Abstract
An outbreak of human immunodeficiency virus (HIV) among people who inject drugs in Glasgow, Scotland started in 2014. We describe 156 cases over 5 years and evaluate the impact of clinical interventions using virological and phylogenetic analysis. We established (1) HIV services within homeless health facilities, including outreach nurses, and (2) antiretroviral therapy (ART) via community pharmacies. Implementation of the new model reduced time to ART initiation from 264 to 23 days and increased community viral load suppression rates to 86%. Phylogenetic analysis demonstrated that 2019 diagnoses were concentrated within a single network. Traditional HIV care models require adaptation for this highly complex population.
Keywords: antiretroviral therapy, ART, HIV, outbreak, PWID
Large-scale outbreaks of human immunodeficiency virus (HIV) among people who inject drugs (PWID) have been reported in Europe and the United States in recent years [1]. These outbreaks have been associated with homelessness, economic recession, stimulant injecting, suboptimal drug treatment coverage, and injecting equipment provision (IEP). Response to these outbreaks has been focused on improving harm reduction services, such as engagement in opioid agonist therapy (OAT), enhanced IEP, and increasing HIV awareness alongside antiretroviral therapy (ART) for those diagnosed [1]. Although there have been HIV outbreaks among PWID in Scotland in the 1980s [2], the most recent was in 1993, with 14 cases identified [3]. Before 2014, there were between 15 and 20 PWID diagnosed with HIV each year in Scotland [4], approximately 10 per year in the NHS Greater Glasgow and Clyde Health Board Area (hereafter “Glasgow”). In Scotland, free IEP saw over 4.4 million needles and syringes distributed in 2016/2017 [5]. Free-to-access services also provide specialist drug treatment, including OAT, with almost 12 000 individuals presenting for assessment in 2016/2017 nationally [6]. Low HIV incidence among PWID in Scotland was attributed to comprehensive IEP and OAT coverage over the last 30 years [7]. Despite this, since 2014, Glasgow has experienced a significant rise in HIV diagnoses among PWID, closely linked to Glasgow City center. Recent epidemiological studies demonstrated that HIV prevalence has increased from 1.1% in 2011–2012 to 10.8% in 2017–2018 in this population in Glasgow City center. This is associated with homelessness, incarceration, injecting cocaine [7], and injecting in outdoor locations or “public places” [8], and it represents the largest HIV outbreak among this population in the United Kingdom (UK) for over 30 years.
Traditionally, HIV care in Glasgow was delivered via a centralized hospital-based outpatient service, with the exception of the blood-borne virus (BBV) prison in-reach service. All patients had to travel to 1 main hospital located in the west of the city, approximately 4 miles from Glasgow City center, to receive HIV care. It is recognized that PWID are less likely to remain engaged in HIV care and on ART than other cohorts [9]. Before 2015, among PWID with HIV in Glasgow, nonattendance levels at this clinic were high and ART adherence was poor, evidenced by patients not returning to the clinic for repeat ART prescriptions and not achieving viral suppression. Antiretroviral therapy was available directly from the hospital or via a home delivery service, the latter requiring a stable address and telephone contact. In people whose accommodation is in flux, receiving and storing medications is a challenge. Stigma associated with HIV infection is commonly expressed in this group even among their peers. The challenges in providing high-quality, holistic HIV care in this population required a paradigm shift in our local HIV care model and approach.
Furthermore, there was an awareness that HIV testing rates in drug treatment services, emergency departments, prisons, and IEP sites were low. Estimates from public health surveillance suggest that rates before the outbreak were approximately 30% tested in the last 12 months [10]. An Incident Management Team was convened in early 2015 by the public health department, and a broad range of strategies were used to address the outbreak.
AIM
Public health and clinical responses were initiated to control this HIV epidemic among PWID in Glasgow. This study will describe the clinical response, named Glasgow Enhanced Care HIV Outreach (GECHO). We aim to (1) describe the sociodemographic characteristics of PWID diagnosed with HIV in Glasgow between June 1, 2014 and June 30, 2019, (2) describe the clinical interventions implemented in response to the HIV outbreak among PWID in Glasgow, and (3) assess the impact of the clinical interventions implemented, using clinical outcome markers and phylogenetic analysis.
METHODS
Setting
In February 2015, the West of Scotland Specialist Virology Centre (WoSSVC) in Glasgow reported to the Public Health Protection Unit that an unusually high number of HIV diagnoses had been made, via dry blood spot tests sent from drug treatment services in the city, in the latter part of 2014 and early 2015. Between June 1, 2014 and June 30, 2019, 156 people were diagnosed with HIV and were identified as part of the Glasgow outbreak. Injecting drug use, or sexual contact with someone known to inject drugs, were identified as the main risk factors for HIV acquisition. Diagnoses over these years were made through the promotion of regular (3 monthly) testing for all those attending drug treatment services for OAT and through extensive contact tracing of those diagnosed. Phylogenetic analysis demonstrated that this strain of subtype C virus, with primary nonnucleoside reverse-transcriptase inhibitor (NNRTI) mutations E138A and V179E, had been transmitted recently and rapidly among Scottish PWID but had not yet spread anywhere else in the UK [11]. The characteristics of PWID in Glasgow have been previously described [7, 10] with HIV uninfected PWID being predominantly male and reporting high levels of heroin and cocaine use, despite the majority being prescribed OAT, a marker for engagement with specialist drug treatment services.
INTERVENTIONS AND TIMELINE
Education and Awareness
Education and awareness-raising initiatives were immediately developed with input from the target population of people currently injecting drugs in the city. This included the following steps. (1) Posters were displayed in areas frequented by the target population, such as homeless accommodations and pharmacies to raise awareness. (2) Leaflets or postcards were shared with the target population through third-sector organizations (nongovernmental organizations), the police, and NHS services. (3) Various communications and BBV training were provided to third-sector organizations, prison healthcare staff, and other NHS services who would come into contact with the target population. (4) Communications with the general public were provided through media release.
HUMAN IMMUNODEFICIENCY VIRUS SERVICE DELIVERY RESPONSE TO THE OUTBREAK
It was acknowledged that the affected population struggled to engage in HIV care when delivered through a hospital model due to social and psychological difficulties. A different approach to address ongoing HIV viremia would benefit individuals and crucially would reduce onward transmission.
Homeless health services in Glasgow are based in 1 location in the city center and encompass specialist drug treatment services and general practice, with visiting services from a range of other healthcare teams. This existing homeless health building was geographically close to the target population, and patients were familiar with it. An intervention model, GECHO, was developed that supported a BBV clinical nurse specialist initially, and later an HIV consultant led service within this setting. Pharmacy services were adapted to enhance ART adherence by providing ART alongside OAT, which in the majority of patients was delivered daily, supervised by their community pharmacist.
JUNE 2016
1. PART-TIME NURSE SUPPORT
Funding was provided by the Glasgow health board for a 0.5 full-time equivalent dedicated clinical nurse specialist (approximately £18 000 GBP per year). Their key role is patient engagement in BBV care and includes liaising with multiagency partners such as voluntary organizations and other healthcare teams. Outreach work includes locating patients who are street homeless, in hostels or temporary accommodation, delivering ART, with treatment monitoring (venepuncture) and broader holistic care, as well as signposting to other services for mental health support, OAT, wound care, other medical issues, general support, and advocacy.
2. COMMUNITY PHARMACY ANTIRETROVIRAL THERAPY MODEL
We developed a model in which ART could be dispensed daily in any Glasgow community pharmacy with or without OAT, and supervised if required. There are 291 pharmacies in Glasgow that can provide this service. Therefore, the majority of patients can receive their daily dose of ART alongside OAT, supervised by the pharmacist, in a community pharmacy, close to their accommodation. This service further allows assessment of ART adherence. For those who declined supervised dispensing of ART, ART uptake was assessed based on prescription and self-report of adherence.
SEPTEMBER 2017
3. FULL-TIME NURSE AND WEEKLY CONSULTANT CLINIC
Additional funding for a further 0.5 full-time equivalent clinical nurse specialist and a 0.2 full-time equivalent dedicated consultant time was provided by the Glasgow health board at a further cost of approximately £27 000 GBP per year. The consultant time provides comprehensive BBV care as well as sexual and reproductive health and specialist infectious diseases management.
DATA SOURCES AND COLLATION
We retrospectively analyzed data collected as part of routine care including social, clinical, and virological information from multiple sources (Supplementary Table 1). All individuals in Scotland can be uniquely identified via their Community Health Index number, which is used as the main identifier for each of these data sources. Data were collected until June 30, 2019 and anonymized for analysis. Date of death or transfer of care was captured to account for censoring. To estimate viral suppression, a community viral load measurement was calculated, as per the Centers for Disease Control and Prevention guidance [12], reporting on the proportion of cases who achieved viral suppression (viral load <200 copies/mL) in each 3-month period (1 quarter). If more than 1 HIV viral load result was available in a quarter, the highest result was used. If no HIV viral load measurement was available for 1 quarter, the case was only considered to be virally suppressed if the measurements immediately before and after that quarter were <200 copies/mL. Cases were included in the cohort from the date of HIV diagnosis and censored at date of death or move. Drug-related mortality was defined as per the National Records of Scotland definition [13].
Where data were available, we compared our cohort to self-reported HIV uninfected PWID from Glasgow using data from the Needle Exchange Surveillance Initiative (N. Palmateer, PhD, 15 September 2019, written personal communication), a biennial, national, anonymous bio-behavioral study of PWID in Scotland.
LABORATORY METHODS
All laboratory testing was carried out at the WoSSVC, Glasgow. Samples (venous blood or dried blood spot samples [DBS]) were screened for HIV using the Architect HIV Ag/Ab combo assay (modified for DBS) (Abbott, Chicago, IL). Additional confirmatory testing was performed using an alternative fourth-generation assay (VIDAS; Biomérieux, Marcy l’Etoile, France) and/or the Geenius HIV1/2 assay (BioRad, Marnes-la-Coquette, France). Confirmed HIV-positive samples were further tested using an in-house HIV avidity assay that determines recency of infection (ie, whether infection was likely to have been acquired within the last 4 months) [14]. Baseline resistance testing was performed using a Sanger sequencing-based method described previously [11]. Human immunodeficiency virus viral load testing was performed using the Abbott HIV-1 real-time PCR assay.
Samples were also screened for hepatitis C virus (HCV) using the Abbott Architect Anti-HCV assay (modified for DBS). Positive venous samples were tested using the Abbott real-time HCV ribonucleic acid assay or the Abbott ARCHITECT HCV Ag assay, whereas DBS were tested using an in house real-time PCR assay [15].
Genetic Sequencing and Phylogenetic Analysis
As a routine component of clinical care, the WoSSVC sequences the pol region of all new HIV diagnoses (HXB2 positions 2253 to 3549), as described previously [11]. In July 2019, all subtype C sequences with both drug-resistant mutations, diagnosed between mid-2012 up until 30 June 2019, were collated for analysis (n = 151). Non-C subtypes were not included in the phylogenetic analysis even if they were diagnosed during this time period. We added sequences from the UK HIV Drug Resistance Database found in our earlier analysis to be linked to the current outbreak (n = 28) [11]. We ran HIV-TRACE at a range of thresholds (0.5%–2%) to identify the lowest threshold that would capture most of the epidemiologically defined outbreak [16]. Previously, the outbreak had been entirely linked using a single-linkage approach at 1%, meaning that each sequence was linked to at least 1 other at a genetic distance threshold of 1%, but a genetic distance of 2% remains indicative of membership in the same transmission chain [17]. At a genetic distance of 2%, 175 of 179 sequences analyzed were linked. A phylogeny was reconstructed for the genetically linked outbreak (n = 175), using RaxML [18] under a Generable Time Reversible model with 4 gamma rates. We then time-resolved the phylogeny using the treedater package, available in R [19], using sample times as tip dates.
STATISTICAL ANALYSIS
Comparing the Glasgow outbreak cohort to HIV-negative PWID in Glasgow, we conducted Fisher’s tests for associations between being HIV positive and sex, age, previous homelessness, or having injected within the 6 months before diagnosis, sharing of injection equipment, prescription of OAT, and hepatitis C status. The Needle Exchange Surveillance Initiative (NESI) data used are aggregate rather than individual-based; therefore, we could not test for interactions between predictors. The Mann-Whitney U test was used to compare time from HIV diagnosis to ART initiation before and after January 1, 2017.
ETHICAL APPROVAL
Advice was sought from the West of Scotland Research Ethics Service and there was no requirement for formal ethical review.
RESULTS
Of the 156 new diagnoses of HIV among PWID or sexual contacts of PWID in Glasgow, from June 1, 2014 to June 30, 2019, routine viral sequencing showed 1 of 156 to be subtype A1, 15 of 156 to be subtype B virus, 2 of 156 unknown (elite controller/low viral load), and 138 of 156 (88.5%) subtype C virus with identical primary NNRTI mutations (E138A and V179E), indicating a common source for the subtype C outbreak.
Table 1 shows sociodemographic characteristics of the HIV outbreak cohort, compared with HIV uninfected PWID in Glasgow. The Glasgow HIV outbreak cohort compared with HIV-negative PWID in Glasgow (NESI participants [10]) were more likely to be women (odds ratio [OR] = 1.63, P < .05), younger (P < .01), more likely to have experienced homelessness within the 6 months before diagnosis (OR = 5.2, P < .0001), more likely to have injected within the 6 months before diagnosis (OR = 2.4, P < .0001), more likely to be injecting cocaine and heroin (P < .001), more likely to share injection equipment (OR = 21.12, P < .0001), and more likely to be HCV coinfected or to have had HCV in the past (P < .001). There was no significant different in use of OAT.
Table 1.
Sociodemographic Characteristics of the HIV Outbreak Cohort Compared With HIV Uninfected PWID in Glasgow
| HIV Outbreak Cohort (n = 156) | HIV Uninfected PWID (n = 780) | P Value | ||
|---|---|---|---|---|
| Social Characteristics | Sex | |||
| Female | 53/156 (34%) | 186/780 (24%) | ||
| Male | 103/156 (66%) | 590/780 (76%) | ||
| Unknown | 0/156 (0%) | 4/780 (1%) | ||
| <.05 | ||||
| Age | ||||
| <35 | 39/156 (25%) | 111/780 (14%) | ||
| 35–39 | 49/156 (31%) | 196/780 (25%) | ||
| 40–44 | 31/156 (20%) | 217/780 (28%) | ||
| 45+ | 37/156 (24%) | 256/780 (33%) | ||
| <.01 | ||||
| Homelessness (defined as no tenancy) | ||||
| Yes | 100/156 (64%) | 198/780 (25%) | ||
| No | 56/156 (36%) | 576/780 (74%) | ||
| Unknown | 0/156 (0%) | 6/780 (1%) | ||
| <.0001 | ||||
| In the 6 Months Before HIV Diagnosis | ||||
| Incarceration | Not available | |||
| Yes | 54/156 (35%) | |||
| No | 102/156 (65%) | |||
| Risk Behaviors | Injecting Drug Use | |||
| Yes | 130/156 (81%) | 523/780 (67%) | ||
| No | 26/156 (19%) | 251/780 (32%) | ||
| Unknown | 0/156 (0%) | 6/780 (1%) | ||
| <.0001 | ||||
| Main Drug Injected | ||||
| Cocaine | 18/130 (14%) | 114/523 (22%) | ||
| Heroin | 51/130 (39%) | 366/523 (70%) | ||
| Heroin and cocaine | 61/130 (47%) | 15/523 (3%) | ||
| Other | 0/130 (0%) | 28/523 (5%) | ||
| <.001 | ||||
| Self-Reported Sharing of Needles or Syringes | ||||
| Yes | 62/130 (48%) | 52/523 (10%) | ||
| No | 26/130 (20%) | 465/523 (89%) | ||
| Unknown | 42/130 (32%) | 6/523 (1%) | ||
| <.0001 | ||||
| Sexual Intercourse | Not available | |||
| Yes | 93/156 (60%) | |||
| No | 55/156 (35%) | |||
| Unknown | 8/156 (5%) | |||
| Type of HIV Acquisition Risk Disclosed From Contact Tracing (Some Reported More Than 1 Type of Risk) | Not applicable | |||
| Injecting drug use/shared injecting equipment contacts | 97/156 (62%) | |||
| Sexual contacts | 72/156 (46%) | |||
| Sexual and sharing injecting equipment contacts | 40/156 (26%) | |||
| Harm Reduction | Prescribed opioid agonist therapy | |||
| Yes | 128/156 (82%) | 627/780 (80%) | ||
| No | 28/156 (18%) | 147/780 (19%) | ||
| Unknown | 0/156 (0%) | 6/780 (1%) | ||
| .823 | ||||
| Acute Presentations | Glasgow acute hospital unscheduled presentations | Not available | ||
| None | 54/156 (34%) | |||
| 1–5 | 87/156 (56%) | |||
| 6–10 | 12/156 (8%) | |||
| >10 | 3/156 (2%) | |||
| BBV Status | HIV infection duration (recent infection defined as IgG avidity <40%) | Not applicable | ||
| Early | 53/156 (34%) | |||
| Chronic | 101/156 (65%) | |||
| Unknown | 2/156 (1%) | |||
| HCV status at diagnosis | ||||
| Ab positive, HCV PCR/Ag negative | 58/156 (37) | 190/780 (24) | ||
| Ongoing/active HCV infection | 92/156 (59) | 268/780 (34) | ||
| HCV negative (Ab and Ag/PCR) | 6/156 (4) | 262/780 (34) | ||
| Unknown | 0/156 (0%) | 60/780 (8) | ||
| <.001 |
Abbreviations: Ab, antibody; Ag, antigen; BBV, blood-borne virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; PCR, polymerase chain reaction; PWID, people who inject drugs.
For those with HIV immunoglobulin (Ig)G avidity >40%, indicating infection acquired more than 4 months before diagnosis (n = 101), the mean CD4 count was 442 cells/mm3 (standard deviation = 244 cells/mm3). Forty-five percent (45 of 101) had a baseline CD4 count <350 cells/mm3, and, of those, the mean CD4 percentage count was 25% (standard deviation = 10%). Within those, 22% (22 of 101) had a baseline CD4 count <200 cells/mm3. Thirty-two percent (7 of 22) of these were diagnosed with acute HIV infection (IgG avidity <40% or negative HIV test within preceding 6 months). Of the remaining 15 of 22, the mean CD4 percentage count was 17% (standard deviation = 8%). No patients have had a clinical acquired immune deficiency syndrome (AIDS)-defining illness. Eighteen percent (28 of 156) of the cohort died. Full information on cause of death for 2019 is not yet available, but those with information available (93%, 26 of 28) show that 50% (14 of 28) were drug-related deaths, with an additional 3 of 28 (11%) due to trauma. There have been no known AIDS-related deaths.
Human Immunodeficiency Virus Testing
Data from public health surveillance have shown that self-reported uptake of HIV testing among PWID in Glasgow has increased from 30% in 2013–2014 to 50% in 2017–2018 [10].
Antiretroviral Therapy Uptake and Time to Treatment
Those who died or moved away from Glasgow before initiating ART (n = 7) were censored from this analysis. Of the remaining patients, all 149 have been commenced on ART, although some have had intermittent adherence. The numbers of patients diagnosed in each year and included in this analysis are as follows: 2014, 4; 2015, 47; 2016, 31; 2017, 34; 2018, 19; 2019, 14 (n = 149).
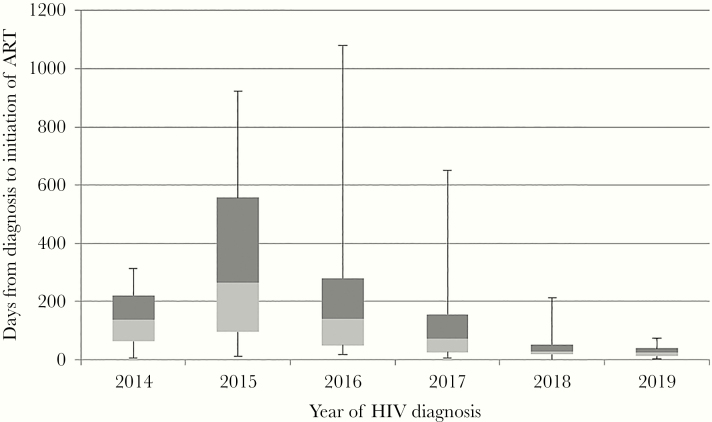
There has been a reduction in the median time from HIV diagnosis to starting ART from 264 days in 2015 (interquartile range [IQR], 94–556) to 23 days (IQR, 12–38) in 2019 (Figure 1). A comparison of time from diagnosis to ART start in those diagnosed from June 1, 2014 to December 31, 2016 to those diagnosed from January 1, 2017 to June 30, 2019 showed a significant reduction in the median time to ART (Mann-Whitney U test, P < .00001).
Figure 1.
Time from human immunodeficiency virus (HIV) diagnosis to antiretroviral therapy (ART) initiation, by year of HIV diagnosis. Box and whisker plot showing minimum, first quartile, median, third quartile, and maximum time in days.
Viral Suppression
One hundred forty-nine patients who started ART were included in an analysis of viral load measurements, and those who moved or died were censored at date of death or move (n = 31). The majority of patients were commenced on a combination of an integrase inhibitor with dual nucleoside reverse-transcriptase inhibitors (NRTIs).
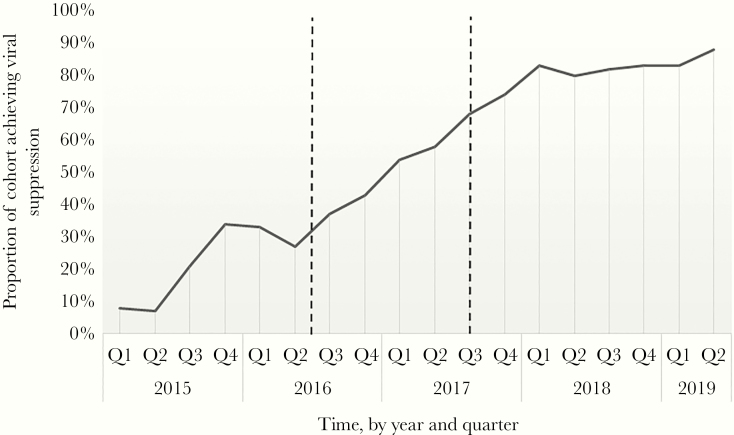
The proportion of patients achieving viral suppression (HIV viral load <200 copies/mL) in each 3-month period (quarters) is shown in Figure 2. The mean proportion of the cohort achieving viral suppression in each year is as follows: 2015, 18%; 2016, 35%; 2017, 64%; 2018, 82%; 2019, 86%. This represents a statistically significant increase each year (χ 2 test, P < .05), except for the last year, for which data are incomplete.
Figure 2.
Line graph representing the proportion of patients in the human immunodeficiency virus (HIV) outbreak cohort achieving viral suppression (HIV viral load <200 copies/mL) in each quarter of each year. The dotted lines mark the dates of the clinical interventions.
Antiretroviral Therapy via Community Pharmacies
Sixty-one percent (91 of 149) of patients who commenced ART in the cohort have ever received ART via community-based pharmacy care since June 2016. In June 2018, this proportion was 59% (77 of 130), and in June 2017 it was 29% (25 of 84); therefore, the proportion of the cohort using this method is increasing over time. Reasons for cessation of ART delivery via this method included death, incarceration, patient choice to receive via other methods, or intermittently stop ART. In addition, transient interruption may occur due to unexpected changes in location of accommodation and therefore local community pharmacy.
Phylogenetic Analysis
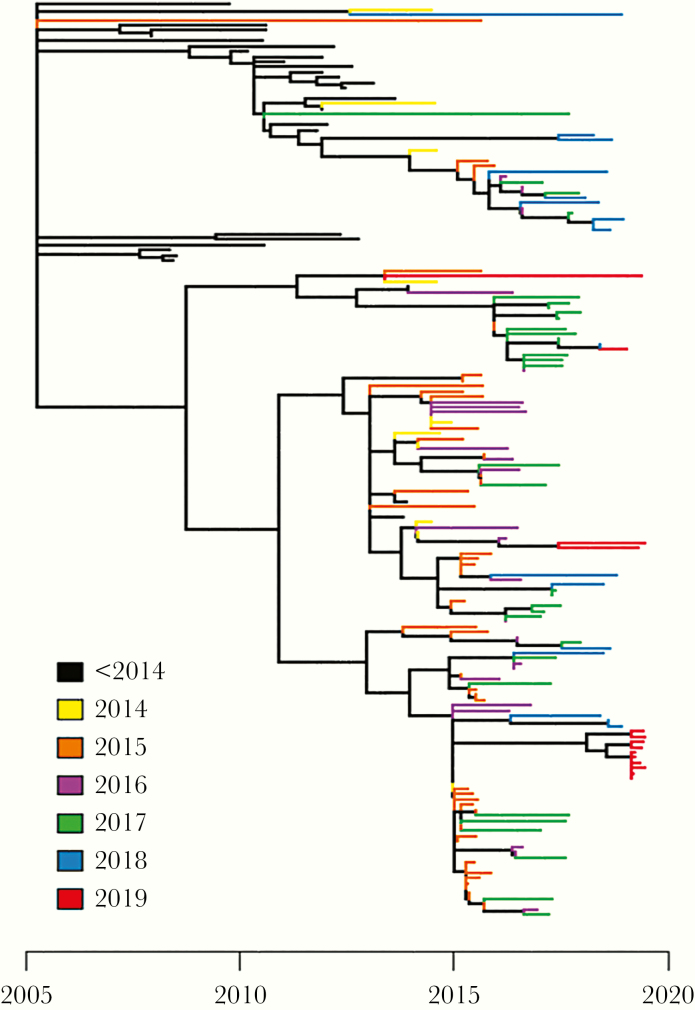
We found 175 individuals whose HIV sequences linked at 2% genetic distance in HIV-TRACE, including 37 individuals diagnosed before June 1, 2014. Inclusion of older sequences improves the resolution of time-measured phylogenies, and so we chose to include all genetically linked sequences in our phylogenetic analysis. We observed new diagnoses in all 3 subclusters observed in our previous analysis [11]. Despite the high number of diagnoses in 2019 (n = 14), the majority of these sequences (n = 10) were part of a single large cluster, likely representing a single social network.
DISCUSSION
Despite comprehensive and free to access IEP and specialist drug treatment services, HIV spread rapidly among PWID in Glasgow from 2014 onwards with prevalence now exceeding 10% within this vulnerable city center population [7]. We compared the outbreak cohort with PWID from Glasgow who report to be HIV negative to identify predictors for HIV acquisition. This comparator group was current, local, and had data available for comparison. Using a case-control analysis, we have shown that differences in drug injecting behavior (such as sharing injecting equipment), higher levels of homelessness, and HCV infection are associated with HIV positivity (Table 1). To address the outbreak, parallel interventions including education of healthcare workers and third-sector organizations and awareness raising among the target population were introduced early. Improvements to IEP, including provision of lower dead space syringes and expanded access in the evenings and at the weekend, were added to the existing harm-reduction armamentarium. Planned developments within the drug treatment services were also implemented, as recommended by a health needs assessment [20]. Despite a focus on harm reduction and comprehensive treatment from drug recovery services (OAT programs), injecting and sharing of drug injecting equipment persisted. This is partly related to the rapid rise in cocaine use and the limited interventions to manage this. Given its short half-life there is an increase injecting frequency and, in turn, blood-borne virus transmission risk through reduced likelihood of using clean equipment at each injection [21, 22]. Drug treatment services also reviewed optimizing OAT dosing, and a heroin-assisted treatment program opened in Glasgow in October 2019. Furthermore, risk-taking behavior can be influenced by structural factors including environmental conditions such as homelessness [23, 24].
A large number of the cohort reported sexual risks for HIV acquisition, and, in Scotland, HIV pre-exposure prophylaxis (PrEP) is provided free of charge from sexual health services for those at risk of HIV via sexual risk [25]. The HIV-negative sexual partners of PWID are generally from similarly multi-disadvantaged backgrounds and face barriers to accessing HIV prevention services [26]. The PrEP delivery among PWID has required modification of this service, and early evaluation suggests acceptability among the small group engaged. In Glasgow, at time of writing, of 20 “at risk” PWID offered HIV PrEP, 75% had commenced it (R. Metcalfe, FRCP, 25 September 2019, written personal communication).
A centralized, hospital-based service was developed many years ago to support HIV care in a holistic way through a colocated, multidisciplinary team. This group was already disadvantaged, and the need to travel without financial means and set appointment times that offered little flexibility proved to be significant barriers to engagement. To address this, a specific HIV clinic was set up within the homeless health services building, but it was poorly utilized in this location reportedly due to HIV-related stigma. Responding to this feedback, the HIV clinic was changed to a general BBV clinic, which was more acceptable. This clinic now has the ability to manage and treat all BBVs, provide comprehensive sexual and reproductive healthcare, and support outpatient management of physical health conditions including serious soft tissue infections.
In modeling studies, HIV treatment as prevention (TasP) has been shown to be economically beneficial [27]. In Scotland, as per national guidelines, ART is started as soon as possible, for individual health benefit and TasP, and is free of charge. However, it is recognized that concerns about drug interactions (including with nonprescribed “street drugs”), perceived or actual poor engagement in care, poor adherence, and the potential development of antiretroviral resistance historically has led to reluctance to prescribe ART to those with ongoing drug use [9]. The vast majority of this population were infected with a primary resistant subtype C HIV virus, and there was concern about progressive resistance given the likelihood of poor adherence.
Before July 2016, ART could only be obtained via the hospital pharmacy or via a home delivery service. We developed a model in which ART could be dispensed daily in any Glasgow community pharmacy with or without OAT, supervised if required. Avoidance of drug interactions was a key concern given high levels of street drug use and other comedications, due to high levels of physical and mental comorbidities. Therefore, protease inhibitors were generally avoided, in addition to NNRTIs, given the primary resistance mutations identified. The combination of a favorable drug interaction profile, high genetic barrier to resistance, and an accelerated reduction in HIV viral load makes the integrase inhibitor class a first-line choice for this cohort. In 2015, the UK treatment guidelines had twice-daily raltegravir as the integrase of choice, and dolutegravir was approved for use in Scotland (in 2014). With the benefits of once-daily dosing, the majority of patients have been prescribed a dolutegravir-based regimen, alongside dual NRTIs.
There are high rates of intermittently poor adherence to ART despite GECHO and focused counseling around adherence. This cohort move accommodation and community pharmacy location frequently. Interruptions in OAT can interfere with daily pharmacy visits. The ART-dispensing model has the flexibility to move between community pharmacies as well as hospital pharmacies as their needs change.
This requires significant input from hospital HIV pharmacy services who oversee the individual prescription and ensure ART continuation in those circumstances. A temporary prescription can be delivered to the patient by the outreach nurse until a stable prescribing situation can be regained. Support from third-sector organizations can help in locating the patients rapidly when they fall out of care, and these links with a multitude of healthcare providers are essential. The rate at which ART is commenced has improved due a multiplicity of factors, but the team aims to start patients on ART now on the same day as a new diagnosis result is returned (Figure 1). No HIV-related deaths have occurred, but there are high rates of unscheduled hospital care due to significant comorbidities, and mortality within the cohort is high [28].
It is challenging to justify the financial input required to achieve treatment goals where a service already exists. However, the data collected and analyzed in Figures 1 and 2 have shown that GECHO, as a response to this health inequality, can achieve results comparable with other HIV-positive cohorts. These metrics can also be used as an indicator for reduction in estimated community viral load and used to evaluate outbreak interventions such as TasP. The success of GECHO is further supported by phylogenetic analysis, highlighting that the majority of infections in 2019 (10 of 14) were concentrated within a single cluster, in contrast to widespread transmission between 2014 and 2018 (Figure 3). Transmission has been interrupted in all but a single network. Phylogenetic analysis in real-time has, alongside partner notification, informed the public health response, allowing targeting of resources to key groups.
Figure 3.
Time-resolved human immunodeficiency virus phylogeny for the Glasgow outbreak (n = 175). Tips are colored by year of diagnosis.
However, the development of the GECHO model of clinical care has been a significant challenge, especially in the current UK environment of austerity. In this group, it was clear that engagement in care required relationship building, between healthcare worker and patient, but also between existing services that do not traditionally work closely together, eg, hospital clinicians and nongovernmental organizations. There are no authoritative guidelines or local health economic analyses to inform a healthcare team and their sponsors on the required support and funding to clinically manage an HIV outbreak among PWID. Upfront investment of significant HIV specialist clinical time for a relatively small number of patients from a group with poor self-esteem needs continued advocacy. The recent outbreak in Athens [29] demonstrated the urgency with which a care model should be implemented to stem onward transmission. We have demonstrated that by adapting traditional models of care, successful clinical outcomes and public health intervention, including TasP, are achievable with relatively little investment in staff costs. However, the timelines suggest that a more urgent response may have improved outcomes at an earlier date.
This outbreak occurred in a setting that provided a high standard of drug recovery service and high level of IEP for PWIDs, but it lacked in comprehensive BBV testing and monitoring of HIV incidence rates. The experience in Glasgow should highlight to public health teams with a similar demographic to be vigilant to an HIV outbreak despite good harm reduction services. Rigorous testing programs are required.
Limitations
This study is an observational retrospective cohort study, and there are limitations to the data collection, which was reliant on accurate recording by healthcare workers and accurate self-reporting from patients and therefore potentially subject to bias. The HIV viral load measurements were not always available every 3 months due to patient disengagement. For those with chronic infection, we are unable to determine when acquisition occurred, and so the risk factors described may not have been present at the time of HIV infection. Many variables may have impacted on virological suppression and engagement in care that cannot be easily measured. The use of community viral load was chosen as the most reliable estimate of viral load for the cohort, including those not in care, but it has limitations in that it does not account for variability of HIV viral load between measurements and does not take into account the viral load measurements of those who are undiagnosed [12].
Because the cohort can move between community-dispensed ART and hospital dispensed, we are unable to compare viral suppression levels in the 2 groups. Using the NESI cohort for the case-control analysis may not be considered the best comparison group because the participants’ inclusion in NESI may indicate that they are more likely to be using needle exchange than the members of the outbreak cohort. However, members of the outbreak were also using needle exchange facilities, and this convenience sample was the best group available for comparison.
CONCLUSIONS
Responding to HIV outbreaks in a PWID population requires an urgent clinical response team in addition to wider public health interventions. Advocacy for rapid investment in a GECHO model of care would be easier with a more detailed health economic analysis and supporting guideline or policy document from a recognized body. The adaptation of clinical services is vital to improve health outcomes and reduce onward transmission when managing an HIV epidemic in this highly complex and multiply disadvantaged group. We believe that this model can be replicated in other locations, but to deliver good clinical and virological HIV outcomes, appropriate and rapid investment, especially in staff costs, is required.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge the advice and support from Professor Sharon Hutchinson (Glasgow Caledonian University), Dr. Emma Thomson, (University of Glasgow), and Revathy Raajaravi (NHS Greater Glasgow and Clyde).
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Des Jarlais DC, Sypsa V, Feelemyer J, et al. HIV outbreaks among people who inject drugs in Europe, North America, and Israel. Lancet HIV 2020; 7:e434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robertson JR, Bucknall AB, Welsby PD, et al. Epidemic of AIDS related virus (HTLVIII/LAV) infection among intravenous drug abusers. BMJ 1986; 292:527–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor A, Goldberg D, Emslie J, et al. Outbreak of HIV infection in a Scottish prison. BMJ 1995; 310:289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Services Scotland. Surveillance report. HIV infection in Scotland: Quarterly report to 31 December 2017 Available at: https://hpspubsrepo.blob.core.windows.net/hps-website/nss/2425/documents/1_hiv-infection-quarterly-dec-2017.pdf Accessed 28 August 2018.
- 5. Information Services Division, Scotland. Injecting Equipment Provision in Scotland 2016/17 7 August 2018. Available at: https://www.isdscotland.org/Health-Topics/Drugs-and-Alcohol-Misuse/Publications/2018-08-07/2018-08-07-IEP-Tables.xlsx. Accessed 2 September 2019.
- 6. Scottish Drug Misuse Database Annual Report. Available at: https://www.isdscotland.org/Health-Topics/Drugs-and-Alcohol-Misuse/Publications/2018-06-26/2018-06-26-SDMD-Summary.pdf Accessed 19 July 2019.
- 7. McAuley A, Palmateer N, Goldberg DJ, et al. Re -emergence of HIV related to injecting drug use despite a comprehensive harm reduction environment: a cross-sectional analysis. Lancet HIV 2019; 6:315–24. [DOI] [PubMed] [Google Scholar]
- 8. Trayner KMA, McAuley A, Palmateer NE, et al. Increased risk of HIV and other drug-related harms associated with injecting in public places: national bio-behavioural survey of people who inject drugs. Int J Drug Policy 2020; 77:102663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. Int J Drug Policy 2007; 18:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Health Protection Scotland. Needle Exchange Surveillance Initiative (NESI) 2008–09 to 2017–18 April 2, 2019. Available at: https://www.hps.scot.nhs.uk/web-resources-container/needle-exchange-surveillance-initiative-nesi-2008-09-to-2017-18/. Accessed 28 August 2019.
- 11. Ragonnet-Cronin M, Jackson C, Bradley-Stewart A, et al. Recent and rapid transmission of HIV among people who inject drugs in scotland revealed through phylogenetic analysis. J Infect Dis 2018; 217:1875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. Guidance on community viral load: a family of measures, definitions, and method for calculation. Available at: https://stacks.cdc.gov/view/cdc/28147. Accessed 3 September 2019. [Google Scholar]
- 13. National Records of Scotland. Information Services. Drug related deaths in Scotland in 2018 July 16, 2019. Available at: https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/deaths/drug-related-deaths-in-scotland/2018. Accessed 17 September 2019.
- 14. Shepherd SJ, McAllister G, Kean J, et al. Development of an avidity assay for detection of recent HIV infections. J Virol Methods 2015; 217:42–9. [DOI] [PubMed] [Google Scholar]
- 15. Bennett S, Gunson RN, McAllister GE, et al. Detection of hepatitis C virus RNA in dried blood spots. J Clin Virol 2012; 54:106–9. [DOI] [PubMed] [Google Scholar]
- 16. Kosakovsky Pond SL, Weaver S, Leigh Brown AJ, Wertheim JO. HIV-TRACE (TRAnsmission Cluster Engine): a tool for large scale molecular epidemiology of HIV-1 and other rapidly evolving pathogens. Mol Biol Evol 2018; 35:1812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wertheim JO, Kosakovsky Pond SL, Forgione LA, et al. Social and genetic networks of HIV-1 transmission in New York City. PLoS Pathog 2017; 13:e1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006; 22:2688–90. [DOI] [PubMed] [Google Scholar]
- 19. Volz EM, Frost SDW. Scalable relaxed clock phylogenetic dating. Virus Evol 2017; 3(2):vex025. doi: 10.1093/ve/vex025 [DOI] [Google Scholar]
- 20. Tweed EJ, Rodgers M, Priyadarshi S, Crighton E. “Taking away the chaos”: a health needs assessment for people who inject drugs in public places in Glasgow, Scotland. BMC Public Health 2018; 18:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hudgins R, McCusker J, Stoddard A. Cocaine use and risky injection and sexual behaviors. Drug Alcohol Depend 1995; 37:7–14. [DOI] [PubMed] [Google Scholar]
- 22. Joe GW, Simpson DD. HIV risks, gender, and cocaine use among opiate users. Drug Alcohol Depend 1995; 37:23–8. [DOI] [PubMed] [Google Scholar]
- 23. Strathdee SA, Hallett TB, Bobrova N, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet 2010; 376:268–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med 2005; 61:1026–44. [DOI] [PubMed] [Google Scholar]
- 25. Health Protection Scotland. Implementation of HIV PrEP in Scotland: First Year Summary and Key points February 2019. Available at: https://hpspubsrepo.blob.core.windows.net/hps-website/nss/2716/documents/1_implementation-of-hiv-prep-in-scotland-first-year-report-summary-and-key-points.pdf. Accessed 17 September 2019.
- 26. UNAIDS. Prevention Gap Report 2016. Available at: https://www.unaids.org/sites/default/files/media_asset/2016- prevention-gap-report_en.pdf. Accessed 23 September 2019.
- 27. Bernard CL, Owens DK, Goldhaber-Fiebert JD, Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med 2017; 14:e1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Metcalfe R, McAuley A, Priyadarshi S, et al. High mortality rate amongst HIV infected people who inject drugs (PWID) in Scotland. [CROI Abstract 894]. In Special Issue: Abstracts From the 2020 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med 2020; 28:483. [Google Scholar]
- 29. Paraskevis D, Nikolopoulos G, Fotiou A, et al. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS One 2013; 8:e78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.