Abstract
Introduction
Systemic sclerosis-associated interstitial lung disease (SSc-ILD) places a substantial burden on patients and healthcare systems. The objectives of this study were to describe clinical characteristics and assess healthcare resource utilization and costs of patients with SSc-ILD in England, compared with patients with non-pulmonary organ involvement related to SSc (SSc-OOI).
Methods
This population-based retrospective study used data from the Clinical Practice Research Datalink linked to Hospital Episode Statistics. Data were extracted from medical records dated January 1, 2005 to March 31, 2016. Patients with SSc were identified and placed in subgroups based on organ involvement: SSc-ILD, SSc-OOI, and both (SSc-ILD-OOI). Patients with SSc-ILD-OOI were included in both the SSc-ILD and SSc-OOI subgroups. All-cause healthcare costs, excluding medication costs, were calculated to 2016 British pounds sterling (£).
Results
This study included 675 patients with SSc: 174 (26%) had neither ILD nor other organ involvement (OOI); 127 (19%) had SSc-ILD; 477 (71%) had SSc-OOI; 103 (15%) had SSc-ILD-OOI. Age-weighted median [interquartile range (IQR)] annual healthcare costs per patient were: £1496 (£664–£2817) in SSc only; £6375 (£3451–£15,041) in SSc-ILD; £4084 (£1454–£10,105) in SSc-OOI; £6632 (£4023–£17,009) in SSc-ILD-OOI. In multivariate analysis, older age at diagnosis, diagnosis of anemia, and number of comorbid diseases were associated with higher yearly healthcare costs.
Conclusion
The annual healthcare cost for patients with SSc-ILD is substantial, and higher than that of patients with SSc-OOI or SSc only. These results quantify the economic burden of SSc-ILD in a real-world setting, and highlight the need for treatment of this disease.
Electronic supplementary material
The online version of this article (10.1007/s12325-020-01330-0) contains supplementary material, which is available to authorized users.
Keywords: CPRD, Electronic health records, Healthcare utilization, Interstitial lung disease, Observational study, SSc-ILD, Systemic sclerosis
Plain Language Summary
SSc is a rare disease that causes fibrosis, or thickening, of the skin. In some patients, SSc can also affect the lungs (‘SSc-ILD’) or other organs, e.g., the heart (‘SSc-OOI’). Patients with SSc-ILD typically have high healthcare costs; however, it is not clear how costs for SSc-ILD compare with those for SSc-OOI. To investigate this, we evaluated the costs associated with SSc-ILD and compared them with those for SSc only or SSc-OOI. In this England-based study, the annual healthcare costs for patients with SSc-ILD were approximately 50% higher than for those without lung disease (SSc only) or SSc-OOI. These results highlight the importance of promptly diagnosing and treating patients with lung fibrosis complicating SSc.
Electronic supplementary material
The online version of this article (10.1007/s12325-020-01330-0) contains supplementary material, which is available to authorized users.
Key Summary Points
| Systemic sclerosis (SSc) is a rare autoimmune disease involving the skin and internal organs, ultimately leading to fibrosis and organ dysfunction. |
| As part of the systemic involvement, the disease affects the lungs (SSc-ILD), other organs (SSc-OOI), or both the lungs and other organs (SSc-ILD-OOI). |
| SSc-ILD is a substantial burden on patients and healthcare services. The aim of this real-world observational study was to evaluate the clinical characteristics and healthcare resource utilization of patients with SSc, SSc-ILD, and SSc-OOI in England. |
| Patients with SSc-ILD have high annual healthcare costs that exceed those of patients with SSc or SSc-OOI. |
| These data show that SSc-ILD represents a high economic burden, and highlight the need for timely diagnosis and treatment to minimize healthcare costs. |
Introduction
Systemic sclerosis (SSc) is a rare, heterogeneous, chronic autoimmune disease that is characterized by fibrosis of the skin and is associated with high mortality [1, 2]. The prevalence of SSc is estimated to be 7.2–33.9 and 13.5–44.3 per 100,000 individuals in Europe and North America, respectively [3, 4]. Patients can develop organ involvement associated with SSc, including pulmonary hypertension, interstitial lung disease (ILD), gastrointestinal disorders, and cardiac and renal involvement [2].
Systemic sclerosis-associated interstitial lung disease (SSc-ILD), in which patients experience parenchymal infiltration leading to lung fibrosis and worsening respiratory symptoms, is a leading cause of SSc-related deaths [5, 6]. It is estimated that 30–52% of patients with SSc develop SSc-ILD [3]. Nintedanib is the first treatment approved by the U.S. Food & Drug Administration that slows the rate of decline in pulmonary function in patients with SSc-ILD [7, 8]. In addition, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion for nintedanib as a therapy for adult patients with SSc-ILD [9]. Several immunosuppressants are used in SSc-ILD based on varying levels of evidence [10], and a 2020 Delphi consensus recommends treatment with cyclophosphamide, mycophenolate mofetil, or nintedanib [11]. Whilst immunosuppression is important in SSc management, there is also a need to address lung fibrosis in SSc-ILD, as it is a major cause of mortality.
Available data suggest that SSc is associated with a substantial clinical and economic burden. A 2017 review identified six studies reporting on SSc-associated costs to patients and/or healthcare systems in five countries (Canada, France, Italy, Spain, USA) [12]. Although limited data are available regarding the healthcare resource utilization (HCRU) of patients with SSc-ILD, recent work in the USA suggests that care of these patients is associated with greater healthcare-related costs than the care of patients with SSc who do not have lung involvement [13, 14]. No data are available regarding the economic burden of SSc or SSc-ILD in England.
The aims of this population-based cohort study were: to describe the clinical characteristics of patients diagnosed with SSc, SSc-ILD, and SSc with other organ involvement (SSc-OOI); to estimate HCRU and costs among patients with SSc, SSc-ILD, and SSc-OOI; and to identify predictors for total costs among patients with SSc-ILD.
Methods
Study Design
This retrospective database analysis used both primary care records from the Clinical Practice Research Datalink (CPRD) GOLD database, and secondary care records relating to the admitted patient care, accident and emergency (A&E), and outpatient care from the Hospital Episode Statistics (HES) database between January 1, 2005 and March 31, 2016 (defined as the study period).
Generic ethical approval for observational research using the CPRD with approval from Independent Scientific Advisory Committee (ISAC) was granted by a Health Research Authority (HRA) Research Ethics Committee (REC) (East Midlands—Derby, REC reference number 05/MRE04/87). The protocol for this research was approved by the ISAC for the Medicines and Healthcare products Regulatory Agency (MHRA) Database Research (protocol number 16_262R2). Informed consent was not needed because of the anonymity of the CPRD data. This study was performed in adherence to the Declaration of Helsinki.
Patient Population
Patients were included in this study if they were: (1) listed in the CPRD database and their record could be linked with HES; (2) registered with a practice of acceptable standard as defined in the CPRD; (3) first diagnosed with SSc within the study period, with at least 12 months of available data in CPRD/HES before and after diagnosis; and (4) at least 18 years old at time of diagnosis. The date of first diagnosis was the index date for the follow-up period, which lasted for the remainder of the study period. Patients entering the database after March 31, 2015 or participating in a clinical trial during the study period were excluded. Due to the requirement for HES linkage, the population was limited to patients from England.
Patients with SSc, with or without ILD and OOI, were identified using the National Health Service’s (NHS) Read Code [15, 16] and the International Classification of Diseases (10th edition) [17] for primary care and secondary care records, respectively, in combination with modified European League Against Rheumatism (EULAR) classification criteria (Table S1 and Table S2) [18]. Included EULAR criteria were: skin thickening of the fingers, fingertip pitting scars, Raynaud’s phenomenon, pulmonary arterial hypertension (PAH), and/or ILD. The date of the last recorded EULAR symptom code was included as the diagnosis and index date.
Two distinct subgroup analyses were performed: the clinical subgroup analysis and the diagnosis subgroup analysis. The clinical subgroup analysis aimed to assess the costs associated with SSc-ILD and to compare these with the costs associated with SSc-OOI. Data from all patients were used in both subgroup analyses. For the clinical subgroup analysis, subgroups were defined based on the presence and nature of organ complications: SSc-ILD, SSc-OOI, and SSc-ILD and SSc-OOI (SSc-ILD-OOI). Patients were considered to have OOI if they had one or more of: angina, atrial fibrillation (AF), coronary heart disease (CHD), difficulty swallowing, diarrhea, dry mouth, heartburn, motor disturbances and dyspepsia, pericardial effusions, or renal crisis. The SSc-ILD and SSc-OOI subgroups were not mutually exclusive; patients with SSc-ILD-OOI were included in both the SSc-ILD and SSc-OOI subgroups, as well as the SSc-ILD-OOI subgroup. For the diagnosis subgroup analysis, three subgroups were defined based on the healthcare setting in which patients received their diagnosis: primary setting, secondary setting, and both settings. The primary and secondary setting subgroups were not mutually exclusive; patients who received diagnoses in both primary and secondary care were included in the primary and secondary setting subgroups, as well as the both settings subgroup.
Outcomes
The primary outcomes of this study were the descriptive statistics for demographics, patient and clinical characteristics, including comorbidities (according to the Charlson Comorbidity index), medical history, and pharmacotherapies prescribed in primary care 90 days before and 12 months after diagnosis, by subgroup.
As secondary outcomes, HCRU statistics were captured under the following categories: inpatient stays, A&E visits, general practitioner (GP) visits, prescriptions issued, and outpatient visits. For each of these, the number and percentage of patients with at least one outcome, the number of unique instances of each outcome, overall costs, and per patient costs were collected. The average length of inpatient stay was recorded, and attendances were grouped by Healthcare Resource Group (HRG) codes. Predictors for total costs by clinical subgroup were calculated. Therapies were identified from primary care prescription records/codes and grouped by drug class. Data regarding pharmacotherapies were only analyzed in the CPRD. Cost estimates were derived from NHS reference costs [19] and are given in 2016 British pounds sterling (£).
Statistical Methodology
Predictors of total cost in the overall population were derived using a multivariate generalized linear model with a gamma distribution and log-link function to allow for non-normal costs. Backward selection was used to identify a parsimonious model for the association between our identified covariates and total healthcare costs. Backward variable selection was performed using an α = 0.05 criterion. Inclusion of “age” and “gender” was forced, as these variables were a priori considered important. A set of variables, corresponding to the variables remaining in the model at the end of the selection procedure, was then created.
Statistical comparisons between subgroups in the clinical and diagnostic analyses were not conducted due to overlap between subgroups (a patient with SSc-ILD and OOI would be counted in the SSc-ILD, SSc-OOI, and SSc-ILD-OOI groups; similarly, a patient with a diagnosis in primary and secondary settings would be counted in the primary, secondary, and both settings subgroups). No p values are presented and comparisons are made using absolute numbers with no invocation of statistical significance.
Results
Patient Demographics and Characteristics
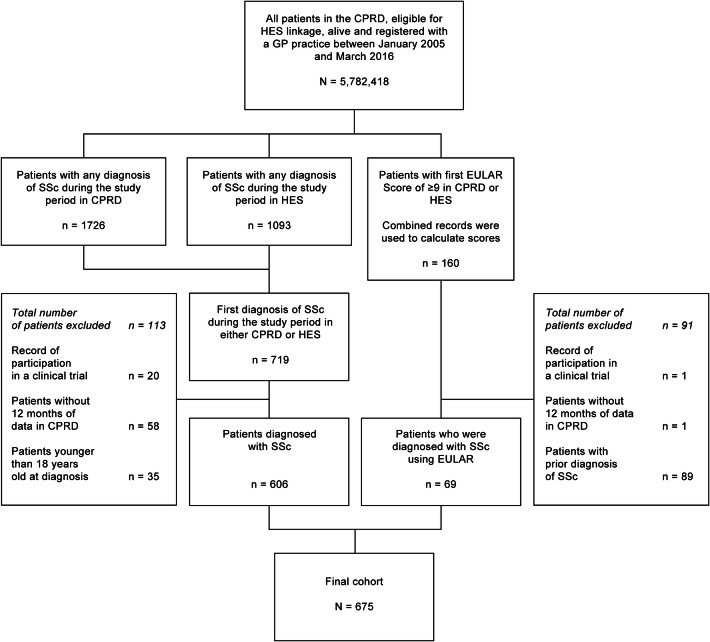
In total, 675 patients met the inclusion criteria (Fig. 1). Demographics, patient and clinical characteristics—including comorbidities—overall and by clinical and diagnosis subgroups, are presented in Table 1. The mean [standard deviation (SD)] age was 62 (17) years; 79% of the population were women. Most patients were white (85%) and lived in the south of England (65%), and half (50%) were overweight (body mass index > 25 kg/m2).
Fig. 1.
Cohort selection flowchart. CPRD Clinical Practice Research Datalink, EULAR European League Against Rheumatism, GP general practitioner, HES Hospital Episode Statistics, SSc systemic sclerosis
Table 1.
Demographics and clinical characteristics of patients diagnosed with SSc; SSc-ILD, SSc-OOI, SSc-ILD-OOI; in primary care, secondary care, or both
| SSc (n = 675) | Clinical subgroup analysis | Diagnostic subgroup analysis | |||||
|---|---|---|---|---|---|---|---|
| SSc-ILD (n = 127) | SSc-OOI (n = 477) | SSc-ILD and SSc-OOI (n = 103) | Primary care diagnosis (n = 485) | Secondary care diagnosis (n = 264) | Diagnosis in both care settings (n = 74) | ||
| Female | 531 (79) | 95 (75) | 379 (79) | 76 (74) | 385 (79) | 202 (77) | 56 (76) |
| Age at diagnosis, years (mean ± SD) | 62 ± 17 | 71 ± 13 | 65 ± 18 | 74 ± 13 | 62 ± 17 | 62 ± 17 | 62 ± 17 |
| Race | |||||||
| White | 572 (85) | 104 (82) | 419 (88) | 86 (83) | 405 (84) | 236 (89) | 69 (93) |
| Black | 18 (3) | 5 (4) | 14 (3) | 5 (5) | 12 (2) | 8 (3) | < 5 (3) |
| Asian | 26 (4) | 9 (7) | 18 (4) | 8 (8) | 16 (3) | 11 (4) | < 5 (1) |
| Other | 13 (2) | 6 (5) | 9 (2) | < 5 (2) | 7 (1) | 7 (3) | < 5 (1) |
| Missing | 46 (7) | < 5 (2) | 17 (4) | < 5 (2) | 45 (9) | < 5 (1) | < 5 (1) |
| BMI | |||||||
| Underweight (< 18.5 kg/m2) | 34 (5) | 8 (6) | 24 (5) | 6 (6) | 24 (5) | 14 (5) | < 5 (5) |
| Normal (18.5–24.9 kg/m2) | 275 (41) | 43 (34) | 200 (42) | 38 (37) | 208 (43) | 100 (38) | 33 (45) |
| Overweight (≥ 25 kg/m2) | 339 (50) | 72 (57) | 234 (49) | 55 (53) | 239 (49) | 133 (50) | 33 (45) |
| Missing | 27 (4) | < 5 (3) | 19 (4) | < 5 (4) | 14 (3) | 17 (6) | < 5 (5) |
| Smoking history | |||||||
| Non-smoker | 243 (36) | 39 (31) | 165 (35) | 32 (31) | 193 (40) | 71 (27) | 21 (28) |
| Current | 97 (14) | 12 (9) | 68 (14) | 8 (8) | 74 (15) | 39 (15) | 16 (22) |
| Ex-smoker | 144 (21) | 31 (24) | 106 (22) | 28 (27) | 103 (21) | 54 (20) | 13 (18) |
| Missing | 191 (28) | 45 (35) | 138 (29) | 35 (34) | 115 (24) | 100 (38) | 24 (32) |
| Region (UK) | |||||||
| North | 131 (19) | 22 (17) | 99 (21) | 16 (16) | 98 (20) | 50 (19) | 17 (23) |
| South | 436 (65) | 84 (66) | 301 (63) | 69 (67) | 309 (64) | 171 (65) | 44 (59) |
| East or West | 108 (16) | 21 (17) | 77 (16) | 18 (17) | 78 (16) | 43 (16) | 13 (18) |
| Comorbidities | |||||||
| Calcinosis | 15 (2) | < 5 (2) | 11 (2) | < 5 (1) | 11 (2) | < 5 (2) | 0 (0) |
| Raynaud’s phenomenon | 389 (58) | 88 (69) | 285 (60) | 71 (69) | 303 (62) | 155 (59) | 69 (93) |
| Esophageal dysmotility | 93 (14) | 20 (16) | 91 (19) | 20 (19) | 65 (13) | 37 (14) | 9 (12) |
| Sclerodactyly | 14 (2) | < 5 (2) | 10 (2) | < 5 (3) | 10 (2) | 5 (2) | < 5 (1) |
| Telangiectasia | 26 (4) | < 5 (2) | 15 (3) | < 5 (2) | 20 (4) | 12 (5) | 6 (8) |
| PAH | 109 (16) | 52 (41) | 94 (20) | 43 (42) | 72 (15) | 68 (26) | 31 (42) |
| GERD | 324 (48) | 72 (57) | 284 (60) | 67 (65) | 230 (47) | 129 (49) | 35 (47) |
| Anemia | 191 (28) | 49 (39) | 159 (33) | 42 (41) | 123 (25) | 90 (34) | 22 (30) |
| Low hemoglobin | 143 (21) | 40 (32) | 118 (25) | 34 (33) | 90 (19) | 76 (29) | 23 (31) |
| MCV | 25 (4) | 7 (6) | 17 (4) | < 5 (3) | 16 (3) | 15 (6) | 6 (8) |
| Acute RF | 6 (1) | < 5 (3) | 5 (1) | < 5 (4) | < 5 (1) | < 5 (1) | < 5 (1) |
| Dysrhythmia | 128 (19) | 43 (34) | 114 (24) | 38 (37) | 81 (17) | 71 (27) | 24 (32) |
| Organ transplant | 7 (1) | < 5 (2) | 5 (1) | < 5 (1) | 6 (1) | 5 (2) | < 5 (5) |
| Right heart catheterization | < 5 (0) | < 5 (2) | < 5 (0) | < 5 (2) | 0 (0) | < 5 (1) | 0 (0) |
| CT scan | 105 (16) | 37 (29) | 86 (18) | 33 (32) | 79 (16) | 47 (18) | 21 (28) |
| Hospitalizations in the year before diagnosis | |||||||
| 0 | 273 (40) | 34 (27) | 159 (33) | 27 (26) | 272 (56) | 7 (3) | 6 (8) |
| 1–19 | 338 (50) | 73 (57) | 265 (56) | 59 (57) | 190 (39) | 199 (75) | 51 (69) |
| ≥ 20 | 64 (9) | 20 (16) | 53 (11) | 17 (17) | 23 (5) | 58 (22) | 17 (23) |
Data are shown in numbers (%), unless otherwise indicated
BMI body mass index, CT computed tomography, GERD gastroesophageal reflux disease, MCV mean corpuscular volume, PAH pulmonary arterial hypertension, RF respiratory failure, SD standard deviation, SSc systemic sclerosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, SSc-OOI systemic sclerosis with other organ involvement, UK United Kingdom
Comorbidities and manifestations of SSc were frequent in the overall population (Table 1). Raynaud’s phenomenon was recorded in 58% of patients. Gastroesophageal reflux disease (GERD) was recorded in 48% of patients, and anemia was recorded in 28%. Most patients (60%) were hospitalized at least once in the year before diagnosis. Only 16% of patients had a computed tomography (CT) scan at diagnosis.
Clinical Subgroup Analysis
Within the overall patient population, 127 patients (19%) were included in the SSc-ILD subgroup and 477 (71%) in the SSc-OOI subgroup (Table 1). The SSc-ILD-OOI subgroup contained 103 patients (15%). Patients with both ILD and OOI comprised 81% of the SSc-ILD subgroup and 22% of the SSc-OOI subgroup. Of 675 patients with SSc, 174 (26%) had neither ILD nor OOI.
GERD was reported more frequently in patients with SSc-ILD or SSc-OOI than in the overall population of patients with SSc. PAH was more common in patients with SSc-ILD than in patients with SSc or SSc-OOI. A higher proportion of the SSc-ILD subgroup presented with Raynaud’s phenomenon compared with the overall SSc population and the SSc-OOI subgroup (Table 1).
Diagnosis Subgroup Analysis
Of all patients (N = 675) diagnosed with SSc, 485 (72%) had their diagnosis recorded in primary care, 264 (39%) had their diagnosis recorded in secondary care, and 74 (11%) had their diagnosis recorded in both (Table 1). A smaller proportion of patients in the primary setting subgroup were hospitalized in the year prior to diagnosis (n = 213, 44%), compared with the secondary (n = 257, 97%) and both settings (n = 68, 92%) subgroups.
In the primary and secondary setting subgroups, the most commonly reported comorbidities were Raynaud’s phenomenon, GERD, and anemia. In the both settings subgroup, Raynaud’s phenomenon, GERD, and PAH were the most commonly reported comorbidities.
Pharmacotherapies Prescribed in Primary Care
Similar proportions of patients in each subgroup were prescribed pharmacotherapies (by treatment type), in both the clinical and diagnosis subgroup analyses (Table 2). In the 90 days prior to diagnosis, 345 patients (51%) did not receive pharmacotherapy in primary care. Glucocorticoids and calcium channel blockers were the most commonly prescribed pharmacotherapies, both before and after SSc diagnosis. In the 12 months after recorded diagnosis, 41% of patients were prescribed glucocorticoids. All types of glucocorticoids were prescribed more frequently after diagnosis, compared with before (Supplementary Table S3). In contrast, the recorded use of mycophenolate mofetil following SSc diagnosis was low (fewer than 5 patients in total).
Table 2.
Treatment prescribed in primary care 90 days before and 12 months after diagnosis to patients diagnosed: with SSc, SSc-ILD, SSc-OOI, or SSc-ILD and SSc-OOI (A); or with SSc in primary care, secondary care or both (B)
| A | SSc (n = 675) | SSc-ILD (n = 127) | SSc-OOI (n = 477) | SSc-ILD and SSc-OOI (n = 103) | ||||
|---|---|---|---|---|---|---|---|---|
| 90 days before diagnosis | 12 months after diagnosis | 90 days before diagnosis | 12 months after diagnosis | 90 days before diagnosis | 12 months after diagnosis | 90 days before diagnosis | 12 months after diagnosis | |
| CC | 174 (26) | 218 (32) | 37 (29) | 48 (38) | 138 (29) | 164 (34) | 32 (31) | 41 (40) |
| ERAs | < 5 (0) | < 5 (0) | 0 (0) | < 5 (1) | < 5 (0) | 6 (1) | 0 (0) | < 5 (1) |
| PDE-5 | < 5 (0) | 10 (1) | < 5 (1) | < 5 (3) | < 5 (0) | 7 (1) | < 5 (1) | < 5 (4) |
| Prostaglandins | 16 (2) | 21 (3) | < 5 (3) | 5 (4) | 13 (3) | 16 (3) | < 5 (3) | < 5 (4) |
| MMF | < 5 (1) | < 5 (1) | < 5 (2) | < 5 (2) | < 5 (1) | < 5 (1) | < 5 (1) | < 5 (3) |
| Azathioprine | 14 (2) | 21 (3) | 8 (6) | 12 (9) | 12 (3) | 19 (4) | 7 (7) | 12 (12) |
| Methotrexate | 24 (4) | 44 (7) | < 5 (2) | 5 (4) | 16 (3) | 27 (6) | < 5 (3) | < 5 (4) |
| Glucocorticoids | 183 (27) | 274 (41) | 58 (46) | 81 (64) | 136 (29) | 199 (42) | 48 (47) | 67 (65) |
| Oxygen therapy | < 5 (1) | < 5 (0) | < 5 (2) | < 5 (1) | < 5 (1) | < 5 (0) | < 5 (1) | < 5 (1) |
| Other treatment | 11 (2) | 7 (1) | 9 (7) | 6 (5) | 7 (1) | 6 (1) | 5 (5) | < 5 (5) |
| No pharmacotherapy | 345 (51) | NA | 41 (32) | NA | 226 (47) | NA | 30 (29) | NA |
| B | SSc (n = 675) | Primary care diagnosis (n = 485) | Secondary care diagnosis (n = 264) | Diagnosis in both care settings (n = 74) | ||||
|---|---|---|---|---|---|---|---|---|
| CC | 174 (26) | 218 (32) | 124 (26) | 167 (35) | 66 (25) | 65 (25) | 16 (22) | 14 (19) |
| ERAs | < 5 (0) | < 5 (0) | < 5 (0) | < 5 (1) | 0 (0) | < 5 (0) | 0 (0) | < 5 (1) |
| PDE-5 | < 5 (0) | 10 (1) | 0 (0) | 6 (1) | < 5 (1) | < 5 (2) | 0 (0) | 0 (0) |
| Prostaglandins | 16 (2) | 21 (3) | 11 (2) | 14 (3) | 7 (3) | 9 (3) | < 5 (3) | < 5 (3) |
| MMF | < 5 (1) | < 5 (1) | < 5 (1) | < 5 (0) | < 5 (1) | < 5 (1) | < 5 (3) | < 5 (1) |
| Azathioprine | 14 (2) | 21 (3) | 8 (2) | 14 (3) | 9 (3) | 12 (5) | < 5 (4) | 5 (7) |
| Methotrexate | 24 (4) | 44 (7) | 17 (4) | 34 (7) | 9 (3) | 12 (5) | < 5 (3) | < 5 (3) |
| Glucocorticoids | 183 (27) | 274 (41) | 124 (26) | 192 (40) | 95 (36) | 117 (45) | 36 (49) | 35 (47) |
| Oxygen therapy | < 5 (1) | < 5 (0) | < 5 (1) | < 5 (0) | < 5 (2) | < 5 (0) | < 5 (5) | < 5 (1) |
| Other treatment | 11 (2) | 7 (1) | 6 (1) | < 5 (1) | 9 (3) | 6 (2) | < 5 (5) | < 5 (3) |
| No pharmacotherapy | 345 (51) | NA | 257 (53) | NA | 115 (44) | NA | 27 (36) | NA |
Data are shown in numbers (%), unless otherwise indicated. Other treatment includes acetylcysteine, pirfenidone, rituximab, and nintedanib
CC calcium channel blocker, ERA endothelin receptor antagonist, MMF mycophenolate mofetil, NA data not available, PDE-5 phosphodiesterase-5 inhibitor, SSc systemic sclerosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, SSc-OOI systemic sclerosis with other organ involvement
Healthcare Resource Utilization
The rate of healthcare resource usage (GP visits, outpatient and inpatient stays, A&E attendances) among patients with SSc-ILD, OOI, or both was consistently higher than in patients with only SSc (Table 3). Patients with SSc-ILD and SSc-ILD-OOI had the highest rates of inpatient stays (2.7 and 2.8, respectively), outpatient visits (9.5 and 9.7), A&E attendances (0.8 and 0.8), and GP visits (16.2 and 16.9) per person-year.
Table 3.
Resource use by patients diagnosed: with SSc; SSc-ILD, SSc-OOI, or SSc-ILD and SSc-OOI (A); in primary care, secondary care, or both (B)
| SSc (n = 675) | A | B | ||||||
|---|---|---|---|---|---|---|---|---|
| SSc only (n = 174) | SSc-ILD (n = 127) | SSc-OOI (n = 477) | SSc-ILD and SSc-OOI (n = 103) | Primary care diagnosis (n = 485) | Secondary care diagnosis (n = 264) | Diagnosis in both care settings (n = 74) | ||
| Inpatient stays | ||||||||
| Rate per person-year (95% CI) | 1.6 (1.5–1.6) | 0.8 (0.7–0.9) | 2.7 (2.6–2.9) | 1.9 (1.8–2.0) | 2.8 (2.7–3.0) | 1.3 (1.2–1.3) | 2.6 (2.5–2.7) | 2.3 (2.1–2.6) |
| Total admissions, n | 3701 | 522 | 1037 | 3023 | 881 | 2306 | 1692 | 297 |
| SSc admissions, n (%) | 233 (6) | 59 (11) | 82 (8) | 154 (5) | 62 (7) | 162 (7) | 73 (4) | 2 (1) |
| A&E attendances | ||||||||
| Rate per person-year (95% CI) | 0.5 (0.5–0.6) | 0.3 (0.3–0.3) | 0.8 (0.7–0.8) | 0.6 (0.6–0.7) | 0.8 (0.7–0.9) | 0.5 (0.4–0.5) | 0.9 (0.9–1.0) | 1.5 (1.3–1.7) |
| Total attendances, n | 1231 | 190 | 296 | 998 | 253 | 829 | 589 | 187 |
| General practitioner visits | ||||||||
| Rate per person-year (95% CI) | 13.1 (12.9–13.2) | 9.5 (9.3–9.8) | 16.2 (15.8–16.6) | 14.6 (14.4–14.7) | 16.9 (16.4–17.4) | 12.0 (12.0–12.0) | 16.0 (15.6–16.2) | 15.1 (14.5–15.8) |
| Total visits, n | 30,271 | 6394 | 6124 | 23,009 | 5256 | 21,816 | 10,378 | 1923 |
| Outpatient stays | ||||||||
| Rate per person-year (95% CI) | 6.5 (6.4–6.6) | 4.4 (4.2–4.5) | 9.5 (9.2–9.9) | 7.3 (7.2–7.4) | 9.7 (9.4–10.1) | 6.2 (6.1–6.3) | 8.0 (7.8–8.2) | 9.5 (9.0–10.1) |
| Total appointments, n | 15,062 | 2926 | 3611 | 11,552 | 3027 | 11,077 | 5198 | 1213 |
A&E accident and emergency services, CI confidence interval, SSc systemic sclerosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, SSc-OOI systemic sclerosis with other organ involvement
Patients with a recorded diagnosis in secondary care, or secondary and primary care had higher HCRU rates than patients with a diagnosis in primary care.
Healthcare Costs
Age-weighted median [interquartile range (IQR)] healthcare costs per patient-year in the clinical subgroup analysis were higher for patients with SSc-ILD [£6375 (£3451–£15,041)] or SSc-ILD and OOI [£6632 (£4023–£17,009)] than for patients with SSc-OOI [£4084 (£1454–£10,105)] or SSc only [£1496 (£664–£2817)] (Fig. 2a). Results were similar for crude data.
Fig. 2.
Median all-cause healthcare costs per patient-year (a); after excluding patients with CVD (b); among patients who died during follow-up (c); and among patients with anemia (d). All-cause healthcare costs are shown in British pounds sterling (£) and do not include medication. All data in b–d are age-weighted. CVD was defined as AF, angina, or CHD. AF atrial fibrillation, CHD coronary heart disease, CVD cardiovascular disease, SSc systemic sclerosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, SSc-OOI systemic sclerosis with other organ involvement
Each patient with SSc-ILD had, on average, 10 outpatient visits per year, at a total yearly median cost of £813. In comparison, patients with SSc-OOI averaged seven outpatient visits at a total yearly median cost of £608 (Tables S4 and S5).
Results of sensitivity analyses showed that healthcare costs were consistently the highest for patients with SSc-ILD (with or without OOI), after excluding patients with cardiovascular disease (CVD; defined as angina, AF, and CHD) (Fig. 2b), as well as among patients who died during follow-up (Fig. 2c) and those with anemia (Fig. 2d). Overall, median age-weighted healthcare costs were higher in patients with CVD [£5507 (£1660–£14,886)], anemia [£5915 (£2368–£14,259)], and in those who died [£9751 (£4550–£26,645)] compared with patients who did not have CVD [£2874 (£1060–£7316)], anemia [£2321 (£888–£6447)], or did not die [£2068 (£936–£5201)] (Fig. 2).
An investigation into potential predictors of total healthcare costs in the overall population, using a multivariate generalized linear model, showed a significant association between costs and older age at diagnosis, a diagnosis of anemia, and the total number of comorbidities based on incremental increases in the Charslon Comorbidity index (all p < 0.0001).
Inpatient Stays and Costs
Inpatient stays were the main contributor to healthcare costs in the entire cohort of patients with SSc [median (IQR)], £1627 (£109–6023); Table S5] and across all clinical subgroups except for the SSc-only subgroup, in which outpatient stays were the major cost contributor. This was true for patients with CVD and SSc, and patients with SSc only. In patients with age-weighted annual costs of ≥ £15,000 (n = 77), inpatient stays were still the main contributor to overall cost, including in patients (n = 6) with SSc only (Table S5E). Of all 790 inpatient attendances, 414 (52%) were day admissions, 287 (36%) were non-elective admissions, and 89 (11%) were ordinary elective admissions (pre-planned with a duration of more than 1 day). The proportions of the different admission types were consistent across all clinical subgroups (data not shown).
The median length of inpatient stay was less than 1 day, indicating that most patients who were hospitalized left the same day (Table 4). This was consistent across diagnostic and clinical subgroups. The median duration of elective stays was 5 days (IQR 3–7), as was the median duration of non-elective stays (IQR 3–11). These findings were also consistent across all clinical subgroups (data not shown). Based on grouping of attendances according to HRG subchapter codes, the main drivers of inpatient costs appeared to be related to connective tissue disorders (23%), the respiratory system (13%), cardiac surgery and primary cardiac conditions (13%), the digestive system (10%), and the vascular system (7%).
Table 4.
Length of inpatient stays by patients diagnosed: with SSc; SSc-ILD, SSc-OOI, or SSc-ILD and SSc-OOI (A); in primary care, secondary care, or both (B)
| SSc (n = 675) | A. Clinical subgroup analysis | B. Diagnostic subgroup analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| SSc only (n = 174) | SSc-ILD (n = 127) | SSc-OOI (n = 477) | SSc-ILD and SSc-OOI (n = 103) | Primary care diagnosis (n = 485) | Secondary care diagnosis (n = 264) | Diagnosis in both settings (n = 74) | ||
| Inpatient stays | ||||||||
| Total admissions, n | 3701 | 522 | 1037 | 3023 | 881 | 2306 | 1692 | 297 |
| Length of stay in days, median (IQR) | 0 (0–2) | 0 (0–1) | 0 (0–3) | 0 (0–2) | 0 (0–3) | 0 (0–1) | 0 (0–2) | 0 (0–3) |
| Inpatient day admissions (0 days duration), n (%) | 2460 (66) | 347 (66) | 667 (64) | 2002 (66) | 556 (63) | 1562 (68) | 1048 (62) | 150 (51) |
| Inpatient stays, 1–2 days duration, n (%) | 426 (12) | 58 (11) | 104 (10) | 346 (11) | 82 (9) | 265 (11) | 233 (14) | 72 (24) |
| Inpatient stays, ≥ 3 days duration, n (%) | 815 (22) | 117 (22) | 266 (26) | 675 (22) | 243 (28) | 479 (21) | 411 (24) | 75 (25) |
IQR interquartile range, SSc systemic sclerosis, SSc-ILD systemic sclerosis-associated interstitial lung disease, SSc-OOI systemic sclerosis with other organ involvement
Discussion
The clinical and economic burden of SSc is substantial [13]. Although it is thought that ILD contributes significantly to the cost of SSc, there is limited evidence regarding the patient characteristics and HCRU associated with SSc-ILD outside of the USA. Through analysis of patient care records from the CPRD and HES databases, this study identified 675 patients with SSc in England. Of these, 19% had ILD and 71% had OOI. The median (IQR) age-weighted annual healthcare cost of a patient with SSc-ILD was estimated to be £6375 (£3451–£15,041), which is larger than that of a patient with SSc-OOI [£4084 (£1454–£10,105)] or SSc alone [£1496 (£664–£2817)]. To date, no other comparisons between HCRU in patients with SSc-ILD and patients with SSc and OOI have been reported, nor have data regarding the HCRU of patients with SSc-ILD in England.
Our finding that SSc-ILD is associated with greater costs than SSc alone is supported by three published studies using US-based data [13, 14, 20]. A study published in 2012 used a national healthcare claims database to assess the burden of SSc from 2003–2008. In addition to finding that patients with SSc had higher healthcare-related costs than matched controls, the authors noted that the cost for patients with SSc-ILD over 1 year was 2.3-times that of patients with SSc alone [20]. An analysis of data from two administrative claims databases also found that patients with SSc-ILD had greater costs than patients with SSc alone during the study period (2003–2014). The largest costs among patients with SSc-ILD and patients with SSc-PAH were for outpatient pharmacy claims [20]. The final study used claims data from 2005–2015 to compare healthcare costs between patients with SSc-ILD and matched controls [14]. Patients with SSc-ILD had higher costs than those without, and results were compared with a study of patients with SSc with otherwise similar methodology. In this comparison, SSc-ILD was associated with higher cost than SSc [14, 21]. Our study differs from these US claims-based studies [13, 14, 20] in that patient inclusion was based on primary and secondary care records generated as part of standard healthcare, and is therefore more likely to reflect the general population. This is in contrast to claims-based studies, which extract data from insured individuals only and therefore may not be representative of the whole population [13, 14, 20].
Two studies have reported analyses of predictors of increased cost in patients with SSc [20, 22]. One study, conducted in Canada, found that, while older age was associated with increasing direct cost, younger age was associated with higher overall cost. This study included indirect costs, and the association between younger age and increased cost reflects the greater lost productivity associated with removal of a younger person from the labor market [22]. The other study, conducted in the USA, found that ages 45–64 years were associated with increased cost compared to ages 18–44 years. The authors also found that ILD, gastrointestinal bleeding, and renal disease were associated with higher cost [20], which is consistent with our finding that a higher number of comorbidities is associated with higher cost. The associations between comorbidities and high cost suggest that prevention and early treatment (where cost-effective) of these disorders could help reduce costs for patients with SSc, including those with SSc-ILD.
Most patients with SSc (61%) in our study had a first recorded diagnosis in primary care. The most frequent clinical manifestations (GERD, Raynaud’s phenomenon, PAH, anemia) were similar across care settings and disease subgroups, which is consistent with observations of early multiorgan involvement [23].
Although no approved pharmacotherapies for SSc-ILD were available during the study period, an analysis of the European Scleroderma Trials and Research patient database suggested that SSc-ILD is frequently managed by off-label use of immunosuppressants, such as cyclophosphamide, mycophenolate mofetil, and glucocorticoids [10]. The European Scleroderma Observational Study found that treatment with cyclophosphamide was associated with significantly greater rates of change in forced vital capacity over time than mycophenolate mofetil, methotrexate, or no immunosuppression in patients with early diffuse cutaneous SSc and pulmonary fibrosis [24]. An analysis of a USA claims database using data from 2009–2014 found that 52% of patients with SSc-ILD received glucocorticoids, 19% received mycophenolate mofetil, and 9% received cyclophosphamide [13]. In our study, the use of mycophenolate mofetil was extremely limited in the overall population and in the SSc-ILD subgroup (both < 5%), and use of cyclophosphamide was not observed. Pharmacotherapy use was derived solely from CPRD records, which include only primary care data. Hence, the infrequent prescribing of recommended immunosuppressants suggests that SSc-ILD is managed outside of a primary care setting. It is also worth noting that mycophenolate mofetil was not included in the 2017 EULAR treatment guidelines, as the first randomized clinical trial of mycophenolate mofetil in SSc-ILD was published in 2016, which was too late for inclusion in the guidelines [25, 26].
In our study, patients with a diagnosis in secondary care had higher HCRU rates compared with patients diagnosed in primary care or both. Most inpatient stays did not result in patients being admitted overnight. Although pharmacotherapy was only directly captured in primary care, these inpatient stays could reflect administration of treatment (such as cyclophosphamide) in secondary care. Therefore, these inpatient stays potentially represent a cost that could be reduced if patients were to be treated with a therapy that does not require administration in a hospital or tertiary care setting. However, these stays could also capture consultant visits, admissions for procedures, administration of other drugs, or disease-related events leading to hospitalization.
Guidelines published by EULAR in 2009 and 2017 do not recommend glucocorticoids in the treatment of SSc or SSc-ILD, and only note that they are associated with an increased risk of scleroderma renal crisis [25, 27]. In the current study, 27% of all patients received glucocorticoids 9 months prior to diagnosis, compared with 41% 12 months after. Of patients with SSc-ILD, 41% received glucocorticoids before diagnosis, compared with 64% after. While glucocorticoids are not recommended for the treatment of SSc-ILD, they are used to treat idiopathic inflammatory myopathies, which can overlap with SSc [1, 28–30]. The treatment of patients with SSc and SSc-ILD in this study could, therefore, represent treatment of patients with musculoskeletal involvement, as well as off-label, unrecommended use of glucocorticoids.
This study had multiple strengths, including the large, real-world dataset of patients with SSc that was used. Data were gathered over a long follow-up period. It is likely that a large proportion of the patient population included in this study comprised patients with clear diagnoses of SSc. Despite this, this study is limited in that these diagnoses were not externally validated. There are likely to have been further patients who were not identified from the dataset due to inadequate coding. Another potential limitation is that CPRD data extraction is dependent on general practices agreeing to participate, and contains data for only 6.9% of the United Kingdom population [31]. However, patients in the CPRD are thought to be broadly representative of the general population [31]. Total healthcare costs were estimated from NHS reference lists and did not include medication costs. Costs arising from prescriptions and tests in secondary care (such as genetic tests or high-resolution CT scans) were not captured.
Conclusions
This observational study characterized the SSc patient population in England for the first time, and found that HCRU costs were higher for patients with SSc-ILD than for patients with SSc but without ILD, irrespective of OOI. The descriptive characteristics and cost estimates reported here may support the development of strategies to facilitate earlier diagnosis and management of SSc and SSc-ILD. These results may also be included as inputs in future economic models to evaluate the cost-effectiveness of SSc-ILD treatment options.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study, the Rpid Service Fee and the Open Access Fee, were funded by Boehringer Ingelheim International GmbH (BI), Germany, which manufactures nintedanib. Writing, editorial support, and formatting assistance for this manuscript was provided by: Ben Daniels (PhD), Laura Schmidt (MRes, MPhil), and Lindsey O’Mahony (PhD) of AMICULUM; and Claire Scott (PhD) of MediTech Media, UK, which was contracted and compensated by BI for these services. BI was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
A. Gayle and D. Clarke are former employees of Boehringer Ingelheim. A. Gayle is a current employee of AstraZeneca, Cambridge, UK. N. Schoof, M. Alves, C. Raabe and P. Das are current employees of Boehringer Ingelheim. F. Del Galdo has received research funding and/or consulting fees or other remuneration from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Actelion, Capella Bioscience, Chemomab and Kymab. T.M. Maher has received research funding and/or consulting fees or other remuneration from GlaxoSmithKline, UCB, Boehringer Ingelheim, AstraZeneca, Roche, Bayer, Biogen Idec, Cipla, Prometic and Samumed, and has stock options or bond holdings in the for-profit corporation Apellis. T.M. Maher is supported by a National Institute for Health Research Clinician Scientist Fellowship (NIHR ref: CS-2013–13-017) and is a British Lung Foundation Chair in Respiratory Research (C17-3).
Compliance with Ethics Guidelines
Generic ethical approval for observational research using the CPRD with approval from Independent Scientific Advisory Committee (ISAC) was granted by a Health Research Authority (HRA) Research Ethics Committee (REC) (East Midlands – Derby, REC reference number 05/MRE04/87). The protocol for this research was approved by the ISAC for the Medicines and Healthcare products Regulatory Agency (MHRA) Database Research (protocol number 16_262R2). Informed consent was not needed because of the anonymity of the CPRD data. This study was performed in adherence to the Declaration of Helsinki.
Data Availability
The data sets analyzed in this investigation were obtained under license from CPRD. Data for this study can be obtained by making an application to CPRD.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12046224.
References
- 1.Allanore Y, Simms R, Distler O, et al. Systemic sclerosis. Nat Rev Dis Primers. 2015;1(1):15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 2.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 3.Bergamasco A, Hartmann N, Wallace L, Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol. 2019;11:257–273. doi: 10.2147/CLEP.S191418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royle JG, Lanyon PC, Grainge MJ, Abhishek A, Pearce FA. The incidence, prevalence, and survival of systemic sclerosis in the UK Clinical Practice Research Datalink. Clin Rheumatol. 2018;37(8):2103–2111. doi: 10.1007/s10067-018-4182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottin V, Brown KK. Interstitial lung disease associated with systemic sclerosis (SSc-ILD) Respir Res. 2019;20(1):13. doi: 10.1186/s12931-019-0980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denton CP, Wells AU, Coghlan JG. Major lung complications of systemic sclerosis. Nat Rev Rheumatol. 2018;14(9):511–527. doi: 10.1038/s41584-018-0062-0. [DOI] [PubMed] [Google Scholar]
- 7.Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis–associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 8.Boehringer Ingelheim GmbH. Ofev (nintedanib) capsules, for oral use. 2014 September 2019 [cited 2019 October 18]. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Ofev/ofev.pdf.
- 9.Boehringer Ingelheim. Boehringer Ingelheim receives positive CHMP opinion for nintedanib for the treatment of systemic sclerosis-associated interstitial lung disease. 2020 28 February 2020 [cited 2020 March 6]. https://www.boehringer-ingelheim.com/press-release/chmpopinionnintedanibssc-ild?v=.
- 10.Adler S, Huscher D, Siegert E, et al. Systemic sclerosis associated interstitial lung disease—individualized immunosuppressive therapy and course of lung function: results of the EUSTAR group. Arthritis Res Ther. 2018;20(1):17. doi: 10.1186/s13075-018-1517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann-Vold A-M, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol. 2020;2(2):e71–e83. doi: 10.1016/S2665-9913(19)30144-4. [DOI] [PubMed] [Google Scholar]
- 12.Fischer A, Zimovetz E, Ling C, Esser D, Schoof N. Humanistic and cost burden of systemic sclerosis: a review of the literature. Autoimmun Rev. 2017;16(11):1147–1154. doi: 10.1016/j.autrev.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Fischer A, Kong AM, Swigris JJ, Cole AL, Raimundo K. All-cause healthcare costs and mortality in patients with systemic sclerosis with lung involvement. J Rheumatol. 2018;45(2):235–241. doi: 10.3899/jrheum.170307. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Fan Y, Thomason D, et al. Economic burden of illness among commercially insured patients with systemic sclerosis with interstitial lung disease in the USA: a claims data analysis. Adv Ther. 2019;36(5):1100–1113. doi: 10.1007/s12325-019-00929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NHS Digital. Read codes. 2018 [cited 2019 April 17]. https://digital.nhs.uk/services/terminology-and-classifications/read-codes.
- 16.CALIBER. Code lists. 2019 [cited 2019 July 24]. https://www.caliberresearch.org/portal/codelists.
- 17.World Health Organization. International statistical classification of diseases and related health problems 10th revision. 2010 [cited 2019 April 17]. https://icd.who.int/browse10/2016/en.
- 18.van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UK Cabinet Office. NHS reference costs 2015 to 2016. 2019 [cited 2019 April 17]. https://www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016.
- 20.Furst DE, Fernandes AW, Iorga SR, Greth W, Bancroft T. Annual medical costs and healthcare resource use in patients with systemic sclerosis in an insured population. J Rheumatol. 2012;39(12):2303–2309. doi: 10.3899/jrheum.120600. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z, Fan Y, Tang W, et al. Economic burden among commercially insured patients with systemic sclerosis in the United States. J Rheumatol. 2019;46(8):920–927. doi: 10.3899/jrheum.180445. [DOI] [PubMed] [Google Scholar]
- 22.Bernatsky S, Hudson M, Panopalis P, et al. The cost of systemic sclerosis. Arthritis Care Res (Hoboken) 2009;61(1):119–123. doi: 10.1002/art.24086. [DOI] [PubMed] [Google Scholar]
- 23.Jaeger VK, Wirz EG, Allanore Y, et al. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: a longitudinal EUSTAR study. PLoS ONE. 2016;11(10):e0163894. doi: 10.1371/journal.pone.0163894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrick AL, Pan X, Peytrignet S, et al. Treatment outcome in early diffuse cutaneous systemic sclerosis: the European Scleroderma Observational Study (ESOS) Ann Rheum Dis. 2017;76(7):1207–1218. doi: 10.1136/annrheumdis-2016-210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327–1339. doi: 10.1136/annrheumdis-2016-209909. [DOI] [PubMed] [Google Scholar]
- 26.Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowal-Bielecka O, Landewé R, Avouac J, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR) Ann Rheum Dis. 2009;68(5):620–628. doi: 10.1136/ard.2008.096677. [DOI] [PubMed] [Google Scholar]
- 28.McGrath ER, Doughty CT, Amato AA. Autoimmune myopathies: updates on evaluation and treatment. Neurotherapeutics. 2018;15(4):976–994. doi: 10.1007/s13311-018-00676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldroyd A, Lilleker J, Chinoy H. Idiopathic inflammatory myopathies—a guide to subtypes, diagnostic approach and treatment. Clin Med (Northfield Il) 2017;17(4):322–328. doi: 10.7861/clinmedicine.17-4-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker UA, Clements PJ, Allanore Y, et al. Muscle involvement in systemic sclerosis: points to consider in clinical trials. Rheumatology. 2017;56(Suppl 5):v38–v44. doi: 10.1093/rheumatology/kex196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analyzed in this investigation were obtained under license from CPRD. Data for this study can be obtained by making an application to CPRD.