Conspectus
Renewable energy resources are mostly intermittent and not evenly distributed geographically; for this reason, the development of new technologies for energy storage is in high demand.
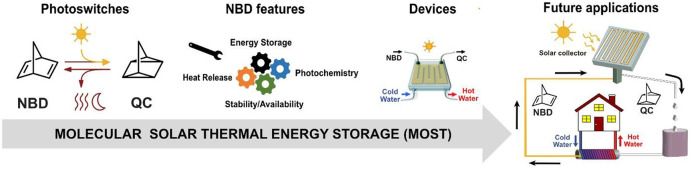
Molecules that undergo photoinduced isomerization reactions that are capable of absorbing light, storing it as chemical energy, and releasing it as thermal energy on demand are referred to as molecular solar thermal energy storage (MOST) or solar thermal fuels (STF). Such molecules offer a promising solution for solar energy storage applications. Different molecular systems have been investigated for MOST applications, such as norbornadienes, azobenzenes, stilbenes, ruthenium derivatives, anthracenes, and dihydroazulenes. The polycyclic strained molecule norbornadiene (NBD), which photoconverts to quadricyclane (QC), is of great interest because it has a high energy storage density and the potential to store energy for a very long time. Unsubstituted norbornadiene has some limitations in this regard, such as poor solar spectrum match and low quantum yield. In the past decade, our group has developed and tested new NBD systems with improved characteristics. Moreover, we have demonstrated their function in laboratory-scale test devices for solar energy harnessing, storage, and release.
This Account describes the most impactful recent findings on how to engineer key properties of the NBD/QC system (photochemistry, energy storage, heat release, stability, and synthesis) as well as examples of test devices for solar energy capture and heat release. While it was known that introducing donor–acceptor groups allows for a red-shifted absorption that better matches the solar spectrum, we managed to introduce donor and acceptor groups with very low molecular weight, which allowed for an unprecedented solar spectrum match combined with high energy density. Strategic steric hindrance in some of these systems dramatically increases the storage time of the photoisomer QC, and dimeric systems have independent energies barriers that lead to an improved solar spectrum match, prolonged storage times, and higher energy densities. These discoveries offer a toolbox of possible chemical modifications that can be used to tune the properties of NBD/QC systems and make them suitable for the desired applications, which can be useful for anyone wanting to take on the challenge of designing efficient MOST systems.
Several test devices have been built, for example, a hybrid MOST device that stores sunlight energy and heat water at the same time. Moreover, we developed a device for monitoring catalyzed QC to NBD conversion resulting in the possibility to quantify a significant macroscopic heat generation. Finally, we tested different formulations of polymeric composites that can absorb light during the day and release the energy as heat during the night for possible use in future window coating applications. These lab-scale realizations are formative and contribute to pushing the field forward toward the real-life application of MOST systems.
1. Introduction
Energy from the sun is fundamental to life on earth. Since the emergence of photosynthesis, plants have converted solar energy into stored chemical energy. Higher organisms consume chemical energy, which eventually allowed for the formation of intelligent life forms. The increased consumption rate of energy from wood enabled early humans to keep warm at night and to cook food. The industrial revolution was made possible through access to coal, and in our modern society, we use a broad range of fossil fuels such as coal, oil, and gas at an ever-increasing pace. Today, we consume fossil fuels (coal, oil, and gas) at a rate of 11 743 MtOE/year, approximately 3 900 000 times faster5 than it has taken to store the energy.5a,5b This consumption is not sustainable in the long run, and a new emission-free energy system needs to be developed, not only for power production but also for heating and cooling, which together amount to ≈50% of energy consumption in the European Union.8
Molecular solar thermal (MOST) systems are based on reversible photoswitches, which are molecules that undergo photoinduced modifications (as isomerizations, dimerizations, and rearrangements). Photoswitches that can absorb and store solar energy and release it as heat on demand have been considered as candidates for MOST applications. These systems have attracted increasing attention in the last years due to their possible use in emission-free energy storage systems.9−23
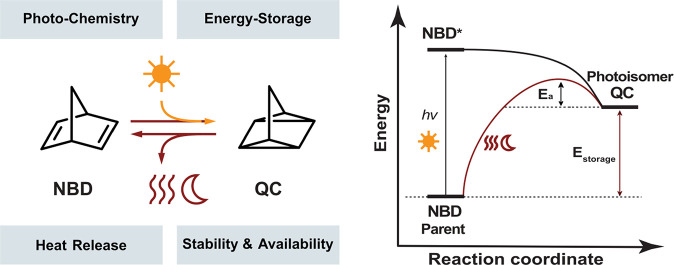
Yoshida in 1985, Bren et al. in 1991, and Dubonosov et al. in 2002 summarized the main findings of early works with photoswitches for MOST applications, presenting the basis for the current development of the MOST systems.24−26 According to these findings, efficient MOST systems should have specific properties that can be classified into four main groups: (i) Photochemistry: the photoswitches should absorb sunlight and convert solar energy into chemical energy through the isomerization of the parent molecule to a high energy photoisomer. (ii) Energy storage: the photoisomer should have a high energy density (Estorage) relative to the parent molecule. Moreover, the photoisomer should be stable for long periods of time that can be expressed as the storage half-life (t1/2), which is related to the energy barrier (Ea) for the back conversion from the photoisomer to the parent molecule. (iii) Heat release: after efficient storage of the energy over time, the energy can be released when the photoisomer back converts to the parent molecule; the process can be triggered by an external stimulus, such as a catalyst,3 electrochemistry,27 heat, or light.28−30 (iv) Stability and availability: the system should be simple to prepare, economically feasible, and robust over extended periods and many cycles. All the properties together constitute a complex challenge for molecular design (Figure 1).
Figure 1.
Basic features of a MOST system; the NBD/QC structure is shown as an example.
A number of different compounds are currently being considered for MOST applications, including norbornadiene/quadricyclane (NBD/QC),2 azobenzene isomers (Azo),31 dihydroazulene/vinylheptafulvene (DHA/VHF),32 and others.23 Several of these systems have been integrated into devices, demonstrating solar energy capture and storage and heat release, both in the liquid1,3 and in the solid state.22,30,33 While each system has challenges and advantages, none of them have yet fulfilled the requirements presented above to the extent that they can be used in real-world applications. In our group, the main focus to this point has been on the molecular design and device demonstrations using the NBD/QC system, which is the topic of this Account.
2. Synthesis
Our first objective was to synthesize new molecular structures and characterize their properties to understand structure–property relationships that can be used to design more efficient systems. A second synthetic objective has been to provide access to large amounts of materials in a scalable, sustainable, and affordable way. The synthesis of NBD derivatives has been done following two main strategies: palladium-catalyzed stepwise synthesis or Diels–Alder reaction.
The first one is a palladium-catalyzed cross-coupling reaction starting from 2-bromo-3-chloronorbornadiene, which has been previously synthesized from commercially available norbornadiene and 1,2-dibromoethane34 as a brominating agent, which is a well-known carcinogenic. Looking for a new synthetical alternative to get 2-bromo-3-chloronorbornadiene, we developed a one-pot route using p-toluenesulfonyl halides as halogenating source.35 These 2-bromo-3-halogenated variants can thus be the starting point of further synthesis using palladium-catalyzed cross-coupling reactions (Scheme 1).15
Scheme 1. Formation of 2-Bromo-3-halogenated-NBD Derivatives and Its Further Reactions to Get Donor–Acceptor NBDs.
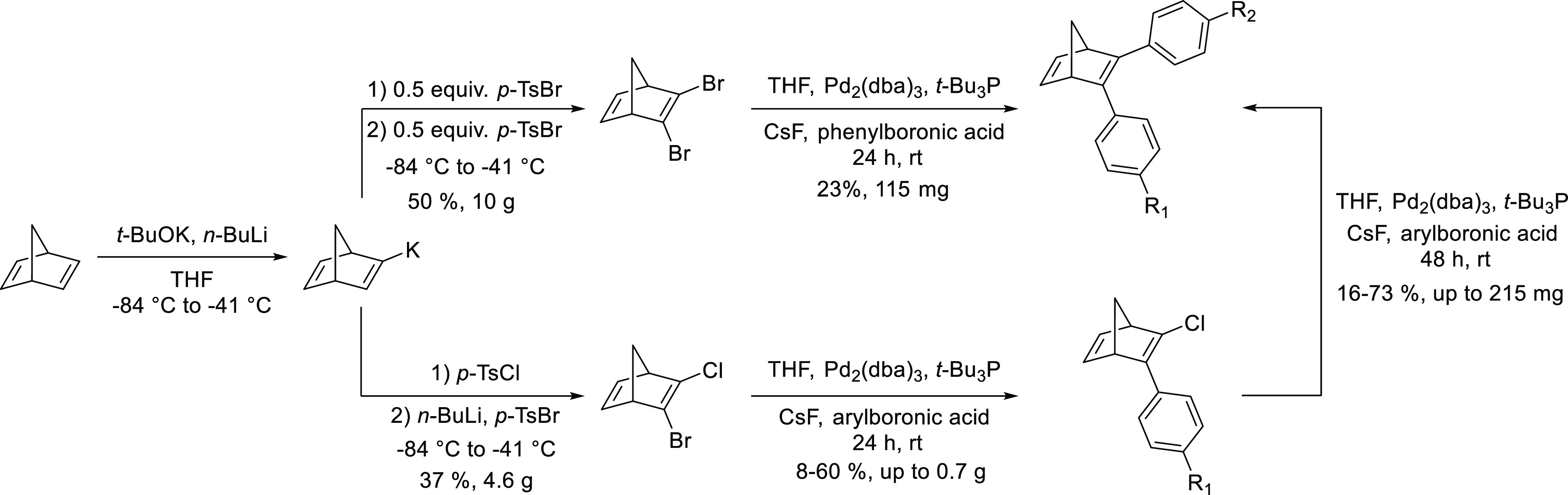
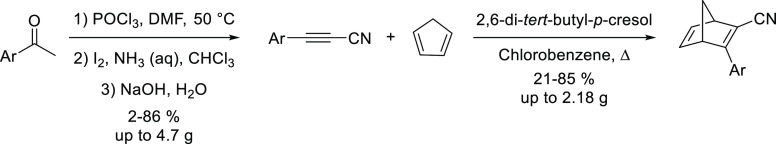
An attractive and economical synthetic alternative to create NBD systems is via a Diels–Alder reaction between acetylene derivatives and cyclopentadiene. In this case, the challenge is to form acetylene precursors bearing functional groups of interest and, at the same time, retaining a good reactivity toward cycloaddition with cyclopentadiene. We have, in this context, developed a simple synthetic route from acetophenone precursors to form a broad range of donor–acceptor (DA) systems without the use of any expensive catalysts or reagents (Scheme 2).2
Scheme 2. NBD Synthesis from Readily Available Acetophenone to Form DA Systems.
3. Molecular Engineering of NBD/QC for MOST Applications
An ideal molecule candidate for MOST applications should fulfill several requirements, such as photochemistry, high energy density, and excellent stability (vide supra). These parameters can be measured in terms of (i) absorption onset of light (Aonset) in the 300–600 nm range to match the solar radiation and (ii) high quantum yields of photoisomerization (φiso) from the parent molecule (NBD) to the photoisomer (QC). (iii) The molecular weight should be low (it has been suggested 140 g/mol),36 targeting an energy density above 0.3 MJ/kg (83.3 Wh/kg).25 (iv) For specific applications, the photoisomer should be stable enough to retain a long storage half-life (t1/2).37 In this section, we show molecular modification strategies that allow for engineering of the different properties in NBD/QC systems.
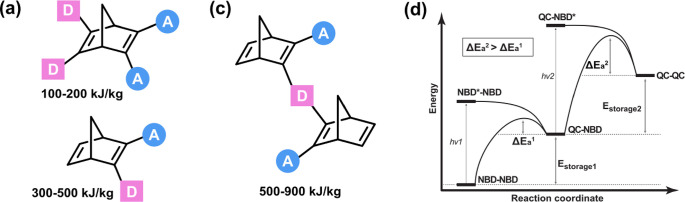
For MOST applications, the absorption band of NBD should be broad toward the visible region. The original approach to red-shift the absorption of the NBDs (Figure 2a) was based on the concept of through-space interactions of donor–acceptor systems (homoconjugation), and it was first extensively investigated by Yoshida and others around 1985.26 These attempts demonstrated that the systems could have absorption onset (Aonset) up to 558 nm, unfortunately often at the expense of increased molecular weight and in turn reduced energy storage density.26,38,39 To shift the norbornadiene absorption onset closer to the optimal 590 nm37 while keeping the molecular weight low, we employed a push–pull conjugation on one of the double bonds. This approach leads to Aonset up to 460 nm (Figure 2b), often with satisfying energy storage density but unfortunately usually at the expense of reduced energy storage times and quantum yields.2,15,16 A third strategy used to achieve a better solar spectrum match is through the synthesis of donor–acceptor dimeric NBD systems (Figure 2c). These exhibit an absorption onset red-shifted up to 410 nm compared to their monomeric analogues.40 One of the main advantages of this last strategy is the possibility to engineer the stability of the high energy photoisomer by coupling two photoswitchable moieties with a specific individual thermal conversion barrier (Figure 2d).
Figure 2.
Red-shifting NBD approaches: (a) donor–acceptor through-space strategy, (b) donor–acceptor in a double bond strategy, and (c) donor–acceptor dimeric strategy. (d) Schematic representation of two switching events in dimeric systems.
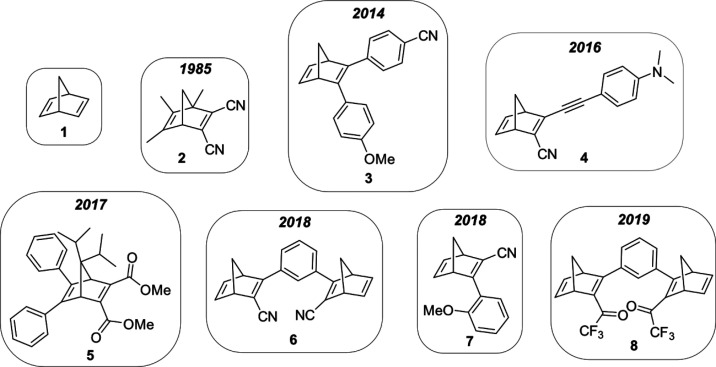
By using these concepts, different libraries of NBDs have been synthesized and characterized; these have allowed for insights into how to engineer important properties of the system. Table 1 shows examples of different NBD systems 1–8 (Figure 3); it is possible to see the structural evolution of the molecules (Figure 3, NBDs 3–8) with a significant improvement of parameters like absorption of light, quantum yield, energy density, and half-life time compared with the standard NBD 1 and structures proposed by Yoshida et al. (NBD 2).2,4,13,15,16,25,26,39,40
Table 1. MOST Parameters of NBDs 1–8.
| NBD | MW (g/mol) | λonseta (nm) | φ | t1/2b (days) | ΔEstorage (kJ/mol) |
|---|---|---|---|---|---|
| 1(25,39) | 92 | 300 | 0.05 | 89 | |
| 2(26) | 184 | 360c | 0.96 | 124d | 88 |
| 3(15) | 299 | 431 | 0.62 | 8.7 | |
| 4(16) | 260 | 456 | 0.28 | 0.2 | 103 |
| 5(13) | 445 | 414 | 0.88 | 2.3 | 46 |
| 6(40) | 308 | 362 | 0.53 | 49 | 173 |
| 7(2) | 223 | 368 | 0.70 | 2273 | 89 |
| 8(4) | 450 | 466 | 0.77 | 0.7 | 216 |
Absorption onset defined as log ε = 2.
Half-lives determined at 25 °C.
Absorption onset estimated from the UV–vis spectrum provided in ref (33).
Half-life determined at 33.5 °C.
Figure 3.
Structural evolution of the NBDs 3–8 developed and characterized in our group compared with the unsubstituted NBD 1 and the NBD 2 studied by Yoshida et al.
The concept of conjugated donor–acceptor systems, as seen in NBD 3 (Table 1), allows one to significantly shift the absorption in a range that better matches the solar radiation. Nevertheless, low molecular weight (MW suggested of 140 g/mol)36 is also an important factor because it affects the energy storage density (ED) due to a negative correlation between these two parameters (eq 1).41
| 1 |
Where Estorage is the energy stored after the photoisomerization process form NBD to QC, χm is the molar fraction of the photoisomer QC, wp is the mass fraction of the parent molecule NBD in the material, and MW is the molecular weight of the parent molecule NBD.41
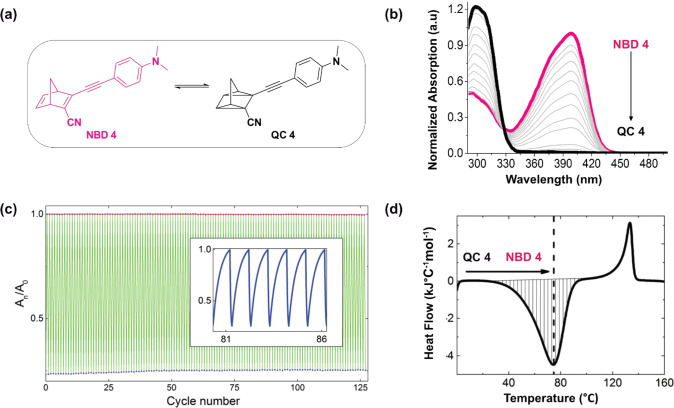
Thus, a new generation of systems was designed where the acceptor p-aryl-substituted group (MW≈ 90 g/mol) was substituted by a cyano unit (MW ≈ 26 g/mol) in a series of compounds, of which NBD 4 is reported here (Figure 3). This set of low molecular weight compounds has red-shifted absorption onset (up to 456 nm for NBD 4, Figure 4a), the values of which are similar to those presented by the higher molecular weight NBD compounds such as NBD 3 (a variation of NBD 3 absorbs up to 462 nm).15 Moreover, NBD 4 have high energy storage density (up to 0.4 MJ/kg ≈ 111 Wh/kg, Figure 4d), higher than the previously defined goal of 0.3 MJ/kg.25 Computational modelling demonstrated that the systems with ethynyl moiety have low distortions, allowing a better π-conjugation, which results in a red-shifted and more narrow absorption peak. The series here represented by NBD 4 achieved a better solar spectrum match, higher energy density, improved stability, and astounding cyclability (negligible degradation over 127 cycles, Figure 4c); these improvements came unfortunately at the expense of reduced storage time (t1/2) of the photoisomers, which are from 5 to 22 h at room temperature.16
Figure 4.
(a) Chemical structures of compounds NBD 4 and QC 4. (b) Stepwise photoisomerization of NBD 4 (in pink) to QC 4 (in black). (c) Cycling tests, photoconversion, followed by thermal back reaction at 60 °C for 127 cycles. The inset shows a detail of the normalized absorption (a.u.) of cycles 81 to 86. (d) DSC thermogram of QC 4 thermally back-isomerizing to NBD 4, showing the associated exothermic peak (downward, hatched). The endothermic peak (upward) associated with the melting of NBD 4 is also observed, centered at about 120 °C. (b) Adapted with permission from ref (16). Copyright 2017 Royal Society of Chemistry. (c, d) Adapted with permission from ref (1). Copyright 2016 Wiley-VCH.
In general, the red-shifting of the absorption of the NBD is often accompanied by the reduced half-life (t1/2) of QC photoisomer.13,37 One way to increase t1/2 is by stabilizing the QC isomer and enhancing the activation energy; in this way, the energy storage density can be affected.13 This trend is observed in a series of NBDs (represented by NBD 5, Figure 3), which have donor–acceptor groups interacting through space and increasingly bulky groups on the one-carbon bridge, hydrogens, methyl, or isopropyl groups as shown (in NBD 5). This set of compounds has red-shift absorption up to Aonset of 414 nm in acetonitrile; t1/2 and quantum yields increase when the molecules are more sterically hindered on the one-carbon bridge (t1/2 from 6.3 to 53 h and φ from 0.73 to 0.88). Variable temperature NMR studies of NBD 5 showed that the signals of isopropyl groups at low temperatures are broad averaged signals in the parent NBD and split to two sets of resolved peaks in the QC photoisomer, indicating the presence of hindered rotation in the NBD. This effect is not observed in the less crowded molecules (bearing hydrogens and methyl groups on the one-carbon bridge, and it causes a higher negative entropy of activation in the isomerization of QC 5 to NBD 5, which makes QC 5 having a longer half-life than the less crowded analogues. This entropic effect on the activation energy of the isomerization is a very powerful tool to engineer t1/2 of QC. At the same time, it is worth mentioning that the energy storage density is decreasing when the steric hindrance increases in the NBD series (from 85.3 to 46.4 kJ/mol) due to the destabilization of the NBD isomers, which partially reduce the energy difference between NBD and QC.13
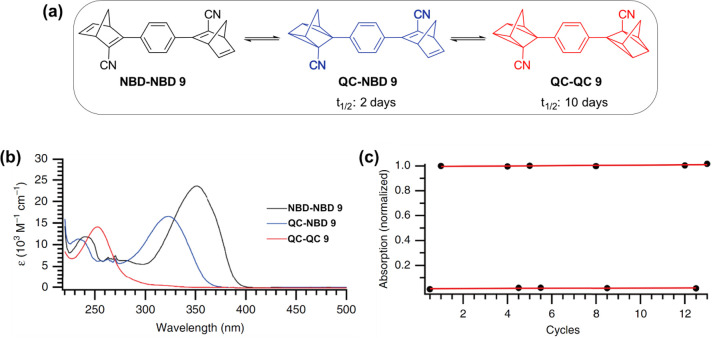
Dimeric and trimeric NBD systems like compound 6 (Figure 3) have been designed to improve storage time and energy density.40 The photophysical properties were measured, showing red-shifted absorption spectra and nearly quantitative quantum yields of photoconversion up to 94% per NBD unit. Interestingly, the para-phenylene bridge NBD dimer 9 has a different absorption between NBD-NBD 9 and NBD-QC 9, indicating a stepwise process in both the forward and backward reaction. Stepwise isomerization and back conversion is an interesting property for the harvesting of solar light due to a blue shift from the first photoisomerization (NBD-NBD 9 to NBD-QC 9) to the second photoswitching step with higher energy (NBD-QC 9 to QC-QC 9), allowing a broad selection of the solar spectrum (Figure 5b). Another property observed in these dimeric systems was a prolonged half-life for the first isomerization from QC-QC 9 to QC-NBD 9 (t1/2 = 10 days). In comparison, the second discharge isomerization from QC-NBD 9 to NBD-NBD 9 is faster (t1/2 = 2 days, Figure 5a). The calculated and measured energy densities of the dimer and trimer systems are up to 0.9 MJ/kg (250 Wh/kg), significantly exceeding the initial target for the energy density of above 0.3 MJ/kg (83.3 Wh/kg)25,37 as well as the values of their monomeric analogues.40 The stability of the dimer NBD-NBD 9 after cycles of photoisomerization and thermal back conversion at 70 °C was examined; when the degradation per cycle was very low (0.11%), the dimeric system demonstrated high robustness (Figure 5c).40 Overall, NBD oligomers have improved photophysical properties, energy density, and stability parameters, highlighting this design concept as promising for future applications as MOST (Figure 5).40
Figure 5.
(a) Stepwise conversion of dimer 9. (b) UV–vis spectra of dimer 9 and its respective photoisomers. Black line: NBD-NBD 9, blue line: QC-NBD 9, red line: QC-QC 9. (c) Cyclability for dimer 9 showing the normalized absorbance at 350 nm in cyclohexane at 70 °C irradiated at 340 nm. Adapted with permission from ref (40). Copyright 2018 Springer Nature.
With the aim of obtaining QC photoisomers with a half-life longer than that of NBD 4, more variations of norbornadienes with cyano groups were synthesized (represented by NBD 7 in Figure 3).2 Different substituted aryl groups have been introduced on the double bond to have push–pull systems with red-shifted absorption spectra. Analysis of the photophysical properties revealed that ortho substituents on the aromatic moiety are correlated with extremely long half-lives, typically 100 times more stable than the non-ortho-substituted isomers, translating to QC isomers metastable for up to 18 years.2 To gain insight into this trend, computational calculations were done where the QC ortho-substituted are sterically constrained, hampering the rotational motion of the side group (Figure 6). Due to the conformational energy landscape and the 3D shape of the conical intersection at the transition state, the sterically hindered isomers experience a higher activation entropy for the back conversion. These findings are a valuable tool to design the half-life time of QCs without compromising other properties, most notably solar spectrum match and energy storage density.2
Figure 6.
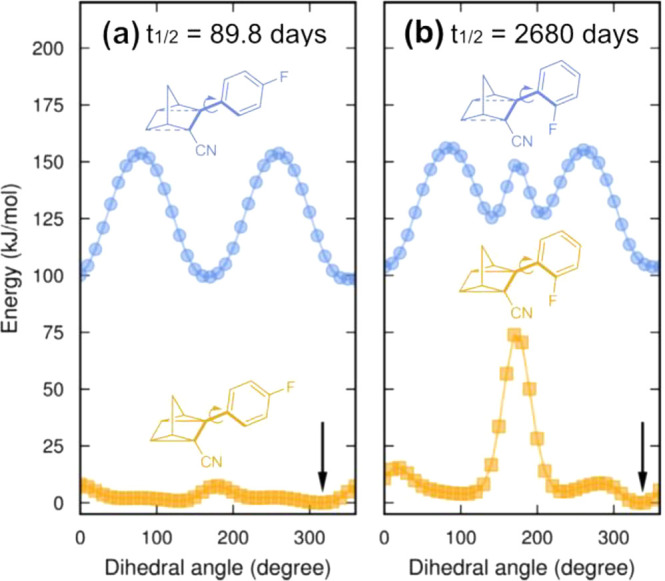
Calculated energy landscapes of the donor side group rotational angle in the QC form (orange) and the TS (blue). (a) Para-substituted group. (b) For the ortho-substituted, the QC landscape is drastically affected, but not in the transition state landscape. The minimum energy in the landscape of the QC is indicated by the black arrow. Adapted with permission from ref (2). Copyright 2018 Wiley-VCH.
4. Application of NBD/QC in Solar Thermal Energy Storage Devices
While in the previous sections we discussed how molecular design can be used to tailor the properties of NBD/QC systems, we will now turn our attention toward how to use demonstration devices and new materials based on NBD/QC photoswitches by testing, simulating, and characterizing the performance of MOST systems.
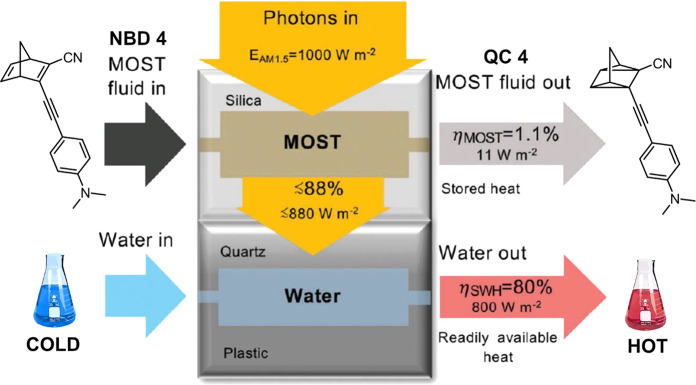
A practical challenge for many photochemical reactions is the efficient harnessing of solar energy. To address this issue, we designed a doubled layer device.1 In the top layer, a solution of NBD 4 was flowed to absorb the sun photons and store them as QC 4; in the lower layer, water was used to harness the transmitted light in the form of readily available heat. Remarkably, the highest measured total solar storage efficiency of the MOST layer (1.1%) was 2-fold higher with respect to any previously reported value.1,42 In the lower part of the device, a 3D printed flow reactor was used to heat water. In combination, the hybrid system demonstrates the utilization of the sunlight at ≈80% total efficiency (Figure 7). This concept was later employed in other geometries and 900 cm2 solar collectors in outdoor test facilities.3
Figure 7.
Hybrid solar energy conversion device. The upper side is used for photoisomerization of NBD 4, and the bottom side is used for water heating. Adapted with permission from ref (1). Copyright 2019 Royal Society of Chemistry.
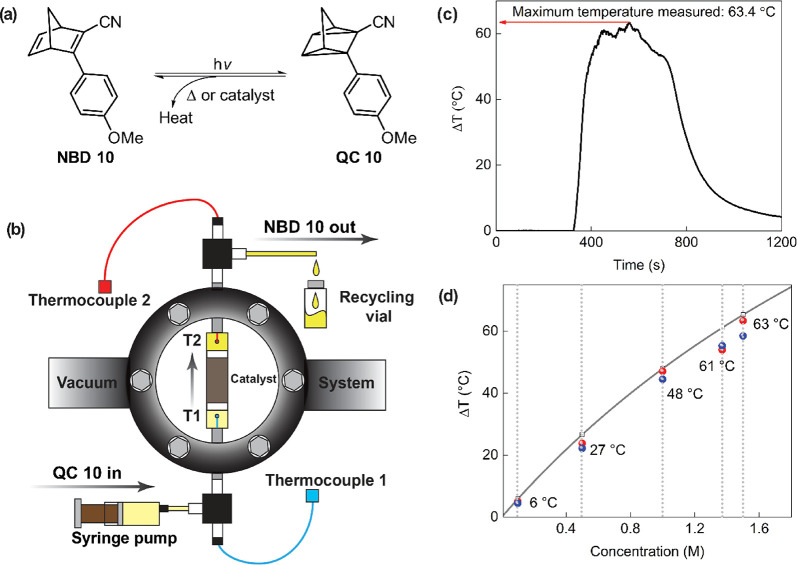
To complete the energy storage and release cycle, an effective means of demonstrating the heat release of the system had to be realized. From a molecular design perspective, solubility becomes a factor because for practical use, it is not the energy density of the molecules that is the determining factor, but the energy density of the full system. In the case of systems in solution, it becomes important to have a high weight percent of molecules in the solvent. The 1-cyano-2-p-methoxyphenyl-norbornadiene 10 was designed to have increased solubility in toluene, and indeed it could be dissolved at up to 1.5 M (334 g/L, Figure 8a). By testing several catalyst candidates, it was found that CoPc could be used to trigger the release of energy from QC 10 to the NBD 10 form of the molecule. To integrate the system into a small device, the catalyst was impregnated onto a solid activated carbon support (Figure 8b). The device was then placed in a high vacuum environment to minimize heat losses and featured temperature gradients of up to 63 °C (85 °C actual temperature, Figure 8c, d). Another attractive feature of the system was its rapid ramp-up time of less than 1 min, which means that future use perhaps could be in load leveling together with other heat sources in various applications.
Figure 8.
(a) Photoisomerization reaction of NBD 10 to QC 10. (b) Illustration of the vacuum chamber. The temperature was measured by thermocouples before (Thermocouple 1) and after the catalytic center (Thermocouple 2). (c) Heat release thermogram of a 1.5 M toluene solution of QC 10 showing the highest temperature gradient of 63.4 °C. (d) Graph of heat release vs concentration, where the theoretical simulation is the gray line and the experimental data are the red and blue dots.3 Adapted with permission from ref (3). Copyright 2019 Royal Society of Chemistry.
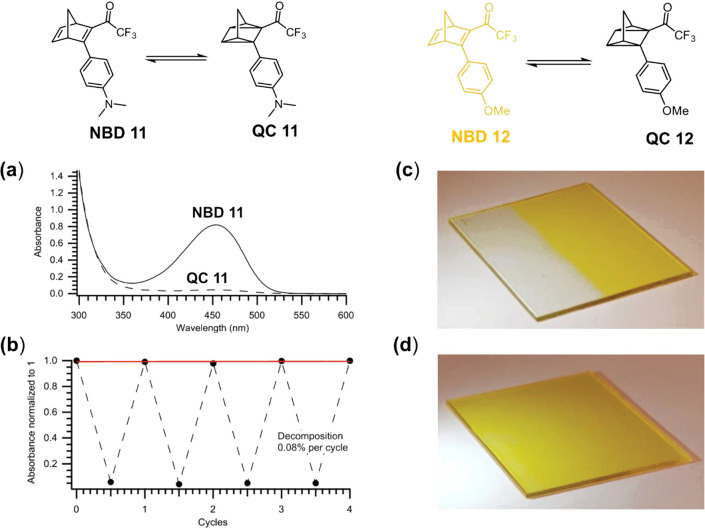
Monomeric and dimeric NBDs with cyano (represented by NBD 6 and NBD 10) and trifluoroacetyl acceptor groups (represented by NBD 8) were tested in polymer composites with application in energy-storing coatings. The systems with a strong acceptor trifluoroacetyl group presented more red-shifted absorption (Aonset up to 529 nm) compared with the cyanide analogues (Aonset up to 400 nm), allowing a better overlapping with the solar spectrum (Figure 9a).4 The half-lives (t1/2 between 0.64 h to 3 days) of NBDs with trifluoroacetyl group are more suitable for applications that require daily cycles compared to their cyano variants (t1/2 between 7 h to 55 days).4 Another derivative with trifluoroacetyl acceptor group, NBD 11 in Figure 8a, was loaded in polystyrene at different concentrations, and the absorption spectra and cyclability tests showed a decomposition per cycle of 0.02–0.45% (Figure 9b). However, studies in more concentrate composites were not carried out due to the fast back conversion rate that makes it impossible to have a full conversion of these NBDs in the composites. To illustrate the potential application of NBD-polystyrene composites in windows, NBD 12/polystyrene composite (0.8 wt % NBD 12) was coated onto a glass substrate. These films presented a fast conversion in the presence of sunlight, demonstrating promising real-life performance as window coatings (Figure 9c, d). The heat released by the NBD/polystyrene composites (0.8 wt %) was measured in the range of 2.7–3.0 kJ/kg.4
Figure 9.
Use of NBD 11 and 12 in the polymer matrix for window coating applications. (a) Absorption spectra of NBD/QC 11 in polystyrene (0.1 wt %). (b) Photothermal cyclability test of NBD 11 in the composite (0.5 wt %). Half of the glass coated with NBD 12/polystyrene composite (0.8 wt %) under light exposition (c) and after back conversion (d). Adapted with permission from ref (4). Copyright 2019 Wiley-VCH.
5. Conclusions and Future Opportunities
In this Account, we have presented some of our findings and rationalizations of molecular design and device demonstrations for efficient MOST based on the NBD/QC system. We have shown how we can use specific molecular design strategies to tune the NBD/QC properties toward desired goals. These findings are rationalized and generalized and will be of interest for future designs of efficient MOST systems. We achieved improved solar spectrum match toward the targeted 590 nm, energy density up to 0.9 MJ/kg ≈ 250 Wh/kg, and tuning of the storage times from very short to very long periods of time (from 1 h up to 18 years), which could suit different applications. We have also developed laboratory-scale test devices to evaluate MOST materials properties and to measure the released energy, which gave insights into MOST performances.
While our initial focus has been on liquid systems, solid-state functional coatings are a new promising direction. The development of new solid MOST materials based on the design principles presented above, which can store incoming sunlight and be integrated into functional coatings for future window applications, is currently under research. Future research directions, which we think will have exciting developments could be related to how to trigger the QC to NBD isomerization utilizing external stimuli. Catalysts or heat have shown to be effective in the past; moreover, we have recently demonstrated that modified NBD/QC systems can be optically modulated to convert both from QC to NBD and from NBD to QC, thus transforming the system from being a photothermal switch to a complete photoswitchable system.28 Very interestingly, in recent years, Libuda and coworkers have been studying electrochemically controlled NBD/QC isomerization.15 Our vision for the future is to develop a heat-generating MOST battery that, unlike traditional photovoltaics and battery combinations, utilizes a carbon-based molecular material for solar energy capture, storage, and heat generation.
Acknowledgments
The authors would like to thank the Knut and Alice Wallenberg Foundation and the Swedish research council FORMAS for financial support.
Biographies
Jessica Orrego-Hernández was born in Apartadó, Colombia. She studied chemistry at Universidad Nacional at Bogotá-Colombia. She received her M. Sc. degree and a Ph.D. degree in chemistry at the Andes University (Bogotá, Colombia) in 2013 and 2018, respectively, where she specialized in the synthesis of heterocycles as fluorescent chemosensors for detection of ions in water. In 2019, she joined the research group of Kasper Moth-Poulsen as a postdoctoral researcher working on the scaling up synthesis of norbornadienes for MOST applications.
Ambra Dreos was born in Trieste, Italy. She studied organic and biomolecular chemistry at the University of Trieste (IT) and graduated summa cum laude in 2014. During the Master’s program, she conducted six months of study in the group of Jan O. Jeppessen at the University of Southern Denmark. In 2015, she joined the research group of Kasper Moth-Poulsen as a Ph.D. student working with MOST systems and related techniques. After graduation in 2019, she has been working as a postdoctoral scholar at Gothenburg University in the J. Hanrieder and M. Schöll group, characterizing new fluorophores for the study of Alzheimer’s pathology.
Kasper Moth-Poulsen was born in Copenhagen, Denmark. He studied chemistry at the University of Copenhagen and graduated with a Ph.D. in 2007 on the topic of single molecular electronics. In 2009–2010, he continued his studies abroad as a postdoc at UC Berkeley, working with new concepts for solar energy storage. Since 2011, he has led his research group at the Chalmers University of Technology in Sweden. His current research interest is MOST systems and nanomaterials chemistry.
The authors declare no competing financial interest.
References
- Dreos A.; Börjesson K.; Wang Z.; Roffey A.; Norwood Z.; Kushnir D.; Moth-Poulsen K. Exploring the potential of a hybrid device combining solar water heating and molecular solar thermal energy storage. Energy Environ. Sci. 2017, 10, 728–734. 10.1039/C6EE01952H. [DOI] [Google Scholar]
- Jevric M.; Petersen A. U.; Mansø M.; Kumar Singh S.; Wang Z.; Dreos A.; Sumby C.; Nielsen M. B.; Börjesson K.; Erhart P.; Moth-Poulsen K. Norbornadiene-Based Photoswitches with Exceptional Combination of Solar Spectrum Match and Long-Term Energy Storage. Chem. - Eur. J. 2018, 24, 12767–12772. 10.1002/chem.201802932. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Roffey A.; Losantos R.; Lennartson A.; Jevric M.; Petersen A. U.; Quant M.; Dreos A.; Wen X.; Sampedro D.; Börjesson K.; Moth-Poulsen K. Macroscopic heat release in a molecular solar thermal energy storage system. Energy Environ. Sci. 2019, 12, 187–193. 10.1039/C8EE01011K. [DOI] [Google Scholar]
- Petersen A. U.; Hofmann A. I.; Fillols M.; Mansø M.; Jevric M.; Wang Z.; Sumby C. J.; Müller C.; Moth-Poulsen K. Solar Energy Storage by Molecular Norbornadiene–Quadricyclane Photoswitches: Polymer Film Devices. Adv. Sci. 2019, 6, 1900367. 10.1002/advs.201900367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a World total proved reserves of fossil fuels. Coal: 1 054 782 million tonnes = 703 188 MtOE. Natural gas: 196.9 trillion cubic meters = 169 334 MtOE. Crude oil: 1729.7 thousand million barrels = 235 931 MtOE. Total: 1 108 453 MtOE. Data from BP Statistical Review of World Energy, 68th ed.; BP p.l.c.: London, 2019. [Google Scholar]; b It took 360 million years to produce the fossil fuels that we currently use. Data from:Acevedo M. F.: Introduction to Renewable Power Systems and the Environment with R; CRC Press: Boca Raton, 2018. [Google Scholar]
- Möller B.; Wiechers E.; Persson U.; Grundahl L.; Lund R. S.; Mathiesen B. V. Heat Roadmap Europe: Towards EU-Wide, local heat supply strategies. Energy 2019, 177, 554–564. 10.1016/j.energy.2019.04.098. [DOI] [Google Scholar]
- Kucharski T. J.; Ferralis N.; Kolpak A. M.; Zheng J. O.; Nocera D. G.; Grossman J. C. Templated assembly of photoswitches significantly increases the energy-storage capacity of solar thermal fuels. Nat. Chem. 2014, 6, 441–447. 10.1038/nchem.1918. [DOI] [PubMed] [Google Scholar]
- Cho E. N.; Zhitomirsky D.; Han G. G. D.; Liu Y.; Grossman J. C. Molecularly Engineered Azobenzene Derivatives for High Energy Density Solid-State Solar Thermal Fuels. ACS Appl. Mater. Interfaces 2017, 9, 8679–8687. 10.1021/acsami.6b15018. [DOI] [PubMed] [Google Scholar]
- Masutani K.; Morikawa M.-a.; Kimizuka N. A liquid azobenzene derivative as a solvent-free solar thermal fuel. Chem. Commun. 2014, 50, 15803–15806. 10.1039/C4CC07713J. [DOI] [PubMed] [Google Scholar]
- Saydjari A. K.; Weis P.; Wu S. Spanning the Solar Spectrum: Azopolymer Solar Thermal Fuels for Simultaneous UV and Visible Light Storage. Adv. Energy Mater. 2017, 7, 1601622. 10.1002/aenm.201601622. [DOI] [Google Scholar]
- Jorner K.; Dreos A.; Emanuelsson R.; El Bakouri O.; Fdez Galván I.; Börjesson K.; Feixas F.; Lindh R.; Zietz B.; Moth-Poulsen K.; Ottosson H. Unraveling factors leading to efficient norbornadiene–quadricyclane molecular solar-thermal energy storage systems. J. Mater. Chem. A 2017, 5, 12369–12378. 10.1039/C7TA04259K. [DOI] [Google Scholar]
- Spivack K. J.; Walker J. V.; Sanford M. J.; Rupert B. R.; Ehle A. R.; Tocyloski J. M.; Jahn A. N.; Shaak L. M.; Obianyo O.; Usher K. M.; Goodson F. E. Substituted Diarylnorbornadienes and Quadricyclanes: Synthesis, Photochemical Properties, and Effect of Substituent on the Kinetic Stability of Quadricyclanes. J. Org. Chem. 2017, 82, 1301–1315. 10.1021/acs.joc.6b02025. [DOI] [PubMed] [Google Scholar]
- Gray V.; Lennartson A.; Ratanalert P.; Börjesson K.; Moth-Poulsen K. Diaryl-substituted norbornadienes with red-shifted absorption for molecular solar thermal energy storage. Chem. Commun. 2014, 50, 5330–5332. 10.1039/C3CC47517D. [DOI] [PubMed] [Google Scholar]
- Quant M.; Lennartson A.; Dreos A.; Kuisma M.; Erhart P.; Börjesson K.; Moth-Poulsen K. Low Molecular Weight Norbornadiene Derivatives for Molecular Solar-Thermal Energy Storage. Chem. - Eur. J. 2016, 22, 13265–13274. 10.1002/chem.201602530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciarini M.; Skov A. B.; Jevric M.; Hansen A. S.; Elm J.; Kjaergaard H. G.; Mikkelsen K. V.; Brøndsted Nielsen M. Towards Solar Energy Storage in the Photochromic Dihydroazulene–Vinylheptafulvene System. Chem. - Eur. J. 2015, 21, 7454–7461. 10.1002/chem.201500100. [DOI] [PubMed] [Google Scholar]
- Blanco-Lomas M.; Martínez-López D.; Campos P. J.; Sampedro D. Tuning of the properties of rhodopsin-based molecular switches. Tetrahedron Lett. 2014, 55, 3361–3364. 10.1016/j.tetlet.2014.04.054. [DOI] [Google Scholar]
- Wang Z.; Losantos R.; Sampedro D.; Morikawa M.-a.; Börjesson K.; Kimizuka N.; Moth-Poulsen K. Demonstration of an azobenzene derivative based solar thermal energy storage system. J. Mater. Chem. A 2019, 7, 15042–15047. 10.1039/C9TA04905C. [DOI] [Google Scholar]
- Schuschke C.; Hohner C.; Jevric M.; Ugleholdt Petersen A.; Wang Z.; Schwarz M.; Kettner M.; Waidhas F.; Fromm L.; Sumby C. J.; Görling A.; Brummel O.; Moth-Poulsen K.; Libuda J. Solar energy storage at an atomically defined organic-oxide hybrid interface. Nat. Commun. 2019, 10, 2384. 10.1038/s41467-019-10263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz A.; Heindl A. H.; Dreos A.; Wang Z.; Moth-Poulsen K.; Becker J.; Wegner H. A. Intermolecular London Dispersion Interactions of Azobenzene Switches for Tuning Molecular Solar Thermal Energy Storage Systems. ChemPlusChem 2019, 84, 1145–1148. 10.1002/cplu.201900330. [DOI] [PubMed] [Google Scholar]
- Gerkman M. A.; Yuan S.; Duan P.; Taufan J.; Schmidt-Rohr K.; Han G. G. D. Phase transition of spiropyrans: impact of isomerization dynamics at high temperatures. Chem. Commun. 2019, 55, 5813–5816. 10.1039/C9CC02141H. [DOI] [PubMed] [Google Scholar]
- Edel K.; Yang X.; Ishibashi J. S. A.; Lamm A. N.; Maichle-Mössmer C.; Giustra Z. X.; Liu S.-Y.; Bettinger H. F. The Dewar Isomer of 1,2-Dihydro-1,2-azaborinines: Isolation, Fragmentation, and Energy Storage. Angew. Chem., Int. Ed. 2018, 57, 5296–5300. 10.1002/anie.201712683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bren V. A.; Dubonosov A. D.; Minkin V. I.; Chernoivanov V. A. Norbornadiene–quadricyclane — an effective molecular system for the storage of solar energy. Russ. Chem. Rev. 1991, 60, 451–469. 10.1070/RC1991v060n05ABEH001088. [DOI] [Google Scholar]
- Dubonosov A. D.; Bren V. A.; Chernoivanov V. A. Norbornadiene–quadricyclane as an abiotic system for the storage of solar energy. Russ. Chem. Rev. 2002, 71, 917–927. 10.1070/RC2002v071n11ABEH000745. [DOI] [Google Scholar]
- Yoshida Z.-i. New molecular energy storage systems. J. Photochem. 1985, 29, 27–40. 10.1016/0047-2670(85)87059-3. [DOI] [Google Scholar]
- Brummel O.; Waidhas F.; Bauer U.; Wu Y.; Bochmann S.; Steinrück H.-P.; Papp C.; Bachmann J.; Libuda J. Photochemical Energy Storage and Electrochemically Triggered Energy Release in the Norbornadiene–Quadricyclane System: UV Photochemistry and IR Spectroelectrochemistry in a Combined Experiment. J. Phys. Chem. Lett. 2017, 8, 2819–2825. 10.1021/acs.jpclett.7b00995. [DOI] [PubMed] [Google Scholar]
- Tebikachew B. E.; Edhborg F.; Kann N.; Albinsson B.; Moth-Poulsen K. Turn-off mode fluorescent norbornadiene-based photoswitches. Phys. Chem. Chem. Phys. 2018, 20, 23195–23201. 10.1039/C8CP04329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreos A.; Wang Z.; Tebikachew B. E.; Moth-Poulsen K.; Andréasson J. Three-Input Molecular Keypad Lock Based on a Norbornadiene–Quadricyclane Photoswitch. J. Phys. Chem. Lett. 2018, 9, 6174–6178. 10.1021/acs.jpclett.8b02567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.; Xue C.; Weis P.; Suzuki Y.; Huang S.; Koynov K.; Auernhammer G. K.; Berger R.; Butt H.-J.; Wu S. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat. Chem. 2017, 9, 145–151. 10.1038/nchem.2625. [DOI] [PubMed] [Google Scholar]
- Han G. G. D.; Li H.; Grossman J. C. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat. Commun. 2017, 8, 1446. 10.1038/s41467-017-01608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen J.; Christensen O.; Kilde M. D.; Abildgaard M.; Metz L.; Kadziola A.; Jevric M.; Mikkelsen K. V.; Nielsen M. B. Molecular Solar Thermal Energy Storage Systems with Long Discharge Times Based on the Dihydroazulene/Vinylheptafulvene Couple. Eur. J. Org. Chem. 2019, 2019, 1986–1993. 10.1002/ejoc.201801776. [DOI] [Google Scholar]
- Hu J.; Huang S.; Yu M.; Yu H. Flexible Solar Thermal Fuel Devices: Composites of Fabric and a Photoliquefiable Azobenzene Derivative. Adv. Energy Mater. 2019, 9, 1901363. 10.1002/aenm.201901363. [DOI] [Google Scholar]
- Kenndoff J.; Polborn K.; Szeimies G. Generation and trapping of 1,5-dehydroquadricyclane. J. Am. Chem. Soc. 1990, 112, 6117–6118. 10.1021/ja00172a031. [DOI] [Google Scholar]
- Lennartson A.; Quant M.; Moth-Poulsen K. A Convenient Route to 2-Bromo-3-chloronorbornadiene and 2,3-Dibromonorbornadiene. Synlett 2015, 26, 1501–1504. 10.1055/s-0034-1380417. [DOI] [Google Scholar]
- Kucharski T. J.; Tian Y.; Akbulatov S.; Boulatov R. Chemical solutions for the closed-cycle storage of solar energy. Energy Environ. Sci. 2011, 4, 4449–4472. 10.1039/c1ee01861b. [DOI] [Google Scholar]
- Börjesson K.; Lennartson A.; Moth-Poulsen K. Efficiency Limit of Molecular Solar Thermal Energy Collecting Devices. ACS Sustainable Chem. Eng. 2013, 1, 585–590. 10.1021/sc300107z. [DOI] [Google Scholar]
- Harel Y.; Adamson A. W.; Kutal C.; Grutsch P. A.; Yasufuku K. Photocalorimetry. 6. Enthalpies of isomerization of norbornadiene and of substituted norbornadienes to corresponding quadricyclenes. J. Phys. Chem. 1987, 91, 901–904. 10.1021/j100288a027. [DOI] [Google Scholar]
- Miki S.; Asako Y.; Yoshida Z.-i. Photochromic Solid Films Prepared by Doping with Donor–Acceptor Norbornadienes. Chem. Lett. 1987, 16, 195–198. 10.1246/cl.1987.195. [DOI] [Google Scholar]
- Mansø M.; Petersen A. U.; Wang Z.; Erhart P.; Nielsen M. B.; Moth-Poulsen K. Molecular solar thermal energy storage in photoswitch oligomers increases energy densities and storage times. Nat. Commun. 2018, 9, 1945. 10.1038/s41467-018-04230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.-L.; Wang C.; Boulatov R. Applications of Photoswitches in the Storage of Solar Energy. ChemPhotoChem. 2019, 3, 268–283. 10.1002/cptc.201900030. [DOI] [Google Scholar]
- Moth-Poulsen K.; Ćoso D.; Börjesson K.; Vinokurov N.; Meier S. K.; Majumdar A.; Vollhardt K. P. C.; Segalman R. A. Molecular solar thermal (MOST) energy storage and release system. Energy Environ. Sci. 2012, 5, 8534–8537. 10.1039/c2ee22426g. [DOI] [Google Scholar]