Abstract
It has become clear since the pandemic broke out that SARS-CoV-2 virus causes reduction of smell and taste in a significant fraction of COVID-19 patients. The olfactory dysfunction often occurs early in the course of the disease, and sometimes it is the only symptom in otherwise asymptomatic carriers. The cellular mechanisms for these specific olfactory disturbances in COVID-19 are now beginning to be elucidated. Several very recent papers contributed to explaining the key cellular steps occurring in the olfactory epithelium leading to anosmia/hyposmia (collectively known as dysosmia) initiated by SARS-CoV-2 infection. In this Viewpoint, we discuss current progress in research on olfactory dysfunction in COVID-19 and we also propose an updated model of the SARS-CoV-2-induced dysosmia. The emerging central role of sustentacular cells and inflammatory processes in the olfactory epithelium are particularly considered. The proposed model of anosmia in COVID-19 does not answer unequivocally whether the new coronavirus exploits the olfactory route to rapidly or slowly reach the brain in COVID-19 patients. To answer this question, new systematic studies using an infectious virus and appropriate animal models are needed.
Keywords: Anosmia, Hyposmia, COVID-19, Olfactory epithelium, SARS-CoV-2, ACE2, Brain infection
1. Considered Scenarios for COVID-19-Associated Anosmia/Hyposmia
Initial hospital observations and early studies have suggested several possible mechanisms for the development of anosmia in COVID-19, including olfactory cleft syndrome, nasal obstruction and rhinorrhea, cytokine storm, direct damage to olfactory receptor neurons (ORNs), and impairment of the olfactory perception centers in the brain. Olfactory cleft syndrome has been reported solely in rare cases. On the other hand, rhinorrhea or associated nasal obstruction which could block nasal air flow is known to be much less often observed in COVID-19 as compared to the frequency of olfactory deficits. Moreover, there is no solid data supporting the rapid damage to olfactory cortical areas in the brain, considering that anosmia is often detected very early on in the disease both in mild cases and even asymptomatic patients. Thus, excessive and systemic inflammatory response in the brain unlikely plays a causative role in the developing anosmia. Finally, most obvious cause of COVID-19-associated anosmia may be direct damage to olfactory receptor neurons (ORNs), as other human coronaviruses (e.g., OC43) were previously shown to directly bind to ORNs. It is a bit surprising, however, that although anosmia is often linked with many human viruses causing common cold (e.g., influenza and coronaviruses), its exact cellular and molecular mechanism has not yet been clearly established. The high incidence of olfactory dysfunction in COVID-19 and its possible implications on the brain have directed part of current research on COVID-19 toward a thorough understanding of this process at the mechanistic level.
2. Sustentacular Cells Are Entering the Game
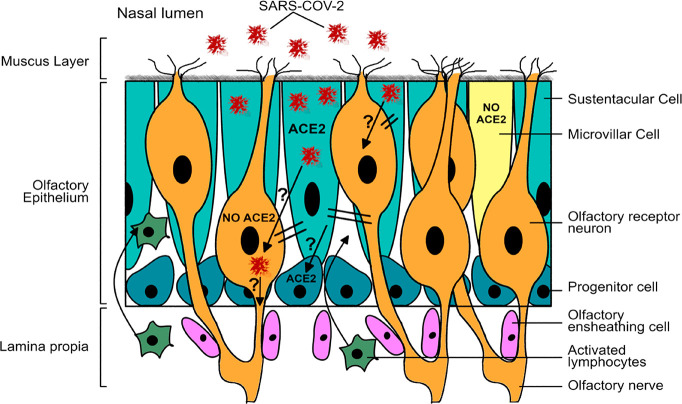
Shortly after the recognition of olfactory dysfunction as a COVID-19 symptom, it was proposed by Butowt and Bilinska1 that non-neuronal cell-specific mechanism operating within the olfactory epithelium (OE) could play a major role. This hypothesis was based on an in silico analysis of available microarrays and RNaseq transcriptome data for SARS-CoV-2 host receptors, ACE2 and TMPRSS2. Preliminary analysis has shown that there is no ACE2 expression in olfactory receptor neurons present in the OE. However, the type of non-neuronal cells that highly express ACE2 were not initially identified.1 Recently, three independent groups reported the identification of sustentacular cells (SUS) present in the OE as those expressing high levels of ACE2 and TMPRSS2.2−4 Initial data based on single cell RNaseq using murine and human OE3,4 were supported by even more convincing results obtained at a protein level.2 The above studies also showed that, in addition to SUSs, progenitor/stem cells and Bowman’s glands are also expressing ACE2. It is widely accepted that SARS-CoV-2 infectivity and its entry into the body depends on binding to the ACE2 host receptor. Hence, the results of the above studies suggest a scenario in which SUS cells , exposed to the external environment, are the SARS-CoV-2 primary target in the OE. More importantly, damage to SUS cells can most likely lead to impaired olfactory perception even without SARS-CoV-2 virus transfer to ORNs. This is due to the fact that SUS cells are functionally and anatomically tightly linked with ORNs. First, SUS cells protect olfactory neurons by detoxifying volatile chemicals by expressing enzymes from the cytochrome P450 family. Second, SUS cells endocytose the odorant-binding proteins–odor complexes after initiation of signal transduction at the neurons’ cilia to allow the next round of odor binding to the receptor. Finally, SUS cells supply ORNs cilia, where olfactory receptors are located, with additional glucose. This process is essential to complement the high-energy demands of the olfactory transduction cascade. Taking all of the above facts into account, the impairment of SUS cells caused by viral infection can easily inhibit the perception of odorants in adjacent ORNs (Figure 1).
Figure 1.
Model for SARS-CoV-2-induced anosmia/hyposmia in COVID-19 based on results obtained from patients and from animal models. Sustentacular cells (SUSs) express ACE2 and are infected first. Impairment of SUS negatively affects olfactory receptor neurons (ORNs), leading to the inhibition of odor perception cascade (double lines). Simultaneously, rapid immune response is induced in a subset of ORNs and in microvillar cells (MVCs). This leads to activation of lymphocytes and macrophages and their infiltration into the OE as well as secretion of proinflammatory cytokines. It is not currently known whether SARS-CoV-2 passes to ORNs as these neurons do not express ACE2 (question mark). The possibility of infection of progenitor cells requires examination, since these cells express ACE2 and are in close contact with SUS cells (question mark). Stem cell infection may potentially explain why a small fraction of COVID-19 patients experience long-term dysosmia.
3. SUS Infection Results in a Rapid Loss of Neuronal Cilia Containing the Olfactory Receptors
As stated above, the determination of the spatiotemporal expression pattern for ACE2 and TMPRSS2 allowed the formulation of a first consistent hypothesis explaining the mechanism leading to dysosmia in COVID-19. Verification of this hypothesis requires experimental data showing the location of the SARS-CoV-2 virus in the OE at various times after infection. The latest work of Bryche and collaborators5 using a hamster model indeed showed the virus accumulation in SUS cells and not in ORNs. This study clearly indicates that very soon after infection specialized neuronal cilia containing olfactory receptors are lost, which is certainly synonymous with the loss of conduction of olfactory stimuli.5 It seems, therefore, that even though there is a massive elimination of cells within the OE during the first days after infection, the sudden loss of smell is caused by an immediate damage to the cilia structure.5 It is now accepted that the perception of smell is restored in most patients within 1–2 weeks. During this time, differentiation of progenitor cells to SUSs and mature ORNs take place. This timing seems a bit short for full regeneration of OE, but SUSs regenerate faster than mature ORNs do, and some mature ORNs can renew not only from progenitor cells but also from pre-existing immature ORNs. The above considerations do not apply to rare COVID-19 patients who experience a sense of smell disturbance for much longer.
Additionally, in this study, there was no obvious acute SARS-CoV-2 transfer from SUS cells to ORNs, at least within 1–2 weeks after infection. Therefore, the results of Bryche and colleagues provide convincing in vivo data supporting the hypothesis that it is the primary impairment of SUS cell function that leads to dysosmia in COVID-19 patients.
This almost perfect image has been somewhat blurred by recent work of Meinhardt and colleagues6 analyzing for the first time OE and brain samples derived from 32 patients who died of COVID-19. The authors interpreted their data as evidence for the presence of SARS-CoV-2 in ORNs. However, at the moment, they should be taken with considerable skepticism because single immunocytochemical images without double staining using cell type-specific markers do not allow one to clearly distinguish between neuronal and non-neuronal cells within the OE, particularly in older samples derived from autopsies performed often as late as 100 h after death. The data presented by Meinhardt et al.6 provide rather weak evidence for rapid (acute) SARS-CoV-2 penetration to the brain along the olfactory nerve because only 3 out of 32 olfactory bulb samples were positive for viral RNA. In contrast, roughly half of OE samples had abundant amounts of viral RNA in this study. Lack of information on which patients experienced anosmia also makes it difficult to interpret these results unambiguously.
4. Inflammatory Processes—Other Side of the Same Coin?
Excessive systemic immune response plays a significant role in multiorgan damage in patients with severe COVID-19. Therefore, SARS-CoV-2-induced immune response may damage ORNs and for this reason lead to olfactory dysfunction. But the fact that dysosmia is often reported in mild cases and even asymptomatic patients argues against the existence of a SUS-independent mechanism based on the immune response. However, the involvement of a local immune response cannot be ruled out as a contributing factor to SUS-mediated mechanism. Recently, Torabi et al.7 showed elevated levels of the proinflammatory cytokine TNF-α in the olfactory epithelium in patients with COVID-19, but no differences in IL-1β were seen.
In another study, small clusters of infiltrating, activated macrophages and granulocytes in the OE upon SARS-CoV-2 infection have been documented.6 It is worth noting, however, that both of the above studies used tissue material from autopsies and artifacts associated with widely known phenomenon of release of proinflammatory cytokines after death cannot be excluded. Nevertheless, a recent study using a hamster model also showed massive infiltration of immune cells, such as macrophages, into the OE shortly after SUS cells infection.5 Deep transcriptional profiling of flow-cytometry-sorted OE cell types suggests that microvillous cells (MVCs) and a small subset of TRPM5-expressing ORNs are likely involved in the inflammatory response to viral infection.8
Considering the above results, an image emerges in which SUS cell infection directly interferes with ORN functions and simultaneously initiates a rapid immune response in at least a subset of ORNs and MVCs (Figure 1). This leads to the migration of activated lymphocytes to the OE as well as induces the production of proinflammatory cytokines. Thus, a local innate immune response may also participate in the process leading to anosmia in COVID-19 (Figure 1). However, it must be established whether SARS-CoV-2 may initiate an immune response in the OE only in an ACE-2- and SUS-dependent manner, as better supported by current data. After all, it cannot be excluded that there is an ACE2-independent pathway mediated by not yet identified alternative viral receptors expressed by MVC and/or ORN, as it was recently shown for rhabdovirus in the fish olfactory epithelium.1
5. Conclusions and Future Direction
The current model of olfactory dysfunction in COVID-19 is based on the already proven observation that SUS cells are the primary target of the virus and that SUSs infection initiates a series of events leading to dysosmia. This is a completely new look at virus-induced anosmia, since it has never been clearly shown for any other virus that the infection of SUS cells interferes with the perception of smell. Such a model of olfactory dysfunction in COVID-19 does not explicitly imply whether SARS-CoV-2 travels from the OE to the brain along olfactory axons. This issue is at the center of current research in this field. No rapid axonal transport of SARS-CoV-2 to the brain has been demonstrated in the hamster model during the first 2 weeks after infection,5 and no preferential virus accumulation/persistence has been reported in olfactory regions of the brain in autopsy material from COVID-19 patients.6 On the other hand, rapid SARS-CoV-2 accumulation in the brain after intranasal injection was recently shown using the new humanized ACE2 knock-in mouse.9 Yet, this is not synonymous with transport along olfactory axons as other routes are also possible. If SARS-CoV-2 indeed travels within the olfactory axons, this would require ACE2-independent passage of the virus from SUSs to ORNs within the OE (Figure 1). In addition, it would be relevant to examine progenitor/stem cells infection, since these OE cells also express significant levels of ACE2 (Figure 1). Moreover, the latest epidemiological analysis revealed that host genetic factors likely play a role in individual susceptibility to anosmia in COVID-19, and characterization of such factors should be of high interest.10 There are many reasons that in the near future the above-mentioned key issues will be the focus of research on chemosensory deficits in COVID-19.
Acknowledgments
The authors would like to thank Christopher von Bartheld (University of Nevada, Reno) and Michal Szpinda (Nicolaus Copernicus University) for their valuable and critical comments.
Author Contributions
Conceptualization, R.B.; graphics, K.B.; writing, K,B, and R,B,; final review and editing, R.B.
Funding support was provided by the “Excellence Initiative -Research University” programme at the Nicolaus Copernicus University.
The authors declare no competing financial interest.
References
- Butowt R.; Bilinska K. (2020) SARS-CoV-2: Olfaction, brain infection and the urgent need for clinical samples allowing earlier virus detection. ACS Chem. Neurosci. 11 (9), 1200–1203. 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- Bilinska K.; Jakubowska P.; von Bartheld C. S.; Butowt R. (2020) Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: Identification of cell types and trends with age. ACS Chem. Neurosci. 11 (11), 1555–1562. 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D. H., Tsukahara T., Weinreb C., Logan D. W., and Datta S. R. (2020) Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRxiv, April 9, 2020, ver. 1. 10.1101/2020.03.25.009084. [DOI] [Google Scholar]
- Fodoulian L., Tuberosa J., Rossier D., Landis B. N., Carleton A., and Rodriguez I. (2020) SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRxiv, April 2, 2020, ver. 1. 10.1101/2020.03.31.013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryche B., Saint-Albin A., Murri S., Lacôte S., Pulido C., Ar Gouilh M., Lesellier S., Servat A., Wasniewski M., Picard-Meyer E., Monchatre-Leroy E., Volmer R., Rampin O., Le Goffic R., Marianneau P., and Meunier N. (2020) Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. bioRxiv, June 16, 2020, 10.1101/2020.06.16.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Mothes R., Franz J., Laue M., Schneider J., Brünink S., Hassan O., Stenzel W., Windfassen M., Rößler L., Goebel H.-H., Martin H., Nitsche A., Schulz- Schaeffer W. J., Hakroush S., Winkler M. S., Tampe B., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold Heppner B., Stadelmann C., Drosten C., Corman V. M., Radbruch H., and Heppner F. L. (2020) Olfactory transmucosal SARS-CoV-2 invasion as port of Central Nervous System entry in COVID-19 patients. bioRxiv, June 4, 2020 10.1101/2020.06.04.135012. [DOI] [PubMed] [Google Scholar]
- Torabi A.; Mohammadbagheri E.; Akbari Dilmaghani N.; Bayat A. H.; Fathi M.; Vakili K.; Alizadeh R.; Rezaeimirghaed O.; Hajiesmaeili M.; Ramezani M.; Simani L.; Aliaghaei A. (2020) Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia [published online ahead of print, 2020 Jun 11]. ACS Chem. Neurosci. 11, 1909. 10.1021/acschemneuro.0c00249. [DOI] [PubMed] [Google Scholar]
- Baxter B. D., Larson E. D., Feinstein P., Polese A. P., Bubak A. N., Niemeyer C. S., Merle L., Shepherd D., Ramakrishnan V. R., Nagel M. A., and Restrepo D. (2020) Transcriptional profiling reveals TRPM5-expressing cells involved in viral infection in the olfactory epithelium. bioRxiv, May 15, 2020 10.1101/2020.05.14.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.-H.; Chen Q.; Gu H.-J.; Yang G.; Wang Y.-X.; Huang X.-Y.; Liu S.-S.; Zhang N.-N.; Li X.-F.; Xiong R.; Guo Y.; Deng Y.-Q.; Huang W.-J.; Liu Q.; Liu Q.-M.; Shen Y.-L.; Zhou Y.; Yang X.; Zhao T.-Y.; Fan C.-F.; Zhou Y.-S.; Qin C.-F.; Wang Y.-C. (2020) A mouse model of SARS-CoV-2 infection and pathogenesis [published online ahead of print, 2020 May 27]. Cell Host Microbe 28, 1–10. 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld C. S., Hagen M. M., and Butowt R. (2020) Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. medRxiv, June 17, 2020 10.1101/2020.06.15.20132134. [DOI] [PMC free article] [PubMed] [Google Scholar]