Abstract
Introduction
This analysis explored laboratory mineral and bone disorder parameters and management of secondary hyperparathyroidism in patients undergoing hemodialysis in Belgium, Canada, China, France, Germany, Italy, Japan, Russia, Saudi Arabia, Spain, Sweden, the UK, and the USA.
Methods
Analyses used demographic, medication, and laboratory data collected in the prospective Dialysis Outcomes and Practice Patterns Study (2012–2015). The analysis included 20,612 patients in 543 facilities. Descriptive data are presented as regional mean (standard deviation), median (interquartile range), or prevalence, weighted for facility sampling fraction. No testing of statistical hypotheses was conducted.
Results
The frequency of serum intact parathyroid hormone levels > 600 pg/mL was lowest in Japan (1%) and highest in Russia (30%) and Saudi Arabia (27%). The frequency of serum phosphorus levels > 7.0 mg/dL was lowest in France (4%), the UK (6%), and Spain (6%), and highest in China (27%). The frequency of serum calcium levels > 10.0 mg/dL was highest in the UK (14%) and China (13%) versus 2% to 9% elsewhere. Dialysate calcium concentrations of 2.5 mEq/mL were common in the USA (78%) and Canada (71%); concentrations of 3.0–3.5 mEq/L were almost universal at facilities in Italy, France, and Saudi Arabia (each ≥ 99%).
Conclusions
Wide international variation in mineral and bone disorder laboratory parameters and management practices related to secondary hyperparathyroidism suggests opportunities for optimizing care.
Keywords: Bone mineral density, CKD, Dialysis, ESRD, Hyperparathyroidism, Mineral metabolism
Key Summary Points
| Why carry out this study? |
| The prevalence of chronic kidney disease (CKD) is increasing globally, which may result in more patients receiving dialysis despite current resource limitations. |
| Effective management is important because of the morbidity and mortality associated with secondary hyperparathyroidism (SHPT) in patients with CKD stage 5. |
| What was learned from the study? |
| This analysis explored laboratory mineral and bone disorder parameters and management of SHPT from multiple dialysis facilities globally, and aimed to identify differences in practices that contribute to outcomes. |
| Wide international variation in mineral and bone disorder laboratory parameters and management practices related to secondary hyperparathyroidism suggests opportunities for optimizing care. |
Introduction
The prevalence of chronic kidney disease (CKD), including progression to CKD stage 5, is increasing globally, including in the Middle East [1–4], China [5], and Russia [6], presumably due to rising prevalence of diabetes and hypertension [7–9]. More patients with CKD in resource-limited countries are expected to receive dialysis in the future, despite current gaps between need and availability in lower-income countries [10]. Secondary hyperparathyroidism (SHPT) is a major complication of CKD resulting from the failure of one or more components of the calcium homeostatic mechanism [11]. SHPT is an early complication of CKD; serum intact parathyroid hormone (iPTH) levels begin to rise when the glomerular filtration rate (GFR) decreases to less than approximately 60 mL/min/1.73 m2 (stage 3 CKD), although serum calcium and phosphorus levels may be normal [12]. The estimated prevalence of SHPT, defined as iPTH > 300 pg/mL, in dialysis populations varies from approximately 30–50% across Europe, Asia, Oceania, and the Americas [13].
A majority of patients with CKD stages 3–5 have CKD mineral and bone disorder (MBD) [14]. Parathyroid hormone is considered a uremic toxin [15], and early diagnosis and treatment of SHPT is essential because disorders of mineral metabolism, i.e., hyperphosphatemia, hypercalcemia, SHPT, and elevated calcium–phosphorus product (Ca × P), are associated with an increased risk of mortality (all-cause and cardiovascular [CV]-related) and hospitalization (all-cause, CV-related, and fracture-related) in patients with end-stage renal disease (ESRD) undergoing dialysis [16, 17].
The Dialysis Outcomes and Practice Patterns Study (DOPPS) is an international, prospective cohort study of hemodialysis practice and patient outcomes [18], with a main goal of identifying practices that improve survival and quality of life for patients undergoing hemodialysis [19]. In an analysis of DOPPS phases 1–3 (1996–2007), which included 25,882 patients [20], hyperphosphatemia (serum phosphorus > 6.1–7.0 mg/dL), hypercalcemia (serum calcium > 10 mg/dL), and elevated iPTH (> 600 pg/mL) were identified as three independent risk factors for all-cause and CV mortality, with hazard ratios (HRs) of 1.18 and 1.61 for phosphorus 6.1–7.0 mg/dL, 1.43 and 1.81 for phosphorus > 7.0 mg/dL, 1.16 and 1.24 for calcium, and 1.21 and 1.17 for iPTH, respectively. Moreover, it has been established that a Ca × P product > 55 mg2/dL2 is associated with an increased risk of cardiac calcification [21]. More recently, using DOPPS phase 1–4 data (1996–2011), an iPTH elevation to > 600 pg/mL (HR 1.23) and even milder elevation of iPTH (301–450 pg/mL) were associated with mortality compared with the reference range of 150–300 pg/mL (HR 1.09) [22]. Initial findings from DOPPS phase 5 (2012–2015) in Russia and countries of the Middle East that are part of the Gulf Cooperation Council (GCC; Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and United Arab Emirates) have demonstrated variation in practices and opportunities for improvement of care [23, 24].
The objectives of this analysis are to identify differences in practices that contribute to outcomes across broad regions (Europe, Asia, the Middle East, and North America) and, as a result, to encourage regional collaboration as a means to standardize treatment targets to improve global outcomes.
Methods
Study Design, Patients, and Data Collection
The DOPPS international, prospective cohort study of adult patients (≥ 18 years) undergoing in-center hemodialysis randomly enrolled patients from a sample of hemodialysis facilities within each nation at the start of each study phase, as described previously [18, 25]. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The study was approved by a central Institutional Review Board in the USA (Ethical & Independent Review Services). Outside of the USA, copies of IRB approvals and/or exemptions were obtained for each participating study site. Additional study approval and patient consent were obtained as required by national and local ethics committee regulations.
The analysis described here includes data collected in the initial prevalent cross-section of patients enrolled in DOPPS phase 5 from 2012 to 2015 in 20,612 patients in 543 facilities across Europe (Belgium, France, Germany, Italy, Spain, Sweden, and the UK), Russia, Asia (China and Japan), Saudi Arabia (results for additional GCC countries have been published separately [23]), and North America (Canada and the USA). Specific parameters analyzed to assess the management of SHPT included biochemical measures and the use of SHPT medications. Demographic data, comorbid conditions, laboratory values, and medications were abstracted from patient records.
Statistical Methods
All analyses were conducted using SAS® software, version 9.4 (SAS institute, Cary, NC). Descriptive data are presented as regional mean (SD), median (interquartile range), or prevalence, weighted for facility sampling fraction. No testing of statistical hypotheses was conducted.
Results
Demographics
Median facility size varied across countries, ranging from 52 patients per facility in the USA to 100 patients per facility in China. Other facility characteristics were comparable among countries (Table 1). Mean patient ages were noticeably lower in Russia (52.7 years) and Saudi Arabia (51.2 years) compared with other countries (overall 63.0 years; range 58.6–69.7 years). The median length of receiving dialysis was higher in France (5.0 years) and Japan (6.4 years) compared with other countries (overall 3.3 years; range 2.6–3.9 years). A majority of patients were men (58%; range 54–64%). The mean body mass index of patients in countries other than China (21.8 kg/m2) and Japan (21.5 kg/m2) indicated that they were overweight (overall 25.9 kg/m2; range 25.0–28.4 kg/m2). Across all countries, serum calcium and phosphorus were measured for > 90% of patients in the 4 months before DOPPS enrollment (overall, 97% and 98%, respectively; range 92–100%). However, serum iPTH was collected less frequently in some countries, with at least 20% of patients not having a measurement in the 4 months before DOPPS enrollment in Russia (27%), the UK (24%), Saudi Arabia (20%), and Japan (20%); iPTH data were missing in 6–19% of patients in other countries.
Table 1.
Facilities and patient characteristics by DOPPS country (2012–2015)
| Europe | Rus | Asia and Middle East | North America | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bel | Fra | Ger | Ita | Spa | Swe | UK | Chi | Jpn | KSA | Can | USA | ||
| Facility characteristics | |||||||||||||
| No. of facilities | 19 | 12 | 22 | 19 | 22 | 18 | 20 | 20 | 45 | 57 | 21 | 24 | 244 |
| Median [IQR] no. of patients per facility | 58 [37, 104] | 88 [52, 121] | 77 [66, 106] | 63 [49, 89] | 68 [57, 78] | 62 [41, 75] | 70 [57, 90] | 94 [53, 128] | 100 [52, 144] | 78 [54, 129] | 53 [34, 85] | 83 [52, 172] | 52 [31, 80] |
| Total no. of sample patients | 506 | 303 | 641 | 540 | 673 | 503 | 484 | 478 | 1186 | 1706 | 432 | 571 | 12,589 |
| Demographics | |||||||||||||
| Mean (SD) age, years | 69.7 (24.0) | 67.4 (27.6) | 67.5 (25.3) | 68.0 (22.6) | 66.2 (23.9) | 66.8 (20.6) | 63.1 (27.2) | 52.7 (23.5) | 58.6 (32.5) | 65.4 (22.5) | 51.2 (31.8) | 65.5 (33.8) | 63.1 (16.7) |
| Percentage of male patients | 62 | 57 | 61 | 57 | 63 | 64 | 63 | 56 | 54 | 64 | 55 | 56 | 55 |
| Median [IQR] years on dialysis | 3.4 [1.5, 6.1] | 5.0 [2.7, 8.3] | 3.3 [1.7, 6.6] | 3.9 [1.9, 7.8] | 3.4 [1.6, 7.2] | 3.0 [1.6, 5.7] | 2.8 [1.5, 6.7] | 3.0 [1.1, 5.9] | 3.5 [1.5, 6.4] | 6.4 [2.9, 13.4] | 2.9 [0.8, 7.2] | 2.6 [1.2, 5.5] | 2.8 [1.2, 5.4] |
| Mean (SD) BMI, kg/m2 | 25.9 (8.6) | 25.4 (10.4) | 27.2 (9.7) | 25.0 (8.2) | 26.1 (8.0) | 26.7 (8.5) | 26.5 (10.7) | 25.5 (9.4) | 21.8 (7.9) | 21.5 (6.3) | 25.6 (13.1) | 28.1 (13.4) | 28.4 (7.8) |
Bel Belgium, BMI body mass index, Can Canada, Chi China, DOPPS Dialysis Outcomes and Practice Patterns Study, Fra France, Ger Germany, IQR interquartile range, Ita Italy, Jpn Japan, KSA Kingdom of Saudi Arabia, Rus Russia, Spa Spain, Swe Sweden
Patient data are weighted for the facility sampling fraction
Dialysate Calcium Values
Dialysate calcium concentrations varied among countries, being lower in North America (the USA and Canada) and the UK compared with other countries, with 2.5 mEq/L predominantly used (78%, 71%, and 59% of patients, respectively) and < 2.5 mEq/L also used in 17% of US patients. Dialysate calcium concentrations of 3.0 mEq/L were especially common in Russia (74%), Italy (95%), France (91%), and China, Japan, and Saudi Arabia (67% for each), while 3.5 mEq/L was most frequently used in Saudi Arabia (32%) and Russia (15%; Fig. 1).
Fig. 1.
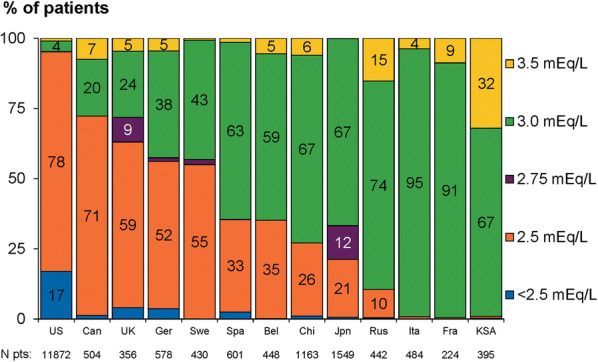
Dialysate calcium values by DOPPS country (2012–2015), weighted by facility sampling fraction and sorted by proportion of patients in category of 2.5 mEq/L. Bel Belgium, Can Canada, Chi China, DOPPS Dialysis Outcomes and Practice Patterns Study, Eq equivalent, Fra France, Ger Germany, Ita Italy, Jpn Japan, KSA Kingdom of Saudi Arabia, pt patient, Rus Russia, Spa Spain, Swe Sweden
Average Laboratory MBD Values
Mean values for total calcium were similar across countries (range 8.7–9.2 mg/dL; Table 2). The mean value for phosphorus was higher in China (6.0 mg/dL) compared with other countries (range of mean values 4.4–5.4 mg/dL). Mean iPTH values were higher in Russia (571 pg/mL) and Saudi Arabia (540 pg/mL), yet lower in Japan (150 pg/mL), compared with other countries (range of mean values 262–439 pg/mL).
Table 2.
Laboratory values by DOPPS country (2012–2015)
| Laboratory value | Europe | Rus | Asia and Middle East | North America | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bel | Fra | Ger | Ita | Spa | Swe | UK | Chi | Jpn | KSA | Can | USA | ||
| Mean (SD) total calcium, mg/dL | 9.0 (1.1) | 8.8 (1.3) | 8.8 (1.4) | 8.9 (1.3) | 8.9 (1.2) | 9.1 (1.1) | 9.2 (1.4) | 8.8 (1.7) | 9.0 (2.1) | 8.8 (1.3) | 8.7 (1.8) | 8.8 (1.7) | 9.0 (0.8) |
| Mean (SD) phosphorus, mg/dL | 4.6 (2.4) | 4.4 (2.7) | 5.4 (2.8) | 4.8 (2.3) | 4.6 (2.3) | 5.2 (2.2) | 4.8 (2.6) | 5.3 (3.0) | 6.0 (4.3) | 5.4 (2.4) | 5.2 (4.5) | 5.0 (3.3) | 5.1 (1.7) |
| Mean (SD) iPTH, pg/mL | 346 (561) | 399 (661) | 262 (429) | 313 (506) | 336 (512) | 379 (497) | 392 (741) | 571 (1182) | 439 (1050) | 150 (233) | 540 (1221) | 431 (812) | 408 (424) |
| Median [IQR] iPTH, pg/mL | 240 [121, 450] | 298 [179, 540] | 192 [92, 357] | 209 [113, 400] | 247 [145, 417] | 287 [161, 468] | 286 [142, 485] | 395 [162, 728] | 284 [138, 518] | 118 [64, 193] | 357 [165, 641] | 332 [190, 532] | 303 [184, 495] |
Bel Belgium, Can Canada, Chi China, DOPPS Dialysis Outcomes and Practice Patterns Study, Fra France, Ger Germany, IQR interquartile range, Ita Italy, Jpn Japan, KSA Kingdom of Saudi Arabia, iPTH intact parathyroid hormone, Rus Russia, Spa Spain, Swe Sweden
Patient data are weighted for the facility sampling fraction
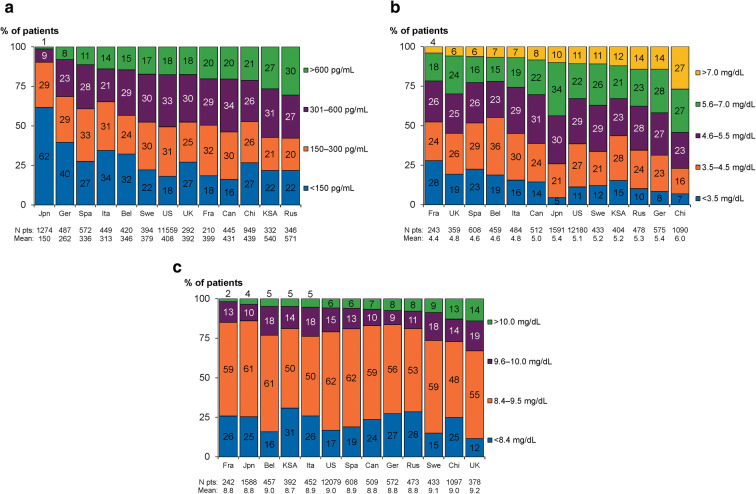
Categorical results for laboratory assessments of serum analytes relevant to management of patients with SHPT are shown in Fig. 2. Serum iPTH levels > 300 and > 600 pg/mL were lowest in Japan (10% and 1%), highest in Saudi Arabia (58% and 27%) and Russia (57% and 30%), and intermediate in other countries (range 31–54% and 8–21%), while iPTH levels < 150 pg/mL were most commonly seen in Japan (62%) and Germany (40%; Fig. 2a). Serum phosphorus levels ≥ 5.6 and > 7.0 mg/dL were lowest in several European countries and Canada (France [22% and 4%], UK [30% and 6%], Spain [22% and 6%], Belgium [22% and 7%], Italy [26% and 7%], and Canada [30% and 8%]), highest in China (54% and 27%), and ranged from 33–44% and 10–14%, respectively, in other countries (Fig. 2b). Serum calcium levels were similar across countries, although there were greater proportions of patients at the highest levels (≥ 9.6 and > 10.0 mg/dL) in the UK (33% and 14%), China (27% and 13%), and Sweden (27% and 9%), and at < 8.4 mg/dL in Saudi Arabia (31%; Fig. 2c).
Fig. 2.
Serum concentrations of MBD-related laboratory assessments by DOPPS country (2012–2015), weighted by facility sampling fraction and sorted by proportion of patients in the highest category. a iPTH. b Phosphorus. c Total calcium. Bel Belgium, Can Canada, Chi China, DOPPS Dialysis Outcomes and Practice Patterns Study, Fra France, Ger Germany, Ita Italy, Jpn Japan, KSA Kingdom of Saudi Arabia, MBD mineral and bone disorder, pt patient, iPTH intact parathyroid hormone, Rus Russia, Spa Spain, Swe Sweden
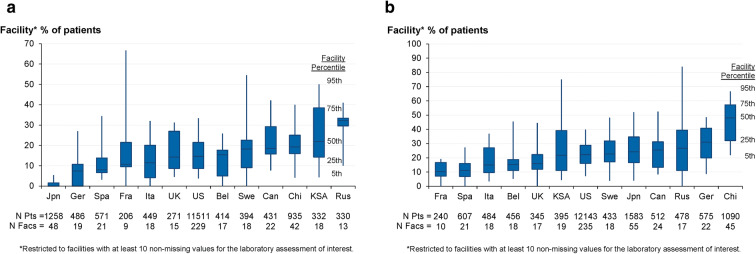
The percentage of patients in each facility who had at least one serum laboratory assessment above a cutoff value is shown in Fig. 3. The facility percentage of patients with serum iPTH > 600 pg/mL varied widely across countries, as well as within most countries, with nearly all facilities in Japan having none of their patients with such elevated iPTH values in contrast to Russia, where 50% of the facilities had at least 32% of their patients with iPTH > 600 pg/mL. For phosphorus, 50% of the facilities in China had at least 48% of their patients with values > 6 mg/dL.
Fig. 3.
Distribution of the percentage of patients in each facility who had a serum laboratory assessment higher than the upper limit value by DOPPS country (2012–2015), sorted by median proportion of patients. a iPTH > 600 pg/mL. b Phosphorus > 6 mg/dL. Bel Belgium, Can Canada, Chi China, DOPPS Dialysis Outcomes and Practice Patterns Study, Fac facility, Fra France, Ger Germany, Ita Italy, Jpn Japan, KSA Kingdom of Saudi Arabia, pt patient, iPTH intact parathyroid hormone, Rus Russia, Spa Spain, Swe Sweden. *Restricted to facilities with ≥ 10 non-missing values for the laboratory assessment of interest
Medication Use for Mineral and Bone Disorder Management
The use of vitamin D and vitamin D analogues for managing MBD differed dramatically across countries (Table 3). Cinacalcet was prescribed most often in Saudi Arabia (29%), European countries (range 13–34%), Japan (24%), and the USA (18%), but infrequently prescribed in China (1%), Russia (6%), and Canada (7%). Most patients in the USA (65%) and Spain (57%) but minorities in other countries (range 2–38%) received vitamin D intravenously. Conversely, use of oral vitamin D administration was low in the USA (15%) and Spain (4%) compared with other countries (range 21–73%). Most patients received vitamin D by only one route; among patients prescribed any vitamin D, the proportions who received vitamin D both intravenously and orally were low across countries (range 1–12%). Among types of vitamin D, alfacalcidol monotherapy predominated in five countries (the UK [96%], Sweden [92%], Saudi Arabia [89%], Russia [85%], and France [82%]) but was used less elsewhere (range 0–62%). Among patients prescribed vitamin D, calcitriol monotherapy was common in China (79%) and Canada (57%) but not in other countries (range 1–35%). Paricalcitol monotherapy use was highest in Spain (87%), Italy (61%), and the USA (45%) but low elsewhere (range 0–13%). Use of other vitamin D types or combinations was common only in Japan (39%) compared with other countries (range 2–14%).
Table 3.
Medications by DOPPS country (2012–2015) as a percentage of patients
| Europe | Rus | Asia and Middle East | North America | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bel | Fra | Ger | Ita | Spa | Swe | UK | Chi | Jpn | KSA | Can | USA | |||
| Medications prescribed* | ||||||||||||||
| Cinacalcet | 15 | 27 | 16 | 21 | 34 | 24 | 13 | 6 | 1 | 24 | 29 | 7 | 18 | |
| Phosphate binder | 77 | 75 | 82 | 76 | 82 | 88 | 72 | 83 | 59 | 84 | 86 | 78 | 65 | |
| IV vitamin D | 2 | 4 | 10 | 38 | 57 | 14 | 3 | 5 | 2 | 37 | 28 | 4 | 65 | |
| Oral vitamin D | 32 | 21 | 46 | 26 | 4 | 70 | 73 | 48 | 57 | 41 | 51 | 61 | 15 | |
| Medications details† | ||||||||||||||
| Vitamin D route | ||||||||||||||
| Oral only | 94 | 83 | 81 | 37 | 5 | 84 | 96 | 91 | 96 | 50 | 60 | 94 | 16 | |
| IV only | 5 | 16 | 15 | 57 | 94 | 13 | 2 | 7 | 1 | 46 | 28 | 5 | 81 | |
| IV + oral | 2 | 1 | 4 | 6 | 2 | 3 | 2 | 3 | 2 | 5 | 12 | 2 | 3 | |
| Vitamin D (IV or oral) type | ||||||||||||||
| Alfacalcidol only | 62 | 82 | 51 | 0 | 3 | 92 | 96 | 85 | 17 | 38 | 89 | 41 | 0 | |
| Calcitriol only | 29 | 4 | 30 | 35 | 4 | 1 | 1 | 2 | 79 | 24 | 7 | 57 | 10 | |
| Paricalcitol only | 0 | 0 | 13 | 61 | 87 | 6 | 0 | 7 | 0 | 0 | 0 | 0 | 45 | |
| Other vitamin D or combination | 10 | 14 | 5 | 4 | 6 | 2 | 4 | 7 | 4 | 39 | 4 | 2 | 3 | |
| Phosphate binder type | ||||||||||||||
| Calcium-based only | 48 | 36 | 30 | 14 | 21 | 21 | 42 | 69 | 89 | 43 | 64 | 60 | 36 | |
| Sevelamer only | 15 | 25 | 14 | 45 | 27 | 34 | 25 | 0 | 1 | 6 | 11 | 12 | 37 | |
| Calcium-based + sevelamer only | 24 | 22 | 11 | 14 | 10 | 21 | 8 | 2 | 0 | 13 | 19 | 15 | 13 | |
| Other binder or combination | 13 | 18 | 45 | 28 | 42 | 24 | 25 | 30 | 10 | 38 | 6 | 13 | 14 | |
Bel Belgium, Can Canada, Chi China, DOPPS Dialysis Outcomes and Practice Patterns Study, Fra France, Ger Germany, Ita Italy, IV intravenous, Jpn Japan, KSA Kingdom of Saudi Arabia, Rus Russia, Spa Spain, Swe Sweden
*Prescription at DOPPS enrollment or in the month before DOPPS enrollment; vitamin D restricted to active vitamin D (calcitriol or one of its synthetic analogues)
†Restricted to patients prescribed the drug class of interest
Patient data are weighted for the facility sampling fraction
Phosphate binders were used less in China (59%) compared with other countries (range 65–88%; Table 3). Among patients prescribed phosphate binders, calcium-based monotherapy was most prevalent in China (89%), Russia (69%), Saudi Arabia (64%), and Canada (60%); in other countries, minorities of patients received this therapy (range 14–48%). Sevelamer monotherapy use was most frequent in Italy (45%), the USA (37%), and Sweden (34%) and least frequent in Russia (0%) and China (1%); in other countries, 6–27% of patients received sevelamer monotherapy. Less than one-quarter of patients prescribed a phosphate binder in each country received calcium-based phosphate binder plus sevelamer dual treatment (range 0–24%). Other binders or combinations of phosphate binders were most commonly used in Germany (45%), Spain (42%), Japan (38%), and Russia (30%) and 6% to 28% in other countries.
Targets for Serum Laboratory Parameters
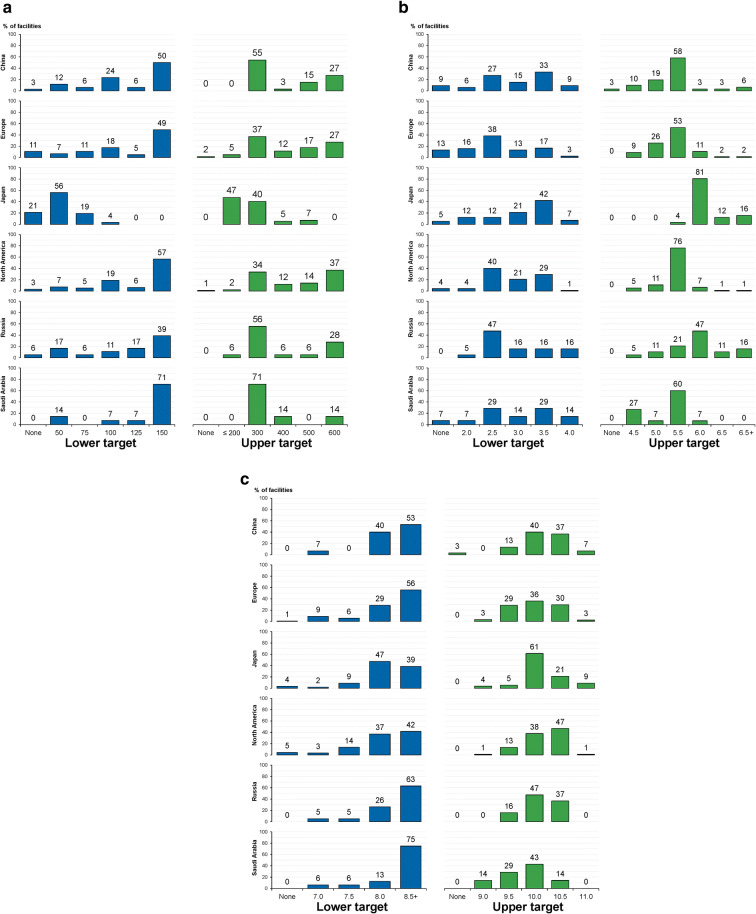
Target values for MBD laboratory assessments, as reported by facility medical directors across regions, are shown in Fig. 4. The lower and upper targets for serum iPTH were usually 150 and 300 pg/mL, respectively; however, the most common lower and upper targets in Japan were 50 and ≤ 200 pg/mL, respectively (Fig. 4a). Lower targets for serum phosphorus were generally between 2.5 and 3.5 mg/dL; upper targets usually were 5.5 or 6.0 mg/dL (Fig. 4b). There was a broad range between the most common lower and upper targets in Russia (2.5 and 6.0 mg/dL, respectively). Lower targets for serum calcium were commonly 8.0 or ≥ 8.5 mg/dL, with ≥ 8.5 mg/dL strongly favored in Russia and Saudi Arabia; upper targets were broadly distributed and usually centered at 10.0 mg/dL (but higher in North America at 10.5 mg/dL; Fig. 4c).
Fig. 4.
MBD lower and upper targets for serum concentrations of laboratory assessments, as reported by medical directors by DOPPS country or region (2012–2015). a iPTH, pg/mL. b Phosphorus, mg/dL. c Total calcium, mg/dL. DOPPS Dialysis Outcomes and Practice Patterns Study, MBD mineral and bone disease, iPTH intact parathyroid hormone. Europe includes Belgium, France, Germany, Italy, Spain, Sweden, and the UK. North America includes Canada and the USA
Discussion
Optimizing the management of SHPT in patients with CKD stage 5 receiving hemodialysis presents an opportunity to decrease morbidity and mortality. Published guidelines are inconsistent regarding optimal target laboratory values, resulting in lack of consensus on treatment targets [26–31]. Although clinical trial data are lacking [32], iPTH concentrations > 300–600 pg/mL have been associated with increased risk of all-cause and CV mortality, and all-cause and CV-related hospitalizations in patients undergoing hemodialysis [16, 20, 22, 33]. Elevated levels of iPTH may also contribute to worsening hypercalcemia and hyperphosphatemia in patients receiving hemodialysis [34]. Conversely, it is expected that more effective control of iPTH would not only enable good control of phosphate and calcium levels but also translate to reduced mortality and morbidity.
The ultimate goal of optimizing iPTH levels is to improve bone health in this population. The assessments and treatment practices observed in DOPPS phase 5 among countries and regions have possible implications for patient outcomes due to identification of best practices. The iPTH targets in most countries are consistent with the 2009 and 2017 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, which both advocate a target of 2–9 times the upper limit of normal [14, 26], as well as the National Kidney Foundation Kidney Diseases Outcomes Quality Initiative (KDOQI) clinical practice guidelines, which suggest a target of 150–300 pg/mL [27]. However, Japanese guidelines recommend a more stringent iPTH target range of 60–240 pg/mL [35], which was associated with most Japanese patients achieving iPTH < 600 pg/mL. Additionally, more aggressive use of medications, including vitamin D and cinacalcet, in Japan (available since 2008) may have been beneficial.
Although hyperphosphatemia resulting from poor urinary excretion is to be expected as CKD progresses [11], management remains challenging because of poor adherence to dietary phosphate restriction and oral phosphate binders [36]. Often the pill burden increases as CKD progresses [37], and dietary restrictions become more stringent, resulting in worsening compliance over time. Observational studies suggest that hyperphosphatemia alone is associated with an increased risk of all-cause and CV mortality in patients undergoing hemodialysis [16, 20, 33], which suggests that more effective control of hyperphosphatemia might reduce mortality rates. In our analysis, the proportion of patients with hyperphosphatemia was highest in China, perhaps reflecting relatively low use of effective phosphate binders. This is consistent with a cross-sectional study of patients receiving dialysis in China, which reported that uncontrolled hyperphosphatemia was common and possibly related to a phosphorus-rich diet, lack of nutritionist support at most dialysis centers, and inadequate use of phosphate binders [38].
The proportions of DOPPS phase 5 patients with hypercalcemia in China (possibly related to predominant use of calcium-based phosphate binders) and the UK poses an additional concern because hypercalcemia has been significantly associated with cardiac arrhythmias and the risk of death, independent of phosphorus levels. Our analysis warrants a call to action by highlighting inconsistent practices and outcomes concerning SHPT management. Our results are consistent with prior reports demonstrating worsening control of SHPT over time. Specifically, iPTH levels have steadily increased from 1996 to 2011 in patients on long-term hemodialysis in Europe, Australia, New Zealand, and North America [22], pointing to the need for greater awareness and better management of SHPT in patients with CKD and ESRD. Because SHPT is an early and progressive [12, 39] complication of CKD, timely diagnosis and treatment is critical.
By conducting clinical audits of their own practices [40], nephrologists may discover ways to improve their use of medications and testing, thus resulting in improved disease control and favorable outcomes. For example, a survey of medical directors of dialysis centers in Saudi Arabia [41] revealed opportunities to increase understanding of SHPT management. A committee of nephrologists from the Middle East subsequently reviewed the 2009 KDIGO guidelines for the management of CKD-MBD [14] to formulate practical, standardized regional recommendations [1]. Encouragingly, an analysis of the management of patients undergoing hemodialysis in Saudi Arabia from 2011 to 2013 found trends over time for better utilization of drugs and improvement in laboratory parameters [42]. Similarly, the Chinese guidance for CKD-MBD management from 2013 is scheduled for update in 2019 with the inclusion of more data specific to China. In Russia, a group of experts prepared national guidelines for the treatment of CKD-MBD based on international recommendations [43].
Individual country and center practices described in this study may be translated for use elsewhere. Although the data from this analysis are retrospective and associative, there may be opportunities for implementation of best practices to improve clinical outcomes. In Japan, ambitious targets for iPTH were associated with success in controlling levels of that hormone, a strategy that could be applied to other parameters and in other countries. Importantly, stable maintenance of iPTH at ≤ 600 pg/mL has been associated with a lower rate of mortality in patients undergoing hemodialysis [44]. Hyperphosphatemia was least common in France and most common in China; although multiple factors could lead to hyperphosphatemia, the use of non-calcium-based phosphate binders may be a contributing factor to success. This has also been demonstrated in previous analyses, in which patients undergoing hemodialysis who were receiving calcium-based phosphate binders had decreased risk of mortality after initiating treatment with sevelamer [45].
A limitation of this analysis is that PTH levels were not measured in a centralized laboratory, and PTH levels were not normalized to account for the differences in iPTH assays used globally and between local hospitals in the same country. Furthermore, the variability in a country’s public spending on health per capita may have influenced the type of guidelines in local use, thereby affecting the availability of resources, management practices, and laboratory parameters. Variability within a country is affected by the sample size, which was not uniform. An additional caveat is that, because of the design of this analysis, patient outcomes were not assessed, thus restricting conclusions about whether the results for laboratory measures had clinically meaningful implications. Nonetheless, given the known complications associated with suboptimal management of SHPT, the findings of this work are expected be relevant to outcomes.
In summary, the management of SHPT is complex, and effective management is important because of the morbidity and mortality associated with SHPT in patients with CKD stage 5. The differences among countries observed in this analysis suggest that aspects of the management of SHPT are not universally accepted. The clearest single factor associated with positive laboratory outcomes in this study was lower target levels of iPTH, which had beneficial effects on control of serum phosphorus and calcium. Other gains can be made by more intense use of vitamin D and cinacalcet and more effective use of phosphate binders. However, differences in available resources between countries may limit the extent to which management may be adjusted. In conclusion, within the constraints of their individual situation, dialysis centers have an opportunity to achieve good patient outcomes by adjusting their targets to achieve treatment goals with whatever means are available to them.
Acknowledgements
Global support for the ongoing DOPPS Programs is provided without restriction on publications. See https://www.dopps.org/AboutUs/Support.aspx for more information. AbbVie and the authors thank the patients and all study investigators for their contributions.
Funding
The DOPPS program and Arbor Research Collaborative for Health were paid by AbbVie to conduct the analyses; authors from Arbor were not compensated for their time spent in their authorship role. AbbVie Inc. participated in the study design, research, interpretation of data, reviewing, and approval of the publication, and funded the journal’s rapid service and open access fees.
Medical Writing, Editorial, and other Assistance
Medical writing assistance, funded by AbbVie, was provided by Michael J. Theisen, PhD, and Janet E. Matsuura, PhD, of Complete Publication Solutions, LLC (North Wales, PA), a CHC Group company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
All authors were involved in the design or conception of the work or in data acquisition or interpretation, contributed important intellectual content during manuscript drafting or revision, and approved the final version. Each author accepts personal accountability for his or her own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Disclosures
Mario Cozzolino has received consulting fees from AbbVie, Amgen, Shire, Baxter, and Vifor; and grants from AbbVie, Shire, Baxter, and Keryx. Masafumi Fukagawa has received consulting fees from Kyowa-Hakko Kirin and Ono Pharmaceutical; lecture fees from Kyowa-Hakko Kirin, Bayer Japan, Torii Pharmaceutical, and Ono Pharmaceutical; and grants from Kyowa-Hakko Kirin and Bayer Japan. Saeed M.G. Al-Ghamdi has received lecture fees and travel support from AbbVie and Amgen and research grants from Sanofi. Bhadrish Vallabh is an employee and shareholder of AbbVie Inc. Deepa H. Chand is a former employee and shareholder of AbbVie Inc. Her current affiliation is AveXis, Inc., Bannockburn, IL. Eugeniy Shilov, Zuo Li, Ronald Pisoni, and Brian Bieber have nothing to disclose.
Compliance with Ethics Guidelines
The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The study was approved by a central Institutional Review Board in the USA (Ethical & Independent Review Services). Outside of the USA, copies of IRB approvals and/or exemptions were obtained for each participating study site. Additional study approval and patient consent were obtained as required by national and local ethics committee regulations.
Data Availability
All data generated or analyzed during this study are included in this article.
Footnotes
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12155313.
References
- 1.Al Rukhaimi M, Al Sahow A, Boobes Y, et al. Adaptation and implementation of the "Kidney Disease: Improving Global Outcomes (KDIGO)" guidelines for evaluation and management of mineral and bone disorders in chronic kidney disease for practice in the Middle East countries. Saudi J Kidney Dis Transpl. 2014;25:133–148. doi: 10.4103/1319-2442.124536. [DOI] [PubMed] [Google Scholar]
- 2.Hassanien AA, Al-Shaikh F, Vamos EP, Yadegarfar G, Majeed A. Epidemiology of end-stage renal disease in the countries of the Gulf Cooperation Council: a systematic review. JRSM Short Rep. 2012;3:38. doi: 10.1258/shorts.2012.011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farag YM, Kari JA, Singh AK. Chronic kidney disease in the Arab world: a call for action. Nephron Clin Pract. 2012;121:c120–c123. doi: 10.1159/000345149. [DOI] [PubMed] [Google Scholar]
- 4.Al Shamsi S, Al Dhanhani A, Sheek-Hussein MM, Bakoush O. Provision of care for chronic kidney disease by non-nephrologists in a developing nation: a national survey. BMJ Open. 2016;6:e010832. doi: 10.1136/bmjopen-2015-010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System . 2017 USRDS annual data report: epidemiology of kidney disease in the United States. MD: Bethesda; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 8.Abuyassin B, Laher I. Diabetes epidemic sweeping the Arab world. World J Diabetes. 2016;7:165–174. doi: 10.4239/wjd.v7.i8.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y, Chen G, Tian H, et al. Prevalence of hypertension in China: a cross-sectional study. PLoS One. 2013;8:e65938. doi: 10.1371/journal.pone.0065938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 12.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 13.Hedgeman E, Lipworth L, Lowe K, Saran R, Do T, Fryzek J. International burden of chronic kidney disease and secondary hyperparathyroidism: a systematic review of the literature and available data. Int J Nephrol. 2015;2015:184321. doi: 10.1155/2015/184321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009:S1–130 [DOI] [PubMed]
- 15.Rodriguez M, Lorenzo V. Parathyroid hormone, a uremic toxin. Semin Dial. 2009;22:363–368. doi: 10.1111/j.1525-139X.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 16.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 17.Ganesh SK, Stack AG, Levin NW, Hylbert-Shearon T, Port FK. Association of elevated serum PO4, Ca × PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 18.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57:S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 19.Robinson BM, Bieber B, Pisoni RL, Port FK. Dialysis Outcomes and Practice Patterns Study (DOPPS): its strengths, limitations, and role in informing practices and policies. Clin J Am Soc Nephrol. 2012;7:1897–1905. doi: 10.2215/CJN.04940512. [DOI] [PubMed] [Google Scholar]
- 20.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis. 2000;35:1226–1237. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]
- 22.Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Salmi I, AlRukhaimi M, AlSahow A, et al. Mineral bone disorder and its management among hemodialysis patients in the Gulf Cooperation Council: initial findings from the Dialysis Outcomes and Practice Patterns Study (2012–2015) Saudi J Kidney Dis Transpl. 2016;27:62–80. doi: 10.4103/1319-2442.194902. [DOI] [PubMed] [Google Scholar]
- 24.Bikbov B, Bieber B, Andrusev A, et al. Hemodialysis practice patterns in the Russia Dialysis Outcomes and Practice Patterns Study (DOPPS), with international comparisons. Hemodial Int. 2017;21:393–408. doi: 10.1111/hdi.12503. [DOI] [PubMed] [Google Scholar]
- 25.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44:7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;2017(7):1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- 28.Burton JO, Goldsmith DJ, Ruddock N, Shroff R, Wan M. Renal association commentary on the KDIGO (2017) clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of CKD-MBD. BMC Nephrol. 2018;19:240. doi: 10.1186/s12882-018-1037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borrelli S, Chiodini P, De Nicola L, et al. Prognosis and determinants of serum PTH changes over time in 1–5 CKD stage patients followed in tertiary care. PLoS One. 2018;13:e0202417. doi: 10.1371/journal.pone.0202417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cozzolino M, Bolasco P, Ronco C, et al. Clinical management of chronic kidney disease patients in Italy: results from the IRIDE Study. Nephron. 2018;140:39–47. doi: 10.1159/000490769. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Castelao A, Gorriz JL, Bover J, et al. Consensus document for the detection and management of chronic kidney disease. Nefrologia. 2014;34:243–262. doi: 10.3265/Nefrologia.pre2014.Feb.12455. [DOI] [PubMed] [Google Scholar]
- 32.Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the diagnosis, evaluation, and treatment of CKD-mineral and bone disorder (CKD-MBD) Am J Kidney Dis. 2010;55:773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
- 33.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Molnar MZ, Zaritsky JJ, et al. Correlates of parathyroid hormone concentration in hemodialysis patients. Nephrol Dial Transplant. 2013;28:1516–1525. doi: 10.1093/ndt/gfs598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–288. doi: 10.1111/1744-9987.12058. [DOI] [PubMed] [Google Scholar]
- 36.Umeukeje EM, Mixon AS, Cavanaugh KL. Phosphate-control adherence in hemodialysis patients: current perspectives. Patient Prefer Adherence. 2018;12:1175–1191. doi: 10.2147/PPA.S145648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4:1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong X, Zhang L, Zhang L, et al. Mineral and bone disorder in Chinese dialysis patients: a multicenter study. BMC Nephrol. 2012;13:116. doi: 10.1186/1471-2369-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman WG, Quarles LD. Development and progression of secondary hyperparathyroidism in chronic kidney disease: lessons from molecular genetics. Kidney Int. 2008;74:276–288. doi: 10.1038/sj.ki.5002287. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin A. Audit: how to do it in practice. BMJ. 2008;336:1241–1245. doi: 10.1136/bmj.39527.628322.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souqiyyeh MZ, Shaheen FA. Survey of attitudes of physicians toward the current evaluation and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Saudi J Kidney Dis Transpl. 2010;21:93–101. [PubMed] [Google Scholar]
- 42.Karkar A, Sinha AK, Abdelrahman M, et al. Trends of elevated parathormone serum titers in hemodialysis patients on intensive therapy for bone disease: a multicenter study. Saudi J Kidney Dis Transpl. 2014;25:1166–1177. doi: 10.4103/1319-2442.144249. [DOI] [PubMed] [Google Scholar]
- 43.Nephrology Association, Scientific Society of Nephrology of Russia. Mineral and bone disturbances in chronic kidney disease (MBD-CKD) [in Russian]. https://nonr.ru/?page_id=3178. Accessed Sep 6, 2019
- 44.Yamamoto S, Karaboyas A, Komaba H, et al. Mineral and bone disorder management in hemodialysis patients: comparing PTH control practices in Japan with Europe and North America: the Dialysis Outcomes and Practice Patterns Study (DOPPS) BMC Nephrol. 2018;19:253. doi: 10.1186/s12882-018-1056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komaba H, Wang M, Taniguchi M, et al. Initiation of sevelamer and mortality among hemodialysis patients treated with calcium-based phosphate binders. Clin J Am Soc Nephrol. 2017;12:1489–1497. doi: 10.2215/CJN.13091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.