Abstract
Introduction
Currently available second-line (2L) therapies for advanced/metastatic esophageal squamous cell carcinoma (adv/met ESCC) include the taxanes paclitaxel and docetaxel. In clinical trials, such therapies have provided only modest improvements in survival. Few studies have assessed outcomes in routine clinical practice in the USA. We compared real-world clinical outcomes in the US for patients receiving taxane or non-taxane 2L therapy for adv/met ESCC.
Methods
The Flatiron Health database was used to identify patients diagnosed with adv/met ESCC (1 January 2011–31 January 2019) who received 2L therapy; index date was date of adv/met diagnosis. Baseline variables and treatment regimens received were identified. Overall survival (OS; 2L start until death or last recorded medical activity) and duration of therapy (DoT; start of 2L therapy until last administration date of 2L therapy) in patients receiving taxane vs. non-taxane-based therapies in the 2L setting were estimated by Kaplan-Meier method.
Results
There were no clear differences in baseline characteristics between patients who received 2L taxane therapy (n = 37) and 2L non-taxane therapy (n = 49). Median (95% CI) 2L OS was significantly longer with 2L taxanes (7.3 [5.9–11.5] months) vs. 2L non-taxanes (5.1 [2.9–7.6] months); median (95% CI) 2L DoT was 2.1 (1.8–3.0) months vs. 3.3 (2.6–6.7) months, respectively.
Conclusion
Survival was generally poor in patients receiving 2L therapy for adv/met ESCC and was longer in patients receiving 2L taxanes than 2L non-taxane therapy. Efficacious, tolerable therapies for ESCC in the 2L setting are urgently needed.
Electronic Supplementary Material
The online version of this article (10.1007/s12325-020-01394-y) contains supplementary material, which is available to authorized users.
Keywords: Electronic health records, Esophageal squamous cell carcinoma, Locally advanced, Metastatic, Oncology, Recurrent
Key Summary Points
| Why carry out this study? |
| The prognosis of patients with unresectable, locally advanced or metastatic esophageal squamous cell carcinoma (adv/met ESCC) remains poor in the USA. |
| Few studies have assessed the outcomes of patients receiving second-line (2L) therapy for adv/met ESCC. |
| What was learned from the study? |
| In this real-world analysis of electronic health records, few patients diagnosed with adv/met ESCC subsequently receive 2L (23.0%) therapy. |
| Treatments received by patients do not appear to adhere closely to clinical guidelines: |
| Relatively few patients received 1L fluoropyrimidines plus platinum therapy (30.2%), and taxanes were the most frequently received 1L regimen (54.7%). |
| Less than half of patients (43.0%) received 2L taxane therapy. |
| Median overall survival in patients receiving 2L therapy for ESCC was generally poor and was longer in patients receiving 2L taxane therapy (7.3 months) compared with 2L non-taxane therapies (5.1 months). |
| The low proportion of patients receiving 2L therapy, and the poor survival outcomes and short DoT seen in patients who do receive 2L therapy, highlights an urgent unmet need for efficacious, tolerable therapies for ESCC in the 2L setting. |
Introduction
Worldwide, esophageal cancer is the ninth most common cancer and is the sixth most frequent cause of cancer-related death [1]. In the USA, esophageal cancers were predicted to account for 17,650 new cases and 16,080 deaths in 2019 [2]. Esophageal cancers are often diagnosed at a relatively late stage and are consequently associated with high mortality; over a third of patients are diagnosed at the metastatic stage [3], and 5-year survival rates are approximately 5% [2]. Although esophageal adenocarcinoma (EAC) is the most common esophageal cancer subtype in the US, esophageal squamous cell carcinoma (ESCC) accounts for approximately 40% of US esophageal cancer cases [3] and is the most common esophageal cancer subtype worldwide [1]. Recent genomic analyses have shown that ESCC is molecularly distinct from EAC [4]. In the US, ESCC is more prevalent among non-Hispanic blacks and Asians than whites and Hispanic whites [3]. The prevalence of ESCC is higher in men than in women [5].
For locally advanced, unresectable, recurrent or metastatic (adv/met) ESCC, NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) recommend two-drug cytotoxic combination treatment as first-line (1L) therapy. The preferred regimens are fluoropyrimidine-based (fluorouracil or capecitabine) and platinum-based (cisplatin or oxaliplatin) combinations [6]. For second-line (2L) and subsequent lines of therapy, docetaxel, paclitaxel and irinotecan (with or without fluorouracil) are the recommended options (category 1 recommendation) [6]. Despite the widespread availability of these agents and common usage in the 2L setting, they provide only modest improvements in survival, with overall survival (OS) ranging from 5.2 to 9.5 months in several phase II and phase III clinical trials [7–10], and taxanes are associated with substantial toxicities [11, 12]. Furthermore, much of the evidence base for 2L treatment guidelines focuses on gastric cancers and EAC and does not specifically consider ESCC. The NCCN Guidelines® have added the programmed death 1 (PD‐1) inhibitor pembrolizumab as a recommended 2L therapy option (category 1) for patients with ESCC tumors that express the programmed death‐ligand 1 (PD-L1) by combined positive score of ≥ 10 [6].
Another PD-1 inhibitor, nivolumab, has demonstrated promising efficacy as therapy for patients with ESCC refractory or intolerant to standard chemotherapy in the international, open-label, phase III ATTRACTION-3 trial comparing nivolumab with taxane (paclitaxel or docetaxel) therapy [13]. Given the relative paucity of studies reporting real-world data from patients with ESCC, this is an opportune time to characterize 2L treatment patterns and clinical outcomes in patients with ESCC preceding the approval of immuno-oncology agents (beginning with the approval of pembrolizumab in September 2019). As no clear 2L standard of care exists, we assessed patients receiving 2L paclitaxel or docetaxel as generally representative of standard 2L therapy, in line with NCCN Guidelines and the comparator group in ATTRACTION-3. This study was conducted to evaluate the baseline characteristics, treatment patterns and clinical outcomes in an ethnically diverse population of patients with adv/met ESCC receiving 2L taxanes versus non-taxane-based therapy in US real-world clinical practice.
Methods
Study Design
This retrospective, observational study used Flatiron Health’s longitudinal, demographically and geographically diverse database, which contains electronic health record data from > 265 cancer clinics including > 2 million active US cancer patients [14]. The Flatiron database includes all patients with a recorded diagnosis of advanced or metastatic disease, as indicated by de-identified patient-level unstructured data collected via technology-enabled chart abstraction from physician notes and other documents, in addition to structured data. Structured data include demographics, diagnosis codes (International Classification of Diseases, 9th Revision [ICD-9]/International Classification of Diseases, 10th Revision [ICD-10]), laboratory visits, medications and Eastern Cooperative Oncology Group (ECOG) performance status (PS). Unstructured data include date of initial diagnosis, stage at initial diagnosis, primary tumor characteristics, biomarker testing and, importantly, limited information on surgeries.
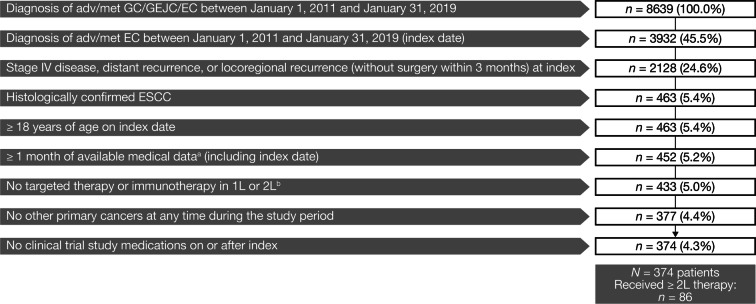
Patients diagnosed with adv/met ESCC in routine clinical practice from 1 January 2011 through 31 January 2019 were included; index date was the date of adv/met ESCC diagnosis. Patients were eligible if they were ≥ 18 years of age, had a histologically confirmed diagnosis of adv/met ESCC and had ≥ 1 month of available medical data after index. Eligibility criteria and patient attrition are presented in Fig. 1. Patients were categorized by mutually exclusive treatment subgroups, based on whether they received taxane-based or non-taxane-based therapy in the 2L setting. Patients were followed until death, discontinuation from database or the end of the study period, whichever came earlier. This article reports on a retrospective, observational study using de-identified anonymized patient-level data. Therefore, informed consent was not obtained, and no institutional review board approval was required.
Fig. 1.
Patient identification and attrition. aMedical data were defined as clinical data in the patient record from outpatient physician office visits, non-facility visits, laboratory visits, treatment/procedure visits or medication administration. Patients who died within 1 month were not excluded. bThe following therapies were excluded: atezolizumab, avelumab, durvalumab, ipilimumab, nivolumab, pembrolizumab and trastuzumab. Adv/met unresectable locally advanced, recurrent or metastatic, EC esophageal cancer, ESCC esophageal squamous cell carcinoma, GC gastric cancer, GEJC gastroesophageal junction cancer
Outcomes
Baseline variables included age, sex, race, disease stage at initial diagnosis (including those before the index diagnosis of adv/met disease) and ECOG PS at the start of 2L therapy. Individual 1L and 2L treatment regimens received were identified and summarized. Clinical outcomes were assessed for all patients who received 2L therapy and by treatment subgroup: taxane-based or non-taxane-based therapy. OS was defined as the time from start of 2L therapy until death or date of last recorded medical activity. Duration of therapy (DoT) was defined as time from start of 2L therapy until last administration date of 2L therapy.
Statistical Analyses
Baseline demographics, patient characteristics, and 1L and 2L treatment patterns were reported descriptively: frequencies and proportions were reported for categorical data, and means (standard deviations) and medians (ranges) were provided for continuous data. Median OS and DoT were estimated by Kaplan-Meier method and presented with 95% CIs. A multivariable Cox proportional hazard model was used to assess potential factors that were significantly associated with OS from start of 2L treatment.
Owing to the absence of data on radiation therapy in the Flatiron database, a two-step sensitivity analysis of survival outcomes was conducted. First, patients coded as having had “locoregional recurrence and no surgery at time of locoregional recurrence” with a record of prior esophagectomy were excluded. Second, patients who received weekly carboplatin plus paclitaxel (i.e., all doses received within 6–8 days in each cycle) were assumed to have received concurrent radiotherapy and were excluded. Kaplan-Meier survival analysis and multivariable Cox proportional hazard model analysis were then used to assess survival outcomes in the 2L taxane and 2L non-taxane subgroups after exclusion of patients meeting these criteria.
Results
After applying inclusion and exclusion criteria, 374 patients with adv/met ESCC were identified. Of these, 263 (70.3%) received ≥ 1L therapy, 86 (23.0%) received ≥ 2L therapy, and 29 (7.8%) received ≥ 3L therapy. Of the 263 patients who received 1L therapy, 123 (46.8%) died before receiving 2L therapy, and a further 54 (20.5%) were censored before 2L therapy.
Baseline Demographics and Characteristics
Patients who received ≥ 2L therapy (n = 86) were mostly male (70.9%), white (60.5%) and had a median (range) age of 64 (36–83) years (Table 1). The majority of patients who received ≥ 2L therapy had stage IV disease at their initial diagnosis (72.1%); among patients with available ECOG PS data at the start of 2L therapy (n = 53), 75.5% had ECOG PS of 0–1, and 24.5% had ECOG PS of ≥ 2. There were no clear differences in baseline demographics or disease characteristics between patients who received 2L taxane therapy (n = 37) and 2L non-taxane therapy (n = 49). Median (range) potential length of follow-up from 2L start date until data cutoff in patients who received at least two lines of therapy was 39.4 (24.5–91.0) months.
Table 1.
Baseline demographics and disease characteristics of patients receiving at least second-line therapy for adv/met ESCC, stratified by 2L taxane groupings
| All ≥ 2L patients (n = 86) | 2L taxane therapy (n = 37) | 2L non-taxane therapy (n = 49) | |
|---|---|---|---|
| Median (range) age at index, years | 64 (36–83) | 63 (36–81) | 65 (37–83) |
| Age group at index, n (%) | |||
| < 65 years | 45 (52.3) | 21 (56.8) | 24 (49.0) |
| ≥ 65 years | 41 (47.7) | 16 (43.2) | 25 (51.0) |
| Male, n (%) | 61 (70.9) | 29 (78.4) | 32 (65.3) |
| Race, n (%) | |||
| White | 52 (60.5) | 22 (59.5) | 30 (61.2) |
| Black or African American | 14 (16.3) | 7 (18.9) | 7 (14.3) |
| Asian | 6 (7.0) | 2 (5.4) | 4 (8.2) |
| Other race | 9 (10.5) | 4 (10.8) | 5 (10.2) |
| Missing | 5 (5.8) | 2 (5.4) | 3 (6.1) |
| Geographic region, n (%) | |||
| South | 37 (43.0) | 18 (48.6) | 19 (38.8) |
| Northeast | 22 (25.6) | 7 (18.9) | 15 (30.6) |
| West | 12 (14.0) | 5 (13.5) | 7 (14.3) |
| Midwest | 10 (11.6) | 3 (8.1) | 7 (14.3) |
| Unknown | 3 (3.5) | 2 (5.4) | 1 (2.0) |
| Other regions | 2 (2.3) | 2 (5.4) | 0 |
| Benefit plan type at indexa, n (%) | |||
| Commercial health plan | 25 (29.1) | 11 (29.7) | 14 (28.6) |
| Other payer (unknown) | 21 (24.4) | 11 (29.7) | 10 (20.4) |
| Medicare | 19 (22.1) | 7 (18.9) | 12 (24.5) |
| Medicaid | 6 (7.0) | 4 (10.8) | 2 (4.1) |
| Other government | 3 (3.5) | 1 (2.7) | 2 (4.1) |
| Patient assistance | 1 (1.2) | 0 | 1 (2.0) |
| Self-pay | 1 (1.2) | 1 (2.7) | 0 |
| Missing | 27 (31.4) | 8 (21.6) | 19 (38.8) |
| Practice type, n (%) | |||
| Community | 83 (96.5) | 35 (94.6) | 48 (98.0) |
| Academic | 3 (3.5) | 2 (5.4) | 1 (2.0) |
| Disease stage at initial diagnosis, n (%) | |||
| Stage I | 5 (5.8) | 2 (5.4) | 3 (6.1) |
| Stage II | 7 (8.1) | 5 (13.5) | 2 (4.1) |
| Stage III | 9 (10.5) | 3 (8.1) | 6 (12.2) |
| Stage IV | 62 (72.1) | 25 (67.6) | 37 (75.5) |
| Unknown | 3 (3.5) | 2 (5.4) | 1 (2.0) |
| ECOG at start of 2Lb, n (%) | |||
| Data available | 53 (61.6) | 21 (57) | 32 (65.3) |
| 0 | 21 (39.6) | 6 (28.6) | 15 (46.9) |
| 1 | 19 (35.8) | 10 (47.6) | 9 (28.1) |
| 2 | 10 (18.9) | 5 (23.8) | 5 (15.6) |
| 3 | 3 (5.7) | 0 | 3 (9.4) |
| Data not available | 33 (38.4) | 16 (43) | 17 (34.7) |
Adv/met unresectable locally advanced, recurrent or metastatic, EC esophageal cancer, ECOG PS Eastern Cooperative Oncology Group performance score, SD standard deviation
aPatients could be enrolled in more than one insurance provider type
bClosest ECOG value on or before start of second-line treatment
Treatment Patterns
In the 2L setting, 43.0% received taxanes, 29.1% received fluoropyrimidine in combination with platinum therapies, and 16.3% received fluoropyrimidine monotherapy (Table 2). In patients receiving taxane regimens (n = 37), the most frequently used regimen was carboplatin plus paclitaxel (56.8% of 2L taxane-based regimens). Among patients who received non-taxane therapies (n = 49), the most frequently used regimen was FOLFOX (42.9% of 2L non-taxane-based regimens) followed by capecitabine monotherapy (12.2%). Among the 21 patients who received 2L FOLFOX, 17 patients had received carboplatin and paclitaxel in the 1L setting. Among the 37 patients who received 2L taxane regimens, 12 patients had received FOLFOX in the 1L setting (9 patients had received carboplatin plus paclitaxel, and 9 patients had received fluorouracil plus cisplatin).
Table 2.
Second-line treatment patterns in patients who received at least second-line therapy of adv/met ESCC
| All ≥ 2L patients (n = 86) | 2L taxane therapy (n = 37) | 2L non-taxane therapy (n = 49) | |
|---|---|---|---|
| Taxanes | 37 (43.0) | 37 (100.0) | 0 |
| Fluoropyrimidine–platinum | 25 (29.1) | 0 | 25 (51.0) |
| Fluoropyrimidine | 14 (16.3) | 0 | 14 (28.6) |
| ECF/DCF | 2 (2.3) | 0 | 2 (4.1) |
| Other | 8 (9.3) | 0 | 8 (16.3) |
Adv/met unresectable locally advanced, recurrent or metastatic, DCF docetaxel, cisplatin and fluorouracil, ECF epirubicin, cisplatin and fluorouracil
Clinical Outcomes
In all patients who received 2L therapy (n = 86), median (95% CI) OS from start of 2L was 6.7 (5.1–8.3) months, and the 12-month survival rate was 28.4% (Table 3; Fig. 2a). Median (95% CI) OS was 7.3 (5.9–11.5) months in patients who received 2L taxane-based therapy (n = 37) and 5.1 (2.9–7.6) months in patients who received 2L non-taxane-based therapy (n = 49); 12-month survival rates were similar (29.3% vs. 28.0%, respectively) (Table 3; Fig. 2b). Based on multivariable analysis, factors associated with significantly longer OS from start of 2L treatment included 2L taxane therapy (vs. 2L non-taxane therapy), female patients (vs. male patients), patients with stage I–III disease at initial diagnosis (vs. disease stage unknown) and patients who had ECOG PS 0–1 at start of 2L therapy (vs. ECOG PS 2–4) (Table 4). In all patients receiving ≥ 2L therapy, median (95% CI) 2L DoT was 2.6 (2.1–3.7) months (Table 3) and was numerically shorter in patients who received 2L taxane therapy than in patients who received 2L non-taxane therapy (2.1 [1.8–3.0] months vs. 3.3 [2.6–6.7] months).
Table 3.
Clinical outcomes in patients receiving at least second-line therapy for adv/met ESCC
| All ≥ 2L patients (n = 86) | 2L taxane therapy (n = 37) | 2L non-taxane therapy (n = 49) | |
|---|---|---|---|
| Median (range) follow-up from start of 2L, months | 5.1 (0.03–74.0) | 6.0 (0.03–74.0) | 4.4 (0.1–47.4) |
| Deaths, n (%) | 63 (73.3) | 26 (70.3) | 37 (75.5) |
| Median (95% CI) OS from start of 2L, months | 6.7 (5.1–8.3) | 7.3 (5.9–11.5) | 5.1 (2.9–7.6) |
| 12-month (SE) survival | 28.4 (5.5) | 29.3 (8.6) | 28.0 (7.2) |
| 24-month (SE) survival | 19.5 (5.0) | 18.3 (7.3) | 21.0 (6.9) |
| Median (95% CI) DoT, months | 2.6 (2.1–3.7) | 2.1 (1.8–3.0) | 3.3 (2.6–6.7) |
Adv/met unresectable locally advanced, recurrent or metastatic, CI confidence interval, DoT duration of therapy, ESCC esophageal squamous cell carcinoma, SE standard error
Fig. 2.
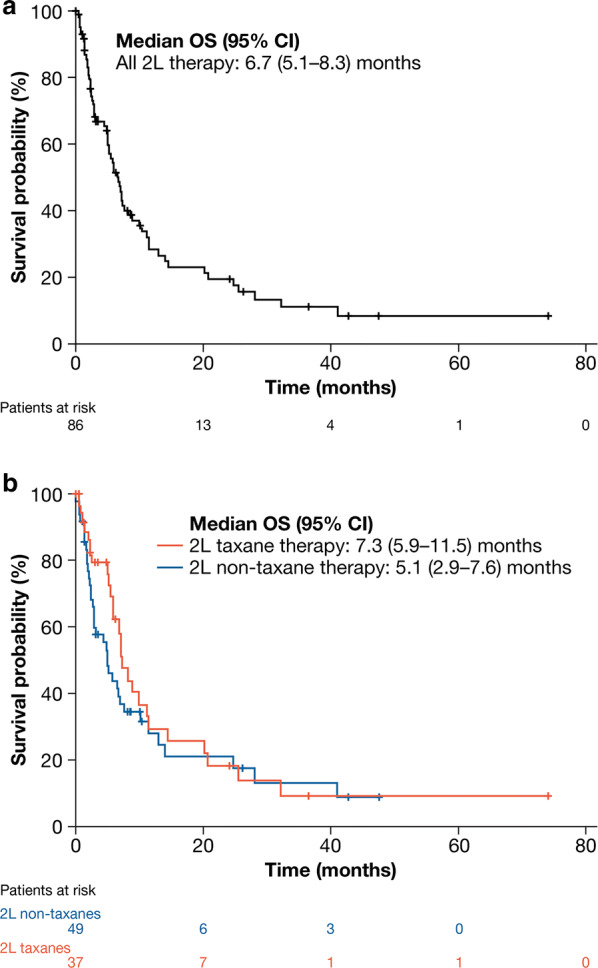
Kaplan-Meier analysis of OS from start of 2L therapy in: a all patients (n = 86); b patients who received taxane-based (n = 37) and non-taxane-based (n = 49) 2L therapy. CI confidence interval, OS overall survival
Table 4.
Cox regression hazard ratios for survival from start of 2L therapy
| Comparator | Reference | HR (95% CI) | p value |
|---|---|---|---|
| Treatment | |||
| 2L non-taxane therapy | 2L taxane therapy | 2.46 (1.29–4.66) | <0.01 |
| Age | |||
| ≥ 65 years | < 65 years | 0.56 (0.30–1.05) | 0.07 |
| Sex | |||
| Female | Male | 0.41 (0.20–0.83) | 0.01 |
| Race | |||
| Black or African American | White | 0.79 (0.35–1.77) | 0.56 |
| Asian | 0.57 (0.14–2.34) | 0.43 | |
| Other | 0.63 (0.24–1.66) | 0.35 | |
| Missing | 1.38 (0.39–4.95) | 0.62 | |
| US geographic region | |||
| South | Midwest | 1.92 (0.68–5.42) | 0.22 |
| Northeast | 0.94 (0.31–2.81) | 0.91 | |
| West | 1.85 (0.50–6.86) | 0.36 | |
| Unknown | 1.45 (0.32–6.48) | 0.63 | |
| Disease stage at initial diagnosis | |||
| Stage IV | Stage I–III | 1.46 (0.72–2.94) | 0.30 |
| ECOG status at 2L start | |||
| ECOG PS 2–4 | ECOG PS 0–1 | 2.85 (1.16–6.97) | 0.02 |
| Missing | 1.01 (0.53–1.92) | 0.99 | |
CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance score, HR hazard ratio
In a sensitivity analysis aimed at identifying patients who may have potentially received 1L carboplatin and paclitaxel as part of a chemoradiotherapy regimen, 16 potential chemoradiotherapy patients (2L taxane group, n = 5; 2L non-taxane group, n = 11) were identified. Exclusion of these patients on the grounds that they may have been receiving chemoradiotherapy made little difference to the survival outcomes (Supplementary Tables 1, 2).
Discussion
This analysis used a real-world data source to identify a cohort of US patients receiving ≥ 2L therapy for adv/met ESCC in routine clinical practice. Among patients diagnosed with adv/met ESSC, 70% of patients received ≥ 1L therapy, and only 23% received ≥ 2L therapy. The few patients who did not receive any therapy after adv/met diagnosis may have received palliative care, elected not to receive 1L therapy or received treatment in clinics not captured in the Flatiron database. However, relatively few patients progressed from 1L therapy to 2L therapy in this analysis. The high attrition from 1L to 2L likely reflects a combination of high mortality (almost half of patients who received 1L therapy died before receiving 2L therapy) and high toxicity and/or low efficacy. Although limited real-world data are available, it has been reported that approximately 30–40% of patients who received 1L chemotherapy for adv/met esophageal cancer subsequently received 2L therapy [15]. This aligns closely with the 67% attrition seen between 1L therapy (n = 263) and 2L (n = 86) therapy in our analysis. The most frequent non-taxane 2L therapies were fluoropyrimidines with or without platinum (approximately 80%).
In Cox proportional hazards analysis, receipt of 2L taxane therapy was associated with significantly longer survival compared with 2L non-taxanes (7.3 vs. 5.1 months; HR [95% CI], 2.46 [1.29–4.66]). This observation appears broadly in line with results from other comparative and single-arm published studies assessing 2L taxanes in patients who had previously received treatment for adv/met esophageal cancer, all of which assessed outcomes in Japanese patients; median OS across these studies ranged from 5.3 to 10.4 months [16–21]. In the current study, there were no clear differences in demographic or disease characteristics between patients who received 2L taxane-based regimens compared with patients who received 2L non-taxane therapy. The low proportion of patients who received 2L therapy, and the poor survival outcomes and short DoT seen in patients who did receive 2L therapy, highlights an urgent unmet need for efficacious, tolerable therapies for ESCC in the 2L setting.
In recent years, elevated PD-L1 has been associated with prognosis and disease progression in patients with ESCC [22]. Consequently, immunotherapy with PD-1/PD-L1 inhibitors has recently been considered a potential treatment for these patients [23]. In the phase III KEYNOTE-181 study, the median OS in patients receiving investigator’s choice chemotherapy was identical to the real-world OS in patients receiving 2L therapy of ESCC observed in the present study (6.7 months); pembrolizumab conferred a significant survival advantage in patients with ESCC expressing PD-L1 (9.3 months) [24]. More recently, the phase III ATTRACTION-3 trial compared nivolumab with taxane therapy in patients with ESCC refractory or intolerant to previous fluoropyrimidine-based and platinum-based chemotherapy [13]. Median OS observed in patients receiving taxane therapy in ATTRACTION-3 (8.4 months) was generally comparable to that seen in the present analysis (7.3 months); 2L nivolumab therapy appeared to confer a substantial survival advantage (10.9 months) compared with taxanes. However, as few non-Asian patients participated in ATTRACTION-3, the present analysis provides context for that study, reporting results from a cohort of US real-world patients receiving 2L therapies, including taxanes, in a period immediately preceding the approval and use of immuno-oncology therapies.
This is to our knowledge the largest study to assess real-world outcomes with 2L therapy in patients with adv/met ESCC in the US. Despite this, several limitations must be considered. The sample size remains relatively small, and extreme caution must be taken when making any inferences from the results in these subgroups. In particular, our statistical interpretations, based on the Cox proportional hazards model, must be viewed within the context of the small sample. Additionally, several weaknesses inherent to real-world analyses are also present, such as the lack of clinical detail in the electronic health records and missing and/or miscategorized data. Notably, the presence of two or more metastatic sites in each patient has been demonstrated to be an independent predictor of poor prognosis [19], but these data were unavailable in the Flatiron electronic health record dataset. Lines of therapy (and the treatment regimens used in each line) are determined algorithmically in line with previous studies of real-world outcomes in patients with adv/met GC/GEJC [25, 26]; however, their accuracy cannot be confirmed, and reasons for discontinuation are unknown. Furthermore, data on prior surgeries are unavailable; prior surgery would have obvious implications on peri- and postoperative treatment selection. Additionally, relatively few patients (approximately 60%) had ECOG PS data available at the start of 2L therapy. However, availability of ECOG PS did not appear to be systematically different between taxane subgroups, and we do not believe any such bias to account for the differences in survival outcomes observed. Real-world data do provide important insights into routine care of patients and provide important context for clinical trial data [27].
Conclusions
Real-world survival outcomes in US patients receiving 2L therapy for adv/met ESCC are poor, and relatively few patients receive therapy consistent with clinical guidelines. In the 2L setting, taxanes significantly improved survival compared with non-taxane therapies, but only to a modest degree. Additional safe and effective therapies are needed to improve survival outcomes for patients receiving 2L therapy of adv/met ESCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study, the journal’s Rapid Service and Open Access Fees were sponsored by Bristol-Myers Squibb.
Medical Writing and Editorial Assistance
The authors thank Martin Bell, PhD, of Envision Pharma Group, for providing writing and editorial assistance, which was funded by Bristol-Myers Squibb.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to and approved the final manuscript.
Disclosures
Pranav Abraham, Joe Gricar, and Ying Zhang are employees of, and hold stock in, Bristol-Myers Squibb. Veena Shankaran has received research funding from Astellas, AstraZeneca, Bristol-Myers Squibb, and Merck.
Compliance with Ethics Guidelines
This article reports on a retrospective, observational study using de-identified anonymized patient-level data. Therefore, informed consent was not obtained, and no institutional review board approval was required.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Digital Features To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12326687.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Ren Y, Du XL, et al. Incidence and survival differences in esophageal cancer among ethnic groups in the United States. Oncotarget. 2017;8:47037–47051. doi: 10.18632/oncotarget.16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network, Asan University Analysis Working Group, B. C. Cancer Agency, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–75. [DOI] [PMC free article] [PubMed]
- 5.Wong MCS, Hamilton W, Whiteman DC, et al. Global Incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries. Sci Rep. 2018;8:4522. doi: 10.1038/s41598-018-19819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for esophageal and esophagogastric junction cancers. ©National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed April 01, 2020. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
- 7.Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 8.Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–4444. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 9.Sym SJ, Hong J, Park J, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol. 2013;71:481–488. doi: 10.1007/s00280-012-2027-3. [DOI] [PubMed] [Google Scholar]
- 10.Assersohn L, Brown G, Cunningham D, et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004;15:64–69. doi: 10.1093/annonc/mdh007. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez P, Pathak A, Phan AT. The role of taxanes in the management of gastroesphageal cancer. J Gastrointest Oncol. 2011;2:240–249. doi: 10.3978/j.issn.2078-6891.2011.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muro K, Hamaguchi T, Ohtsu A, et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. 2004;15:955–959. doi: 10.1093/annonc/mdh231. [DOI] [PubMed] [Google Scholar]
- 13.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 14.Flatiron Health. About us. Accessed January 1, 2020. https://flatiron.com/about-us/.
- 15.Thallinger CM, Raderer M, Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. J Clin Oncol. 2011;29:4709–4714. doi: 10.1200/JCO.2011.36.7599. [DOI] [PubMed] [Google Scholar]
- 16.Mizota A, Shitara K, Kondo C, et al. A retrospective comparison of docetaxel and paclitaxel for patients with advanced or recurrent esophageal cancer who previously received platinum-based chemotherapy. Oncology. 2011;81:237–242. doi: 10.1159/000334057. [DOI] [PubMed] [Google Scholar]
- 17.Nakatsumi H, Komatsu Y, Sawada K, et al. P-168: retrospective comparison of efficacy and safety of docetaxel and weekly-paclitaxel as 2nd-line chemotherapy for patients with unresectable or recurrent esophageal cancer. Ann Oncol. 2016;27:ii50. doi: 10.1093/annonc/mdw199.162. [DOI] [Google Scholar]
- 18.Sakamoto T, Takegawa N, Kushida S, et al. P2-6-3: a retrospective study of weekly paclitaxel as second-line treatment for advanced or recurrent esophageal cancer. Ann Oncol. 2014;25:v93. doi: 10.1093/annonc/mdu436.80. [DOI] [Google Scholar]
- 19.Shirakawa T, Kato K, Nagashima K, et al. A retrospective study of docetaxel or paclitaxel in patients with advanced or recurrent esophageal squamous cell carcinoma who previously received fluoropyrimidine- and platinum-based chemotherapy. Cancer Chemother Pharmacol. 2014;74:1207–1215. doi: 10.1007/s00280-014-2597-3. [DOI] [PubMed] [Google Scholar]
- 20.Tsushima T, Motoo N, Iwasa S, et al. P1–5–18: re-introduction of taxane for patients with esophageal squamous cell carcinoma refractory to 5-FU, CDDP, and a taxane. Ann Oncol. 2015;26:vii115. doi: 10.1093/annonc/mdv472.42. [DOI] [Google Scholar]
- 21.Kato K, Tahara M, Hironaka S, et al. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol. 2011;67:1265–1272. doi: 10.1007/s00280-010-1422-x. [DOI] [PubMed] [Google Scholar]
- 22.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 23.Raufi AG, Klempner SJ. Immunotherapy for advanced gastric and esophageal cancer: preclinical rationale and ongoing clinical investigations. J Gastrointest Oncol. 2015;6:561–569. doi: 10.3978/j.issn.2078-6891.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima T, Muro K, Francois E, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study. J Clin Oncol. 2019;37:2. doi: 10.1200/JCO.2019.37.4_suppl.2. [DOI] [PubMed] [Google Scholar]
- 25.Le DT, Ott PA, Korytowsky B, et al. Real-world treatment patterns and clinical outcomes across lines of therapy in patients with advanced/metastatic gastric or gastroesophageal junction cancer. Clin Colorectal Cancer. 2020;19(1):32–38.e3. [DOI] [PubMed]
- 26.Chau I, Le DT, Ott PA, et al. Developing real-world comparators for clinical trials in chemotherapy-refractory patients with gastric cancer or gastroesophageal junction cancer. Gastric Cancer. 2020;23:133–141. doi: 10.1007/s10120-019-01008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan R. Real world data: additional source for making clinical decisions. Int J Appl Basic Med Res. 2015;5:82. doi: 10.4103/2229-516X.157148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.