Abstract
Introduction
Janus kinase (JAK) inhibitors are a class of targeted therapies for rheumatoid arthritis (RA) with established clinical efficacy. However, little is known about their efficacy compared with each other. This network meta-analysis (NMA) estimated the comparative efficacy of JAK inhibitors currently approved for RA.
Methods
A targeted literature review was conducted for phase III randomized controlled trials (RCTs) evaluating the efficacy of three approved JAK inhibitors (tofacitinib, baricitinib, and upadacitinib) as monotherapy or combination therapy among patients with moderate-to-severe RA who had inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARD-IR). Using Bayesian NMA, American College of Rheumatology (ACR) 20/50/70 responses and clinical remission (defined as DAS28-CRP < 2.6) were evaluated separately at 12 and 24 weeks.
Results
Eleven RCTs were identified and included in the NMA. All JAK inhibitors demonstrated significantly better efficacy than csDMARD. Among combination therapies, upadacitinib 15 mg had the highest 12-week ACR50 responses (median [95% credible interval]: 43.4% [33.4%, 54.5%]), followed by tofacitinib 5 mg (38.7% [28.6%, 49.8%]), baricitinib 2 mg (37.1% [25.0%, 50.6%]), and baricitinib 4 mg (36.7%, [27.2%, 47.0%]). Similar results were observed for ACR20/70 and at week 24. Upadacitinib 15 mg + csDMARD was also found to have the highest clinical remission rates at week 12 (29.8% [16.9%, 47.0%]), followed by tofacitinib 5 mg (24.3%, [12.7%, 40.2%]), baricitinib 4 mg (22.8%, [11.8%, 37.5%]), and baricitinib 2 mg (20.1%, [8.6%, 37.4%]). Similar results were seen at week 24. Among monotherapies, upadacitinib had a higher ACR50 response (38.5% [25.3%, 53.2%]) than tofacitinib (30.4% [18.3%, 45.5%]). The differences in efficacy measures were not statistically significant between the JAK inhibitors.
Conclusions
The NMA found that upadacitinib 15 mg once daily had numerically higher efficacy in terms of ACR response and clinical remission among approved JAK combination therapies and monotherapies for csDMARD-IR patients with RA.
Electronic Supplementary Material
The online version of this article (10.1007/s12325-020-01303-3) contains supplementary material, which is available to authorized users.
Keywords: Clinical remission, Disease-modifying antirheumatic drugs, Janus kinase inhibitors, Network meta-analysis, Rheumatoid arthritis, Rheumatology
Key Summary Points
| Why carry out this study? |
| JAK inhibitors are a class of targeted therapies for treating patients with moderate-to-severe rheumatoid arthritis (RA) who have an inadequate response to csDMARDs (csDMARD-IR). |
| Because of the absence of direct head-to-head studies, comparative efficacy data between the currently approved JAK inhibitors - tofacitinib, baricitinib, and upadacitinib is limited. |
| This network meta-analysis (NMA) compared ACR20/50/70 and DAS28-CRP remission outcomes at 12- and 24-weeks for all JAK inhibitors currently approved for the treatment of RA. |
| What was learned from the study? |
| The results indicated that upadacitinib 15 mg once daily + csDMARD had numerically the highest ACR responses and DAS28-CRP remission rates among all combination therapies at both 12 and 24 weeks and among monotherapies, ACR responses with upadacitinib 15 mg once daily were numerically higher than those with tofacitinib 5 mg twice daily. |
| The study helps in better understanding of the comparative efficacy of the three approved JAKs via an indirect treatment comparison approach. Given the lack of H2H trials between the three JAKs, the study findings study findings could provide insights to physicians and payers in treatment and reimbursement decision making. |
INTRODUCTION
Janus kinase (JAK) inhibitors are small molecules that block the JAK family enzymes (i.e., JAK1, JAK2, JAK3, and tyrosine kinase 2 [TYK2]) that play a role in the cell-signaling processes leading to inflammation and immune responses observed in rheumatoid arthritis (RA) [1]. Currently, three JAK inhibitors (tofacitinib, baricitinib, and upadacitinib) have been approved by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for use in moderate-to-severe RA. All inhibitors have been approved for use either as monotherapy or in combination with methotrexate (MTX), one of the most commonly used conventional synthetic disease-modifying antirheumatic drugs (csDMARD).
Tofacitinib was the first JAK inhibitor approved by the FDA in 2012 and subsequently by the EMA in 2017 for use in patients with moderate-to-severe RA and a prior inadequate response (IR) to MTX (MTX-IR) or csDMARDs (csDMARD-IR) at a dose of 5 mg twice daily (BID). Tofacitinib predominantly inhibits JAK3, JAK1, and to a lesser degree, JAK2. In human cells, tofacitinib preferentially inhibits signaling associated with JAK3 and/or JAK1, with functional selectivity over pairs of JAK2 [2]. Baricitinib mainly inhibits JAK2 [3, 4]. In cell-free isolated enzyme assays, baricitinib had greater inhibitory potency at JAK1, JAK2, and TYK2 relative to JAK3 [5]. Baricitinib 2 mg once daily (QD) (as monotherapy or combination therapy) was approved for RA patients with inadequate response to one or more tumor necrosis factor antagonist therapies in the US and for csDMARD-IR in Canada, while baricitinib 2 mg and 4 mg QD (as monotherapy or combination therapy) were approved for RA patients with csDMARD-IR in Europe. Upadacitinib is a selective and reversible JAK inhibitor engineered to have greater inhibitory potency for JAK1 versus JAK2, JAK3, and TYK2. In human cellular assays, upadacitinib preferentially inhibits signaling by JAK1 or JAK1/3 with functional selectivity over cytokine receptors that signal via pairs of JAK2. In engineered cellular assays, upadacitinib demonstrated approximately 40-fold higher potency for JAK1 over JAK2, 130-fold for JAK1 over JAK3, and 190-fold for JAK1 over TYK2 [6, 7]. Upadacitinib 15 mg QD (monotherapy or combination therapy) was approved in 2019 to treat moderate-to-severe patients with MTX-IR RA by the FDA and with csDMARD-IR RA by the EMA and other agencies including those in Canada, Japan, and Australia.
In phase III randomized controlled trials (RCTs), all three approved JAK inhibitor combination therapies demonstrated significantly improved American College of Rheumatology (ACR) response and Disease Activity Score for 28 joints (DAS28) versus placebo and csDMARDs [8–13]. Similarly, upadacitinib monotherapy also showed significantly better efficacy than csDMARDs [8]. While there are no studies of direct comparisons between JAK inhibitors, all the approved JAK inhibitors have been studied in direct head-to-head studies with adalimumab, which have yielded different outcomes. Both upadacitinib 15 mg + MTX and baricitinib 4 mg + MTX have demonstrated clinical superiority over adalimumab + MTX at 12 weeks, albeit for different end points—ACR50 responses and improvements in pain severity and physical function for upadacitinib [14]—and ACR20 and change in DAS28-C-reactive protein (DAS28-CRP) for baricitinib [15]. However, only upadacitinib + MTX demonstrated significantly higher remission rates (DAS28-CRP < 2.6, Clinical Disease Activity Index < 2.8, and Boolean) compared with adalimumab + MTX [14]. In contrast, tofacitinib + MTX demonstrated non-inferiority (based on ACR50) compared with adalimumab + MTX [16, 17]. The observed differences in trial results imply that individual JAK inhibitors may exhibit distinct efficacy profiles. However, in the absence of any direct head-to-head studies, the comparative effectiveness of the JAK inhibitors remains unclear.
The lack of comparative efficacy evidence may create uncertainty for physicians when determining which JAK inhibitor is most suitable for their patients with RA. The comparative efficacy among JAK inhibitors can provide insights to payers for decision making. To this end, the present network meta-analysis (NMA) aimed to evaluate the comparative efficacy of upadacitinib, baricitinib, and tofacitinib (including monotherapy and combination therapy) for csDMARD-IR patients with moderate-to-severe RA.
METHODS
Study Identification and Selection
A targeted literature review (TLR) was conducted for phase III RCTs evaluating the efficacy of tofacitinib, baricitinib, and upadacitinib among csDMARD-IR patients with moderate-to-severe RA.
The literature review was conducted by searching the MEDLINE, MEDLINE In-Process, EMBASE, and Cochrane databases for articles published prior to 1 April 2019. All databases were searched through the Ovid platform using full-text terms and Medical Subject Heading terms to identify clinical trials for all FDA/EMA-approved RA treatments. Electronic searches were supplemented by hand searching using the trial registry number (i.e., National Clinical Trial number) and trial name of included studies, relevant conference proceedings, and registry websites. All articles identified in the initial database search were screened for relevance based on title, abstract, and the full text.
To be included in the network, an RCT had to meet the criteria for the study population, interventions, comparators, and outcomes described in Table 1. Specifically, the trial had to include a comparison between csDMARDs and a JAK inhibitor or a comparison between different JAK inhibitors. In addition, the study population was required to be biologic-naïve or contain only a small proportion of patients with prior use of biologics (≤ 20%) [18, 19]. All RCTs that met the criteria were included in the ACR and DAS28 remission networks. Accordingly, all combination therapies for the three JAK therapies were included at both 12- and 24-week time points for both networks. Due to variability in the time points at which monotherapy outcomes were reported, the only monotherapies that could be compared were upadacitinib and tofacitinib on ACR outcomes at 12 weeks. There were no identified RCTs of baricitinib monotherapy in csDMARD-IR; hence, baricitinib monotherapy could not be compared with other monotherapies. Upadacitinib monotherapy data were not available at 24 weeks for either ACR or DAS28 outcomes. Therefore, no comparison could be made between upadacitinib and tofacitinib monotherapies at week 24 for ACR or DAS28 outcomes.
Table 1.
Study selection criteria for inclusion in the network meta-analysis
| Characteristic | Inclusion criteria |
|---|---|
| Population | Adult patients (≥ 18 years of age) meeting the ACR classification criteria for moderate-to-severe RA and had an inadequate response or were intolerant to at least one csDMARD. Trials reporting a small proportion of patients with prior use of bDMARDs (≤ 20%) were included |
| Interventions or comparators | csDMARD |
| Baricitinib oral 2 mg once daily | |
| Baricitinib oral 4 mg once daily | |
| Baricitinib oral 2 mg once daily + csDMARD | |
| Baricitinib oral 4 mg once daily + csDMARD | |
| Tofacitinib oral 5 mg twice daily | |
| Tofacitinib oral 5 mg twice daily + csDMARD | |
| Upadacitinib 15 mg once daily | |
| Upadacitinib 15 mg once daily + csDMARD | |
| Outcomes | ACR 20/50/70 response rate at 12 weeks (12–14 weeks) or 24 weeks (24–26 weeks) |
| Clinical remission (DAS28-CRP < 2.6) response rates at 12 weeks (12–14 weeks) or 24 weeks (24–26 weeks) | |
| Study design | Phase III randomized controlled trial |
ACR American College of Rheumatology, bDMARD biologic disease-modifying antirheumatic drug, csDMARD conventional synthetic disease-modifying antirheumatic drug, DAS28-CRP disease activity scored based on 28 joints and C-reactive protein, RA rheumatoid arthritis
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Outcome Measures
ACR Outcomes
ACR response evaluates the relative improvement of the RA disease condition associated with the treatment and is one of the primary outcomes used by the FDA to evaluate new treatments for RA [20]. ACR20, ACR50, and ACR70 were the primary outcomes in the majority of the identified RCTs and therefore were selected as co-primary outcomes in the NMA. ACR20 is defined as a minimum of 20% improvement both in the number of swollen and tender joints and in three of the additional five measures: patient global assessment, physician global assessment, Health Assessment Questionnaire, visual analog pain scale, and erythrocyte sedimentation rate or CRP [21]. ACR50 and ACR70 outcomes were similarly defined, with improvement levels of 50% and 70%, respectively.
DAS28-CRP Remission
DAS28-CRP is a composite score based on 28 tender joint counts, 28 swollen joint counts, patient global health assessment, and CRP (a biomarker for inflammation). It is reported as a continuous score ranging from 0 to 10. Lower DAS28-CRP scores indicate lower disease activity and therefore better treatment outcomes. In the clinical management of RA, clinical remission typically serves as the ultimate goal in the treat-to-target strategy recommended by the ACR and the European League Against Rheumatism [22–25]. Multiple disease activity measures could be used to define clinical remission. Among those, DAS28-CRP remission (defined as DAS28-CRP < 2.6) was the most commonly reported in the clinical trials of JAK inhibitors and thus was considered in the current study.
For this analysis, DAS28-CRP scores were classified into three categories, clinical remission (< 2.6), low disease activity (LDA) (2.6 – < 3.2), and medium/high disease activity (MDA/HDA) (> 3.2). All three DAS28-CRP categories were modeled as a multinomial outcome in the NMA. Among these, clinical remission is the most clinically meaningful outcome as it serves as the ultimate therapeutic target in RA management.
Outcomes (ACR and DAS28-CRP remission) reported between week 12 and 14 were used for the 12-week analysis, and data between week 24 and 26 were used for the 24-week analysis.
NMAs
Four separate NMAs were conducted—one for each of the combinations of time points (12 and 24 weeks) and outcomes (ACR and DAS28-CRP). NMA combines data from several different randomized studies of treatment comparisons to deliver an internally consistent set of estimates while respecting the randomization within each trial. Given that the ACR outcomes (i.e., ACR20, ACR50, and ACR70) and DAS28-CRP (i.e., remission, low-disease activity, and medium/high disease activity) were ordered multinomial, Bayesian NMA was conducted using an ordered multinomial likelihood with a probit link function to estimate the probabilities of achieving each of the response categories. This model allowed the three outcomes to be analyzed jointly and further assumed that each treatment had the same effect on each outcome category on the probit scale [26].
A random-effects model was chosen because it provided a means of quantifying the between-trials heterogeneity and is a conservative approach. Random-effects models provide a generalizable set of results by treating the selected studies as random samples from a larger population. In addition, we observed similar values of the deviance information criteria for the random-effects models and fixed-effects models, which also supports our model selection.
For each NMA, the model was run with three chains and 50,000 posterior samples per chain. The posterior distribution for the probability of achieving ACR responses and DAS28-CRP outcomes was summarized using posterior medians and the associated 95% credible intervals (CrI). The odds ratios (ORs) relative to csDMARD were calculated for both the ACR and DAS28-CRP outcomes. The surface under the cumulative ranking curve (SUCRA) was used to assess the overall ranking of each treatment. SUCRA can be interpreted as the average proportion of comparators that would have worse efficacy than the treatment of interest [27]. The higher the SUCRA value, the higher the likelihood that a therapy is in the top rank (or one of the top ranked). The closer to 0 the SUCRA value, the higher the likelihood that a therapy is in the bottom rank (or one of the bottom ranks) (17). To compare each pair of treatments, the posterior probability for one treatment being associated with a higher ACR response/DAS28 remission rate than the other was calculated.
All analyses were implemented using the statistical software R (v3.5.1) and Just Another Gibbs Sampler, also known as JAGS.
RESULTS
Evidence Base
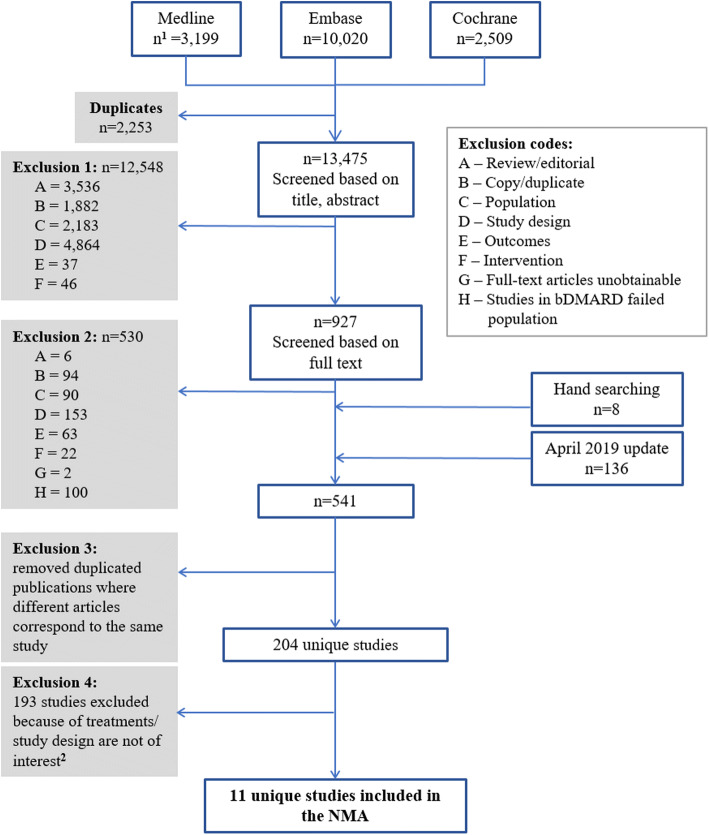
Of the 13,475 unique publications identified from the TLR, 11 RCTs met the eligibility criteria and were included in the NMA (Fig. 1). The details of all included studies are described in Supplemental Table 1. No trials were identified for baricitinib (2 mg or 4 mg QD) monotherapy in the csDMARD-IR population for either ACR response or DAS28-CRP remission outcomes.
Fig. 1.
PRISMA diagram showing the selection of RA trials included in the NMA. Abbreviations: bDMARD biologic disease-modifying antirheumatic drugs, PRISMA preferred reporting items for systematic reviews and meta-analyses, NMA network meta-analysis, RA rheumatoid arthritis. Notes: 1n represents the number of identified publications. 2Studies were excluded because of one of the following reasons: not a phase III trial, no outcome of interest reported, or did not include the treatment or dosage of interest (i.e., JAK inhibitors with approved dosage)
In terms of inclusion/exclusion criteria, all studies included csDMARD-IR patients with moderate-to-severe RA and no prior usage of biologic DMARD (bDMARD), with the exception of four studies that enrolled < 20% patients with prior bDMARD use (Supplemental Table 1). Other inclusion and exclusion criteria for study participants, such as age and disease severity, were generally similar across trials. Extracted trial data are provided in Supplemental Tables 3 and 4.
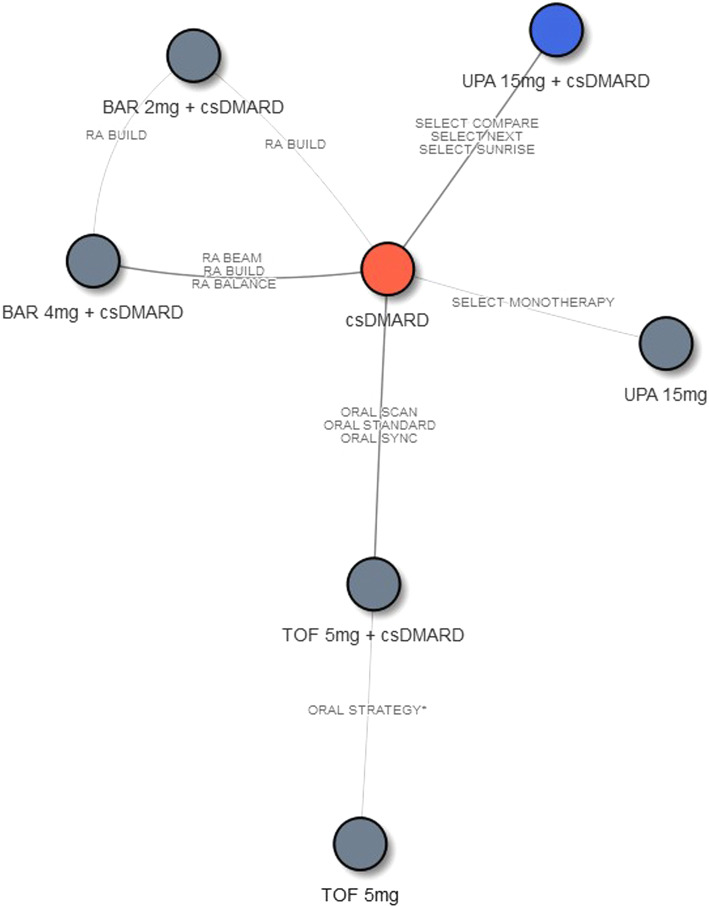
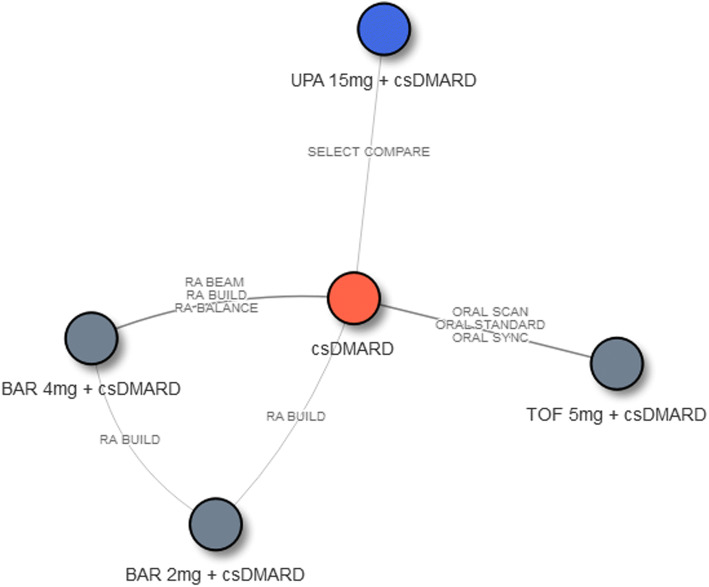
The network diagrams for NMAs of efficacy outcomes assessed at week 12 and week 24 are presented in Figs. 2 and 3, respectively. Treatments of interest were compared mainly through their connections with csDMARD. One exception is tofacitinib 5 mg BID, which was connected through tofacitinib 5 mg BID + csDMARD to the week 12 evidence network. For ACR, the 12-week NMA included 7 interventions from 11 RCTs, and the 24-week NMA included 5 interventions from 7 RCTs. For DAS28-CRP remission, the 12-week NMA included 5 interventions from 9 RCTs, and the 24-week NMA included 5 interventions from 7 RCTs.
Fig. 2.
Network diagram of studies contributing ACR outcomes (N = 11) and DAS28-CRP remission (N = 9) at week 12. Abbreviations: ACR American College of Rheumatology, BAR baricitinib, csDMARD conventional synthetic disease-modifying antirheumatic drug, csDMARD-IR inadequate response to csDMARD, RA rheumatoid arthritis, TOF tofacitinib, UPA upadacitinib. Note: 1ORAL Strategy and SELECT-MONOTHERAPY were not included in the DAS28 network
Fig. 3.
Network diagram of studies contributing ACR outcomes and DAS28-CRP remission at week 24 (N = 7). Abbreviations: ACR American College of Rheumatology, BAR baricitinib, csDMARD conventional synthetic disease-modifying antirheumatic drug, csDMARD-IR inadequate response to csDMARD, RA rheumatoid arthritis, TOF tofacitinib, UPA upadacitinib
Combination Therapies
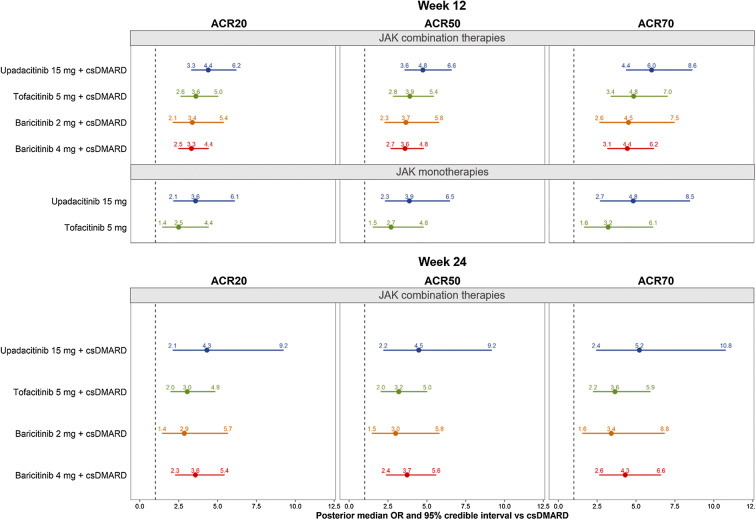
Among JAK combination therapies, upadacitinib 15 mg QD had the numerically highest estimated median ACR50 at 12 weeks (median [95% CrI]: 43.4% [33.4%, 54.5%]), followed by tofacitinib 5 mg BID (38.7% [28.6%, 49.8%]), baricitinib 2 mg QD (37.1% [25.0%, 50.6%]), and baricitinib 4 mg QD (36.7%, [27.2%, 47.0%]). In contrast, csDMARD were estimated to have median ACR50 of 13.9% (95% CrI: 10.0%, 18.8%) at week 12. The corresponding SUCRA values among JAK combination therapies ranged from 0.516 (the lowest) for baricitinib 4 mg QD to 0.898 (the highest) for upadacitinib 15 mg QD (Table 2). Similar results were seen for ORs of achieving ACR responses versus csDMARD. The highest ORs for ACR50 were achieved by upadacitinib 15 mg QD (median [95% CrI]: 4.8 [3.6, 6.6]), and the lowest ORs for ACR50 were seen for baricitinib 4 mg QD (3.6 [2.7, 4.8]) among combination therapies in the 12-week network (Fig. 4).
Table 2.
ACR outcomes and SUCRA scores at week 12/24 in the csDMARD-IR RA population
| Treatment | Median ACR20% (95% CrI)1 | Median ACR50% (95% CrI)1 | Median ACR70% (95% CrI)1 | SUCRA2 |
|---|---|---|---|---|
| Week 12 network3 | ||||
| csDMARD | 35.9 (28.9, 43.4) | 13.9 (10.0, 18.8) | 4.3 (2.7, 6.4) | 0.001 |
| JAK combination therapies4 | ||||
| Upadacitinib 15 mg + csDMARD | 71.1 (61.6, 79.8) | 43.4 (33.4, 54.5) | 21.1 (14.3, 30.0) | 0.898 |
| Tofacitinib 5 mg + csDMARD | 66.8 (56.3, 76.3) | 38.7 (28.6, 49.8) | 17.7 (11.4, 26.0) | 0.649 |
| Baricitinib 2 mg + csDMARD | 65.3 (51.9, 77.0) | 37.1 (25.0, 50.6) | 16.7 (9.5, 26.7) | 0.552 |
| Baricitinib 4 mg + csDMARD | 65.0 (54.7, 74.1) | 36.7 (27.2, 47.0) | 16.5 (10.7, 23.9) | 0.516 |
| JAK monotherapies4 | ||||
| Upadacitinib 15 mg | 66.7 (52.3, 78.9) | 38.5 (25.3, 53.2) | 17.6 (9.6, 28.9) | 0.626 |
| Tofacitinib 5 mg | 58.3 (42.9, 72.9) | 30.4 (18.3, 45.5) | 12.5 (6.1, 22.7) | 0.257 |
| Week 24 network3 | ||||
| csDMARD | 34.8 (27.9, 42.3) | 18.5 (13.6, 24.2) | 7.4 (5.0, 10.7) | 0.004 |
| JAK combination therapies | ||||
| Upadacitinib 15 mg + csDMARD | 69.7 (51.8, 84.0) | 50.4 (32.2, 68.8) | 29.6 (15.6, 47.7) | 0.871 |
| Baricitinib 4 mg + csDMARD | 65.5 (52.6, 76.4) | 45.7 (32.9, 58.3) | 25.6 (16.1, 36.9) | 0.696 |
| Tofacitinib 5 mg + csDMARD | 62.0 (48.8, 74.1) | 42.0 (29.5, 55.6) | 22.7 (13.8, 34.3) | 0.496 |
| Baricitinib 2 mg + csDMARD | 60.4 (41.9, 76.5) | 40.4 (23.8, 58.5) | 21.5 (10.4, 37.0) | 0.433 |
ACR American College of Rheumatology, csDMARD conventional synthetic disease-modifying antirheumatic drug, csDMARD-IR inadequate response to csDMARD, CrI credible interval, JAK Janus kinase, RA rheumatoid arthritis, SUCRA surface under the cumulative ranking curve
1Medians and credible intervals for ACR outcomes were estimated using a random effects multinomial model. The distribution of means and credible intervals were sampled using Monte Carlo methods (150,000 posterior simulations per treatment after 50,000 burn-in, thinning parameter of 10, and 3 chains)
2SUCRA was calculated to assess the overall ranking of each treatment based on ACR20 outcomes. Higher SUCRA values (closer to 1) represent more favorable rankings
3Due to differences in trial design, ACR outcomes were used in the 12-week network if reported between 12 and 14 weeks and used in the 24-week network if reported between 24 and 26 weeks
4JAK combination therapies and monotherapy treatments were analyzed together in the same network for 12-week ACR outcomes
Fig. 4.
Forest plot of week 12 and 24 model results in csDMARD-IR RA: OR of achieving ≥ 20%, ≥ 50%, or ≥ 70% ACR response versus csDMARD(s). Abbreviations: ACR American College of Rheumatology, csDMARD conventional synthetic disease-modifying antirheumatic drug, csDMARD-IR inadequate response to csDMARD, OR odds ratio, RA rheumatoid arthritis. Notes: 1Medians and credible intervals for ACR responses were estimated using a random effects multinomial model. The distribution of means and credible intervals was sampled using Monte Carlo methods (150,000 posterior simulations per treatment after 50,000 burn-in, thinning parameter of 10, and 3 chains). 2Due to differences in trial design, ACR responses were used in the 12-week network if reported between 12 and 14 weeks and used in the 24-week network if reported between 24 and 26 weeks. 3JAK combination therapies and monotherapy treatments were analyzed together in the same network
At week 24, upadacitinib 15 mg QD was still ranked numerically highest for ACR50 response (50.4% [32.2%, 68.8%]) among JAK combination therapies, followed by baricitinib 4 mg QD (45.7% [32.9%, 58.3%], tofacitinib 5 mg BID (42.0% [29.5%, 55.6%]) and baricitinib 2 mg QD (40.4% [23.8%, 58.5%]), higher than csDMARD (18.5% [13.6, 24.2]) with at least 95% probability. The highest SUCRA value calculated based on ACR response was achieved by upadacitinib 15 mg QD (0.871), and the lowest SUCRA value was observed for baricitinib 2 mg QD (0.433) among JAK combination therapies (Table 2). Similar results were seen for ORs, with median values calculated based on ACR50 ranging from 3.0 (95% CrI 1.5, 5.8) for baricitinib 2 mg QD to 4.5 (2.2, 9.2) for upadacitinib 15 mg QD (Fig. 4). Efficacy ranks were consistent for ACR20/70 outcomes.
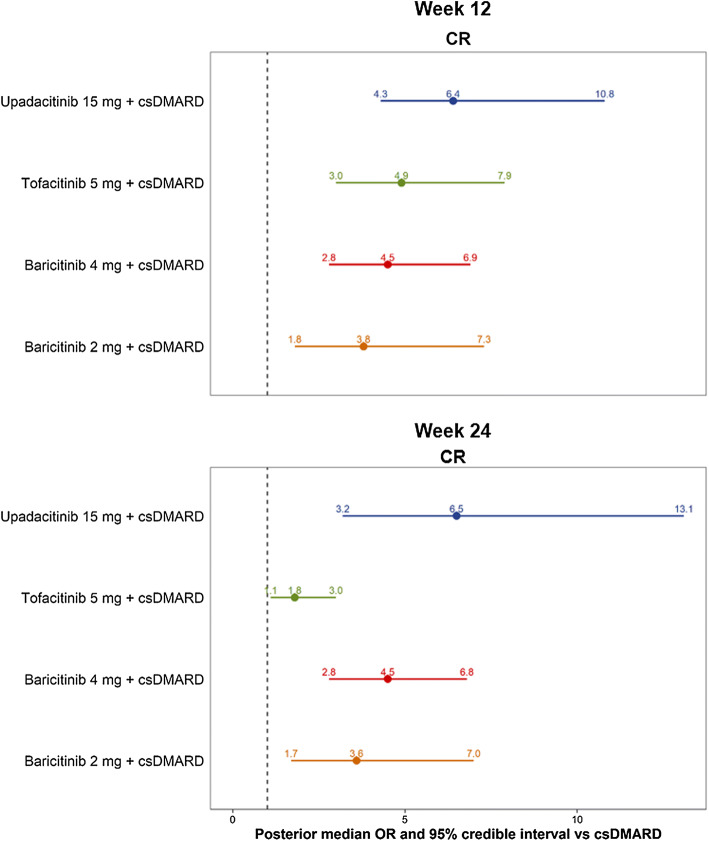
Comparable results were seen for clinical remission (DAS28-CRP < 2.6) outcomes. Among combination therapies, upadacitinib 15 mg QD demonstrated the numerically highest 12-week clinical remission rate (median [95% CrI]: 29.8% [16.9%, 47.0%]), followed by tofacitinib 5 mg BID (24.3% [12.7%, 40.2%]), baricitinib 4 mg QD (22.8% [11.8%, 37.5%]), and baricitinib 2 mg QD (20.1% [8.6%, 37.4%]). Conversely, csDMARD only achieved a clinical remission rate of 6.2% (95% CrI 2.9%, 11.9%) among patients with csDMARD-IR RA at week 12, lower than JAK inhibitors with at least 95% probability. The corresponding SUCRA values maintained the same efficacy ranks, with the highest value achieved by upadacitinib 15 mg QD (0.930) and the lowest value by baricitinib 2 mg QD (0.393) among JAK combination therapies (Table 3). For ORs based on DAS28-CRP remission, upadacitinib 15 mg QD had the highest ORs (6.4, [4.3, 10.8]), and baricitinib 2 mg QD had the lowest OR (3.8, [1.8, 7.3]) in the 12-week network (Fig. 5).
Table 3.
DAS28-CRP remission and SUCRA scores at week 12/24 in the csDMARD-IR RA population
| Treatment | DAS28-CRP remission | |
|---|---|---|
| Median rate, % (95% CrI)2 | SUCRA3 | |
| Week 12 network4 | ||
| csDMARD | 6.2 (2.9, 11.9) | 0.001 |
| JAK combination therapies | ||
| Upadacitinib 15 mg + csDMARD | 29.8 (16.9, 47.0) | 0.930 |
| Tofacitinib 5 mg + csDMARD | 24.3 (12.7, 40.2) | 0.628 |
| Baricitinib 4 mg + csDMARD | 22.8 (11.8, 37.5) | 0.549 |
| Baricitinib 2 mg + csDMARD | 20.1 (8.6, 37.4) | 0.393 |
| Week 24 network4 | ||
| csDMARD | 10.6 (5.5, 18.6) | 0.006 |
| JAK combination therapies | ||
| Upadacitinib 15 mg + csDMARD | 43.4 (24.1, 64.6) | 0.954 |
| Baricitinib 4 mg + csDMARD | 34.7 (20.1, 51.6) | 0.727 |
| Baricitinib 2 mg + csDMARD | 29.6 (14.0, 49.9) | 0.549 |
| Tofacitinib 5 mg + csDMARD | 17.8 (8.7, 31.8) | 0.265 |
CRP C-reactive protein, csDMARD conventional synthetic disease-modifying antirheumatic drug, CrI credible interval, DAS28 disease activity score in 28 joints, RA rheumatoid arthritis, SUCRA surface under the cumulative ranking curve
1DAS28-CRP remission was defined as a score < 2.6
2Medians and credible intervals for outcome categories were estimated using a random effects multinomial model. The distribution of means and credible intervals was sampled using Monte Carlo methods (150,000 posterior simulations per treatment after 50,000 burn-in, thinning parameter of 10, and 3 chains)
3SUCRA was calculated to assess the overall ranking of each treatment based on DAS28-CRP remission rate. Higher SUCRA values (closer to 1) represent more favorable rankings
4Due to differences in trial design, DAS28-CRP remission was used in the 12-week network if reported between 12 and 14 weeks and used in the 24-week network if reported between 24 and 26 weeks
Fig. 5.
Forest plot of week 12 and 24 model results in csDMARD-IR RA: OR of achieving DAS28-CRP remission versus csDMARD. Abbreviations: CR clinical remission, CRP, C-reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; csDMARD-IR, inadequate response to csDMARD; DAS28, disease activity score in 28 joints; OR, odds ratio; RA, rheumatoid arthritis. Notes: 1DAS28-CRP remission was defined as a score < 2.6. 2Medians and credible intervals for response categories were estimated using a random effects multinomial model. The distribution of means and credible intervals was sampled using Monte Carlo methods (150,000 posterior simulations per treatment after 50,000 burn-in, thinning parameter of 10, and 3 chains). 3Due to differences in trial design, DAS28 remission was used in the 12-week network if reported between 12 and 14 weeks and used in the 24-week network if reported between 24 and 26 weeks. 4Upadacitinib and baricitinib are once daily, and tofacitinib is twice daily
At week 24, upadacitinib 15 mg QD had the numerically highest remission rate of 43.4% (95% CrI 24.1%, 64.6%), followed by baricitinib 4 mg QD (34.7% [20.1%, 51.6%]), baricitinib 2 mg QD (29.6% [14.0%, 49.9%]), and tofacitinib 5 mg BID (17.8% [8.7%, 31.8%]). The treatment group of csDMARD had the lowest DAS28-CRP remission (< 2.6) rate of 10.6% (95% CrI 5.5%, 18.6%) among csDMARD-IR patients (Table 3). The clinical remission (DAS28-CRP < 2.6) based SUCRA values maintained the same efficacy ranks, with the highest value achieved by upadacitinib (0.954) and the lowest value by tofacitinib (0.265) among JAK combination therapies (Table 3). Similar results were seen for ORs of achieving clinical remission, ranging from 1.8 (1.1, 3.0) for tofacitinib 5 mg BID to 6.5 (3.2, 13.1) for upadacitinib 15 mg QD (Fig. 5). All JAK combination therapies demonstrated improved ACR responses and DAS28-CRP remission (< 2.6) rates compared with csDMARD with at least 95% probability. However, only numerical differences in efficacy outcomes were seen between JAK combination therapies. Results regarding DAS28-CRP LDA and MDA/HDA outcomes are provided in the Supplemental Table 2.
Monotherapies
For monotherapy, both upadacitinib 15 mg QD and tofacitinib 5 mg BID had improved ACR responses compared with csDMARD with at least 95% probability. At week 12, upadacitinib 15 mg QD had numerically higher 12-week ACR50 (median [95% CrI] 38.5% [25.3%, 53.2%]) than tofacitinib 5 mg BID (30.4% [18.3%, 45.5%]). Efficacy ranks were consistent for ACR20/70 outcomes. The corresponding SUCRA values were 0.626 for upadacitinib and 0.257 for tofacitinib (Table 2). Similarly, upadacitinib 15 mg QD had numerically higher ORs for ACR20/50/70 (ACR20: 3.6; ACR50: 3.9; ACR70: 4.8) than tofacitinib 5 mg BID (ACR20: 2.5; ACR50: 2.7; ACR70: 3.2) in the 12-week network (Fig. 4).
DISCUSSION
This NMA assessed the comparative efficacy of all three approved JAK inhibitors at their approved doses (upadacitinib 15 mg QD, baricitinib 2 and 4 mg QD, and tofacitinib 5 mg BID) among csDMARD-IR patients with moderate-to-severe RA. The results of the NMAs at 12 weeks and 24 weeks indicated that all three approved JAK inhibitor combination therapies demonstrated better efficacy than csDMARD, and upadacitinib and tofacitinib monotherapies also showed improved efficacy compared with csDMARDs. In addition, among combination therapies, upadacitinib 15 mg was associated with numerically higher ACR responses and clinical remission rates (DAS28-CRP < 2.6) compared with tofacitinib 5 mg, baricitinib 2 mg, and baricitinib 4 mg. Among monotherapies, upadacitinib 15 mg showed numerically higher ACR response rates compared with tofacitinib 5 mg.
To comprehensively evaluate the efficacy of JAK inhibitors, the present study considered two different efficacy outcomes: ACR response and DAS28-CRP remission. While the ACR response rates measured the improvement in disease condition relative to patients’ baseline, the DAS28-CRP remission rates assessed the absolute disease activity at any given time. Previous studies have suggested that the concordance between ACR response categories and DAS28-CRP remission categories could be low for patients with a high level of disease activity at baseline [28]. While both efficacy measures are important for depicting a complete picture of the efficacy profile of different JAK agents, the remission outcome has a particular clinical implication as it often serves as the ultimate therapeutic target to achieve. Treat-to-target has been established as a guiding principle for the management of RA and is endorsed by many professional organizations such as ACR, the European League Against Rheumatism, and the National Institute for Health and Care Excellence [22–25]. Following this principle, patients’ disease activities are evaluated every 3 or 6 months against the pre-specified therapeutic target, which should drive decisions to continue or adjust the existing treatment. It is worth noting that the difference between JAK inhibitors is particularly pronounced in DAS28-CRP remission outcomes at week 24. The NMA estimated that 25%–144% more patients were to achieve DAS28-CRP remission at week 24 with upadacitinib 15 mg compared with alternative JAK inhibitors, highlighting its clinical value in the management of RA.
Two previous NMAs have compared RCT-reported efficacy outcomes of JAK inhibitors in RA. Song et al. (whose study did not include baricitinib) concluded upadacitinib 15 mg QD + csDMARD had the highest ACR response rates, followed by tofacitinib 10 mg BID + csDMARD and tofacitinib 5 mg BID + csDMARD among patients with csDMARD-IR RA [29]. Bae and Lee (whose study did not include upadacitinib) concluded that tofacitinib 10 mg BID + csDMARD was the best performing intervention, followed by baricitinib 4 mg QD + csDMARD, baricitinib 2 mg QD + csDMARD, and tofacitinib 5 mg BID + csDMARD [30]. It should be noted that the dose of tofacitanib 10 mg BID is not approved for use in RA by either the FDA or EMA. Nevertheless, the results of these studies were similar to those of the present analyses, despite small variations in the relative rankings between baricitinib combination therapies and tofacitinib 5 mg BID + csDMARD arms in selected networks.
The results of the current study were also in line with the findings of studies using other indirect comparison methods. For example, a matching-adjusted indirect comparison indicated that upadacitinib 15 mg QD + MTX was associated with significantly higher ACR50 response and remission rates compared with tofacitinib 5 mg BID + MTX among patients with csDMARD-IR RA after adjusting for baseline characteristics [31].
The reason for variance in the clinical performance of the three JAK inhibitors observed in this NMA and other studies is unknown, given they all target JAKs. However, it is of note that each has a different inhibitory profile against the four JAK isotypes [32, 33]. Tofacitinib targets JAK1, JAK2, and JAK3; baricitinib targets JAK1 and JAK2 [34]. In human cellular assays, upadacitinib preferentially inhibits signaling by JAK1 or JAK1/3 with functional selectivity over cytokine receptors that signal via pairs of JAK2. In engineered cellular assays, upadacitinib shows greater inhibitory potency at JAK1 over JAK2, JAK3, and TYK2 [6, 7]. Although the different JAK inhibitors vary in their biologic mechanism, the linkage between different target profiles and treatment performance is largely unknown, and direct evidence from population-level studies is limited [35, 36]. Over the next few years, new JAK inhibitors such as filgotinib (a selective JAK1 inhibitor) [37] and peficitinib (a pan-JAK inhibitor) [38] are expected to be approved by the FDA and EMA. Their introduction to the RA treatment landscape should warrant a reevaluation of the comparative efficacy of all JAK inhibitors.
This study is subject to several limitations, many of which are common to all meta-analyses. First, the study designs and patient characteristics of the included trials were heterogeneous. Therefore, there is a risk that the differences in study designs, outcome assessments, statistical handling, and patient characteristics across the studies may have confounded the results of the analyses. To mitigate the impact of different study designs, only phase III RCTs were considered in the NMA that have more comparable trial designs. Second, the current NMA did not adjust for potential differences in placebo arm response rates across included trials, which could be a potential confounder of the estimated treatment effect. Third, the follow-up time was limited to 24 weeks, which may not be adequate to evaluate the longer term effects of the included therapies. Fourth, this study only focused on selected efficacy outcomes without comparing the safety outcomes across different JAK inhibitors. This is mainly because RCTs are usually statistically underpowered to detect the specific harm either by recruitment of a low-risk population or low intensity of ascertainment of event. Therefore, a NMA approach for comparison of safety outcomes across different interventions is subject to methodologic limitations and may not provide meaningful results. In addition, RCTs tend to have limited follow-up duration, which may not be appropriate for evaluating safety outcomes. Future studies evaluating comparative safety using long-term data are warranted to provide a balanced view of the benefit-risk profiles of different JAK inhibitors. Moreover, the results of this NMA were based on data from RCTs and may not be generalizable to the broader RA population managed in the real-world setting with heterogeneous characteristics and treatment plans. Furthermore, this NMA was restricted by the type of data that were available from the included RCTs. Most trials did not report DAS28 remission based on the erythrocyte sedimentation rate, so this outcome could not be studied. Additionally, fewer data were available for monotherapies including the absence of trials evaluating baricitinib monotherapy. Likewise, the placebo response varied in each study and the trial methodology included an “early escape” within trials where, if the primary outcome was not achieved at 12 weeks, then patients were subsequently considered non-responders at further time points. Most of the prior csDMARD(s) used were MTX. Therefore, the results are mostly generalizable to MTX inadequate responders and to a lesser degree inadequate responders to other csDMARDs. Finally, the current NMA was developed to focus on the csDMARD-IR population and among approved JAK inhibitors only. Future studies including other treatment types or focusing on different RA populations (e.g., biologic-IR, MTX-naïve) are warranted.
CONCLUSIONS
This NMA provides a comprehensive assessment of the comparative efficacy across approved JAK inhibitors for moderate-to-severe RA at 12- and 24-week outcomes. All approved JAK inhibitors were more efficacious than csDMARDs with 95% probability. At both time points, upadacitinib 15 mg QD monotherapy and in combination with csDMARD consistently demonstrated numerically higher responses in terms of ACR 20/50/70 and DAS28-CRP remission among patients with csDMARD-IR RA compared with other JAK inhibitors [39].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Financial support for the study was provided by AbbVie. AbbVie participated in interpretation of data, review, and approval of the presentation. All authors contributed to development of the presentation and maintained control over final content. The Rapid Service and Open Access Fees were funded by AbbVie.
Medical Writing, Editorial and Other Assistance
Manuscript drafts were prepared by Shelley Batts, PhD, a professional medical writer employed by Analysis Group, Inc. Support for this assistance was provided by AbbVie, Inc. The authors thank Jordan Cammarota, Akanksha Dua, Henry Lane, Rochelle Sun, Aozhou Wu, and Muhan Yuan, employees of Analysis Group, Inc., for assistance with analysis and preparation of the manuscript. The authors also thank Tim Shaw from AbbVie for his contribution as a clinical expert in reviewing the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Keith A. Betts, Ella X. Du, Cynthia Z. Qi, Yan Song, and Patrick Tang are employees of Analysis Group, Inc., which has received consulting fees from the sponsor. Ruta Sawant and Namita Tundia are employees of AbbVie, Inc., and hold stock/options. Janet Pope has consulted and received honoraria from AbbVie, Amgen, BMS, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sandoz, Sanofi, and UCB.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
This is a meta-analysis that used published data from clinical trials. Data are provided in the Supplemental Tables 3 and 4.
Footnotes
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.11962149.
References
- 1.Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, et al. The mechanism of action of tofacitinib—an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(2):318–328. [PubMed] [Google Scholar]
- 2.European Medicines Agency (EMA). Summary of Product Characteristics. XELJANZ 5 mg and 10 mg film-coated tablets.https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf. Accessed 2 Mar 2020.
- 3.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17(1):78. doi: 10.1038/nrd.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency (EMA). Summary of Product Characteristics. Olumiant 2 mg and 4 mg film-coated tablets. https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf. Accessed 28 Jan 2020.
- 5.United States Food and Drug Administration (FDA). Highlights of prescribing information: OLUMIANT (baricitinib) tablets, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf. Accessed 2 Mar 2020.
- 6.Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) BMC Rheumatol. 2018;2:23. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States Food and Drug Administration (FDA). Highlights of prescribing information: RINVOQ (upadacitinib) extended-release tablets, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211675s000lbl.pdf. Accessed 2 Mar 2020.
- 8.Smolen JS, Pangan AL, Emery P, Rigby W, Tanaka Y, Vargas JI, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303–2311. doi: 10.1016/S0140-6736(19)30419-2. [DOI] [PubMed] [Google Scholar]
- 9.Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, Li Y, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Takeuchi T, Yamaoka K, Oribe M, Kawano M, Zhou Y, et al. A phase 2b/3 randomised, placebo-controlled, double-blind study of upadacitinib, a selective jak1 inhibitor, in Japanese patients with active rheumatoid arthritis and inadequate response to conventional synthetic DMARDs. Ann Rheum Dis. 2018;77(Supplement 2):991–992. [Google Scholar]
- 11.Dougados M, Van Der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: Results from the RA-BUILD study. Ann Rheum Dis. 2017;76(1):88–95. doi: 10.1136/annrheumdis-2016-210094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: Twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559–570. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 13.Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: A randomized trial. Ann Intern Med. 2013;159(4):253–261. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann R, Pangan AL, Song IH, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 15.Taylor PC, Keystone EC, Van Der Heijde D, Weinblatt ME, Del Carmen ML, Gonzaga JR, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 16.Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390(10093):457–468. doi: 10.1016/S0140-6736(17)31618-5. [DOI] [PubMed] [Google Scholar]
- 17.Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Meijide JAG, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health Care and Excellence (NICE). Baricitinib for moderate to severe rheumatoid arthritis: Technology appraisal guidance. 2017; https://www.nice.org.uk/guidance/ta466. Accessed 2 Mar 2020.
- 19.National Institute for Health and Clinical Excellence (NICE). Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed: Technology appraisal guidance. 2016; https://www.nice.org.uk/guidance/ta375. Accessed 2 Mar 2020.
- 20.American College of Rheumatology Committee to Reevaluate Improvement C. A proposed revision to the ACR20: the hybrid measure of American College of Rheumatology response. Arthritis Rheum. 2007;57(2):193–202. [DOI] [PubMed]
- 21.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American college of rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36(6):729–740. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 22.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 23.Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health and Clinical Excellence (NICE). Rheumatoid arthritis in adults: Management (NICE guideline). 2018; https://www.nice.org.uk/guidance/ng100. Accessed 2 Mar 2020.
- 25.van Vollenhoven R. Treat-to-target in rheumatoid arthritis — are we there yet? Nat Rev Rheumatol. 2019;15(3):180–186. doi: 10.1038/s41584-019-0170-5. [DOI] [PubMed] [Google Scholar]
- 26.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Heijde D, Klareskog L, Boers M, Landewé R, Codreanu C, Bolosiu HD, et al. Comparison of different definitions to classify remission and sustained remission: 1 year TEMPO results. Ann Rheum Dis. 2005;64(11):1582–1587. doi: 10.1136/ard.2004.034371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song GG, Choi SJ, Lee YH. Comparison of the efficacy and safety of tofacitinib and upadacitinib in patients with active rheumatoid arthritis: A Bayesian network meta-analysis of randomized controlled trials. Int J Rheumat Dis. 2019;22(8):1563–1571. doi: 10.1111/1756-185X.13616. [DOI] [PubMed] [Google Scholar]
- 30.Bae SC, Lee YH. Comparison of the efficacy and safety of tofacitinib and baricitinib in patients with active rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Z Rheumatol. 2019;78(6):559–567. doi: 10.1007/s00393-018-0531-5. [DOI] [PubMed] [Google Scholar]
- 31.Edwards C, Sawant R, Du E, Cammarota J, Tang P, Garg V, et al. THU0168 A matching-adjusted indirect comparison (MAIC) of upadacitinib versus tofacitinib in csDMARD-IR patients with moderate to severe rheumatoid arthritis (RA) Annals of the Rheumatic Diseases. 2019;78:358. [Google Scholar]
- 32.O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheumat Dis. 2013;72(suppl 2):ii11–ii5. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs. 2014;23(8):1067–1077. doi: 10.1517/13543784.2014.918604. [DOI] [PubMed] [Google Scholar]
- 35.Riese RJ, Krishnaswami S, Kremer J. Inhibition of JAK kinases in patients with rheumatoid arthritis: scientific rationale and clinical outcomes. Best Pract Res Clin Rheumatol. 2010;24(4):513–526. doi: 10.1016/j.berh.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Namour F, Diderichsen PM, Cox E, Vayssiere B, Van der Aa A, Tasset C, et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic modeling of filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of phase IIB dose selection. Clin Pharmacokinet. 2015;54(8):859–874. doi: 10.1007/s40262-015-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavanaugh A, Kremer J, Ponce L, Cseuz R, Reshetko OV, Stanislavchuk M, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2) Ann Rheum Dis. 2017;76(6):1009–1019. doi: 10.1136/annrheumdis-2016-210105. [DOI] [PubMed] [Google Scholar]
- 38.Markham A, Keam SJ. Peficitinib: first global approval. Drugs. 2019;79(8):887–891. doi: 10.1007/s40265-019-01131-y. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Hu J, Bao C, Li X, Li X, Xu J, et al. Efficacy and safety of baricitinib in MTX-IR patients with rheumatoid arthritis: 52 week results from a phase 3 study (RA-BALANCE) Ann Rheum Dis. 2018;77(Suppl 2):969–970. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is a meta-analysis that used published data from clinical trials. Data are provided in the Supplemental Tables 3 and 4.