Abstract
Introduction
Hemorrhoidal disease (HD) is a common and recurrent problem for many adults worldwide. Venoactive drugs, such as micronized purified flavonoid fraction (MPFF; Daflon®), have been used to treat HD and their clinical benefits have been demonstrated in previous meta-analyses of clinical trials. The aim of this study was to evaluate the efficacy of MPFF across the broader spectrum of signs and symptoms following treatment of patients with HD.
Methods
We performed a systematic review of the literature to identify randomized clinical trials in which MPFF treatment was compared to placebo or no treatment for acute HD or for relief of symptoms after patients had undergone medical management or a surgical procedure to remove hemorrhoids. The main endpoints investigated were bleeding, pain, pruritus, discharge or leakage, and overall improvement. There was no limit on treatment duration.
Results
From 351 unique records retrieved, 11 studies reported in 13 articles were included. On the basis of findings from qualitative analysis, MPFF was reported in most studies to be beneficial in treating bleeding, pain, pruritus, anal discharge/leakage, and tenesmus, and in overall improvement. Quantitative meta-analysis of four studies indicated that MPFF treatment provided significant benefits for bleeding (odds ratio [OR] 0.082, 95% confidence interval [CI] 0.027–0.250; P < 0.001), discharge/leakage (OR 0.12, 95% CI 0.04–0.42; P < 0.001), and overall improvement according to patients (OR 5.25, 95% CI 2.58–10.68; P < 0.001) and investigators (OR 5.51, 95% CI 2.76–11.0; P < 0.001). MPFF also tended to decrease pain (OR 0.11, 95% CI 0.01–1.11; P = 0.06).
Conclusion
Taken together, these results suggest that MPFF treatment can improve the most important signs and symptoms of HD.
Keywords: Hemorrhoidal disease, Hemorrhoidectomy, Hemorrhoids, Micronized purified flavonoid fraction, MPFF, Venoactive drugs
Key Summary Points
| Why carry out this study? |
| This systematic review and meta-analysis sought to evaluate the efficacy of micronized purified flavonoid fraction (MPFF) in comparison with placebo or no treatment in patients with hemorrhoidal disease (HD). |
| A wide range of symptoms associated with acute HD or occurring after a medical management or surgical procedure for HD were assessed across randomized controlled studies using qualitative and quantitative analyses. |
| What was learned from this study? |
| Our results suggest that MPFF treatment can improve the most important signs and symptoms of HD including bleeding, pain, pruritus, tenesmus, and anal discharge/leakage. |
Introduction
Hemorrhoidal disease (HD) is a common medical problem among adults worldwide [1–3]. In addition to body mass index, other proposed risk factors include conditions that elevate intra-abdominal pressure such as pregnancy and straining [4].
HD occurs when hemorrhoids become inflamed and swollen with venous blood. External HD involves swelling of the external perianal vasculature and the tissues lining the anal canal below the dentate line; external HD is thus associated with symptoms of pain and pruritus, and less frequently bleeding or thrombosis. Internal HD occurs when the internal hemorrhoids swell and slide toward the anus, causing additional venous dilatation [4]. Although they are often asymptomatic and painless because the tissues are not innervated with somatic nerves, they may be associated with bleeding during defecation.
Internal HD is classified according to degree of prolapse (grade I, no prolapse; grade IV, permanent prolapse below the dentate line). Prolapsed internal hemorrhoids may be removed through various outpatient procedures or surgical excision [4, 5]. Complications of surgical procedures can include pain and profuse bleeding; rare but life-threatening complications include abscess formation, sepsis, and fecal incontinence [6].
HD may also be treated conservatively. This approach includes diet and lifestyle modification to increase fiber and fluid intake, increase physical activity, avoid constipation, and avoid straining during defecation. Topical treatments with creams containing anesthetics, corticosteroids, and anti-inflammatory drugs may provide some symptom relief for HD. Venoactive drugs or phlebotonics, which are available in Europe and Asia for the treatment of chronic venous disease (CVD; e.g., varicose veins), have also been used to treat HD [7]. Of these, flavonoids are the most common agent used. Micronized purified flavonoid fraction (MPFF; Daflon® [Servier, France]) is a well-known and well-studied venoactive drug and is frequently prescribed for CVD symptom relief, especially in France [8]. In CVD patients, MPFF has been shown to improve venous tone and to reduce capillary permeability, vascular endothelial activation, and inflammation [9, 10].
With respect to MPFF treatment in HD, previous non-systematic reviews have found evidence for the efficacy of MPFF not only in reducing pain, bleeding, anal discharge, and prolapse in acute HD but also in preventing relapse and reducing the duration and severity of acute attacks in chronic HD [7, 11, 12]. A 2006 meta-analysis of 14 studies investigating flavonoid treatment (MPFF, diosmin, or rutosides) for hemorrhoids reported that flavonoids reduced the risk of not improving globally by 58%, with apparent reductions in the risks of bleeding, pain, itching, and recurrences [8]. A more recent Cochrane meta-analysis of hemorrhoid treatment with phlebotonics, which covered a wide variety of drugs including MPFF, found that phlebotonics provided statistically significant benefits in bleeding and overall symptom improvement in acute HD and in bleeding after hemorrhoidectomy [13].
To evaluate the efficacy of MPFF across the broader spectrum of signs and symptoms following treatment of patients with HD, we conducted a systematic review to identify studies to be included in a meta-analysis of the magnitude of MPFF treatment effects compared to either placebo or no treatment for the symptoms and signs associated with acute HD or after a medical management or surgical procedure.
Methods
Criteria for Considering Studies in this Review
Study Design
We included all published parallel-group randomized controlled trials (RCTs) investigating the effect of MPFF in relieving the signs and symptoms in patients with acute HD (i.e., within 7 days of the onset of symptoms—usually either bleeding or pain) or after medical or surgical procedure to remove hemorrhoids.
The design of the included studies should be:
For acute HD: double-blind RCTs comparing treatment with MPFF versus placebo
After a medical or surgical procedure for HD: open-label, single-blind or double-blind RCTs comparing treatment with MPFF versus placebo or versus no treatment were accepted
Quasi-randomized studies were excluded.
There was no limit on treatment duration.
Study Participants
Eligible participants were patients with HD of all ages and both sexes, including pregnant or postpartum women, and patients after a procedure for HD.
Study Endpoints
The studies were included if at least one of the following endpoints was reported: bleeding, pain, pruritus, analgesic consumption, discharge or leakage, overall improvement according to patient or investigator, hospitalization duration after a procedure, anal discomfort, edema, tenesmus, and recurrence of HD crisis.
Literature Search Strategy
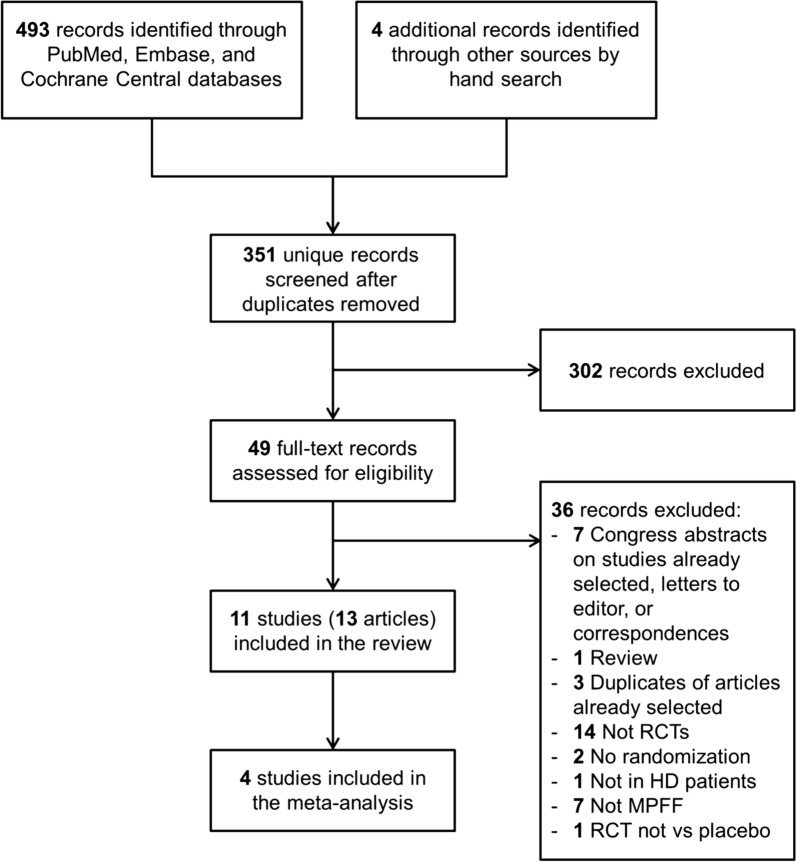
We systematically reviewed the literature to identify articles investigating the effects of oral MPFF treatment for HD. We conducted the search in three different online databases (PubMed, Embase, and the Cochrane Central Register of Controlled Trials) on June 6, 2018. Search terms included hemorrhoid, flavonoid, hesperidin, diosmin, micronized purified flavonoid fraction, MPFF, and Daflon, as well as synonyms and other brand names for MPFF. There was no restriction on language and publication date. In addition, PRISMA guidelines were used to report this review and meta-analysis (Fig. 1) [14].
Fig. 1.
PRISMA flow diagram. HD hemorrhoidal disease, MPFF micronized purified flavonoid fraction RCT randomized controlled trial
After using Citavi® (Swiss Academy Software, version 6.2) to remove duplicates, two reviewers independently screened titles and abstracts of the retrieved records to identify relevant clinical trials and reviews for further analysis, and to exclude clearly noncompliant and ineligible studies. Following the selection of records, full texts were obtained for all selected articles and reviews. To identify additional potentially relevant trials, a manual search was then conducted on the reference list of the selected reviews. A manual search was also performed in a Servier internal database (Pharmanet). The two reviewers independently assessed the full text of the selected articles to determine study eligibility using the predefined inclusion and exclusion criteria. In some cases, authors were contacted for clarification. Conference abstracts, letters, notes, and editorials were excluded. Unpublished studies were not considered. At all stages of the screening process, disagreements between reviewers were resolved by discussion. A last manual search was then conducted on the reference list of the included articles.
Data Extraction and Analysis
Data extraction was performed independently by the two reviewers using specifically designed Excel files. Collected data included study methodology and patient baseline information as well as MPFF efficacy and safety results. Statistical analyses were carried out using SAS® for Windows (SAS Institute Inc., version 9.2).
Main efficacy outcomes were bleeding, pain, and pruritus. Other outcomes analyzed included analgesic consumption, discharge or leakage, overall improvement (according to patient or investigator), anal discomfort, edema, tenesmus, recurrence of acute HD crises, and length of hospital stay after a procedure. For pain, pruritus, anal discomfort, and tenesmus, the patient’s reported outcome was considered first if available, whereas for bleeding, discharge/leakage, and edema, investigator’s reported outcome was considered first if available. These endpoints were analyzed at day 4 (or at day 3 or 5 if not available) and at day 7 (or day 4–10 if not available).
When efficacy endpoints were reported by a single study, only a descriptive analysis was performed (i.e., qualitative analysis). Mean differences or standard mean differences for continuous endpoints and odds ratios (OR) for binary endpoints were calculated with their associated 95% confidence intervals. When efficacy endpoints were reported by more than one study, a meta-analysis on aggregated data was performed (i.e., quantitative analysis). Fixed-effect (Mantel–Haenszel) and random-effects (DerSimonian–Laird) methods were used for binary endpoints [15, 16], whereas inverse-variance fixed-effect and random-effects methods were used for continuous endpoints.
Heterogeneity between studies was measured with the Cochran Q statistic [17]. A non-significant P value for the Q indicator indicated that the treatment effect among the studies was homogeneous. In addition, the degree of heterogeneity between studies was measured with the I2 indicator [18]. If I2 was greater than 50%, a model with random effects was used instead of a model with fixed effects. In one study (Jiang and Cao) [19], the results for binary endpoints excluded the modality “mild”, which required an additional sensitivity analysis.
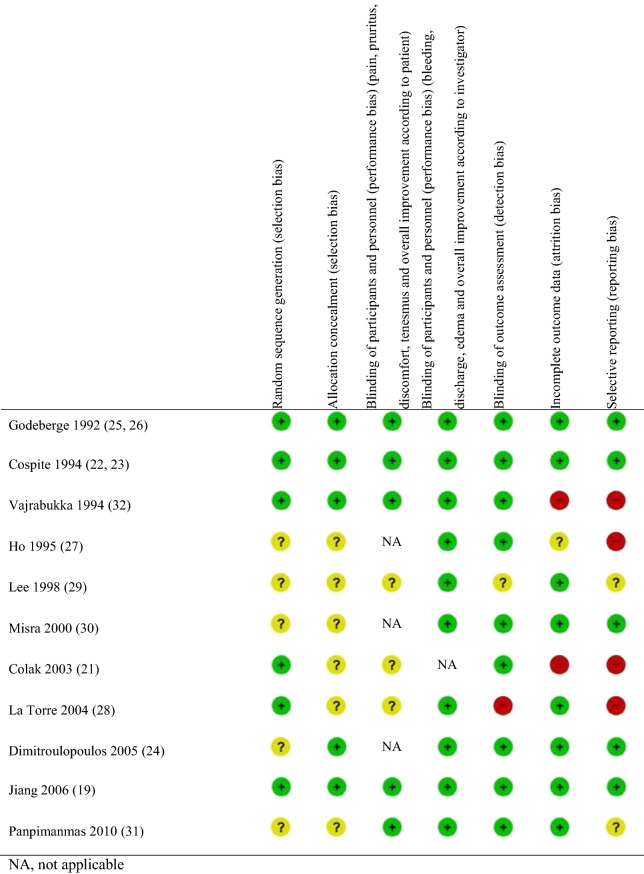
Assessment of Study Quality and Risk of Bias
The two reviewers independently assessed the quality of the selected studies using the Cochrane risk of bias tool [20]. For each study, the reviewers evaluated and graded the risk of bias as low, high, or unclear for each of the following items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias) for symptoms and signs separately, blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias).
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Results of the Literature Search
A total of 493 records were identified through PubMed, Embase, and Cochrane Central databases (Fig. 1). An additional four records were identified through manual search. After removal of duplicates, 351 unique records were screened using titles and abstracts. Of them, 49 potentially relevant records were retrieved for full text assessment. Finally, a total of 11 studies reported in 13 articles [19, 21–32] were included in the systematic review (qualitative analysis). As a result of substantial heterogeneity in the endpoints reported in the studies, only four studies could be included in the meta-analysis (quantitative analysis) [19, 21, 22, 30].
Risk of Bias in Studies Included
For 7 out of 11 studies, the risk of bias was unclear or high in at least two aspects of the study designs (Table 1). The study by Lee et al. appeared to be at the highest risk of bias [29], with unclear risk in five study aspects, although three studies were deemed to be at high risk in two aspects [21, 28, 32]. In four studies, the risk of bias was low in all or most aspects of the study designs [19, 22–26].
Table 1.
Risk of bias summary for the studies included in the systematic review
Study Characteristics
All the studies included in the systematic review were conducted in Europe or Asia and published between 1992 and 2010 (Table 2). Participants were adult patients of both sexes, and the mean age of the study populations ranged between 33 and 57 years. Patients with HD of all grades (I–IV) were included. Six studies investigated the effects of MPFF treatment vs placebo for acute HD and five investigated treatment following a medical management or surgical procedure. Treatment with MPFF was between 1000 and 3000 mg/day for between 5 and 90 days.
Table 2.
Characteristics of the studies included in the systematic review
| Study, year, country/interventions | Study design/N participants | Participants: inclusion and exclusion criteria | Outcomes analyzed (MPFF vs control) | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Day | MPFF | Control | Significant (Y/N) | |||
| Investigations of direct effects of MPFF on acute HD | |||||||
|
Italy MPFF or placebo 3000 mg/day for 3 days, 2000 mg/day for 4 days |
Double-blind RCT N = 100 MPFF: n = 50 Placebo: n = 50 Mean age* 43–45 years 53% women |
Inclusion criteria: adult outpatients or inpatients of any sex and age, with known HD, diagnosed by proctoscopy, suffering from an uncomplicated acute hemorrhoidal crisis without any previous treatment for a maximum of 3 days. The patients had to suffer from symptoms such as bleeding, anal discomfort, pain and pruritus, and objective proctoscopic signs Exclusion criteria: patients requiring a surgical procedure, suffering from anal fistula or rectal prolapse |
Systemic analgesic consumption | 7 | 0.2 ± 0.1 | 1.6 ± 0.2 | Y |
| Paina | 7 | 42/50 (84%) | 8/50 (16%) | Y | |||
| Anal discomfort | 7 | 19/49 (39%) | 3/49 (6%) | Y | |||
| Bleeding | 7 | 19/20 (95%) | 9/18 (50%) | Y | |||
| Discharge/leakage | 7 | 21/26 (81%) | 7/23 (30%) | Y | |||
| Overall improvement | 7 | 48/49 (74.1%) | 34/45 (75.6%) | Y | |||
| Overall satisfaction (patient “satisfied” or “very satisfied”)b | 7 | 42/50 (84%) | 21/50 (42%) | Y | |||
| Overall improvement (investigator “very good”, “good”, “useful”)c | 7 | 45/50 (90%) | 29/50 (58%) | Y | |||
|
France MPFF or placebo 1000 mg/day for 60 days |
Double-blind RCT N = 120 MPFF: n = 60 Placebo: n = 60 Mean age* 46–48 years 80% women |
Inclusion criteria: ambulatory patients, aged over 18 years, with symptomatic HD (1st–3rd degree); with an acute episode in preceding 2 months Exclusion criteria: complicated HD as a result of previous surgery, associated anal fissure, or those requiring immediate surgery; bleeding disorder; treatment with a venotropic agent in the previous 2 months; anocutaneous parasitic infestation, or a rectosigmoidal disorder |
Paind | 60 | 98% | 47% | Y |
| Pruritus | 60 | 86% | 58% | Y | |||
| Tenesmus | 60 | 98% | 50% | Y | |||
| Bleeding | 60 | 91% | 59% | Y | |||
| Discharge/leakage | 60 | 97% | 54% | Y | |||
| Edema | 60 | 98% | 47% | Y | |||
| Erythema | 60 | 95% | 48% | Y | |||
| Recurrence of acute episode on treatment | 60 | 40% | 76% | Y | |||
| Patient satisfaction | 60 | 90% | 40% | Y | |||
|
Jiang (2006) [19] China MPFF or placebo 3000 mg/day for 4 days, then 1500 mg/day for 3 days |
Double-blind RCT N = 90 MPFF: n = 49 Placebo: n = 41 Mean age 43.2 years 46% women |
Inclusion criteria: male or female patients, aged > 18 years, followed up as out- or inpatients, presenting for the first time with an acute hemorrhoidal episode (diagnosis confirmed by clinical and endoscopic examination) of less than 48 h, with symptoms such as pain, itching and edema, and/or signs (bleeding, mucus leakage, and prolapse), having not received other prior therapies (any phlebotropic medication, anticoagulant and antiplatelet agents, analgesic or anti-inflammatory drugs) Exclusion criteria: presence of severe hemorrhoidal manifestations requiring surgery, anal fissure, permanent prolapsed hemorrhoids, and parasitic infection |
Bleedinge (% grades 2 or 3) [mean score] |
4 |
2/49 (4%) [1.3] |
2/41 (4.9%) [1.5] |
Y |
| 7 |
1/49 (2%) [1.2] |
3/41 (7.3%) [1.4] |
Y | ||||
|
Pain (% grades 2 or 3) [mean score] |
4 |
9/49 (18.3%) [2.0] |
8/41 (19.5%) [2.1] |
N | |||
| 7 |
3/49 (6.1%) [1.4] |
6/41 (14.6%) [1.8] |
Y | ||||
|
Itching (% grades 2 or 3) [mean score] |
4 |
1/49 (2%) [1.2] |
2/41 (4.9%) [1.3] |
N | |||
| 7 |
0/49 (0%) [1.1] |
2/41 (4.9%) [1.3] |
Y | ||||
|
Leakage (% grades 2 or 3) [mean score] |
4 |
1/49 (2%) [1.2] |
1/41 (2.4%) [1.2] |
N | |||
| 7 |
0/49 (0%) [1.1] |
1/41 (2.4%) [1.2] |
N | ||||
|
Edema (% grades 2 or 3) [mean score] |
4 |
12/49 (24.5%) [2.1] |
10/41 (24.4%) [2.0] |
N | |||
| 7 |
4/49 (8.1%) [1.6] |
8/41 (19.5%) [1.7] |
N | ||||
|
Prolapse (% grades 2 or 3) [mean score] |
7 |
0/49 (0%) [1.1] |
2/41 (4.9%) [1.3] |
Y | |||
| Overall therapeutic evaluation (patient) (% reporting “good” or “excellent”) | 7 | 75.6% | 39.0% | Y | |||
| Overall therapeutic evaluation (investigator) (% reporting “good” or “excellent”) | 7 | 75.5% | 39.0% | Y | |||
|
Misra (2000) [30] India MPFF or placebo 3000 mg/day for 4 days, then 2000 mg/day for 3 days, then 1000 mg/day for 83 days |
Double-blind RCT N = 100 MPFF: n = 50 Placebo: n = 50 Mean age* 33–35 years 21% women |
Inclusion criteria: Outpatients of either sex, > 18 years old, with past history of HD of < 18 months and presenting with acute rectal bleeding of < 3 days in association with visibly distended or displaced anal cushions conforming to grade 1 or 2 internal hemorrhoids identified on proctoscopic examination Exclusion criteria: patients with anal fissure, inflammatory bowel disease, colorectal cancer or pregnancy; previous laser treatment or use of a flavonoid drug 1 month before inclusion. Previous treatment with analgesics, topical antihemorrhoidal ointments, nonsteroidal anti-inflammatory drugs, steroids, anticoagulants or antiplatelet agents |
Bleeding cessation | 3 | 40/50 (80%) | 19/50 (38%) | Y |
| 4 | 46/50 (92%) | 20/50 (40%) | Y | ||||
| 5 | 46/50 (92%) | 26/50 (52%) | Y | ||||
| 6 | 47/50 (94%) | 29/50 (58%) | Y | ||||
| 7 | 47/50 (94%) | 30/50 (60%) | Y | ||||
| Bleeding cessation to recurrence (interval days) | 53.1 ± 22.7 | 34.2 ± 22.6 | N | ||||
|
Panpimanmas (2010) [31] Thailand CQ, MPFF, or placebo 3000 mg/day for 4 days, then 2000 mg/day for 3 days |
Double-blind RCT N = 570 CQ: n = 191 MPFF: n = 189 Placebo: n = 190 Mean age not reported 49–55% women |
Inclusion criteria: acute rectal bleeding within 5 days Exclusion criteria: previous hemorrhoidectomy, treatment with anticoagulant or acetylsalicylic acid, treatment with other antihemorrhoid drugs at that time, permanent prolapsed internal hemorrhoid requiring surgery, moderate to severe hypertension; cardiovascular diseases; renal failure, cirrhosis, pregnancy, and lactation |
Bleeding (patient)f | 7 | 118/174 (67.8%) | 132/178 (74.2%) | N |
| Pain (patient) | 7 | 57/174 (32.8%) | 58/178 (32.6%) | N | |||
| Discharge (patient) | 7 | 9/174 (5.2%) | 11/178 (6.2%) | N | |||
| Pruritus (patient) | 7 | 12/174 (6.9%) | 17/178 (9.6%) | N | |||
| Prolapse (patient) | 7 | 6/174 (3.4%) | 11/178 (6.2%) | N | |||
| Bleeding (investigator) | 7 | 28/174 (16.1%) | 25/178 (14.0%) | N | |||
| Edema (investigator) | 7 | 54/174 (31.0%) | 68/178 (38.2%) | N | |||
|
Vajrabukka (1994) [32] Thailand MPFF or placebo 3000 mg/day for 4 days, then 2000 mg/day for 3 days |
Double-blind RCT N = 226 MPFF: n = 121 Placebo: n = 105 Mean age* 35–37 years 50% women |
Inclusion criteria: non-pregnant outpatient > 18 years of age with acute symptoms of hemorrhoids no longer than 3 days with pain, discomfort, bleeding and discharge symptoms Exclusion criteria: permanently prolapsed hemorrhoids (grade 4) or associated anorectal diseases such as anal fissure, fistula-in-ano, and abscesses |
Paing | 7 | 69/84 (82.1%) | 66/78 (84.6%) | N |
| Anal discomfort | 7 | 57/85 (67.1%) | 39/69 (56.8%) | N | |||
| Bleeding | 7 | 79/92 (85.9%) | 73/90 (81.1%) | N | |||
| Discharge/leakage | 7 | 40/51 (78.9%) | 27/42 (64.7%) | N | |||
| Investigations of effects of MPFF treatment following a medical/surgical procedure | |||||||
|
Colak (2003) [21] Turkey MPFF (dose not reported) or no treatment for 7 days after hemorrhoidectomy |
Observer-blind RCT N = 112 MPFF: n = 56 No treatment: n = 56 Median age* 41–45 years 40–43% women |
Inclusion criteria: patients with symptomatic third- or fourth-degree hemorrhoids who underwent hemorrhoidectomy Exclusion criteria: patients with concomitant anal disease such as fissure, abscess, fistula, Cohn’s or ulcerative colitis, or rectal cancer, and those taking oral anticoagulants |
Pain (0–10 scale) | 2 | 5 (2–7) | 6 (5–7) | Y |
| 3 | 3.5 (0–5.5) | 5 (3.5–7) | Y | ||||
| 7 | 2 (0–3) | 3.5 (2–4) | Y | ||||
| Analgesic consumption | 7 | 38/56 (67.9%) | 48/56 (85.7%) | Y | |||
| Analgesic consumption duration (days) | 2 (0–3) | 3 (1.5–3) | Y | ||||
| Overall satisfaction by patient (“moderate”, “good” and “excellent”)h | 7 | 25/28 (89.3%) | 21/28 (75.0%) | Y | |||
|
Dimitropoulos (2005) [24] Greece MPFF 3000 mg/day or no treatment for 5 days after IRP |
Observer-blind RCT N = 351 MPFF + IRP: n = 117 IRP: n = 117 Mean age 49.2 years 51% women |
Inclusion criteria: ambulatory male and female patients, over 18 years with rectal bleeding due to grades I, II, and III acute internal hemorrhoids, with no previous treatment for HD within the 6 months preceding the study and without coexistent colon diseases Exclusion criteria: pregnancy, history of acute HD attacks, concomitant large-bowel and anal canal diseases, history of pelvic radiation, use of anticoagulant and antiplatelet agents or nonsteroidal anti-inflammatory drugs |
Bleeding cessation | 5 | 83/111 (74.8%) | 65/117 (55.6%) | Y |
| Bleeding recurrence | 90 | 4/83 (4.8%) | 9/65 (13.8%) | N | |||
|
Ho (1995) [27] Singapore MPFF 3000 mg/day for 3 days then 1500 mg/day for 4 days, or no treatment after hemorrhoidectomy |
Observer-blind RCT N = 228 MPFF: n = 114 No treatment: n = 114 Mean age 39.5 years 46% women |
Inclusion criteria: patients with three columns of symptomatic irreducible prolapsed hemorrhoids | Bleeding (secondary) | 6–15 | 1/114 (0.9%) | 7/114 (6.1%) | Y |
|
La Torre (2004) [28] Italy MPFF 2000 mg/day for 10 days and 1000 mg/day for 20 days, or no treatment after hemorrhoidectomy |
RCT N = 50 MPFF: n = 25 No treatment: n = 25 Mean age* 56–57 years 37% women |
Inclusion criteria: patients > 18 years old with an indication for hemorrhoidectomy, a past history of HD longer than 6 months, symptomatic irreducible prolapsed hemorrhoids Exclusion criteria: fistula or chronic anal fissure, inflammatory bowel disease, diabetes or other metabolic or endocrine disorders, alcoholism, drug abuse, coagulation disorders, and previous anorectal surgery |
Paini | 3 | 3.48 | 6.16 | Y |
| 60 | 3.88 ± 2.28 | 8.32 ± 2.88 | Y | ||||
| Pruritus | 3 | 1.84 | 4.04 | Y | |||
| 60 | 1.96 ± 1.54 | 5.04 ± 2.49 | Y | ||||
| Tenesmus | 3 | 1.48 | 5.36 | Y | |||
| 60 | 1.68 ± 1.82 | 7.21 ± 3.13 | Y | ||||
| Bleeding | 3 | 2 | 4.4 | Y | |||
| 60 | 2.00 ± 1.29 | 5.24 ± 2.8 | Y | ||||
|
Lee (1998) [29] Korea MPFF 3000 mg/day for 4 days, then 2000 mg/day for 3 days, or placebo after hemorrhoidectomy |
RCT N = 54 MPFF: n = 27 Placebo: n = 27 Mean age* 41–43 years 51–56% women |
Inclusion criteria: male or female, over 18 years old, had undergone surgery for hemorrhoids of at least grade 2 at Samsung Medical Center’s General Surgery department from January 1, 1997 to January 1, 1998 Exclusion criteria: patients who were pregnant or nursing; suffering from rectal or colon cancer, gastrointestinal inflammation or infection, anal diseases other than HD; patients taking any other vasoactive agent, anticoagulants, adrenocortical hormones, nonsteroidal anti-inflammatory drugs, antiplatelet agents or other pharmaceutical HD treatment, and patients with compliance of < 80% or > 120% with the study drug |
Painj | 18 | 18/27 (66.7%) | 7/27 (25.9%) | Y |
| Anal discomfort | 18 | 11/27 (40.7%) | 5/27 (18.5%) | N | |||
| Postoperative bleeding | 18 | 24/27 (88.9%) | 6/27 (22.2%) | Y | |||
| Postoperative pus discharge | 18 | 23/27 (85.2%) | 10/27 (37.0%) | Y | |||
CQ Cissus quadrangularis L., IRP infrared photocoagulation, MPFF micronized purified flavonoid fraction
*Mean age was calculated by treatment group
aAll signs and symptoms graded on a 4-point scale: 0 = none; 1 = mild; 2 = significant; 3 = severe. Percentages are percentages of patients with no symptoms at day 7
bThree-item assessment: “very satisfied”, “satisfied”, “not satisfied”
cFour-item assessment: “very good”, “good”, “useful”, “null”
dIll signs and symptoms graded on a 4-point scale from 0 (nil) to 3 (severe). Percentages are percentages of patients with a decrease in score (improvement) after treatment
eAll signs and symptoms graded on a 4-point scale: 0 = absent, 1 = mild, 2 = moderate, 3 = severe
fAll signs and symptoms graded on a 4-point scale: 0 = absent, 1 = mild, 2 = moderate, 3 = severe. Percentages are percentages of patients with a decrease in score (improvement) after treatment
gAll signs and symptoms graded on a 4-point scale: 0 = nil, 1 = notable improvement, 2 = significant improvement, 3 = disappearance or cure. Percentages are percentages of patients with improvement (scored 1, 2, or 3) after treatment
hFour-item assessment: “excellent”, “good”, “moderate”, “poor”. Distributions were compared using Wilcoxon signed-rank test
iAll signs and symptoms graded on a 4-point scale from 0 to 3: 0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe intensity. The values are the global scores which were calculated by adding up mean symptom scores over the observation period. Values at day 60 are tabulated with standard deviations, which were not provided for day 3 data
jAll signs and symptoms graded on a 4-point scale: 0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe intensity. Percentages are percentages of patients with no symptoms at day 18
Effects of Intervention
Bleeding
Four studies reported significant effects of MPFF on bleeding in acute HD. One study showed that the proportion of patients with bleeding at day 7 was significantly lower (P = 0.006) in the MPFF-treated group compared to the placebo group [22, 23]. Another study showed that bleeding scores (assessed using a 4-point scale) at day 4 and day 7 were significantly lower (P < 0.05) [19] in the MPFF-treated group compared to the control group. In addition, the proportion of patients in whom bleeding had improved at day 60 was significantly higher (91% vs 59%, respectively; P < 0.01) [25, 26] with MPFF treatment than with placebo treatment. In another study, significantly more patients had ceased bleeding at day 3 through 7 (P < 0.01) with MPFF treatment than with placebo, and bleeding duration was also significantly shorter by 2.1 (95% CI 1.2–2.9) days with MPFF treatment (P < 0.01) [30]. Two studies reported that neither patient- nor physician-assessed bleeding was significantly improved with MPFF treatment [31, 32].
A pooled meta-analysis of two studies for the presence/absence of bleeding after 7 days of MPFF or placebo treatment for acute HD yielded an OR of 0.082 (95% CI 0.027–0.250; P < 0.001) in favor of MPFF treatment (Fig. 2a) [22, 23, 30]. These results were confirmed in a sensitivity analysis that included a third study (OR 0.101, 95% CI 0.037–0.272; P < 0.001) [19] (Fig. 2b). In this third study, the results for bleeding and the other binary endpoints excluded the modality “mild” in contrast to the other studies, which considered “mild”, “moderate”, or “severe” events. Because this exclusion introduced a bias in the results, this study was added as a sensitivity analysis even though heterogeneity was low (I2 = 0).
Fig. 2.
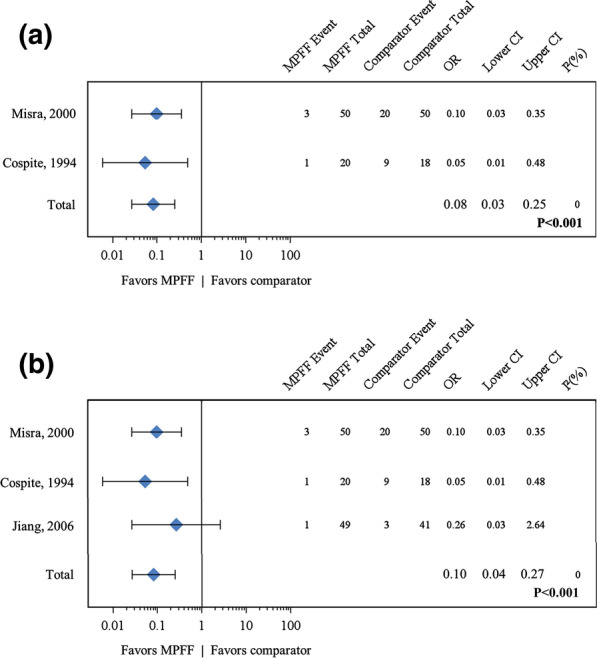
Forest plot comparisons of MPFF versus placebo for bleeding in acute HD after 7 days of treatment. In a pooled analysis of two studies using the fixed-effect (Mantel–Haenszel) method, micronized purified flavonoid fraction (MPFF) treatment was associated with a beneficial and statistically significant effect for bleeding in acute hemorrhoidal disease (odds ratio [OR] 0.08, 95% confidence interval [CI] 0.03–0.25; P < 0.001) with no statistical heterogeneity (I2 = 0%) (a). Results were similar in a sensitivity analysis that included a third study (Jiang 2006) in which the bleeding endpoint was heterogeneous (OR 0.10, 95% CI 0.04–0.27; P < 0.001) (b)
Four studies reported that bleeding after a surgical or outpatient procedure improved significantly with MPFF treatment. MPFF treatment was significantly better than no treatment for complete cessation of bleeding at day 5 after an infrared photocoagulation (IRP) procedure (P = 0.004) [24], secondary bleeding at 7–14 days (P = 0.03) [27] and 18 days (P < 0.005) [29] after hemorrhoidectomy, and for bleeding scores on days 1–3 (P < 0.0001) and at day 60 (P < 0.0001) after hemorrhoidectomy [28].
Pain
Five studies investigated the effects of MPFF treatment on pain in acute HD. Three studies reported statistically significant improvements in pain with MPFF treatment compared to placebo, by pain score at day 7 (P < 0.001 [22, 23]; and P = 0.001 [19]), and by the proportion of patients in whom pain had improved at day 60 (98% vs 47%; P < 0.01) [25, 26]. Two studies reported no significant improvement in pain at day 7 with MPFF compared to placebo [31, 32].
A pooled meta-analysis of two studies for the presence/absence of pain after 7 days of MPFF or placebo treatment yielded an OR of 0.11 (95% CI 0.01–1.11; P = 0.06) indicating that MPFF treatment is associated with a nearly statistically significant benefit for pain (Fig. 3). This result should be viewed in the context of high statistical heterogeneity (I2 = 85%) since the study by Jiang and Cao [19] did not include “mild” as a pain modality.
Fig. 3.
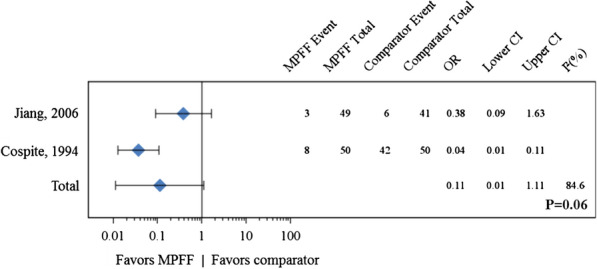
Forest plot comparison of MPFF versus placebo for pain in acute hemorrhoidal disease after 7 days of treatment. In a pooled analysis of two studies, micronized purified flavonoid fraction (MPFF) treatment was associated with a beneficial but not statistically significant effect for pain in acute hemorrhoidal disease (odds ratio [OR] 0.11 95% confidence interval [CI] 0.01–1.11, P = 0.06) with high statistical heterogeneity (I2 = 84.6%)
Three studies reported that MPFF significantly improved pain following hemorrhoidectomy. Patients treated with MPFF compared to those receiving placebo or no treatment, respectively, had significant differences in:
Pruritus
In acute HD, two studies reported significant improvement in pruritus with MPFF, whereas one reported no improvement. Improvement in pruritus was observed at day 60 in 86% of patients who received MPFF and in 58% of patients who received placebo (P < 0.01) [25, 26]. A slight but statistically significant difference in mean pruritus score was observed at day 7 (P < 0.05) but not at day 4 [19]. In contrast, patient-assessed pruritus was not significantly improved with MPFF treatment at day 7 compared to placebo (OR 1.24, 95% CI 0.62–2.49; P = 0.539) [31].
One study reported significant improvement in pruritus after hemorrhoidectomy. Mean pruritus scores were significantly better with MPFF treatment than with no treatment over days 1–3 and at day 60 (P < 0.0001) after surgery [28].
Anal Discomfort
One study reported significant improvement in anal discomfort with MPFF compared to placebo (P < 0.001) at day 7 [22, 23], whereas a second study found no significant improvement [32]. After hemorrhoidectomy, one study reported no significant improvement in anal discomfort with MPFF compared to placebo at days 7 and 18 [29].
Tenesmus
In acute HD, tenesmus at day 60 was improved in significantly more patients treated with MPFF than with placebo (98% vs 50%; P < 0.01) [25, 26]. After hemorrhoidectomy, tenesmus scores were significantly lower with MPFF than with no treatment over days 1–3 and at day 60 (P < 0.0001) [28].
Anal Discharge or Leakage
Three studies reported that anal discharge, defined as an intermittent or continuous expression of liquid from the anus, in acute HD significantly improved with MPFF compared to placebo. The proportion of patients without discharge or mild discharge was higher at day 7 (P < 0.001) [22, 23]. The proportion of patients who had improved by day 60 (97% vs 54%; P < 0.01) [25, 26] was significantly greater with MPFF than with placebo. Discharge improvement by patient assessment was significantly better at day 2 (P = 0.038) and discharge intensity by investigator assessment was significantly lower at day 7 (P = 0.001) [32]. In contrast, discharge was not significantly improved with MPFF in terms of leakage scores at day 4 or 7 [19] or in the number of patients who reported improvement at day 7 (OR 1.35, 95% CI 0.79–3.17; P = 0.473) [31].
A pooled meta-analysis of two studies for the presence/absence of discharge or leakage after treatment for acute HD yielded an OR of 0.12 (95% CI 0.04–0.42; P < 0.001) in favor of MPFF, without statistical heterogeneity (I2 = 0) (Fig. 4) [19, 22, 23]. This result indicates that MPFF treatment is associated with a statistically significant benefit of reducing discharge and leakage.
Fig. 4.
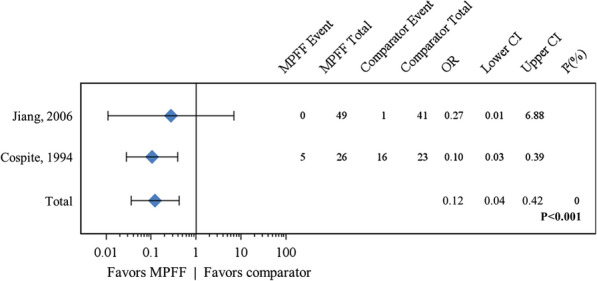
Forest plot comparison of MPFF versus placebo for discharge or leakage after 7 days of treatment. In a pooled analysis of two studies using the fixed-effect (Mantel–Haenszel) method, micronized purified flavonoid fraction (MPFF) treatment was associated with a beneficial and statistically significant effect for discharge or leakage in acute hemorrhoidal disease (odds ratio [OR] 0.12, 95% confidence interval [CI] 0.04–0.42; P < 0.001) with no statistical heterogeneity (I2 = 0%)
Following hemorrhoidectomy, one study reported that the proportion of patients with discharge at day 18 was lower with MPFF treatment than with placebo (14.8% vs 63.0%; P < 0.005) [29].
Edema
Two studies reported significant improvement in edema with MPFF treatment in acute HD. Clinical findings including edema were improved in significantly more patients treated with MPFF than in those treated with placebo after 7 days of treatment (94% vs 58%; P < 0.001) [23] and after 60 days of treatment (98% vs 47%; P < 0.01) [25, 26]. However, in one study, the proportion of patients in whom edema improved after 7 days of treatment with MPFF or with placebo was not significantly different (P = 0.43) [31]. Similarly, mean edema score in patients was not significantly different after 4 or 7 days of treatment with MPFF or placebo [19].
Overall Improvement
In acute HD, patient-assessed overall improvement or satisfaction in five studies was consistently greater with MPFF treatment than with placebo. Improvement scores were significantly greater with MPFF on all days from day 2 to day 7 (P < 0.001) [22, 23]. More patients were satisfied or very satisfied with MPFF than with placebo after 7 days of treatment (P = 0.023) [32] and after 60 days of treatment (P < 0.01) [25, 26]. Overall efficacy was assessed as good or excellent by significantly more patients treated with MPFF (75.6%) than with placebo (39.0%; P = 0.007) [19]. Similarly, significantly more patients reported day-to-day improvement with MPFF (73.5%) than with placebo (41.5%; P = 0.012) [19]. Patient satisfaction after hemorrhoidectomy was also significantly greater with MPFF treatment than with no treatment (P = 0.001) [21].
A pooled meta-analysis of three studies for patient-assessed overall improvement at day 7 in acute HD or after hemorrhoidectomy yielded an OR of 5.25 (95% CI 2.58–10.68; P < 0.001) in favor of MPFF treatment (Fig. 5a) [19, 21–23].
Fig. 5.
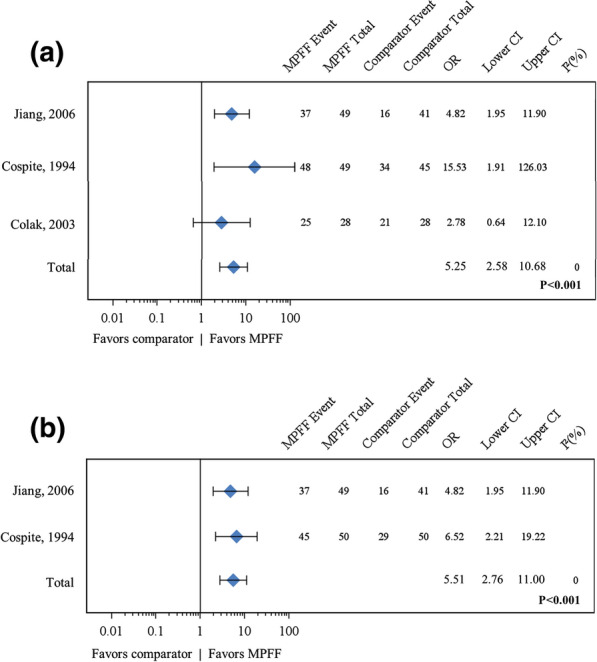
Forest plot comparisons of MPFF versus placebo for overall improvement according to patient and to investigator after 7 days of treatment. In a pooled analysis of three studies using the fixed-effect (Mantel–Haenszel) method, micronized purified flavonoid fraction (MPFF) treatment was associated with a beneficial and statistically significant effect for overall improvement according to patients (odds ratio [OR] 5.25, 95% confidence interval [CI] 2.58–10.68; P < 0.001) with no statistical heterogeneity (I2 = 0%) (a). In a pooled analysis of two studies using the fixed-effect (Mantel–Haenszel) method, MPFF treatment was associated with a beneficial and statistically significant effect for overall improvement according to investigators (OR 5.51, 95% CI 2.76–11.00; P < 0.001) with no statistical heterogeneity (I2 = 0%) (b)
In four studies of acute HD, investigators also considered treatment efficacy to be greater with MPFF than with placebo. After 7 days of treatment, physician investigators in two studies considered therapeutic activity to be good or excellent for significantly more patients treated with MPFF (75.5% versus 39.0%, respectively) than with placebo (32% and, respectively; P < 0.001 and P < 0.006) [19]. Overall activity evaluated by the investigator at day 7 was also significantly better with MPFF than with placebo in two other studies (P < 0.001 and P = 0.009, respectively) [22, 23, 32]. Results were similar after 60 days of treatment, with overall efficacy being considered good or very good for 83% of patients treated with MPFF compared to 37% of those treated with placebo (P < 0.01) [25, 26].
A pooled meta-analysis of two studies for investigator-assessed overall improvement in acute HD at day 7 yielded an OR of 5.51 (95% CI 2.76–11.0; P < 0.001) in favor of MPFF treatment, with no statistical heterogeneity (I2 = 0) (Fig. 5b) [19, 22, 23].
Other Outcomes
Two studies investigated the effect of MPFF treatment on prolapse [19, 31]; however, there were no significant differences between the MPFF arm and the control placebo groups with respect to this outcome.
Two out of three studies that investigated concomitant use of topical and systemic analgesics found statistically significant beneficial effects associated with MPFF treatment. One study found that MPFF treatment significantly reduced the use of topical and systemic analgesics in acute HD from day 3 (P = 0.041 and P = 0.013, respectively) through day 7 (P < 0.001 and P < 0.001, respectively) [22, 23]. One study found that uptake of intramuscular analgesic at 2 and 3 days (P = 0.022 and P = 0.007) after hemorrhoidectomy and overall (P = 0.021) was significantly less with MPFF treatment [21]. One study reported no significant difference in analgesic consumption after hemorrhoidectomy between the MPFF and no treatment groups [27].
Two studies investigated the effects of MPFF treatment on hospital length-of-stay (LOS). One study reported that hospital LOS after hemorrhoidectomy was significantly shorter with MPFF than with no treatment (2 vs 2.5 days, P = 0.001) [21]. One study reported no significant difference in hospital LOS after hemorrhoidectomy between groups receiving MPFF treatment or no treatment [27].
Of the three studies that investigated the effects of MPFF treatment on recurrence of acute episodes of HD, two studies reported statistically significant beneficial effects of MPFF treatment and one study was associated with a beneficial but not statistically significant effect. The proportion of patients who presented with an acute HD episode during 60 days of treatment was significantly smaller with MPFF treatment than with placebo (40% vs 76%; P < 0.05) [25, 26]. The frequency of bleeding relapse in acute HD was reduced by 24% (95% CI 2–46) with MPFF taken as preventive treatment over 90 days compared to placebo treatment (P < 0.05) [30]. In the third study, the bleeding relapse rate within 90 days after IRP tended to be lower with 5 days of MPFF post-procedure treatment (4.8%) than with no treatment (13.8%), but the difference was not statistically significant (P = 0.12) [24].
MPFF was well tolerated and none of these studies showed any serious side effect and the majority of the studies had no secondary effect. Only in a few cases were mild, known secondary effects like gastralgia, diarrhea, and abdominal pain declared.
Discussion
MPFF has a variety of significant anti-inflammatory, antioxidant, and venoprotective actions, which form the basis of its beneficial clinical effects [10, 12, 33]. In a number of experimental models, it reduced venous inflammation by inhibiting leukocyte rolling, adhesion, and migration, and inhibited the synthesis of inflammatory mediators [10, 34–36]. It improves venous tone and lymphatic drainage by modulating noradrenergic signalling and reducing norepinephrine metabolism [37, 38]. MPFF significantly reduces capillary hyperpermeability and improves the capillary resistance in patients with abnormal capillary fragility leading to further improvement of microcirculation [39, 40]. As venous pathologies and diminished venous return play prominent roles in HD, these actions of MPFF provide the rationale for its use in treating HD.
To study the efficacy of MPFF across a broader spectrum of signs and symptoms in patients with HD, we examined data from 11 studies that compared MPFF to placebo or no treatment for the treatment of acute HD or symptoms after a medical or surgical procedure to remove prolapsed hemorrhoids. In more than half of the studies, there was some risk of bias due to incomplete descriptions of randomization, allocation concealment, or blinding of participants to the treatment for subjective patient-determined endpoints such as pain or overall improvement and selective reporting. As a result of heterogeneity in study design, only selected endpoints could be used in pooled meta-analyses to assess the magnitude of effects across the different studies. Quantitative analysis of pooled results indicated that 7 days of MPFF treatment in patients with acute HD was associated with statistically significant beneficial effects for reducing bleeding (P < 0.001), reducing discharge and leakage (P < 0.001), and overall improvement according to patients (P = 0.002) and investigators (P < 0.001). Quantitative analysis also indicated that MPFF treatment tended to decrease pain (P = 0.06). In general, qualitative analysis of the studies indicated that MPFF treatment was beneficial for the most important signs and symptoms of acute HD and for post-hemorrhoidectomy patients.
Four studies in this systematic review reported significant reductions in bleeding compared to placebo after MPFF treatment [19, 22, 23, 25, 26, 30] versus two that reported no significant benefit [31, 32]. Only two of these studies could be combined for meta-analysis [22, 23, 30], with the result showing a significant benefit in favor of MPFF treatment by reducing the risk of bleeding by over 90% (OR 0.08, P < 0.001). Four studies also reported that MPFF treatment showed an apparent benefit in reducing post-procedure bleeding [24, 27–29].
Similar benefits of MPFF treatment in reducing bleeding were reported in two previous meta-analyses. In a Cochrane meta-analysis of the studies by Jiang and Misra [19, 30], Perera et al. found an OR of 0.12 (95% CI 0.04–0.37; P < 0.001) in favor of MPFF over placebo [13]. Whereas in a more recent meta-analysis of the studies by Jiang, Cospite, and Misra, which was published while our study was being conducted, Aziz et al. found a relative risk (RR) of 1.46 (95% CI 1.10–1.93; P = 0.008) in favor of MPFF treatment for reducing bleeding [41]. Our findings confirm these results, indicating that MPFF reduces bleeding effectively and quickly.
Qualitative analysis of MPFF treatment for reducing pain associated with acute HD included three studies reporting significant benefit after treatment [19, 22, 23, 25, 26] and two studies reporting no significant benefit [31, 32]. Pooled meta-analysis indicated that MPFF treatment is associated with a nearly statistically significant benefit for pain (P = 0.06). Results from three previous meta-analyses are varied, with one reporting significant reduction in pain with MPFF or flavonoid (RR 0.35, 95% CI 0.18–0.69) treatment [8], one a nearly significant benefit (P = 0.06) [13], and one no significant benefit (P = 0.47) [41]. The reason for this variability may be that pain is not a universal symptom in internal HD and, if present, it is often at a low level. Thus it may be difficult to demonstrate a statistically significant benefit in studies lacking enough patients to provide sufficient statistical power.
In contrast, MPFF treatment after hemorrhoidectomy demonstrated consistent and statistically significant benefits in pain relief [21, 28, 29]. It is possible that the higher level of pain associated with hemorrhoidectomy allows the pain relief benefit of MPFF to be demonstrated more consistently than with acute HD.
Qualitative analyses suggest that MPFF treatment may provide some benefits for pruritus. Two studies reported significant improvements in anal itching in acute HD [19, 25, 26], although the treatment period in the Godeberge study was long (60 days) and the itching score improvement at day 7 in the Jiang study was small (1.1 vs 1.3). In contrast, a third study reported no significant improvement after 7 days of MPFF treatment [31]. In the one study that investigated pruritus after hemorrhoidectomy, significant benefits were observed in global itching scores [28]. Similarly, meta-analyses conducted by Perera et al. [13] and by Alonso-Coello et al. [8] found significant benefits for pruritus with flavonoid treatment in HD.
Among the five studies that investigated anal discharge or leakage in acute HD, three reported a significant benefit with MPFF treatment [22, 23, 25, 26, 32], whereas two did not [19, 31]. However, quantitative analysis of the results that could be pooled indicated a statistically significant benefit in favor of MPFF. This result was also found in a previous meta-analysis [13] that included the same studies by Jiang and Cospite [19, 22]. Significant benefits of MPFF in leakage or discharge were also observed in the recovery period after hemorrhoidectomy [29]. Taken together, these results could suggest a likely benefit for MPFF treatment in reducing leakage in HD.
There was some evidence for a positive effect of MPFF treatment on tenesmus from two studies [25, 26, 28] but the evidence for improvement of anal discomfort or edema was limited with only one of three studies reporting a significant benefit for each symptom [22, 23, 25, 26]. Such symptoms may be difficult to assess consistently if anal discomfort is not precisely defined and edema is difficult to measure.
Consistent benefits were reported for MPFF treatment in patient-assessed overall improvement and satisfaction either in acute HD [19, 22, 23, 25, 26, 32] or after hemorrhoidectomy [21]. The meta-analysis of three of these studies [19, 21–23] yielded a significant OR of 5.25 in favor of MPFF treatment. These results strongly suggest that patients consider MPFF to be an effective treatment providing quick relief for acute HD and in improving the quality of the recovery period after hemorrhoidectomy. Similarly, investigators in four studies also consistently considered MPFF an effective treatment [19, 22, 23, 25, 26, 32], and this was supported by the accompanying meta-analysis.
Reduction in the need for concomitant analgesic use, shorter hospital stays after hemorrhoidectomy, and prevention of relapses have been identified as indirect benefits of treatment with MPFF in some studies [21–23, 25, 26, 30].
Compared to our meta-analysis, the previously published meta-analyses by Perera et al. [13] and Alonso-Coello et al. [8] did not target any particular venoactive drug. On the other hand, the meta-analysis by Aziz et al. [41] specifically examined the effects of MPFF but excluded trials in patients who underwent surgery/procedures for HD. Moreover, Aziz et al. assessed only pain, bleeding, and itching whereas, in our meta-analysis, a wide range of parameters were investigated. As far as we can ascertain, the initial scope of our literature search was therefore broader than for the other meta-analyses and assessed more parameters which provided further added value.
The chief limitation in this study is that only 11 studies could be included in the systematic review and there was considerable heterogeneity among the endpoints reported in these studies. This heterogeneity limited our ability to pool the studies for meta-analysis. In addition, variability in the results for some of the endpoints, which may have been the consequence of differences in patient populations and assessment methods, made it difficult to draw firm conclusions from the qualitative analyses. Another limitation is that the risk of bias was high or unclear in seven of the 11 studies. Despite the fact that no limit for treatment duration was set, all of the studies which met the selection criteria treatment were of short duration or, if of longer duration, only short-term assessments were performed. One study performed by Godeberge and collaborators [25, 26] measured longer-term efficacy, but in an open-label follow-up. Consequently, no studies assessing the long-term benefit of MPFF versus placebo under blinded conditions have been identified. Lastly, none of the studies evaluated potential benefits of treatment with MPFF on hard clinical endpoints (e.g., less need for transfusions, fewer hospital admissions) after hemorrhoidectomy.
Despite these limitations, findings from qualitative analysis were consistent in suggesting benefits of MPFF treatment in reducing bleeding in acute HD and in patients after hemorrhoidectomy. Quantitative analyses also indicated that MPFF treatment was associated with a significant benefit in reducing discharge and leakage, and a benefit in reducing pain that was nearly statistically significant. Consistent and statistically significant quantitative evidence was also found for the overall improvement in symptoms as assessed by patients and investigators.
Conclusions
The aim of our review was to assess all published randomized placebo-controlled trials investigating the effect of MPFF in relieving the signs and symptoms in patients with acute HD as well as RCTs with patients who underwent medical or surgical procedures. We thus performed an extensive search of several different databases to identify these studies.
Taken together, the results of our qualitative and quantitative analyses suggest that MPFF treatment can improve the most important signs and symptoms of HD including bleeding, pain, pruritus, tenesmus, and anal discharge/leakage.
Acknowledgements
Funding
Financial support for this study, Rapid Service Fee and Open Access Fees was provided by the Institut de Recherches Internationales Servier (Suresnes, France). The sponsor provided support for the literature search, data acquisition, statistical analysis, and medical writing assistance. The sponsor also reviewed the manuscript prior to submission for publication.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance was provided by Dr. Kurt Liittschwager (4Clinics, France) and paid for by the sponsor (Servier, France). Statistical analysis was performed by statisticians employed by the sponsor.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Parvez Sheikh, Varut Lohsiriwat, and Yury Shelygin have received speaker fees from the study sponsor Servier.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Digital Features
This article is published with digital features, including a video abstract, slide deck and summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.12145386.
Change history
6/23/2021
The original article was revised due to update in digital feature statement.
References
- 1.Haas PA, Haas GP, Schmaltz S, Fox TA., Jr The prevalence of hemorrhoids. Dis Colon Rectum. 1983;26(7):435–439. doi: 10.1007/BF02556521. [DOI] [PubMed] [Google Scholar]
- 2.Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology. 1990;98(2):380–386. doi: 10.1016/0016-5085(90)90828-O. [DOI] [PubMed] [Google Scholar]
- 3.Riss S, Weiser FA, Schwameis K, et al. The prevalence of hemorrhoids in adults. Int J Colorectal Dis. 2012;27(2):215–220. doi: 10.1007/s00384-011-1316-3. [DOI] [PubMed] [Google Scholar]
- 4.Sun Z, Migaly J. Review of hemorrhoid disease: presentation and management. Clin Colon Rectal Surg. 2016;29(1):22–29. doi: 10.1055/s-0035-1568144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madoff RD, Fleshman JW. American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology. 2004;126(5):1463–1473. doi: 10.1053/j.gastro.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Cristea C, Lewis CR. Hemorrhoidectomy. StatPearls. Treasure Island (FL). 2019. https://www.ncbi.nlm.nih.gov/books/NBK549864.
- 7.Misra MC. Drug treatment of haemorrhoids. Drugs. 2005;65(11):1481–1491. doi: 10.2165/00003495-200565110-00003. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, et al. Meta-analysis of flavonoids for the treatment of haemorrhoids. Br J Surg. 2006;93(8):909–920. doi: 10.1002/bjs.5378. [DOI] [PubMed] [Google Scholar]
- 9.Kakkos SK, Nicolaides AN. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int Angiol. 2018;37(2):143–154. doi: 10.23736/S0392-9590.18.03975-5. [DOI] [PubMed] [Google Scholar]
- 10.Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018;19(6):1669. doi: 10.3390/ijms19061669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johanson JF. Nonsurgical treatment of hemorrhoids. J Gastrointest Surg. 2002;6(3):290–294. doi: 10.1016/S1091-255X(01)00081-6. [DOI] [PubMed] [Google Scholar]
- 12.Lyseng-Williamson KA, Perry CM. Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63(1):71–100. doi: 10.2165/00003495-200363010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Perera N, Liolitsa D, Iype S, et al. Phlebotonics for haemorrhoids. Cochrane Database Syst Rev. 2012(8):CD004322. [DOI] [PubMed]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint-preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880. doi: 10.1093/ptj/89.9.873. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 17.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang ZM, Cao JD. The impact of micronized purified flavonoid fraction on the treatment of acute haemorrhoidal episodes. Curr Med Res Opin. 2006;22(6):1141–1147. doi: 10.1185/030079906X104803. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011] London: The Cochrane Collaboration; 2011. [Google Scholar]
- 21.Colak T, Akca T, Dirlik M, Kanik A, Dag A, Aydin S. Micronized flavonoids in pain control after hemorrhoidectomy: a prospective randomized controlled study. Surg Today. 2003;33(11):828–832. doi: 10.1007/s00595-003-2604-5. [DOI] [PubMed] [Google Scholar]
- 22.Cospite M. Double-blind, placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology. 1994;45(6 Pt 2):566–573. [PubMed] [Google Scholar]
- 23.Cospite M. Double blind placebo controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute haemorrhoids. Phlebology. 1994;9(Suppl 1):40–43. doi: 10.1177/0268355594009001s12. [DOI] [PubMed] [Google Scholar]
- 24.Dimitroulopoulos D, Tsamakidis K, Xinopoulos D, Karaitianos I, Fotopoulou A, Paraskevas E. Prospective, randomized, controlled, observer-blinded trial of combined infrared photocoagulation and micronized purified flavonoid fraction versus each alone for the treatment of hemorrhoidal disease. Clin Ther. 2005;27(6):746–754. doi: 10.1016/j.clinthera.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Godeberge P. Daflon 500 mg is significantly more effective than placebo in the treatment of haemorrhoids. Phlebology. 1992;7(Suppl. 2):61–63. [Google Scholar]
- 26.Godeberge P. Daflon 500 mg in the treatment of hemorrhoidal disease: a demonstrated efficacy in comparison with placebo. Angiology. 1994;45(6 Pt 2):574–578. [PubMed] [Google Scholar]
- 27.Ho YH, Foo CL, Seow-Choen F, Goh HS. Prospective randomized controlled trial of a micronized flavonidic fraction to reduce bleeding after haemorrhoidectomy. Br J Surg. 1995;82(8):1034–1035. doi: 10.1002/bjs.1800820809. [DOI] [PubMed] [Google Scholar]
- 28.La Torre F, Nicolai AP. Clinical use of micronized purified flavonoid fraction for treatment of symptoms after hemorrhoidectomy: results of a randomized, controlled, clinical trial. Dis Colon Rectum. 2004;47(5):704–710. doi: 10.1007/s10350-003-0119-1. [DOI] [PubMed] [Google Scholar]
- 29.Lee HW, Lee WY, Chun HK. Clinical effects of Venitol® on complications after hemorrhoidectomy prospective randomized and placebo-controlled trial. J Korean Soc Coloproctol. 1998;14(4):761–766. [Google Scholar]
- 30.Misra MC, Parshad R. Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br J Surg. 2000;87(7):868–872. doi: 10.1046/j.1365-2168.2000.01448.x. [DOI] [PubMed] [Google Scholar]
- 31.Panpimanmas S, Sithipongsri S, Sukdanon C, Manmee C. Experimental comparative study of the efficacy and side effects of Cissus quadrangularis L. (Vitaceae) to Daflon (Servier) and placebo in the treatment of acute hemorrhoids. J Med Assoc Thai. 2010;93(12):1360–1367. [PubMed] [Google Scholar]
- 32.Vajrabukka T, Rojanasakul A, Vathanophas V, et al. Therapeutic activity of Daflon 500 mg® in acute episodes of hemorrhoids. Chula Med J. 1994;38(2):77–83. [Google Scholar]
- 33.Katsenis K. Micronized purified flavonoid fraction (MPFF): a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr Vasc Pharmacol. 2005;3(1):1–9. doi: 10.2174/1570161052773870. [DOI] [PubMed] [Google Scholar]
- 34.das Gracas CSM, Cyrino FZ, de Carvalho JJ, Blanc-Guillemaud V, Bouskela E. Protective effects of micronized purified flavonoid fraction (MPFF) on a novel experimental model of chronic venous hypertension. Eur J Vasc Endovasc Surg. 2018;55(5):694–702. doi: 10.1016/j.ejvs.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Friesenecker B, Tsai AG, Allegra C, Intaglietta M. Oral administration of purified micronized flavonoid fraction suppresses leukocyte adhesion in ischemia-reperfusion injury: in vivo observations in the hamster skin fold. Int J Microcirc Clin Exp. 1994;14(1–2):50–55. doi: 10.1159/000178206. [DOI] [PubMed] [Google Scholar]
- 36.Korthuis RJ, Gute DC. Postischemic leukocyte/endothelial cell interactions and microvascular barrier dysfunction in skeletal muscle: cellular mechanisms and effect of Daflon 500 mg. Int J Microcirc Clin Exp. 1997;17(Suppl 1):11–17. doi: 10.1159/000179261. [DOI] [PubMed] [Google Scholar]
- 37.Cotonat A, Cotonat J. Lymphagogue and pulsatile activities of Daflon 500 mg on canine thoracic lymph duct. Int Angiol. 1989;8(4 Suppl):15–18. [PubMed] [Google Scholar]
- 38.McHale NG, Hollywood MA. Control of lymphatic pumping: interest of Daflon 500 mg. Phlebology. 1994;9:23–25. doi: 10.1177/0268355594009001s08. [DOI] [Google Scholar]
- 39.Behar A, Lagrue G, Cohen-Boulakia F, Baillet J. Study of capillary filtration by double labelling I131-albumin and Tc99m red cells. Application to the pharmacodynamic activity of Daflon 500 mg. Int Angiol. 1988;7(2 Suppl):35–38. [PubMed] [Google Scholar]
- 40.Galley P, Thiollet M. A double-blind, placebo-controlled trial of a new veno-active flavonoid fraction (S 5682) in the treatment of symptomatic capillary fragility. Int Angiol. 1993;12(1):69–72. [PubMed] [Google Scholar]
- 41.Aziz Z, Huin WK, Badrul Hisham MD, Tang WL, Yaacob S. Efficacy and tolerability of micronized purified flavonoid fractions (MPFF) for haemorrhoids: a systematic review and meta-analysis. Complement Ther Med. 2018;39:49–55. doi: 10.1016/j.ctim.2018.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.