Abstract
Introduction
Teneligliptin, a dipeptidyl peptidase 4 inhibitor, was approved for the treatment of type 2 diabetes mellitus (T2DM) in Japan in 2012. However, clinical trials of teneligliptin involved limited numbers of elderly patients. Therefore, we investigated the safety and efficacy of teneligliptin in elderly patients with T2DM.
Methods
This 3-year follow-up RUBY surveillance registered patients with T2DM who started treatment with teneligliptin between May 2013 and February 2015 in Japan. Collected data included demographics, treatments, adverse drug reactions (ADRs), and laboratory variables. Data were analysed for patients in three age subgroups (< 65, ≥ 65 to < 75, or ≥ 75 years old). Safety was assessed as the incidence of ADRs and efficacy was assessed in terms of glycaemic control, for up to 3 years.
Results
The ADRs and serious ADRs occurred in 3.35% and 0.65% of 4596 patients aged < 65 years, in 4.42% and 1.22% of 3371 patients aged ≥ 65 to < 75 years, and in 3.99% and 1.69% of 2729 patients aged ≥ 75 years. The most common ADRs in patients aged ≥ 65 to < 75 years and ≥ 75 years were gastrointestinal disorders, but the incidence of these ADRs did not show an age-dependent increase. Hypoglycaemia occurred in 0.24%, 0.56%, and 0.29% of patients in each age subgroup, respectively. The least-squares mean changes in glycosylated haemoglobin (HbA1c) adjusted for baseline were − 0.66 ± 0.02% (n = 2177), − 0.72 ± 0.02% (n = 1689), and − 0.77 ± 0.03% (n = 1161) at 3 years.
Conclusion
There was no clear difference in the number of ADRs among the three age subgroups, although the incidence of serious ADRs was higher in elderly patients than in patients aged < 65 years. We found no additional safety or efficacy concerns among elderly patients beyond those already described in the package insert. The present results support the use of teneligliptin in elderly patients with T2DM in real-world clinical practice.
Trial Registration
Japic Clinical Trials Information identifier, Japic CTI-153047.
Electronic Supplementary Material
The online version of this article (10.1007/s12325-020-01306-0) contains supplementary material, which is available to authorized users.
Keywords: Diabetes, Dipeptidyl peptidase 4 inhibitor, Elderly patients, Post-marketing surveillance, Real-world, Teneligliptin, Type 2 diabetes mellitus
Plain Language Summary
Teneligliptin is an oral drug taken once daily to manage blood glucose levels in people with type 2 diabetes. A number of studies of teneligliptin have investigated its safety and efficacy, but these studies included limited numbers of elderly people, aged 75 years or older. Following the approval of teneligliptin in Japan, post-marketing surveillance was started to monitor its safety and efficacy when prescribed by doctors to people in actual clinical practice. We analysed data from the surveillance to check if the safety and efficacy of teneligliptin differ in younger and older people separately. We found that there was no clear difference in the number of adverse drug reactions among three age subgroups: < 65 years, ≥ 65 to < 75 years, or ≥ 75 years, although the incidence of serious adverse drug reactions was higher in elderly patients than in patients aged < 65 years. Treatment with teneligliptin also lowered blood glucose levels in all three age subgroups, and the changes were maintained for up to 3 years in many individuals in each age subgroup. We found no additional safety or efficacy concerns among elderly patients beyond those already described in the package insert. The present results support the use of teneligliptin for the treatment of elderly patients with type 2 diabetes mellitus in real-world clinical practice.
Electronic Supplementary Material
The online version of this article (10.1007/s12325-020-01306-0) contains supplementary material, which is available to authorized users.
Key Summary Points
| Why carry out this study? |
| The number of elderly patients with type 2 diabetes mellitus (T2DM) is increasing worldwide and in Japan. |
| Teneligliptin is a dipeptidyl peptidase 4 inhibitor that was approved for the treatment of T2DM in Japan in 2012. |
| Clinical trials are often limited in terms of their duration, number of patients, and the background characteristics of patients, notably elderly patients. |
| We examined the long-term, real-world safety and efficacy of teneligliptin in elderly patients (≥ 65 to < 75, and ≥ 75 years old) using data from a 3-year post-marketing surveillance of teneligliptin in Japan. |
| What was learned from this study? |
| We found no additional safety or efficacy concerns among elderly patients beyond those already described in the package insert. The present results support the use of teneligliptin for the treatment of elderly patients with T2DM in real-world clinical practice. |
Introduction
The burden of type 2 diabetes mellitus (T2DM) is rapidly growing worldwide [1] and in Japan [2]. The number of elderly patients with diabetes is also increasing because of the aging of society [1–3]. Societal aging also introduces difficulties when treating patients with diabetes because elderly people are more likely to have clinically significant comorbidities that may need to be managed more intensively or require multiple drugs, increasing the risk of drug–drug interactions with any antidiabetic drugs. Additionally, elderly patients may be at greater risk of adverse events (AEs) or adverse drug reactions (ADRs), especially hypoglycaemia, in part due to impaired renal function and comorbidities [4, 5].
In Japan, dipeptidyl peptidase 4 (DPP4) inhibitors are the most widely prescribed drugs for treating T2DM [6]. Clinicians may favour DPP4 inhibitors in Asian and Japanese patients owing to the pathophysiology of T2DM that is characterised by β-cell dysfunction, less adiposity, and less insulin resistance compared with non-Asian patients [7, 8]. These factors, as well as differences in genetic makeup and diet, may contribute to a greater efficacy of DPP4 inhibitors in Japanese patients, and encourage their use for the treatment of T2DM [8].
Teneligliptin, a DPP4 inhibitor, is currently approved in Japan and Korea for the treatment of T2DM [9–11]. In Japan, it is prescribed at a starting dose of 20 mg once daily, but its dose can be increased to 40 mg once daily if the glucose-lowering effect of 20 mg once daily is insufficient. In addition, teneligliptin can be administered to patients with impaired renal function, including patients on dialysis, without the need for dose adjustment [9–11]. Clinical trials showed teneligliptin to be effective and well tolerated in Japanese patients when administered as monotherapy [12, 13] or in combination with other oral antidiabetic agents or insulin [14–19].
Long-term treatment is invariably necessary for patients with T2DM, but prior clinical trials of teneligliptin were limited to 1 year in duration. Additionally, most of the trials in Japan enrolled patients aged 20–75 years. Therefore, longer-term and elderly patients’ data are needed. Post-marketing surveillance provides an opportunity to evaluate the efficacy and safety of recently approved drugs in real-world settings over a longer term than in clinical trials, and surveillance can include elderly patients who are often underrepresented in clinical trials [20].
The RUBY (ExploRing the long-term efficacy and safety including cardiovascUlar events in patients with type 2 diaBetes treated bY teneligliptin in the real-world) surveillance was therefore performed to examine the long-term (3 years) safety and efficacy of teneligliptin in more than 10,000 patients with T2DM in real-world settings [21, 22]. The results at 3 years were recently obtained [23], and we performed the present analyses to evaluate the long-term safety and efficacy of teneligliptin in elderly patients (≥ 65 to < 75, and ≥ 75 years).
Methods
The design and results of the study have been published [21–23]. As previously described, RUBY was approved by the Ministry of Health, Labour and Welfare of Japan and was performed by Mitsubishi Tanabe Pharma Corporation in accordance with the Japanese ministry directive on Good Post-marketing Study Practice (GPSP). The surveillance used anonymous data collected in clinical practice in Japan. In accordance with Japanese regulations for post-marketing surveillance, it is not necessary to obtain informed consent from patients. RUBY was registered on the Japan Pharmaceutical Information Center clinical trials database (Japic CTI-153047).
Patients who were first prescribed teneligliptin between May 2013 and February 2015 were registered and followed for up to 3 years through to August 2018. All treatments, including teneligliptin and combination therapies, were at the prescribing physician’s discretion according to the package insert [10, 11] or regimen. Teneligliptin was administered with an option to increase to 40 mg once daily from 20 mg once daily if the prescribing physician thought that the hypoglycaemic effect of 20 mg once daily was insufficient.
Data were collected electronically and the database was locked in January 2019 [23]. Laboratory test data, including glycosylated haemoglobin (HbA1c), fasting blood glucose (FBG), and lipids, were recorded at fixed intervals. Safety was assessed in terms of the incidence of ADRs, and efficacy was assessed in terms of glycaemic control, such as HbA1c and FBG, up to 3 years.
Safety was assessed as the incidence of ADRs classified using the Medical Dictionary for Regulatory Activities (MedDRA)/Japanese version 21.1. ADRs were defined as AEs for which a causal relationship with teneligliptin could not be excluded, i.e. related or unknown, as previously reported [21–23]. ADRs of special interest were those related to hypoglycaemia, skin and subcutaneous tissue disorders (including pemphigoid), gastrointestinal disorders (including pancreatitis and intestinal obstruction), hepatic impairment, renal impairment, cardiovascular events, and malignant tumours [22, 23]. Serious hypoglycaemia was defined as blood glucose level of ≤ 50 mg/dL or diagnosis by the prescribing physician. The incidences of cardiovascular- and malignant tumour-related AEs were also evaluated.
Data were analysed for patients divided into three age subgroups (< 65, ≥ 65 to < 75, and ≥ 75 years). The safety analysis set comprised all registered patients who underwent safety assessments and the efficacy analysis set comprised all patients in whom efficacy outcomes were evaluated. Continuous data were summarised as descriptive statistics, and discrete data were summarised as the number and percentage of patients for each category. For ADRs or AEs of special interest, the risk ratio of their incidence and the 95% confidence interval (CI) were calculated for each age subgroup, and the uniformity of the incidence rate was evaluated. Paired t tests were used to compare changes in continuous variables from baseline. A p-value less than 0.05 was considered to be significant. The change in HbA1c was also evaluated as the least-squares (LS) mean change and standard error (SE) by analysis of covariance with the baseline value as a covariate. The last observation carried forward method was used to impute missing data at the final time point. All analyses were performed using SAS statistical software version 9.1.3 or later.
Results
Patients
As previously described [23], 11,677 patients were initially registered, of which 10,696 and 10,249 were included in the safety and efficacy analysis sets, respectively. The 10,696 patients in the safety analysis set were divided into three subgroups (< 65, ≥ 65 to < 75, and ≥ 75 years), which comprised 4596 (43.0%), 3371 (31.5%), and 2729 (25.5%) patients, respectively. There were some differences in patient characteristics at baseline among the three age subgroups, particularly the percentage of male/female patients, the duration of diabetes mellitus, the rates of diabetic and non-diabetic complications, the rate of exercise therapy, and the estimated glomerular filtration rate between elderly patients and patients aged < 65 years (Table 1). HbA1c and FBG tended to be higher in younger patients.
Table 1.
Patient characteristics at baseline
| < 65 years old (n = 4596) | ≥ 65 to < 75 years old (n = 3371) | ≥ 75 years old (n = 2729) | |
|---|---|---|---|
| Sex | |||
| Male | 3141 (68.3%) | 1954 (58.0%) | 1344 (49.2%) |
| Female | 1455 (31.7%) | 1417 (42.0%) | 1385 (50.8%) |
| Age (years) | 53.9 ± 8.4 | 69.3 ± 2.9 | 80.2 ± 4.3 |
| Duration of T2DM (years) | 5.53 ± 5.84 (n = 3288) | 8.35 ± 8.40 (n = 2313) | 9.70 ± 9.51 (n = 1737) |
| BMI (kg/m2) | 26.44 ± 4.76 (n = 3158) | 24.52 ± 3.79 (n = 2258) | 23.95 ± 3.84 (n = 1707) |
| HbA1c (%) | 8.15 ± 1.75 (n = 4223) | 7.57 ± 1.32 (n = 3118) | 7.31 ± 1.14 (n = 2480) |
| FBG (mg/dL) | 157.9 ± 57.6 (n = 1641) | 147.6 ± 46.9 (n = 1188) | 145.7 ± 48.1 (n = 890) |
| eGFR* (mL/min/1.73 m2) | 83.91 ± 23.11 (n = 3979) | 71.31 ± 19.79 (n = 2982) | 61.07 ± 20.02 (n = 2421) |
| Diet therapy | 3586 (78.0%) | 2585 (76.7%) | 1968 (72.1%) |
| Exercise therapy | 2912 (63.4%) | 2032 (60.3%) | 1346 (49.3%) |
| Diabetic complications | |||
| Any | 1057 (23.0%) | 899 (26.7%) | 840 (30.8%) |
| Retinopathy | 449 (9.8%) | 370 (11.0%) | 256 (9.4%) |
| Neuropathy | 379 (8.2%) | 353 (10.5%) | 331 (12.1%) |
| Nephropathy | 722 (15.7%) | 642 (19.0%) | 636 (23.3%) |
| Other complications | |||
| Hypertension | 2477 (53.9%) | 2208 (65.5%) | 2020 (74.0%) |
| Dyslipidaemia | 3127 (68.0%) | 2211 (65.6%) | 1687 (61.8%) |
| Heart disease | 425 (9.2%) | 615 (18.2%) | 809 (29.6%) |
Values are n (%) of patients or mean ± standard deviation
T2DM type 2 diabetes mellitus, BMI body mass index, HbA1c glycosylated haemoglobin, FBG fasting blood glucose, eGFR estimated glomerular filtration rate
*Patients not on dialysis at baseline
Teneligliptin and Combination Therapy
Teneligliptin was administered for a median of 1096 days (i.e. 3 years) in each subgroup at mean daily dose of 20.34–20.50 mg (Table 2). Administration of teneligliptin was discontinued in 34.4%, 31.4%, and 38.1% of patients aged < 65, ≥ 65 to < 75, and ≥ 75 years, respectively, primarily as a result of the patient stopping hospital visits, transfer to another hospital, or an insufficient/ineffective treatment response (Table 2). The dose of teneligliptin was increased to 40 mg once daily in 100 (2.2%), 71 (2.1%), and 49 (1.8%) patients aged < 65, ≥ 65 to < 75, and ≥ 75 years, respectively. The median time to the first dose escalation was 182, 226, and 141 days in patients aged < 65, ≥ 65 to < 75, and ≥ 75 years, respectively, and the median period of administration of the higher dose was 645, 585, and 552 days, respectively.
Table 2.
Teneligliptin administration and concomitant therapies
| < 65 years (n = 4596) | ≥ 65 to < 75 years (n = 3371) | ≥ 75 years (n = 2729) | |
|---|---|---|---|
| Duration of treatment (days) |
1096 (477–1096) 821.48 ± 391.63 (n = 4309) |
1096 (625–1096) 855.34 ± 370.93 (n = 3126) |
1096 (427–1096) 799.59 ± 391.81 (n = 2550) |
| Daily dose (mg) | 20.50 ± 2.98 (n = 4309) | 20.40 ± 2.79 (n = 3126) | 20.34 ± 2.64 (n = 2550) |
| Discontinuation/withdrawal for any reason | 1581 (34.4%) | 1059 (31.4%) | 1040 (38.1%) |
| Did not return to the hospital* | 601 (38.0%) | 318 (30.0%) | 289 (27.8%) |
| Transfer to another hospital* | 329 (20.8%) | 251 (23.7%) | 335 (32.2%) |
| Insufficient/ineffective response* | 315 (19.9%) | 175 (16.5%) | 114 (11.0%) |
| Cure/symptom improvement* | 122 (7.7%) | 81 (7.6%) | 67 (6.4%) |
| AE/ADR* | 78 (4.9%) | 109 (10.3%) | 121 (11.6%) |
| Other* | 176 (11.1%) | 155 (14.6%) | 150 (14.4%) |
| Not reported* | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) |
| Concomitant antidiabetic agents | |||
| Monotherapy | 2076 (45.2%) | 1653 (49.0%) | 1397 (51.2%) |
| Sulfonylureas | 1126 (24.5%) | 868 (25.7%) | 691 (25.3%) |
| Biguanides | 1303 (28.4%) | 652 (19.3%) | 339 (12.4%) |
| α-GIs | 487 (10.6%) | 422 (12.5%) | 342 (12.5%) |
| Thiazolidines | 426 (9.3%) | 289 (8.6%) | 230 (8.4%) |
| Insulin | 353 (7.7%) | 272 (8.1%) | 196 (7.2%) |
| Glinides | 229 (5.0%) | 206 (6.1%) | 161 (5.9%) |
| SGLT2 inhibitors | 304 (6.6%) | 89 (2.6%) | 36 (1.3%) |
| Other concomitant therapies | |||
| Antihypertensive drugs | 1908 (41.5%) | 1806 (53.6%) | 1658 (60.8%) |
| Antidyslipidaemic drugs | 1844 (40.1%) | 1474 (43.7%) | 1118 (41.0%) |
Values are n (%) of patients, median (25th–75th percentile), or mean ± standard deviation
AE adverse event, ADR adverse drug reaction, α-GI α-glucosidase inhibitor, SGLT2 sodium–glucose cotransporter 2
*The denominator is the number of patients in each subgroup who discontinued the study for any reason
Teneligliptin was administered as monotherapy for T2DM in about half of the patients. Sulfonylureas, biguanides, and α-glucosidase inhibitors were the main combination therapies in all three subgroups. The use of metformin and SGLT2 inhibitors was lower in patients aged ≥ 65 to < 75 years and ≥ 75 years than in patients aged < 65 years. The concomitant use of antihypertensive drugs was higher in elderly patients than in patients aged < 65 years: 41.5% in patients aged < 65 years, 53.6% in patients aged ≥ 65 to < 75 years, and 60.8% in patients aged ≥ 75 years.
Safety
ADRs occurred in a similar proportion of patients in each age subgroup, with 176 ADRs in 154 (3.35%) patients aged < 65 years, 184 ADRs in 149 (4.42%) patients aged ≥ 65 to < 75 years, and 129 ADRs in 109 (3.99%) patients aged ≥ 75 years (Table 3). There was a tendency for a higher incidence of serious ADRs in elderly patients than in patients aged < 65 years, with 31 serious ADRs in 30 (0.65%) patients aged < 65 years, 46 serious ADRs in 41 (1.22%) patients aged ≥ 65 to < 75 years, and 56 serious ADRs in 46 (1.69%) patients aged ≥ 75 years.
Table 3.
Incidence of adverse drug reactions of special interest and adverse events related to cardiovascular disorders and malignant tumours
| < 65 years (n = 4596) | ≥ 65 to < 75 years (n = 3371) | ≥ 75 years (n = 2729) | |
|---|---|---|---|
| All ADRs | 154 (3.35%) | 149 (4.42%) | 109 (3.99%) |
| Serious ADRs | 30 (0.65%) | 41 (1.22%) | 46 (1.69%) |
| ADRs/AEs of special interest | |||
| Hypoglycaemia-related events | |||
| All ADRs | 11 (0.24%) | 19 (0.56%) | 8 (0.29%) |
| Per 100 patient-years | 0.10 | 0.23 | 0.13 |
| IRR (95% CI) | 1 | 2.27 (1.08–4.77) | 1.25 (0.50–3.10) |
| Serious | 2 (0.04%) | 3 (0.09%) | 3 (0.11%) |
| Skin and subcutaneous disorder-related events | |||
| All ADRs | 15 (0.33%) | 15 (0.44%) | 14 (0.51%) |
| Per 100 patient-years | 0.14 | 0.18 | 0.22 |
| IRR (95% CI) | 1 | 1.31 (0.64–2.69) | 1.60 (0.77–3.32) |
| Serious | 1 (0.02%) | 1 (0.03%) | 4 (0.15%) |
| Pemphigoid | 1 (0.02%) | 0 | 4 (0.15%) |
| Gastrointestinal disorder-related events | |||
| All ADRs | 25 (0.54%) | 32 (0.95%) | 16 (0.59%) |
| Per 100 patient-years | 0.23 | 0.39 | 0.25 |
| IRR (95% CI) | 1 | 1.68 (1.00–2.84) | 1.10 (0.59–2.06) |
| Serious | 3 (0.07%) | 4 (0.12%) | 5 (0.18%) |
| Pancreatitis | 0 | 1 (0.03%) | 0 |
| Intestinal obstruction (ileus) | 0 | 2 (0.06%) | 2 (0.07%) |
| Hepatic impairment-related events | |||
| All ADRs | 25 (0.54%) | 13 (0.39%) | 9 (0.33%) |
| Per 100 patient-years | 0.23 | 0.16 | 0.14 |
| IRR (95% CI) | 1 | 0.68 (0.35–1.33) | 0.62 (0.29–1.32) |
| Serious | 3 (0.07%) | 0 (0%) | 1 (0.04%) |
| Renal impairment-related events | |||
| All ADRs | 16 (0.35%) | 13 (0.39%) | 5 (0.18%) |
| Per 100 patient-years | 0.15 | 0.16 | 0.08 |
| IRR (95% CI) | 1 | 1.07 (0.51–2.22) | 0.54 (0.20–1.46) |
| Serious | 4 (0.09%) | 1 (0.03%) | 2 (0.07%) |
| Cardiovascular events | |||
| All AEs | 14 (0.30%) | 29 (0.86%) | 42 (1.54%) |
| Per 100 patient-years | 0.13 | 0.35 | 0.66 |
| IRR (95% CI) | 1 | 2.73 (1.44–5.17) | 5.16 (2.82–9.45) |
| All ADRs | 4 (0.09%) | 7 (0.21%) | 7 (0.26%) |
| Per 100 patient-years | 0.04 | 0.08 | 0.11 |
| IRR (95% CI) | 1 | 2.30 (0.67–7.86) | 3.00 (0.88–10.26) |
| Serious AEs | 11 (0.24%) | 25 (0.74%) | 40 (1.47%) |
| Serious ADRs | 1 (0.02%) | 6 (0.18%) | 7 (0.26%) |
| Malignant tumours* | |||
| All AEs | 22 (0.48%) | 48 (1.42%) | 41 (1.50%) |
| Per 100 patient-years | 0.20 | 0.58 | 0.65 |
| IRR (95% CI) | 1 | 2.88 (1.74–4.77) | 3.21 (1.91–5.39) |
| All ADRs | 8 (0.17%) | 9 (0.27%) | 10 (0.37%) |
| Per 100 patient-years | 0.07 | 0.11 | 0.16 |
| IRR (95% CI) | 1 | 1.48 (0.57–3.83) | 2.15 (0.85–5.44) |
| Pancreatic carcinoma, AE | 1 (0.02%) | 6 (0.18%) | 8 (0.29%) |
| Pancreatic carcinoma, ADR | 1 (0.02%) | 3 (0.09%) | 1 (0.04%) |
| Dizziness | |||
| All ADRs | 2 (0.04%) | 5 (0.15%) | 4 (0.15%) |
| Serious | 0 | 0 | 0 |
ADRs were defined as AEs for which a causal relationship with teneligliptin could not be excluded (i.e. causal or unknown)
Values are n (%) of patients, incidence rate per 100 patient-years, or incidence rate ratio (95% CI) versus the < 65-year-old subgroup
ADR adverse drug reaction, IRR incidence rate ratio, CI confidence interval, AE adverse event
*All tumours were classified as serious
After dose escalation to 40 mg, 16 ADRs were reported in 13 patients, including two ADRs in two (2.00%) of 100 patients aged < 65 years, seven ADRs in six (8.45%) of 71 patients aged ≥ 65 to < 75 years, and seven ADRs in five (10.20%) of 49 patients aged ≥ 75 years. The relationship between teneligliptin and the ADR was reported to be unknown for six of seven ADRs in patients aged ≥ 65 to < 75 years and in four of seven ADRs in those ≥ 75 years. Of the other four ADRs that were considered related to teneligliptin, the prescribing physician reported that other factors, such as complications or concomitant agents, could also be involved in two ADRs. In terms of serious ADRs, there was one ADR in one patient aged < 65 years (lung neoplasm malignant), none in patients aged ≥ 65 to < 75 years, and three in two patients aged ≥ 75 years (hepatic function abnormal and pancreatic carcinoma in one patient, and pemphigoid in the other patient). The causal relationship was unknown for all four serious ADRs.
As indicated in Table 3, the incidence of hypoglycaemia-related ADRs was higher in patients aged ≥ 65 to < 75 years than in patients aged < 65 years, occurring in 0.56% vs 0.24% of patients with an incidence rate per 100 person-years of 0.23 vs 0.10. However, compared with patients aged < 65 years, there was no clear difference in the incidence of hypoglycaemia-related ADRs in patients aged ≥ 75 years, with an incidence of 0.29% (0.13 per 100 person-years). Serious hypoglycaemia-related ADRs were reported in three patients aged ≥ 65 to < 75 years and in three patients aged ≥ 75 years. All of these patients were using sulfonylurea or insulin.
There was no significant difference in the incidence of skin and subcutaneous disorder-related ADRs across the three subgroups (Table 3). However, pemphigoid was reported in one patient (0.02%) aged < 65 years and in four patients (0.15%) aged ≥ 75 years.
Gastrointestinal disorders were the most common ADRs of special interest in the elderly patients (Table 3). There was no increase in the frequency of age-dependent ADRs related to gastrointestinal disorders but there tended to be more serious cases among elderly patients, occurring in three (0.07%) patients aged < 65 years, four (0.12%) patients aged ≥ 65 to < 75 years, and five (0.18%) patients aged ≥ 75 years. The serious gastrointestinal ADRs were intestinal obstruction (ileus) (n = 2), inguinal hernia (n = 1), and acute pancreatitis (n = 1) in patients aged ≥ 65 to < 75 years, and intestinal obstruction (ileus) (n = 2), gastric ulcer haemorrhage (n = 1), intestinal ischaemia (n = 1), and large intestine perforation (n = 1) in patients aged ≥ 75 years.
There were no age-dependent increases in the incidences of all or serious hepatic- and renal-related ADRs (Table 3).
The incidences of cardiovascular- and malignant tumour-related AEs were significantly greater in patients aged ≥ 65 to < 75 and ≥ 75 years than in patients aged < 65 years, but there was no significant age-related difference in the rates of these ADRs (Table 3). Pancreatic carcinoma was reported as an AE in six (0.18%) and eight (0.29%) patients aged ≥ 65 to < 75 and ≥ 75 years, respectively.
Dizziness, as an ADR other than those of special interest, occurred in 0.15% of patients in each of the ≥ 65 to < 75 and ≥ 75 years subgroups (Table 3).
HbA1c and FBG
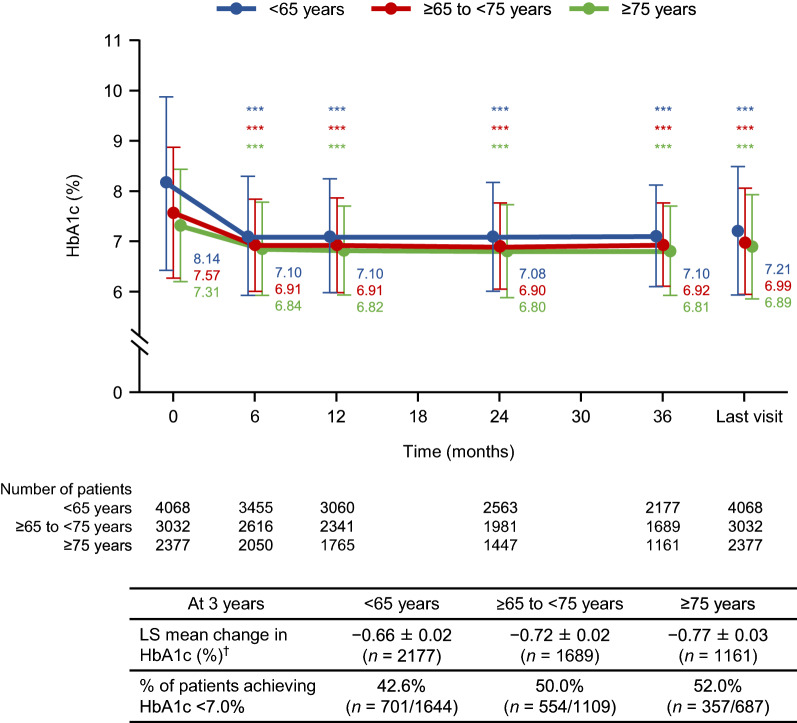
HbA1c decreased significantly at 6 months of treatment in all three subgroups, and these reductions were maintained through to 3 years (Fig. 1). The baseline-adjusted LS mean changes ± SE at 3 years were − 0.66 ± 0.02%, − 0.72 ± 0.02%, and − 0.77 ± 0.03%, respectively, with small but significant differences among the three subgroups (p < 0.001). At 3 years, the mean ± standard deviation HbA1c levels were 7.10 ± 1.01%, 6.92 ± 0.83%, and 6.81 ± 0.89% in patients aged < 65, ≥ 65 to < 75, and ≥ 75 years, respectively. Among patients with HbA1c ≥ 7% at baseline and in whom HbA1c was measured at 3 years, about half in each age subgroup achieved HbA1c < 7% at 3 years (42.6% [701/1644], 50.0% [554/1109], and 52.0% [357/687], respectively).
Fig. 1.
Mean levels and LS mean changes in HbA1c over time in patients aged < 65, ≥ 65 to < 75, or ≥ 75 years. The graph shows mean ± standard deviation at each time-point. ***p < 0.001 vs baseline by paired t test. †LS mean ± standard error, adjusted for baseline HbA1c. HbA1c glycosylated haemoglobin, LS mean least squares mean
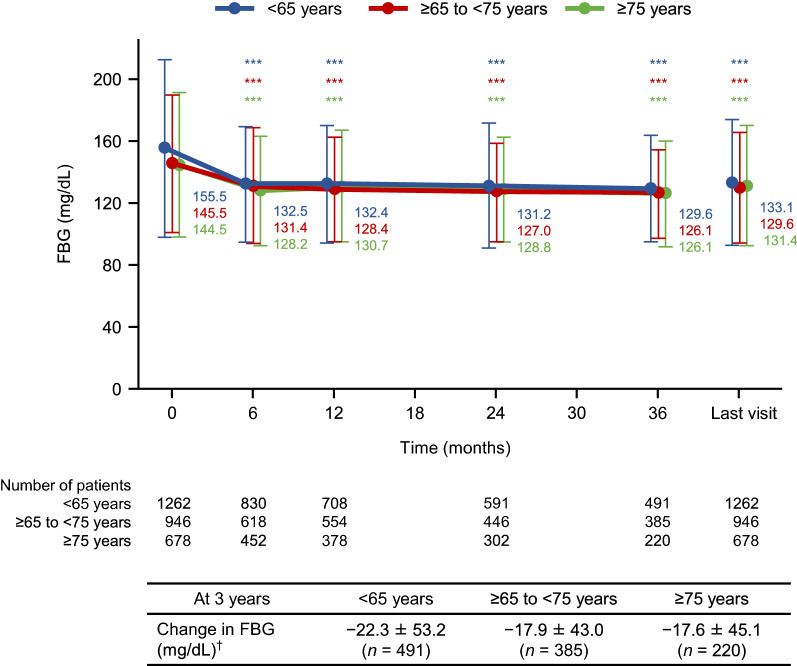
The changes in FBG over time followed similar trends to those observed for HbA1c, with significant reductions in FBG from baseline at each time point (Fig. 2). There were no clear differences in the mean changes ± standard deviation in FBG at 3 years among the three subgroups (− 22.3 ± 53.2 mg/dL, − 17.9 ± 43.0 mg/dL, and − 17.6 ± 45.1 mg/dL, respectively).
Fig. 2.
Mean levels and mean changes in FBG over time in patients aged < 65, ≥ 65 to < 75, or ≥ 75 years. The graph shows mean ± standard deviation at each time point. ***p < 0.001 vs baseline by paired t test. †Mean ± standard deviation. FBG fasting blood glucose
Body Weight and Lipids
Supplemental Fig. 1 shows the changes in body weight and lipids at each time point in the three age subgroups. There were small but significant reductions in body weight over 3 years in each age subgroup (Supplemental Fig. 1a). In terms of lipid levels during the observation period, there were small reductions in triglycerides and low-density lipoprotein cholesterol in each age subgroup, but there were no obvious changes in high-density lipoprotein cholesterol in the three subgroups (Supplemental Fig. 1b–d).
Discussion
This subgroup analysis of the RUBY surveillance was performed to provide insight into the long-term safety and efficacy of teneligliptin in elderly patients. Of note, we found no age-dependency on the overall incidences of ADRs, which occurred in about 3–4% of patients in each age subgroup, although the incidence of serious ADRs was higher in the elderly patients (≥ 65 to < 75 and ≥ 75 years subgroups) than in patients aged < 65 years. Needless to say, it is important to recognize that elderly patients are at risk of serious ADRs. The incidence of ADRs after a dose increase to 40 mg teneligliptin was numerically higher among elderly patients, but the causal relationship was unclear for many of the ADRs in those patients.
Interim results of the RUBY surveillance have been published, including the safety and efficacy for up to 2 years among elderly patients treated with teneligliptin [21]. The results of the present 3-year analysis are generally consistent with these earlier findings in terms of patient background, incidence and type of ADRs, and efficacy outcomes. The current findings extend those findings by showing that the safety profile of teneligliptin is maintained for up to 3 years of treatment with stable glycaemic control over this longer period.
In a 2-year real-world surveillance of anagliptin in Japan [24], the incidence of ADRs was 6.9% (146/2110) in patients aged < 65 years, 8.2% (117/1424) in patients aged ≥ 65 to < 75 years, and 10.2% (106/1039) in patients aged ≥ 75 years. Similarly, in a 78-week interim analysis of surveillance of linagliptin [25, 26], the incidence of ADRs was 8.58% (81/944) in patients aged < 65 years and 12.28% (172/1401) in patients aged ≥ 65 years when prescribed as monotherapy, and 9.01% (134/1488) and 9.76% (234/2398), respectively, when prescribed as combination therapy. Recently, the final results of this post-marketing surveillance based on data up to 156 weeks were reported for patients who started linagliptin monotherapy, with an incidence of ADRs of 8.9% (80/902) in patients aged < 65 years and 12.0% (160/1333) in patients aged ≥ 65 years [27]. In general, elderly patients are at increased risk for serious and non-serious AEs for many reasons, such as renal and/or hepatic function impairment, comorbidities, and multiple drug use [4, 5,28]. As stated in the package insert for teneligliptin [10, 11], it is important to administer it with care while closely monitoring the patient's condition.
We found no increase in the frequency of age-dependent ADRs in terms of ADRs of special interest, but the incidence of serious ADRs tended to be higher in elderly patients, particularly those aged ≥ 75 years.
Hypoglycaemia-related ADRs were most frequent in patients aged ≥ 65 to < 75 years among the three age subgroups, with an incidence rate ratio of 2.27 versus patients aged < 65 years. Perhaps unexpectedly, the incidence of hypoglycaemia-related ADRs in patients aged ≥ 75 years was similar to the corresponding values in patients aged < 65 years. Some possible factors that might contribute to these findings are that patients aged ≥ 75 years might be more likely to develop hypoglycaemia unawareness or that the physicians involved in the treatment of elderly patients were more cautious when prescribing concomitant drugs. All of the patients who experienced serious hypoglycaemia-related ADRs were taking teneligliptin in combination with insulin or sulfonylurea, which was associated with increased risk of hypoglycaemia. Hypoglycaemia contributes to adverse outcomes, such as deterioration of quality of life [29, 30] and increased fracture risk [31], and severe hypoglycaemia is an established risk factor for death [32], cardiovascular disease [32, 33], and dementia [34, 35]. Therefore, physicians should be particularly cautious to avoid hypoglycaemia in elderly patients.
An association between DPP4 inhibitors and pemphigoid has been reported [36, 37]. As a consequence, the package inserts for DPP4 inhibitors, including teneligliptin, were revised to include a precaution regarding pemphigoid in Japan. In this surveillance, most of the patients who developed pemphigoid were elderly, aged ≥ 75 years. Epidemiologically, pemphigoid has been reported to occur more frequently in elderly patients, especially those in their late 70s or older [38]. The findings of the RUBY surveillance are consistent with these earlier results.
Gastrointestinal disorders were the most common types of ADRs, but these occurred in fewer than 1% of patients in each subgroup. Although there was no clear age-dependency of gastrointestinal disorders, the frequency of serious gastrointestinal ADRs tended to be higher among elderly patients.
We also found that the incidences of cardiovascular- and malignant tumour-related AEs, but not ADRs, tended to increase with age. The higher incidence of cardiovascular-related AEs in elderly patients may be due to higher incidences of concurrent heart disease or renal impairment [39] at the start of teneligliptin treatment. The incidence of pancreatic carcinoma, which was the most common malignant tumour-related AE in RUBY [23], tended to increase with age. Advanced age and diabetes were reported to be associated with pancreatic carcinoma [40, 41]. As we previously reported [23], the incidence rates of cardiovascular-related AEs and pancreatic carcinoma were comparable with or lower than those observed in epidemiological surveys. Therefore, the increased incidence of these AEs after treatment with teneligliptin was considered to be due to advancing age.
As a most common ADR other than those of special interest, dizziness occurred in 0.15% of patients in each of the ≥ 65 to < 75 and ≥ 75 years subgroups, with an incidence similar to that reported in the prior clinical trials [10]. Therefore, we found no additional safety concerns among older people beyond those already described in teneligliptin’s package insert.
The efficacy of teneligliptin was evaluated in terms of the time course of HbA1c and FBG in each age subgroup. Improvements in glycaemic control were apparent within 6 months of starting treatment, and were maintained over the course of 3 years. There were no marked or clinically relevant differences in the reductions of HbA1c or FBG at 3 years across the three age subgroups. Some 24- or 52-week clinical trials have revealed that teneligliptin and other DPP4 inhibitors are also effective in elderly patients as monotherapy or in combination with other antidiabetic drugs [42–44]. The findings of the RUBY surveillance are consistent with these earlier studies. Our results also demonstrate the long-term efficacy of teneligliptin for the treatment of elderly patients with T2DM in the real world.
There were almost no changes in body weight at 3 years after the start of teneligliptin in all three age subgroups. Elderly patients with diabetes are prone to sarcopenia [4]. The prevention of sarcopenia is important because it increases the risk of falls, fractures, and deaths, and impairs quality of life [45]. Like other DPP4 inhibitors [46], the impact of teneligliptin on body weight may be neutral.
Limitations
Some possible limitations of this surveillance deserve mention, and have already been acknowledged in our prior report [23], especially the lack of a control group, changes in concomitant agents or lifestyle modifications, the possibility of reporting bias, and laboratory test data being unavailable for a substantial number of patients. These limitations should be taken into account when interpreting the present data. Another limitation specific to the present report relates to the possibility of bias due to the unequal distribution of patients in each age subgroup.
Conclusions
This surveillance provides evidence for the safety and efficacy of teneligliptin in elderly patients, and the results reported here are clinically relevant considering the aging of society and the trend towards increasing prevalence of T2DM in elderly patients. We found no additional safety or efficacy concerns among elderly patients beyond those already described in teneligliptin’s package insert. The present results support the use of teneligliptin for the treatment of elderly patients with T2DM in real-world clinical practice.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to all the physicians and patients involved in this post-marketing surveillance, K. Yoshida (Mitsubishi Tanabe Pharma Corporation) who managed the collection of the case report forms, M. Matsukawa (former employee of Mitsubishi Tanabe Pharma Corporation) for conducting the analyses, and H. Nakamura (Mitsubishi Tanabe Pharma Corporation) and M. Ueno (former employee of Mitsubishi Tanabe Pharma Corporation) for insightful discussions.
Funding
The surveillance was funded by Mitsubishi Tanabe Pharma Corporation and Daiichi Sankyo Co., Ltd. The Rapid Service and Open Access Fees were funded by Mitsubishi Tanabe Pharma Corporation.
Medical Writing Assistance
The authors thank Nicholas D. Smith of EMC K.K. for medical writing support, which was funded by Mitsubishi Tanabe Pharma Corporation.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
T. Kadowaki, M. Haneda, and H. Ito contributed to data interpretation and provided medical advice. K. Sasaki and Y. Yamada contributed to the conception of the surveillance and data interpretation. All authors contributed to manuscript development.
Disclosures
Takashi Kadowaki has received grants and personal fees from Astellas Pharma Inc., MSD Corporation, Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Taisho Pharma Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., and Sanofi K.K.; personal fees from AstraZeneca K.K., Kowa Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Kyowa Kirin Co., Ltd., Nipro Corporation, Medical View Co., Ltd., Musashino Foods Corporation, Medtronic Sofamor Danek Co., Ltd., Johnson & Johnson Co., Ltd., Terumo Co., Ltd., Medical Review Co., Ltd., Medscape Education, Abbott Japan Co., Ltd., Cosmic Corporation Co., Ltd., and Fujifilm Toyama Chemical Co., Ltd.; funding for endowed chair from AstraZeneca Co., Ltd., MSD Corporation, Ono Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., and Asahi Mutual Life Insurance Co.; contract research funding from AstraZeneca Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; and joint research funding from Daiichi Sankyo Co., Ltd. Masakazu Haneda has received clinical research grants from Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., and Johnson & Johnson Co., Ltd.; and personal fees from Astellas Pharma Inc., Taisho Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., MSD Corporation, Novartis Pharma K.K., and Novo-Nordisk Pharma Ltd. Hiroshi Ito has received grants and personal fees from Mitsubishi Tanabe Pharma Corporation and Daiichi Sankyo Co., Ltd. Kazuyo Sasaki and Yuka Yamada are employees of Mitsubishi Tanabe Pharma Corporation.
Compliance with Ethics Guidelines
RUBY was approved by the Ministry of Health, Labour and Welfare of Japan and was performed by Mitsubishi Tanabe Pharma Corporation in accordance with the Japanese ministry directive on Good Post-marketing Study Practice (GPSP). The surveillance used anonymous data collected in clinical practice in Japan. In accordance with Japanese regulations for post-marketing surveillance, it is not necessary to obtain informed consent from patients. RUBY was registered on the Japan Pharmaceutical Information Center clinical trials database (Japic CTI-153047).
Data Availability
The datasets generated and/or analysed during the current surveillance are not publicly available to protect individual patient confidentiality, but are available from the corresponding author on reasonable request.
Footnotes
Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11993877.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health, Labour and Welfare. National Health and Nutrition Survey in Japan 2016. 2016. https://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/kekkagaiyou_7.pdf. Accessed 04 Jan 2020. (In Japanese).
- 3.Goto A, Noda M, Inoue M, Goto M, Charvat H. Increasing number of people with diabetes in Japan: is this trend real? Intern Med. 2016;55(14):1827–1830. doi: 10.2169/internalmedicine.55.6475. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Older adults: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S152–S162. doi: 10.2337/dc20-S012. [DOI] [PubMed] [Google Scholar]
- 5.Japan Diabetes Society/Japan Geriatrics Society Joint Committee on Improving Care for Elderly Patients with Diabetes. Haneda M, Ito H. Glycemic targets for elderly patients with diabetes. Diabetol Int. 2016;7(4):331–333. doi: 10.1007/s13340-016-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–109. doi: 10.1111/jdi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yabe D, Seino Y, Fukushima M, Seino S. Beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15(6):602. doi: 10.1007/s11892-015-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015;6(5):495–507. doi: 10.1111/jdi.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceriello A, De Nigris V, Iijima H, Matsui T, Gouda M. The unique pharmacological and pharmacokinetic profile of teneligliptin: implications for clinical practice. Drugs. 2019;79(7):733–750. doi: 10.1007/s40265-019-01086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TENELIA® 20 mg, 40 mg tablets. Interview form, 14th version. 2019. https://medical.mt-pharma.co.jp/di/file/if/tnl.pdf. Accessed 04 Jan 2020. (In Japanese).
- 11.TENELIA® 20 mg, 40 mg tablets. Package insert. June 2019. https://medical.mt-pharma.co.jp/di/file/dc/tnl.pdf. Accessed 04 Jan 2020. (In Japanese).
- 12.Eto T, Inoue S, Kadowaki T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012;14(11):1040–1046. doi: 10.1111/j.1463-1326.2012.01662.x. [DOI] [PubMed] [Google Scholar]
- 13.Kadowaki T, Kondo K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(9):810–818. doi: 10.1111/dom.12092. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki T, Inagaki N, Kondo K, et al. Efficacy and safety of teneligliptin added to canagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a multicentre, randomized, double-blind, placebo-controlled, parallel-group comparative study. Diabetes Obes Metab. 2018;20(2):453–457. doi: 10.1111/dom.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4(6):576–584. doi: 10.1111/jdi.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab. 2014;16(5):418–425. doi: 10.1111/dom.12235. [DOI] [PubMed] [Google Scholar]
- 17.Kadowaki T, Kondo K, Sasaki N, et al. Efficacy and safety of teneligliptin add-on to insulin monotherapy in Japanese patients with type 2 diabetes mellitus: a 16-week, randomized, double-blind, placebo-controlled trial with an open-label period. Expert Opin Pharmacother. 2017;18(13):1291–1300. doi: 10.1080/14656566.2017.1359259. [DOI] [PubMed] [Google Scholar]
- 18.Kim MK, Rhee EJ, Han KA, et al. Efficacy and safety of teneligliptin, a dipeptidyl peptidase-4 inhibitor, combined with metformin in Korean patients with type 2 diabetes mellitus: a 16-week, randomized, double-blind, placebo-controlled phase III trial. Diabetes Obes Metab. 2015;17(3):309–312. doi: 10.1111/dom.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Kang ES, Jang HC, et al. Teneligliptin versus sitagliptin in Korean patients with type 2 diabetes inadequately controlled with metformin and glimepiride: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2019;21(3):631–639. doi: 10.1111/dom.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asahina Y, Sugano H, Sugiyama E, Uyama Y. Representation of older patients in clinical trials for drug approval in Japan. J Nutr Health Aging. 2014;18(5):520–523. doi: 10.1007/s12603-014-0031-5. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki T, Haneda M, Ito H, et al. Safety and efficacy of long-term treatment with teneligliptin: interim analysis of a post-marketing surveillance of more than 10,000 Japanese patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2018;19(2):83–91. doi: 10.1080/14656566.2017.1420165. [DOI] [PubMed] [Google Scholar]
- 22.Haneda M, Kadowaki T, Ito H, et al. Safety and efficacy of teneligliptin in patients with type 2 diabetes mellitus and impaired renal function: interim report from post-marketing surveillance. Diabetes Ther. 2018;9(3):1083–1097. doi: 10.1007/s13300-018-0416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadowaki T, Haneda M, Ito H, Sasaki H, Matsukawa M, Yamada Y. Long-term, real-world safety and efficacy of teneligliptin: a post-marketing surveillance of more than 10,000 patients with type 2 diabetes in Japan. Adv Ther. 2020;37(3):1065–1086. doi: 10.1007/s12325-019-01189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasahara T, Tabuchi O, Kashiwagi N, Ichikawa K. Safety and efficacy of long-term therapy with dipeptidyl peptidase-4 (DPP-4) inhibitor, anagliptin (Suiny®) in patients with type 2 diabetes mellitus—2-year interim report I of Suiny post-marketing prospective study with type 2 diabetes mellitus in Japan (SWIM-JPN) Jpn Pharmacol Ther. 2018;46(8):1293–1314. [Google Scholar]
- 25.Unno Y, Ikeda R, Ochiai K, Hayashi N. Safety and efficacy of long-term combination therapy with linagliptin (Trazenta® tablet 5 mg), a DPP-4 inhibitor, in patients with type 2 diabetes mellitus–Interim report from a special drug use-results survey. J New Rem Clin. 2018;67(7):799–822. [Google Scholar]
- 26.Unno Y, Ochiai K, Ikeda R, Hayashi H. Long-term safety and efficacy of linagliptin (Trazenta® tablets 5 mg), a DPP-4 inhibitor, in patients with type 2 diabetes mellitus–Interim report from special surveillance in patients who started linagliptin treatment as monotherapy. J New Rem Clin. 2018;67(6):667–688. [Google Scholar]
- 27.Yamamoto F, Unno Y, Okamura T, Ikeda R, Ochiai K, Hayashi N. Long-term safety and effectiveness of linagliptin in Japanese patients with type 2 diabetes mellitus: a 3-year post-marketing surveillance study. Diabetes Ther. 2020;11(1):107–117. doi: 10.1007/s13300-019-00723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med. 2000;160(18):2717–2728. doi: 10.1001/archinte.160.18.2717. [DOI] [PubMed] [Google Scholar]
- 29.Araki A, Ito H. Development of elderly diabetes burden scale for elderly patients with diabetes mellitus. Geriatr Gerontol Int. 2003;3(4):212–214. doi: 10.1111/j.1444-1586.2003.00084.x. [DOI] [Google Scholar]
- 30.Laiteerapong N, Karter AJ, Liu JY, et al. Correlates of quality of life in older adults with diabetes: the Diabetes & Aging Study. Diabetes Care. 2011;34(8):1749–1753. doi: 10.2337/dc10-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston SS, Conner C, Aagren M, Ruiz K, Bouchard J. Association between hypoglycaemic events and fall-related fractures in Medicare-covered patients with type 2 diabetes. Diabetes Obes Metab. 2012;14(7):634–643. doi: 10.1111/j.1463-1326.2012.01583.x. [DOI] [PubMed] [Google Scholar]
- 32.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 33.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 34.Lin CH, Sheu WH. Hypoglycaemic episodes and risk of dementia in diabetes mellitus: 7-year follow-up study. J Intern Med. 2013;273(1):102–110. doi: 10.1111/joim.12000. [DOI] [PubMed] [Google Scholar]
- 35.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301(15):1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka H, Ishii T. Analysis of patients with drug-induced pemphigoid using the Japanese Adverse Drug Event Report database. J Dermatol. 2019;46(3):240–244. doi: 10.1111/1346-8138.14741. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi Y, Shimauchi R, Nishibori N, et al. Dipeptidyl peptidase-4 inhibitors-associated bullous pemphigoid: a retrospective study of 168 pemphigoid and 9,304 diabetes mellitus patients. J Diabetes Investig. 2019;10(2):392–8. doi: 10.1111/jdi.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 39.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 40.Kasuga M, Ueki K, Tajima N, et al. Report of the JDS/JCA Joint Committee on Diabetes and Cancer. Diabetol Int. 2013;4(2):81–96. doi: 10.1007/s13340-013-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cancer Information Service, National Cancer Center, Japan Cancer Registry and Statistics. https://ganjoho.jp/data/reg_stat/statistics/dl/cancer_incidence47pref(2014-2015).xls. Accessed 04 Jan 2020. (In Japanese).
- 42.Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle HJ. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9902):1413–1423. doi: 10.1016/s0140-6736(13)61500-7. [DOI] [PubMed] [Google Scholar]
- 43.Strain WD, Lukashevich V, Kothny W, Hoellinger MJ, Paldanius PM. Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet. 2013;382(9890):409–416. doi: 10.1016/s0140-6736(13)60995-2. [DOI] [PubMed] [Google Scholar]
- 44.Kadowaki T, Marubayashi F, Yokota S, Katoh M, Iijima H. Safety and efficacy of teneligliptin in Japanese patients with type 2 diabetes mellitus: a pooled analysis of two phase III clinical studies. Expert Opin Pharmacother. 2015;16(7):971–981. doi: 10.1517/14656566.2015.1032249. [DOI] [PubMed] [Google Scholar]
- 45.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Diabetes Association Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S98–S110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current surveillance are not publicly available to protect individual patient confidentiality, but are available from the corresponding author on reasonable request.