:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inbathamizh L, Ponnu TM, Mary EJ. In vitro evaluation of antioxidant and anticancer potential of Morinda pubescens synthesized silver nanoparticles. J Pharm Res 2013;6:32–8. [Google Scholar]
- 24. Reshi MS, Bhatia G, Yadav D et al. . Pure silver nanoparticles showed potential anticancer effect on colon and breast cancer cell lines. Octa J Biosci 2016;4:46–8. [Google Scholar]
- 25. Boey A, Ho HK. All roads lead to the liver: metal nanoparticles and their implications for liver health. Small 2020;16:2000153. [DOI] [PubMed] [Google Scholar]
- 26. Iavicoli I, Fontana L, Nordberg G. The effects of nanoparticles on the renal system. Crit Rev Toxicol 2016;46:490–560. [DOI] [PubMed] [Google Scholar]
- 27. Riley V. Adaptation of orbital bleeding technique to rapid serial blood studies. Proc Soc Exp Biol Med 1960;104:751–4. [DOI] [PubMed] [Google Scholar]
- 28. Sharma SK, Krishnamurthy CR. Production of lipidperoxides of brain. J Neurochem 1968;15:147–9. [DOI] [PubMed] [Google Scholar]
- 29. Brehe JE, Burch HB. Enzymatic assay for glutathione. Anal Biochem 1976;74:189–95. [DOI] [PubMed] [Google Scholar]
- 30. Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121–6. [DOI] [PubMed] [Google Scholar]
- 31. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170–5. [PubMed] [Google Scholar]
- 32. Seth PK, Tangri KK. Biochemical effects of newer salicylic acid congeners. J Pharm Pharmacol 1966;18:831–3. [DOI] [PubMed] [Google Scholar]
- 33. Whitaker D, Williams V. Cytopreparatory techniques. In: Woods AE, Ellis RC (eds).. Laboratory Histopathology: A Complete Reference (First ed., Vol. 2, pp. 10.1-1-10.1-26). Edinburgh: Churchill Livingstone, 1994. [Google Scholar]
- 34. Snedecor GW, Cochran WG. Statistical Method, 8th edn. Ames, Iowa: Affiliated East-West Press, 1989, 217–36. [Google Scholar]
- 35. Refaie AA, Ramadan A, Mossa AT. Oxidative damage and nephrotoxicity induced by prallethrin in rat and the protective effect of origanum majorana essential oil. Asian Pac J Trop Med 2014;7:S506–13. [DOI] [PubMed] [Google Scholar]
- 36. Uthra C, Shrivastava S, Jaswal A et al. . Therapeutic potential of quercetin against acrylamide induced toxicity in rats. Biomed Pharmacother 2017;86:705–14. [DOI] [PubMed] [Google Scholar]
- 37. Mayne PD. The Kidneys and Renal Canculi In: Clinical Chemistry in Diagnosis and Treatment. 6th ednLond: Edward Arnold Pub; 1994; 2–14. [Google Scholar]
- 38. Şener G, Şehirli AÖ, Ayanoğlu-Dülger G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study. J Pineal Res 2003;35:61–8. [DOI] [PubMed] [Google Scholar]
- 39. Cekmen M, Ilbey YO, Ozbek E et al. . Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol 2009;47:1480–4. [DOI] [PubMed] [Google Scholar]
- 40. Hart SE, Beierschmitt WP, Wyand DS et al. . Acetaminophen nephrotoxicity in CD-1 mice: I. evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol Appl Pharmacol 1994;126:267–75. [DOI] [PubMed] [Google Scholar]
- 41. El-Maddawy ZK, El-Sayed YS. Comparative analysis of the protective effects of curcumin and N-acetyl cysteine against paracetamol-induced hepatic, renal, and testicular toxicity in Wistar rats. Environ Sci Pollut Res 2018;25:3468–79. [DOI] [PubMed] [Google Scholar]
- 42. Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 2001;31:55–138. [DOI] [PubMed] [Google Scholar]
- 43. Liang H, Feng Y, Cui R et al. . Simvastatin protects against acetaminophen-induced liver injury in mice. Biomed Pharmacother 2018;98:916–24. [DOI] [PubMed] [Google Scholar]
- 44. Acharya M, Lau-Cam CA. Comparison of the protective actions of N-acetylcysteine, hypotaurine and taurine against acetaminophen-induced hepatotoxicity in the rat. J Biomed Sci 2010;17:S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palipoch S. A review of oxidative stress in acute kidney injury: protective role of medicinal plants-derived antioxidants. Afr J Tradit Complement Altern Med 2013;10:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Patra A, Mandal S, Samanta A et al. . Therapeutic potential of probiotic lactobacillus plantarum AD3 on acetaminophen induced uremia in experimental rats. Clin Nutr Exp 2018;19:12–22. [Google Scholar]
- 47. Nirala SK, Bhadauria M. Propolis reverses acetaminophen induced acute hepatorenal alterations: a biochemical and histopathological approach. Arch Pharm Res 2008;31:451. [DOI] [PubMed] [Google Scholar]
- 48. Jaswal A, Sharma M, Raghuvanshi S et al. . Therapeutic efficacy of nigella sativa Linn. Against Antituberculosis drug−induced hepatic injury in Wistar rats. J Environ Pathol Toxicol Oncol 2016;35:59–71. [DOI] [PubMed] [Google Scholar]


