Abstract
Purpose
To compare visual outcomes and treatment burden between intravitreally administered aflibercept (IVT-AFL) and ranibizumab (RBZ) treat-and-extend (T&E) regimens in patients with wet age-related macular degeneration (wAMD) at 2 years.
Methods
A systematic literature review was carried out in Medline, EMBASE, and CENTRAL in October 2018. Matching-adjusted indirect comparison (MAIC) and/or individual patient data meta-regression was used to connect ALTAIR (assessing IVT-AFL T&E) with other studies, adjusting for between-trial differences in baseline visual acuity and age or baseline visual acuity, age, and polypoidal choroidal vasculopathy (PCV) status. Sensitivity analyses were conducted to test the robustness of the results, including direct MAIC between IVT-AFL T&E (ALTAIR) and RBZ T&E (CANTREAT and TREX-AMD trials).
Results
Six randomized controlled trials (RCTs) (ALTAIR, VIEW 1 and 2, CATT, CANTREAT, and TREX) were included in the analysis. IVT-AFL T&E was assessed in one study, ALTAIR (n = 255), while RBZ T&E was assessed in two trials (n = 327). At 2 years, the median difference (95% credibility interval) between IVT-AFL T&E and RBZ T&E regarding the numbers of Early Treatment Diabetic Retinopathy Study (ETDRS) letters gained was not significant (M1: − 2.29 [− 8.10, 3.58]; M2: − 0.55 [− 6.34, 5.29]). IVT-AFL T&E was associated with significantly fewer injections than RBZ-T&E (M1: − 6.12 [− 7.60, − 4.65]; M2: − 5.93 [− 7.42, − 4.45]). Results of the sensitivity analyses were consistent with the main scenarios.
Conclusion
Patients with wAMD receiving an IVT-AFL T&E regimen achieved and maintained improvement in visual acuity with fewer injections over 2 years compared with RBZ T&E. IVT-AFL T&E may therefore serve as the optimal therapy for wAMD, as it was associated with clinical efficacy and minimized treatment burden.
Electronic supplementary material
The online version of this article (10.1007/s12325-020-01298-x) contains supplementary material, which is available to authorized users.
Keywords: Intravitreal anti-vascular endothelial growth factor therapy, Network meta-analysis, Ophthalmology, Wet age-related macular degeneration
Key Summary Points
| Why carry out this study? |
| The goal of anti-vascular endothelial growth factor (VEGF) treatment for wet age-related macular degeneration (wAMD) beyond the first 12 months is to maintain or improve functional and anatomic gains achieved during the 1st year of treatment, while minimizing the burden on patients related to clinical visits and the number of injections. |
| Independently, both intravitreally administered aflibercept (IVT-AFL) treat-and-extend (T&E) and ranibizumab (RBZ) T&E regimens have demonstrated a reduced treatment burden compared with bimonthly and monthly traditional anti-VEGF regimens, while maintaining efficacy in visual acuity (VA) gains in a long-term perspective (e.g., 2 years). |
| Randomized controlled trials (RCTs) describe outcomes with IVT-AFL T&E and RBZ T&E at 1 year; however, head-to-head data on IVT-AFL T&E and RBZ T&E regimens showing the long-term perspective (2 years) is limited. |
| In this analysis, we completed an indirect treatment comparison/network meta-analysis to compare IVT-AFL T&E versus RBZ T&E at 2 years regarding visual outcomes (mean change in Early Treatment Diabetic Retinopathy Study [ETDRS] letters vs baseline) and treatment burden (mean number of injections). |
| What was learned from the study? |
| The analysis indicates that in a clinical trial setting both IVT-AFL T&E and RBZ T&E are efficacious in the treatment of patients with wAMD. Both regimens are associated with comparable efficacy regarding number of ETDRS letters gained vs baseline after 2 years. |
| When assessing the associated treatment burden, patients receiving IVT-AFL T&E can achieve these outcomes with fewer injections. At 2 years, IVT-AFL T&E was associated with, on average, six fewer injections than RBZ T&E. |
| The high between-treatment difference in the number of injections is consistent with individual RCT results and reflects differences in the T&E regimens tested as well as the criteria for extension adopted in the studies included in the analysis. |
| On the basis of in the evidence assessed, IVT-AFL T&E may serve as optimal therapy for wAMD vs RBZ T&E, as it is associated with similar clinical efficacy and reduced treatment burden over the first 2 years of treatment. |
Introduction
Age-related macular degeneration affects 8.7% of the adult population globally [1] and is a leading cause of visual impairment and severe vision loss worldwide [2]. Neovascular (wet) age-related macular degeneration (wAMD) is a chronic and progressive degenerative disorder that is characterized by the presence of neovascularization within the macula and leads to severe vision impairment or loss of vision in older patients [3].
There is no curative treatment for wAMD; however, the progression of visual impairment can be slowed down or reversed to some extent with anti-vascular endothelial growth factor (anti-VEGF) therapies [4]. The inhibition of VEGF prevents neovascularization, decreases capillary leakage, and has proven efficacy in restoring visual acuity [4, 5]. Two anti-VEGF therapies have been approved and are currently in use globally for the treatment of wAMD: ranibizumab treat-and-extend (RBZ T&E; Lucentis; Novartis Pharma AG, Basel, Switzerland) and aflibercept treat-and-extend (IVT-AFL T&E; Eylea; Bayer Consumer Care AG, Basel, Switzerland for use outside of the USA). Both therapies are administered as intravitreal injections based on regimens with fixed or flexible intervals between administrations.
Although anti-VEGF therapy has become the mainstay in the treatment of wAMD, the need for repeated intravitreal injections imposes a serious burden on patients due to treatment-related anxiety, financial considerations, and transport burden [6]. Two strategies allowing for flexibility in the frequency of anti-VEGF administration have been developed. Under a pro re nata (PRN) regimen, the decision regarding whether to give an anti-VEGF injection is made at each visit, based on the results of an optical coherence tomography. Conversely, with treat-and-extent (T&E) regimens, a proactive treatment approach is taken; the patient receives an intravitreal injection at every visit, but intervals between consecutive visits can be adjusted on the basis of disease progression. Intravitreal administration of anti-VEGF agents may be carried out only by qualified personnel and requires highly specialized diagnostic procedures, making the therapy burdensome also for healthcare systems [7]. For this reason, an optimal treatment strategy will allow uncompromised clinical effectiveness with a reduced treatment burden.
In theory, the PRN regimen requires the same number of visits as for the fixed-interval regimen, although patients may receive injections less frequently compared with fixed monthly interval dosing; however, in daily practice PRN is associated with suboptimal efficacy, possibly driven by undertreatment of the disease [8]. It has been observed that patients who were switched from a PRN to a T&E regimen experienced improvement in visual acuity even 8 years after initial treatment [8, 9]. The switch to T&E was associated with a reduced number of visits and a slightly higher number of injections [9]. Therefore, T&E regimens are considered to be optimal strategies for intravitreal anti-VEGF administration, yielding noninferior efficacy and a noticeably decreased treatment-related burden compared with a fixed-interval posology [10–13].
Good treatment adherence has been recognized as another factor associated with optimal visual acuity in patients with wAMD treated with anti-VEGF therapy [14]. In the reality of clinical practice, missed visits or even treatment terminations are more frequent when the patient requires a higher frequency of visits [15, 16].
The publicly available evidence describes efficacy and treatment burden associated with IVT-AFL T&E and RBZ T&E, but there are limited data that could be used to assess the relative efficacy and treatment burden of both drugs. A recently published RIVAL trial did not demonstrate differences between IVT-AFL and RBZ regarding visual outcomes and the mean number of injections at 1 and 2 years [17, 18]. The authors describe that all patients received assigned treatment according to T&E regimen, although the frequency of injections in the AFL arm was higher compared with a fixed 8-week regimen. Thus, in the light of concerns regarding external validity of the RIVAL study, the question and credibility of presented estimates remains. Therefore, we attempted to compare both regimens using validated methods for indirect treatment comparison (ITC), including individual patient data (IPD) meta-regression and matching-adjusted indirect treatment comparison (MAIC) to establish the connection in the network of evidence for a network meta-analysis (NMA).
Methods
This NMA was preceded by a systematic literature review (SLR) of randomized controlled trials (RCTs) assessing efficacy and treatment burden of anti-VEGF therapies administered in patients with wAMD according to the approved posology [19].
In the SLR, the following inclusion criteria were used: at least 40 patients with wAMD, assessment of visual outcomes and/or treatment burden followed up for at least 2 years, and a comparison between at least two of the following regimens: IVT-AFL T&E or IVT-AFL every 8 weeks (Q8W), and RBZ T&E or RBZ every 4 weeks (Q4W), and IVT-AFL or RBZ administered PRN. Studies with a follow-up shorter than 2 years, those using an off-label dosage of IVT-AFL or RBZ or using off-label therapies, and those without relevant outcomes were excluded. The search was conducted according to the guidelines of the Cochrane Collaboration [20] and PRISMA statement [21] and was based on the keywords related to wAMD, assessed interventions, and RCTs combined with appropriate Boolean operators. The Medline® and EMBASE® databases (access via Ovid platform), Cochrane’s CENTRAL database, conference websites, and clinical trial registries were searched up to October 4, 2018, with no restrictions on language and geographical scope (the search strategy is presented in Tables 1 and 2 in the supplementary material). Studies were selected by two independent reviewers with differences resolved by a third reviewer. Data were extracted by one analyst and quality control was performed by another analyst. The Cochrane risk of bias tool checklist was used to assess the quality of the included studies. This study is a secondary analysis based on the results of data collected within eligible clinical trials. The data source included aggregated, clinical results identified from the public domain as well as limited individual patients’ data from ALTAIR and VIEW 1 & 2 trials. Individual patient data were anonymized before sharing, so that the analysts had no access to any personal information allowing one to identify individual patients. For this reason, this study did not require any ethical approval.
Indirect Treatment Comparison for Disconnected Studies
An NMA has been recommended to compare treatments assessed in studies which form a connected network of evidence [22]. The comparison of interventions assessed in disconnected studies requires careful adjustment for baseline differences that could bias the estimation of the relative effect. In this analysis, treatments from disconnected studies were compared, accounting for the differences in the baseline characteristics using methodologies recommended by the Decision Support Unit commissioned by the National Institute for Health and Care Excellence (NICE DSU), including the MAIC and regression analysis [23, 24]. Both methods require the use of patient-level data to calculate the difference between outcomes observed in disconnected arms, adjusting for differences in distributions of baseline characteristics with a potential to modify the treatment effects, referred to as treatment confounders. According to the guidelines, the comparison was carried out between studies on similar populations, which reduces the loss of information and risk of bias [23].
Individual Patient Data Meta-Regression Analysis
The IPD meta-regression analysis included IPD from disconnected studies and adopted multiple linear regression analysis to estimate the difference between disconnected arms. The difference between disconnected regimens was assessed adjusting for the baseline age () and the baseline best-corrected visual acuity () expressed with the Early Treatment Diabetic Retinopathy Study (ETDRS) letters. The outcomes of interest [change in best-corrected visual acuity (BCVA): , and the total number of injections at 1 year: ] were considered as dependent variables, while the assigned treatment () and baseline characteristics were analyzed as independent variables, as presented in the equations below:
Regression coefficients represent intercept, adjusted between-treatment difference, contribution of baseline BCVA, and age, respectively.
Matching-Adjusted Indirect Treatment Comparison
MAIC requires access to patient-level data from one trial, which are matched in terms of baseline characteristics with aggregated data from the other study using propensity score matching technique as described by NICE DSU Technical Support Document 18 [22]. Weights calculated during the matching and effects observed for each individual patient were used to recalculate the adjusted effect in the treatment group. The standard error for the adjusted effect was estimated using the nonparametric bootstrap method described by Gatz and Smith [25]. The adjusted effects were then compared with pooled estimates from the disconnected comparator group using the traditional statistical methodology for a comparison of two population means. The baseline characteristics used for matching included age, BCVA, and polypoidal choroidal vasculopathy (PCV).
An effective sample size (ESS) was calculated and reported for each analysis according to the recommendations of the NICE DSU [23, 24]. Analyses for which the ESS was reduced by more than 50% in comparison with the available IPD were not considered reliable owing to a high risk of bias and were not presented in this paper.
Network Meta-Analysis
An NMA in a Bayesian framework was conducted to compare the IVT-AFL T&E regimen with the RBZ T&E regimen as outlined in the NICE DSU Technical Support Document [22]. Estimates from ITC (meta-regression or MAIC) were incorporated in the network of evidence with the results from RCTs. The NMA was conducted on the mean between-treatment differences for BCVA gain from baseline and the mean differences in the number of intravitreal injections at 2 years using both random- and fixed-effects models. The selection of the model was based on the value of the deviance information criterion (DIC). Results were reported as the median between-treatment differences together with 95% credibility intervals (CrI). Missing standard deviations (SD) were imputed with the SD associated with the same treatments in other included studies. Analyses were conducted using R statistical software and WinBUGS1.4.3.
Results
Systematic Literature Review
The SLR identified 3134 potentially relevant citations, out of which eight (six RCTs) were included in the analysis (a PRISMA diagram is presented in Fig. 1 in the supplementary material). The six RCTs compared approved regimens of IVT-AFL and/or RBZ at 2 years in terms of the BCVA gain from baseline and the number of injections (ALTAIR [26], VIEW 1 and 2 [27], CATT [28], CANTREAT [12], and TREX [13]). IVT-AFL T&E was assessed in one study [26], including a total of 255 patients, while RBZ T&E was assessed in two studies [12, 13], including a total of 327 patients. The mean age at baseline ranged from 73.0 (IVT-AFL T&E 2-week adjustment) to 75.0 (IVT-AFL T&E 4-week adjustment) in the ALTAIR trial and from 76.0 (TREX) to 78.8 (CANTREAT) in trials assessing RBZ T&E. The mean BCVA at baseline ranged from 54.8 (IVT-AFL T&E 2-week adjustment) to 55.3 (IVT-AFL T&E 4-week adjustment) in the ALTAIR trial and from 58.7 (CANTREAT) to 59.9 (TREX) in trials assessing RBZ T&E. At 2 years, the mean BCVA gain from baseline was 6.1 (IVT-AFL T&E 4-week adjustment) and 7.6 (IVT-AFL T&E 2-week adjustment) ETDRS letters in IVT-AFL T&E with 4- and 2-week adjustment arms, respectively, while the visual acuity of patients treated with RBZ T&E improved by 6.4 and 8.7 letters in the CANTREAT and TREX trials, respectively. At 2 years, patients in both ALTAIR arms received 10.4 injections on average, while their counterparts from the CANTREAT and TREX trials were administered 18 and 18.6 injections (Table 1).
Table 1.
Baseline characteristics of populations from identified RCTs included in the ITC
| Study | Treatment arm | Number of patients randomized | Baseline BCVA Mean (SD) |
Baseline age Mean (SD) |
% of PCV |
|---|---|---|---|---|---|
| ALTAIR [26] | IVT-AFL T&E (2-weeks adj) | 124 | 54.8 (13.1) | 73.0 (7.9) | 38.0 |
| ALTAIR [26] | IVT-AFL T&E (4-weeks adj) | 123 | 55.3 (12.0) | 75.0 (8.1) | 37.9 |
| VIEW 1 [27] | IVT-AFL Q8 → PRN | 301 | 55.7 (12.8) | 77.9 (8.4) | 12.0a |
| VIEW 1 [27] | RBZ Q4 → PRN | 304 | 54.0 (13.4) | 78.2 (7.6) | |
| VIEW 2 [27] | IVT-AFL Q8 → PRN | 306 | 51.6 (13.9) | 73.8 (8.6) | |
| VIEW 2 [27] | RBZ Q4 → PRN | 291 | 53.8 (13.5) | 73.0 (9.0) | |
| TREX-AMD [13] | RBZ T&E | 40 | 59.9 (14.2) | 76.0 (n/a) | 8.7a |
| TREX-AMD [13] | RBZ Q4 | 20 | 60.3 (10.7) | 79.0 (n/a) | |
| CANTREAT [12] | RBZ T&E | 287 | 58.7 (14.2) | 58.7 (14.2) | 8.7a |
| CANTREAT [12] | RBZ Q4 | 293 | 59.4 (13.5) | 59.4 (13.5) | |
| CATT [28] | RBZ Q4 | 146 | 59.9 (14.2) | 79.5 (7.4) | Not assessed |
| CATT [28] | RBZ Q4 → PRN | 138 | 60.9 (14.3) | 78.8 (7.5) | Not assessed |
BCVA best-corrected visual acuity, IVT-AFL intravitreally administered aflibercept, PCV polypoidal choroidal vasculopathy, Q4 one injection every 4 weeks, Q4 → PRN one injection every 4 weeks in the first year followed by pro re nata regimen in the second year, Q8 → PRN one injection every 8 weeks in the first year followed by pro re nata regimen in the second year, RBZ ranibizumab, SD standard deviation, T&E treat-and-extend regimen, 2-wk adj treatment interval adjusted every 2 weeks, 4-wk adj treatment interval adjusted every 4 weeks
aValues estimated
Evidence Network
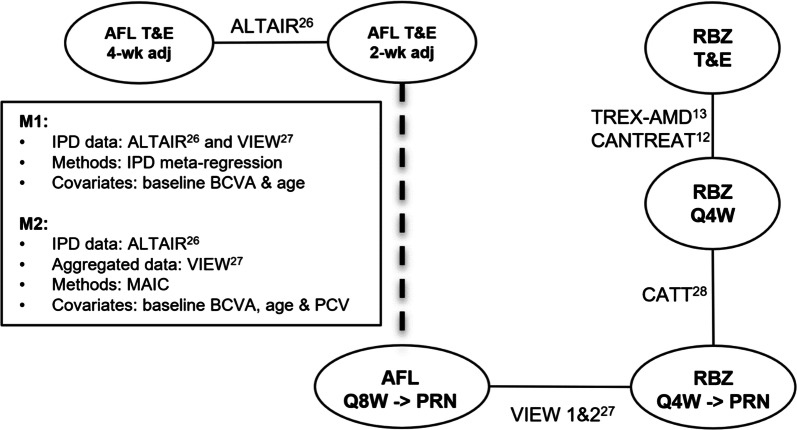
The only RCT on IVT-AFL T&E (ALTAIR, NCT02305238) was designed to compare two IVT-AFL T&E regimens using 2-week and 4-week adjustments. After four initial doses administered at weeks 0, 4, 8, and 16, IVT-AFL T&E was injected at variable treatment intervals ranging from 8 to 16 weeks. The ALTAIR trial was disconnected from the network of other studies. Therefore, the connection between the ALTAIR study and the network of evidence accounting for the differences in the baseline characteristics had to be established in order to allow for quantitative comparison between IVT-AFL T&E and RBZ T&E. The comparison was considered the most appropriate between the ALTAIR and combined data from VIEW 1 and 2 trials, as the distributions of both BCVA and age at baseline and access to patient-level data were similar. Two methods dedicated to comparing disconnected evidence were tested as described in the “Methods” (Fig. 1).
Fig. 1.
Network of evidence. Solid lines indicate head-to-head comparisons within RCTs and a dashed line indicates a reconstituted connection between studies. BCVA best-corrected visual acuity, IPD individual patient data, IVT-AFL intravitreally administered aflibercept, MAIC matching-adjusted indirect comparison, PCV polypoidal choroidal vasculopathy, Q4W one injection every 4 weeks, Q4W → PRN one injection every 4 weeks in the first year followed by pro re nata regimen in the second year, Q8W → PRN one injection every 8 weeks in the first year followed by pro re nata regimen in the second year, RBZ ranibizumab, T&E treat-and-extend regimen, 2-wk adj treatment interval adjusted every 2 weeks, 4-wk adj treatment interval adjusted every 4 weeks
In the main analysis 1 (M1), the meta-regression analysis on IPD was conducted to assess the difference between the ALTAIR study (2-week adjustment) versus IVT-AFL Q8W/PRN in VIEW 1 and 2, adjusting for the baseline age and the baseline BCVA. A sensitivity analysis was conducted pooling both arms of the ALTAIR trial (2-week adjustment and 4-week adjustment), with patients receiving the IVT-AFL Q8W/PRN regimen using the same IPD meta-regression techniques.
MAIC was used as the second method to anchor the ALTAIR trial to the network of evidence through the VIEW trials. In the main analysis 2 (M2), the weights were assigned to patients receiving IVT-AFL T&E (2-week adjustment) in the ALTAIR trial to balance the differences in the baseline characteristics of patients treated with IVT-AFL Q8W/PRN in the VIEW 1 and 2 studies. The baseline characteristics used for matching included age, BCVA, and PCV. Because PCV status was not reported in VIEW trials, it was estimated assuming the same PCV prevalence as in the ALTAIR trial (38%) for the subset of Asian patients and as reported by Lorentzen et al. [29] for those with non-Asian ethnicity (8.75%). On the basis of these assumptions, the overall prevalence of patients with PCV in the VIEW trials was 12%. Several sensitivity analyses were conducted to test the robustness of the results, including matching of IPD from the VIEW studies to adjust for aggregated baseline characteristics from the ALTAIR study, comparison with different IVT-AFL T&E regimens (2-week, 4-week, 2- and 4-week regimens), as well as comparison between the IVT-AFL T&E (ALTAIR) and RBZ T&E regimen (CANTREAT and TREX-AMD). Although the sample of the ALTAIR trial was limited, the baseline characteristics were well matched in terms of mean parameter values and corresponding standard deviations. The ESS following MAIC did not decrease below 50% of initial samples of IPD.
Estimates from ITC (meta-regression or MAIC) were incorporated in the network of evidence with the results from RCTs as described in the “Methods”.
Figure 1 presents the network of evidence used for NMA, showing head-to-head comparisons within RCTs and a reconstituted connection between studies, with most resembling distributions of the baseline BCVA and age (ALTAIR and VIEW). The NMA models with input data are presented in the supplementary material.
Results of Indirect Treatment Comparison of Visual Acuity
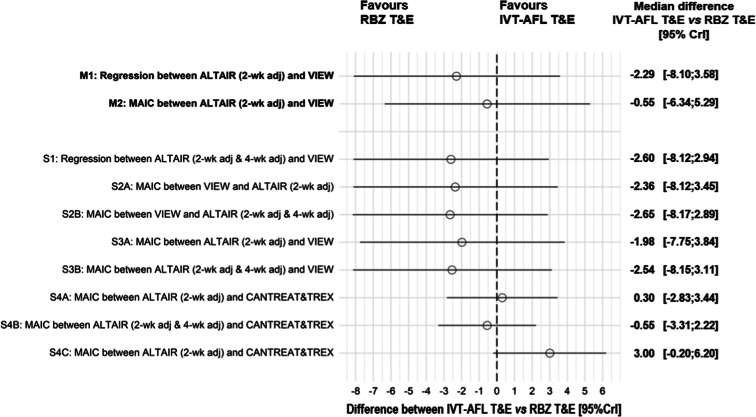
The gain in ETDRS letters reported in the VIEW 1 and 2 and ALTAIR studies at 2 years was 7.6 in both IVT-AFL Q8W/PRN and IVT-AFL T&E (2-week adjustment) groups. The median differences (95% CrI) in visual acuity gain between IVT-AFL Q8W/PRN and IVT-AFL T&E were 0.73 (− 2.23, 3.69) ETDRS letters for the IPD meta-regression adjusted for the baseline BCVA and age (M1) and − 0.99 (− 3.90, 1.93) ETDRS letters for the MAIC of IVT-AFL T&E matched to aggregated IVT-AFL Q8W/PRN on the baseline BCVA, age, and PCV (M2). The results of all sensitivity analyses were consistent with M1 and M2 scenarios and did not demonstrate a significant difference in visual outcomes between IVT-AFL Q8W/PRN and IVT-AFL T&E at 2 years.
The NMA pooling results of the network of RCTs together with the estimated difference between regimens assessed in the ALTAIR and VIEW studies did not demonstrate a significant difference between IVT-AFL T&E and RBZ T&E in terms of improvement in visual acuity (Fig. 2). All sensitivity analyses provided similar conclusions.
Fig. 2.
Difference between IVT-AFL T&E and RBZ T&E in change in BCVA. BCVA best-corrected visual acuity, Clr credibility intervals, IVT-AFL intravitreally administered aflibercept, MAIC matching-adjusted indirect comparison, M1–2 main analyses 1 and 2, RBZ ranibizumab, S1–S4C sensitivity analyses 1–4C, T&E treat-and-extend regimen, 2-wk adj treatment interval adjusted every 2 weeks, 2-wk adj & 4-wk adj treatment interval adjusted either every 2 or 4 weeks
Results of Indirect Treatment Comparison of Number of Injections
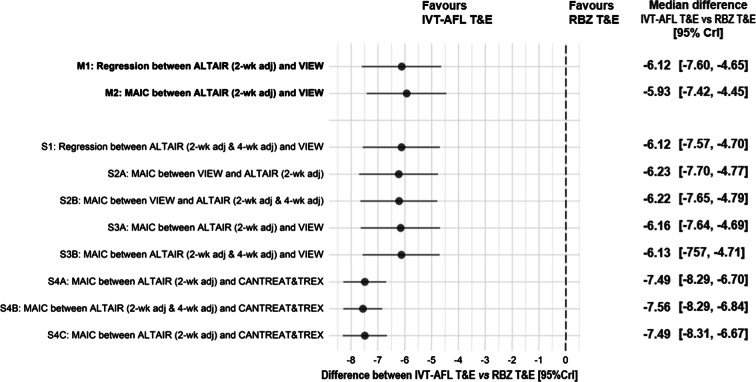
The mean number of injections received by patients treated with IVT-AFL Q8W/PRN and IVT-AFL T&E at week 96 was 11.2 and 10.4. The median differences (95% CrI) in the number of injections between IVT-AFL Q8W/PRN and IVT-AFL T&E were 0.89 (0.35, 1.43) injections for IPD meta-regression adjusted for the baseline BCVA and age (M1) and 0.70 (0.15, 1.26) injections for MAIC of IPD patients receiving IVT-AFL T&E to aggregated IVT-AFL Q8W/PRN with the baseline BCVA, age, and estimated prevalence of PCV as covariates (M2).
The results of all sensitivity analyses were consistent with M1 and M2 scenarios and demonstrated that the IVT-AFL T&E regimen was associated with 0.7–1.0 fewer injections on average compared with IVT-AFL Q8W/PRN at 2 years.
The NMA pooling results of the network of RCTs together with estimated difference between regimens assessed in the ALTAIR and VIEW studies demonstrated that at 2 years, IVT-AFL T&E was associated with significantly fewer injections compared with RBZ T&E, which was confirmed across all sensitivity analyses including direct comparison using MAIC between IVT-AFL T&E and RBZ T&E. The magnitude of the difference between IVT-AFL T&E and RBZ T&E was approximately 6 injections in both main scenarios and reached 7.5 injections in the MAIC directly comparing IVT-AFL T&E and RBZ T&E (Fig. 3).
Fig. 3.
Difference between IVT-AFL T&E and RBZ T&E in change in number of injections. Clr credibility intervals, IVT-AFL intravitreally administered aflibercept, MAIC matching-adjusted indirect comparison, M1–2 main analyses 1 and 2, RBZ ranibizumab, S1–S4C sensitivity analyses 1–4C, T&E treat-and-extend regimen, 2-wk adj treatment interval adjusted every 2 weeks, 2-wk adj & 4-wk adj treatment interval adjusted either every 2 or 4 weeks
Discussion
This analysis was performed to fill gaps in clinical evidence from RCTs on visual outcomes and treatment burden of anti-VEGF T&E therapies in a 2-year perspective. The NMA was performed using six RCTs (ALTAIR, VIEW 1 and 2, CATT, CANTREAT, and TREX). The results of this comparison indicate that over 2 years, IVT-AFL T&E provided visual improvements comparable to those achieved with RBZ T&E with a lower treatment burden based on significantly fewer injections.
The pivotal ALTAIR trial assessing two different regimens of IVT-AFL T&E regimens could not be connected within a network of evidence with the remaining trials assessing anti-VEGF regimen, which precluded the possibility of using standard network meta-analysis to compare between IVT-AFL T&E and RBZ T&E. Therefore, we followed the guidelines issued by the NICE Decision Support Unit describing methods for the population-adjusted indirect comparison between disconnected evidence and incomplete networks [23]. In this analysis we conducted MAIC and adopted regression-based analysis to reconstitute the connection of incomplete networks of evidence allowing one to compare between IVT-AF T&E versus RBZ T&E.
Clinical data comparing the efficacy and treatment burden of IVT-AFL and RBZ in a longer perspective are limited. Results of an NMA reported by Danyliv et al. did not demonstrate significant differences between IVT-AFL fixed regimens versus various RBZ regimens regarding visual outcomes [30]. The estimates presented by Danyliv et al. were based on the two large RCTs (VIEW 1 and 2) designed to compare IVT-AFL versus RBZ [27, 31]. During the first year, patients received intravitreal injections of RBZ every 4 weeks or IVT-AFL every 4 weeks or every 8 weeks after three initial monthly injections. During the second year, patients continued to receive their originally assigned agents. While patients were followed up every 4 weeks and could receive injections at follow-up visits, based on protocol-defined retreatment criteria, only quarterly injections were mandatory. The results of VIEW trials indicate that IVT-AFL 2 mg monthly and RBZ 0.5 mg monthly were equally effective and had comparable safety profiles at 1 year and 2 years, despite that approximately five fewer doses were administered in the 2 mg monthly IVT-AFL group throughout the entire follow-up (16.0 vs 11.2 injections) [27, 31].
Although the VIEW trials demonstrate that IVT-AFL yields visual effects similar to those seen with RBZ, with a lower number of injections, it was not immediately understood how those findings could be extrapolated to T&E regimens [27]. While the RIVAL study is the only clinical trial comparing IVT-AFL and RBZ in an identical proactive treatment regimen, described as T&E, the treatment criteria were geared towards aggressive elimination of any retinal fluid in order to achieve its primary endpoint. This study had been designed to assess treatment differences regarding the change in the area of geographic atrophy from baseline to 24 months as the primary outcome measures. Visual outcomes and the number of injections at 12 and 24 months were defined as secondary objectives. Since the statistical analysis did not adjust for multiple comparisons, the reported estimates should be interpreted with caution. The recently published results from the RIVAL study reported similar mean BCVA gain in IVT-AFL and RBZ groups at 12 months (+ 5.2 vs + 6.9 logMAR letters) and 24 months (+ 5.3 vs + 6.5 logMAR letters) [17, 18]. In the first year, patients received on average 9.7 injections in each group, while over entire 2-year treatment period the mean number of injections was 17.0 and 17.7 in IVT-AFL and RBZ arms, respectively. The external validity of these estimates is questionable not only because of the study design limitation related to primary outcome and statistical analysis but also owing to concerns regarding assessed treatment regimens. All patients received allocated therapies according to the same regimen of three initial monthly doses followed by an extension phase, during which the interval between subsequent injections was adjusted by 2 weeks within a range of a minimum of 4 weeks and a maximum of 12 weeks between administrations. Additionally, the between-treatment interval was shortened to 4 weeks if more than one sign of disease activity was observed, thus limiting the number of possible extensions. The schedule adopted in RIVAL required more frequent IVT-AFL administration compared with the European label for IVT-AFL, which indicates that—after the 3 initial monthly injections, the interval between subsequent IVT-AFL injections should not be shorter than 8 weeks. As a result, patients treated with IVT AFL in the RIVAL study received more intensive treatment than has been described in the ALTAIR trial assessing IVT-AFL regimens (17 injections vs 10.4 injections at 104 and 96 weeks, respectively) and combined IVT-AFL Q8W arms from the VIEW 1 and 2 trials (17 injections vs 11.2 injections at 104 and 96 weeks, respectively), which may constitute overtreatment. Importantly, the pooled results of the VIEW 1 and 2 trials indicate that additional injections with IVT-AFL do not yield additional clinical benefit [17]. Therefore an injection of IVT-AFL when not clinically indicated might not improve its efficacy compared with RBZ T&E but potentially jeopardized the between-treatment comparison regarding the mean number of injections. A more intensive intravitreal regimen may be considered justified to treat patients with suboptimal response during the treatment. For example, the presence of PCV has been considered as a potential predictor of poor response of patients with wAMD treated with anti-VEGF regimens [32–34]. Approved IVT-AFL T&E regimen may not be sufficient to treat non-responders with PCV, who may require more frequent anti-VEGF injections and the use of rescue photodynamic therapy. As demonstrated in the PLANET trial, around 12.1% of participants with PCV required monthly IVT-AFL T&E combined with rescue photodynamic therapy within the first year of treatment [35]. Taking into account that the RIVAL trial recruited predominantly white patients with low baseline risk of PCV, it seems unlikely that more intensive administration of IVT-AFL T&E could be attributed to PCV-related non-responders. Therefore, owing to the serious methodological concerns and limited external validity, the RIVAL trial was excluded from our analysis.
The IVT-AFL T&E regimen has been granted market authorization in the EU, Australia, and Japan on the basis of the outcomes of the ALTAIR study. This study demonstrated the efficacy and treatment burden of two different approaches of IVT-AFL T&E dosing with a 2-week and 4-week adjustment. At 2 years, patients receiving IVT-AFL T&E improved their visual acuity by 6.1–7.6 ETDRS letters with 10.4 injections compared with baseline. The evidence from RCTs on the use of RBZ T&E at 2 years is also limited. Two ongoing studies met the inclusion criteria for this analysis: the CANTREAT trial and small TREX-AMD study. A naive unadjusted comparison of the results from these studies indicate that RBZ T&E provides visual improvement similar to that achieved using IVT-AFL T&E (6.4 ETDRS letter gain in CANTREAT and 8.7 in TREX), but with a higher absolute number of injections (18.0 in CANTREAT and 18.6 in TREX). These results are consistent with the LUCAS trial, which was not included in this analysis because it did not meet the inclusion criteria (assessment of an off-label therapy as comparator), in which at 2 years, patients allocated to the RBZ T&E arm received a mean number of 16.0 intravitreal injections, which was associated with a 6.6 ETDRS letter gain compared with baseline [36].
The current NMA has some limitations: first, the connection between the ALTAIR study and the network was reconstituted using methods for an unanchored comparison, which allows adjustments only for a few treatment modifiers in each analysis. Second, standard deviations were not published in all studies assessing RBZ regimens and had to be imputed to conduct NMA. Third, the proportion of patients with PCV at baseline was available only for the ALTAIR trial and had to be estimated for the remaining trials in order to allow for PCV-adjusted analysis. The estimation of the percentage of patients with PCV was based on the results of epidemiologic meta-analysis, including 11 studies identified through SLR [29]. This estimation is associated with inherent uncertainty, since the prevalence of PCV in studies included in this ITC could differ from the point estimate reported in the meta-analysis. Although there was a tentative estimation of the proportion of PCV, populations enrolled in different studies may vary. For these reasons, the estimates adjusted for PCV should be interpreted with caution. However, the sensitivity analyses demonstrated that the adjustment for baseline PCV may not change the inference of this comparison. The results of all the analyses consistently did not reveal any significant differences between IVT-AFL T&E and RBZ regarding visual acuity gain and demonstrated a noticeably lower number of injections in IVT-AFL T&E group. Models adjusting for baseline PCV slightly favored IVT-AFL T&E in terms of visual acuity compared with models not accounting for PCV although without change of the inference. On the contrary, the estimates for the treatment burden were highly consistent across all sensitivity analyses with or without baseline PCV as a covariate. This is because no interaction between the presence of PCV and injection frequency was observed in the ALTAIR trial.
Fourth, there was a slight discrepancy between trials regarding the exact time point of the data collection for the analysis at 2 years. The data for the IVT-AFL T&E were collected 8 weeks earlier compared with the data for RBZ T&E (96 weeks vs 104 weeks), which may slightly interfere with the comparison between IVT-AFL T&E and RBZ T&E regarding the number of injections. However, the impact of this 8-week difference is limited because of the low frequency of IVT-AFL T&E administration in the second year of the ALTAIR trial (3.7 injections administered between week 54 and week 96). On the basis of this information, it can be assumed that patients would receive less than one additional AFL injection if ALTAIR were extended to 104 weeks. This means that at 104 weeks, IVT-AFL T&E is associated with an estimated five fewer injections compared with RBZ T&E. Finally, the included studies assessing T&E regimens differed regarding the time when the extension was initiated and the possible ranges of injection intervals. It cannot be excluded that the between-study differences regarding T&E posology may, in part, explain the difference in the number of injections between IVT-AFL T&E and RBZ T&E estimated in this analysis.
Administration of anti-VEGF therapy imposes a serious burden on patients due to treatment-related anxiety, financial considerations, and transport burden [37]. Most patients with wAMD have varying degrees of disability due to age and insufficient vision, and thus require help in their daily activities as well as escorts when travelling to the hospital [38, 39]. The disease itself and the treatment frequency are therefore associated with a significant caregiver burden, which can have a negative impact on the relationships between the caregiver and patient [38, 40]. The frequency of injections can be considered a relevant surrogate of a treatment burden. Therefore, the results of our comparison indicate that IVT-AFL T&E may serve as an optimal treatment option, owing to its clinical efficacy, which is comparable to that of RBZ T&E, and a noticeably lower treatment burden [6].
The between-treatment difference in the number of injections is consistent with individual RCT results and reflects differences in the T&E regimens tested and criteria for extension adopted in respective studies. In the ALTAIR trial, IVT-AFL T&E was administered at weeks 0, 4, 8, and 16, followed by the variable treatment interval ranging from 8 to 16 weeks. The interval was adjusted on the basis of physician judgment of vision and/or anatomic outcomes. On the contrary, RBZ T&E monthly treatment could be continued following initial doses (1) until (in the CANTREAT study) visual acuity was deemed stable, indicated by an improvement in visual acuity of at most 3 ETDRS letters gained (or no loss of more than 5 letters) from the prior month; no clinical evidence of lesion growth, fluid, or blood; and no intraretinal or subretinal fluid seen on optical coherence tomography; or (2) until (in the TREX study) achievement of “dry” retina based on resolution of intraretinal and subretinal fluid and all subretinal hemorrhage related to active wAMD. When this was achieved, the treatment interval could be extended by 2 weeks to a maximum of 12 weeks. In the case of recurrent disease activity, the between-injection interval could be shortened to a minimum of 4 weeks.
Finally, this analysis was based on RCTs conducted under experimental conditions with tightly defined treatment algorithms, which might not fully reflect the posology used in real clinical practice. Therefore, the differences in relative efficacy and treatment burden between IVT-AFL T&E and RBZ T&E administered in the real-world settings may potentially differ from the estimates of this analysis.
Conclusions
Patients with wAMD receiving an IVT-AFL T&E regimen can achieve and maintain improvement in visual acuity with fewer injections over a 2-year period compared with those receiving RBZ T&E. Because it is associated with clinical efficacy and a minimized treatment burden, IVT-AFL T&E may therefore serve as the optimal therapy for wAMD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by Bayer AG, Basel. Bayer AG, Basel also funded the Journal Rapid Service and the Open Access Fees.
Medical Writing and Editorial Assistance
Medical writing assistance was provided by Piotr Wojciechowski and editorial assistance in the preparation of this article was provided by Małgorzata Biernikiewicz of Creativ-Ceutical, and was funded by Bayer AG, Basel.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for the manuscript to be published.
Disclosures
Masahito Ohji received financial support from Bayer, Santen, and Novartis; Jean-Francois Korobelnik received consulting fees from Bayer, Novartis, and Allergan; Paolo Lanzetta received consulting fees from Bayer, Centervue, Novartis; Claudia Tuckmantel and Celine Deschaseaux are employees of Bayer. Piotr Wojciechowski and Vanessa Taieb are employees of Creativ-Ceutical which was contracted by Bayer to undertake the data analysis for this study. Vanessa Taieb is now an employee of HEOR consulting. At the time this work was completed she was employed by Creativ-Ceutical. Daniel Janer is now an employee Kodiak Sciences GmbH. At the time this work was completed he was employed by Bayer Consumer Care AG.
Compliance with Ethics Guidelines
This study is a secondary analysis based on the results of data collected within eligible clinical trials. The data source included aggregated, clinical results identified from the public domain as well as limited individual patients’ data from ALTAIR and VIEW 1 & 2 trials. Individual patient data were anonymized before sharing, so that the analysts had no access to any personal information allowing one to identify individual patients. For this reason, this study did not require any ethical approval.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because of the confidential character of individual patient data used for the calculations.
Footnotes
Enhanced Digital Features
To view digital features for this article go to 10.6084/m9.figshare.11967852.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;59(2):74–77. [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:Cd005139. doi: 10.1002/14651858.CD005139.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Progr Retin Eye Res. 2008;27(4):331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23(2):127–140. doi: 10.1080/13548506.2016.1274040. [DOI] [PubMed] [Google Scholar]
- 7.Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725–731.e1. doi: 10.1016/j.ajo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Kvannli L, Krohn J. Switching from pro re nata to treat-and-extend regimen improves visual acuity in patients with neovascular age-related macular degeneration. Acta Ophthalmol. 2017;95(7):678–682. doi: 10.1111/aos.13356. [DOI] [PubMed] [Google Scholar]
- 9.Hatz K, Prunte C. Changing from a pro re nata treatment regimen to a treat and extend regimen with ranibizumab in neovascular age-related macular degeneration. Br J Ophthalmol. 2016;100(10):1341–1345. doi: 10.1136/bjophthalmol-2015-307299. [DOI] [PubMed] [Google Scholar]
- 10.Haga A, Kawaji T, Ideta R, Inomata Y, Tanihara H. Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol. 2018;96(3):e393–e398. doi: 10.1111/aos.13607. [DOI] [PubMed] [Google Scholar]
- 11.Silva R, Berta A, Larsen M, Macfadden W, Feller C, Mones J. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the trend study. Ophthalmology. 2018;125(1):57–65. doi: 10.1016/j.ophtha.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Kertes PJ, Galic IJ, Greve M, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology. 2019126(6):841–48. [DOI] [PubMed]
- 13.Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina. 2017;1(4):314–321. doi: 10.1016/j.oret.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Ehlken C, Helms M, Bohringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20. doi: 10.2147/OPTH.S151611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimes B, Gunnemann F, Ziegler M, et al. Compliance of age related macular degeneration patients undergoing anti-VEGF therapy: analysis and suggestions for improvement. Ophthalmologe. 2016;113(11):925–932. doi: 10.1007/s00347-016-0275-z. [DOI] [PubMed] [Google Scholar]
- 16.Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38(7):620–627. doi: 10.1016/j.jfo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Gillies MC, Hunyor AP, Arnold JJ, et al. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2019;134(4):372–379. doi: 10.1001/jamaophthalmol.2018.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillies MC, Hunyor AP, Arnold JJ, et al. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2019;137(4):372–379. doi: 10.1001/jamaophthalmol.2018.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanzetta P, Ohji M, Korobelnik JF, et al, editors. Clinical evidence among patients with wet age-related macular degeneration treatment with intravitreal aflibercept and ranibizumab: a systematic literature review. In: 19th EURETINA congress; 5–8 September 2019; Paris.
- 20.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration; 2011.
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London: NICE; 2011. [PubMed]
- 23.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. ICE DSU Technical Support Document 18: methods for population-adjusted indirect comparisons in submission to NICE. London: NICE; 2016.
- 24.Faria R, Hernandez Alva AM, Manca A, Wailoo AJ. NICE DSU Technical Support Document 17: the use of observational data to inform estimates of treatment effectiveness for technology appraisal: methods for comparative individual patient data. London: NICE; 2015.
- 25.Gatz DF, Smith L. The standard error of a weighted mean concentration—I. Bootstrapping vs other methods. Atmos Environ. 1995;29(11):1185–1193. [Google Scholar]
- 26.Ohji M, Okada A, Takahashi A, editors. Two different treat and extend dosing regimens of intravitreal aflibercept for wAMD in Japanese patients: 52 weeks results of the ALTAIR study. In: 17th EURETINA congress; 2017; Barcelona.
- 27.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorentzen TD, Subhi Y, Sorensen TL. Prevalence of polypoidal choroidal vasculopathy in white patients with exudative age-related macular degeneration: systematic review and meta-analysis. Retina. 2018;38(12):2363–2371. doi: 10.1097/IAE.0000000000001872. [DOI] [PubMed] [Google Scholar]
- 30.Danyliv A, Glanville J, McCool R, Ferreira A, Skelly A, Jacob RP. The clinical effectiveness of ranibizumab treat and extend regimen in nAMD: systematic review and network meta-analysis. Adv Ther. 2017;34(3):611–619. doi: 10.1007/s12325-017-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009;148(1):70–8.e1. doi: 10.1016/j.ajo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Hatz K, Prunte C. Polypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab response. Br J Ophthalmol. 2014;98(2):188–194. doi: 10.1136/bjophthalmol-2013-303444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palkar AH, Khetan V. Polypoidal choroidal vasculopathy: an update on current management and review of literature. Taiwan J Ophthalmol. 2019;9(2):72–92. doi: 10.4103/tjo.tjo_35_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee WK, Iida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136(7):786–793. doi: 10.1001/jamaophthalmol.2018.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg K, Hadzalic E, Gjertsen I, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the lucentis compared to avastin study treat-and-extend protocol: two-year results. Ophthalmology. 2016;123(1):51–59. doi: 10.1016/j.ophtha.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Smith AG, Kaiser PK. Emerging treatments for wet age-related macular degeneration. Expert Opin Emerg Drugs. 2014;19(1):157–164. doi: 10.1517/14728214.2014.884559. [DOI] [PubMed] [Google Scholar]
- 38.Vukicevic M, Heraghty J, Cummins R, Gopinath B, Mitchell P. Caregiver perceptions about the impact of caring for patients with wet age-related macular degeneration. Eye (Lond) 2016;30(3):413–421. doi: 10.1038/eye.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor DJ, Hobby AE, Binns AM, Crabb DP. How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open. 2016;6(12):e011504. doi: 10.1136/bmjopen-2016-011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gohil R, Crosby-Nwaobi R, Forbes A, Burton B, Hykin P, Sivaprasad S. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLoS One. 2015;10(6):e0129361. doi: 10.1371/journal.pone.0129361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available because of the confidential character of individual patient data used for the calculations.