Abstract
Background
International variation in anemia assessment and management practices in chronic kidney disease (CKD) is poorly understood.
Methods
We performed a cross-sectional analysis of anemia laboratory monitoring, prevalence and management in the prospective Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps). A total of 6766 participants with CKD Stages 3a–5ND from nephrology clinics in Brazil, France, Germany and the USA were included.
Results
Among patients with anemia (hemoglobin <12 g/dL), 36–58% in Brazil, the USA and Germany had repeat hemoglobin measured and 40–61% had iron indices measured within 3 months of the index hemoglobin measurement. Anemia was more common in the USA and Brazil than in France and Germany across CKD stages. Higher ferritin and lower iron saturation (TSAT) levels were observed with lower hemoglobin levels, and higher ferritin with more advanced CKD. The proportion of anemic patients with ferritin <100 ng/mL or TSAT <20% ranged from 42% in Brazil to 53% in France and Germany, and of these patients, over 40% in Brazil, Germany and the USA, compared with 27% in France, were treated with oral or intravenous iron within 3 months after hemoglobin measurement. The proportion of patients with hemoglobin <10 g/dL treated with erythropoiesis-stimulating agents ranged from 28% in the USA to 57% in Germany.
Conclusions
Hemoglobin and iron stores are measured less frequently than per guidelines. Among all regions, there was a substantial proportion of anemic patients with iron deficiency who were not treated with iron, highlighting an area for practice improvement in CKD care.
Keywords: anemia, chronic kidney disease, erythropoiesis-stimulating agents, iron deficiency, iron supplementation
INTRODUCTION
Anemia is a common complication among patients with chronic kidney disease (CKD), and its prevalence rises as estimated glomerular filtration rate (eGFR) decreases [1–3]. Anemia in CKD is associated with decreased quality of life and increased risk of cardiovascular disease and mortality [4–6]. Several mechanisms of CKD-related anemia have been implicated [5], including relative erythropoietin deficiency [7], decreased red cell life span [8], abnormal iron metabolism [9], chronic inflammation [10], metabolic abnormalities [11–16] and effects of medications such as renin angiotensin system (RAS) inhibitors [17–21].
Several practice guidelines provide frameworks to assist clinicians with diagnosis and management of anemia in CKD patients [2, 22–28]. Among Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps) countries, the Kidney Disease: Improving Global Outcomes (KDIGO) anemia guidelines [22] are widely utilized in Brazil and the USA, while the European Renal Best Practice (ERBP) group published a position statement adapting KDIGO anemia recommendations for the European population [25]. Many of the KDIGO recommendations were not graded or had low quality of evidence [22]. KDIGO and ERBP recommendations differ in hemoglobin thresholds for defining anemia and in iron parameter thresholds for initiating iron therapy [25]. In addition to practice variation between countries, we hypothesized there would also be substantial variation at the clinic level based on local practice patterns, individualization of treatment and nephrologist preferences.
Using data from the CKDopps, we evaluated the monitoring frequency, prevalence and management of anemia and iron deficiency in patients with CKD Stages 3–5ND in Brazil, France, Germany and the USA.
MATERIALS AND METHODS
CKDopps is an ongoing international prospective cohort study of nondialysis patients with eGFR <60 mL/min/1.73 m2; study methods have been previously published [29, 30]. Participants were sequentially or randomly selected from national samples of nephrologist-run CKD clinics in Brazil, France, Germany and the USA. Ethics approval for CKDopps was obtained from a central institutional review board (study number: 14004-05).
This investigation utilized data available from 1 January 2013 until 13 April 2018, which included patients entering CKDopps at different time points over this 5.3-year period. Patients were excluded if (i) baseline demographics/medical history questionnaire were not completed, (ii) no laboratory data were reported or (iii) no hemoglobin measurements were reported either during the 6 months prior to enrollment or within 6 months of a reported eGFR during follow-up (see Supplementary data, Figure S1 for study flow diagram).
The hemoglobin measurement used for the cross-sectional analyses (index hemoglobin) was usually the most recent measurement reported during the 6 months prior to the study enrollment date. In patients who did not have hemoglobin reported prior to enrollment, the first available measurement during study follow-up was used, as long as the hemoglobin measurement date was within 6 months of a reported eGFR. For analyses by CKD stage, patients contributed once to the analyses for each CKD stage, utilizing the first hemoglobin measured within 6 months at or after transition to the new CKD stage.
Anemia monitoring by CKD stage was assessed by time from index hemoglobin measurement to the next hemoglobin measurement within a particular CKD stage and presented as cumulative incidence functions. Follow-up time was censored at the earliest of when a patient switched to a different CKD stage, date of last available lab data or end of patient’s follow-up.
Iron status was assessed by the percentage of patients with (i) both ferritin <100 ng/mL and iron saturation (TSAT) <20%; (ii) one of: ferritin <100 ng/mL or TSAT <20% (when both ferritin and TSAT values were available); or (iii) ferritin <100 ng/mL or TSAT <20% (when data for only ferritin or only TSAT were available). The closest TSAT or ferritin measurements within ±3 months of the index hemoglobin were considered concurrent measurements.
Anemia treatment with erythropoiesis stimulating agents (ESAs) and/or iron was based upon relevant prescriptions at or within 3 months after the index hemoglobin measurement; only 3% of patients with hemoglobin measurements were missing data regarding iron or ESA prescription status.
We performed a sensitivity analysis excluding patients with CKD Stage 3a, a subgroup with lower likelihood of metabolic complications of CKD. A subgroup analysis of anemia treatment after two consecutive hemoglobin measurements was also performed (data were available for Brazil, Germany and the USA for this analysis).
Standard descriptive statistics were applied for this investigation. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient characteristics
The study sample was comprised of 6766 CKD Stages 3–5ND patients from 135 CKD clinics across the four countries (Table 1; Supplementary data, Table S1). By study design [29], most patients had CKD Stage 4: 49% (Brazil), 42% (France), 73% (Germany) and 55% (USA). The most common reported primary causes of CKD were diabetes and hypertension in all countries, although the prevalence of diabetic kidney disease was lower in France (22%) compared with other countries (30–37%). Patients with lower hemoglobin levels displayed a higher prevalence of female sex, black race, more advanced CKD, congestive heart failure, other cardiovascular disease, diabetes and peripheral vascular disease.
Table 1.
Patient characteristics, by hemoglobin level and country
| Brazil |
France |
Germany |
USA |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin level, g/dL | <10 | 10 to <12 | ≥12 | All | <10 | 10 to <12 | ≥12 | All | <10 | 10 to <12 | ≥12 | All | <10 | 10 to <12 | ≥12 | All |
| N (% in country) | 91 (13) | 255 (36) | 363 (51) | 709 | 82 (3) | 744 (25) | 2118 (72) | 2944 | 127 (7) | 553 (32) | 1040 (60) | 1720 | 208 (15) | 521 (37) | 664 (48) | 1393 |
| Demographics | ||||||||||||||||
| Age (years) | 64.0 (16.8) | 65.9 (14.1) | 65.7 (14.2) | 65.5 (14.5) | 64.0 (15.9) | 67.6 (13.8) | 66.8 (12.4) | 67.0 (12.9) | 74.1 (11.0) | 74.5 (11.3) | 71.3 (12.7) | 72.5 (12.3) | 67.2 (14.9) | 70.1 (12.9) | 68.2 (11.9) | 68.8 (12.8) |
| Female sex (%) | 54 | 60 | 36 | 47 | 45 | 51 | 29 | 35 | 42 | 54 | 36 | 42 | 57 | 55 | 39 | 48 |
| Black race (%) | 39 | 33 | 26 | 30 | 6 | 3 | 2 | 3 | – | – | – | – | 36 | 24 | 15 | 22 |
| Body mass index (kg/m2) | 27.8 (5.7) | 27.6 (4.8) | 28.2 (5.7) | 27.9 (5.4) | 27.7 (6.6) | 28.8 (6.7) | 28.7 (5.5) | 28.7 (5.9) | 27.8 (5.8) | 29.0 (5.7) | 29.6 (5.4) | 29.2 (5.5) | 31.3 (7.8) | 30.7 (7.6) | 31.5 (6.5) | 31.2 (7.1) |
| CKD stage (%) | ||||||||||||||||
| 3 | 4 | 23 | 43 | 31 | 38 | 35 | 61 | 54 | 12 | 17 | 33 | 26 | 16 | 22 | 42 | 31 |
| 4 | 51 | 50 | 48 | 49 | 44 | 57 | 36 | 42 | 86 | 81 | 67 | 73 | 50 | 62 | 50 | 55 |
| 5 | 45 | 27 | 9 | 20 | 18 | 8 | 3 | 4 | 2 | 2 | 0 | 1 | 34 | 16 | 8 | 15 |
| Reported cause of CKD (%) | ||||||||||||||||
| Diabetes | 39 | 40 | 32 | 36 | 26 | 27 | 19 | 22 | 33 | 32 | 28 | 30 | 44 | 42 | 31 | 37 |
| Glomerular disease | 6 | 10 | 11 | 10 | 30 | 18 | 18 | 19 | 12 | 9 | 10 | 10 | 7 | 9 | 7 | 8 |
| Hypertensiona | 30 | 28 | 33 | 31 | 21 | 29 | 31 | 30 | 29 | 36 | 35 | 35 | 26 | 33 | 38 | 34 |
| Other | 25 | 22 | 24 | 23 | 24 | 26 | 32 | 30 | 26 | 23 | 28 | 26 | 23 | 16 | 24 | 21 |
| Comorbidities (%) | ||||||||||||||||
| Coronary artery disease | 25 | 22 | 24 | 23 | 31 | 27 | 24 | 25 | 35 | 26 | 28 | 28 | 33 | 33 | 27 | 30 |
| Cerebrovascular disease | 14 | 9 | 12 | 11 | 12 | 12 | 12 | 12 | – | – | – | – | 10 | 12 | 12 | 12 |
| Congestive heart failure | 23 | 15 | 15 | 16 | 21 | 14 | 13 | 13 | 16 | 12 | 10 | 11 | 26 | 17 | 13 | 16 |
| Myocardial infarction | 7 | 6 | 9 | 8 | 16 | 14 | 13 | 13 | 8 | 6 | 10 | 9 | 11 | 9 | 7 | 9 |
| Hypertensionb | 91 | 91 | 92 | 92 | 92 | 91 | 91 | 91 | 81 | 84 | 87 | 86 | 93 | 93 | 92 | 93 |
| Peripheral vascular disease | 26 | 23 | 23 | 23 | 24 | 21 | 19 | 20 | – | – | – | – | 24 | 17 | 12 | 16 |
| Other cardiovascular disease | 18 | 12 | 15 | 14 | 29 | 29 | 27 | 28 | 21 | 14 | 13 | 14 | 20 | 19 | 20 | 20 |
| Cancer (nonskin) | 8 | 8 | 10 | 9 | 23 | 20 | 22 | 21 | – | – | – | – | 20 | 14 | 18 | 17 |
| Diabetes | 55 | 52 | 46 | 47 | 46 | 48 | 41 | 43 | 47 | 45 | 43 | 43 | 64 | 62 | 52 | 55 |
| Gastrointestinal bleeding | 7 | 2 | 2 | 3 | 0 | 1 | 1 | 1 | 5 | 2 | 1 | 1 | 2 | 2 | 1 | 1 |
| HIV/AIDS | 2 | 2 | 0 | 1 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 |
| Lung disease | 8 | 5 | 10 | 8 | 19 | 12 | 9 | 10 | 6 | 6 | 7 | 7 | 18 | 13 | 9 | 12 |
| Neurologic disease | 18 | 11 | 12 | 12 | 1 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 5 | 5 | 3 | 4 |
| Psychiatric disorder | 17 | 14 | 11 | 13 | 6 | 10 | 9 | 9 | – | – | – | – | 21 | 15 | 14 | 15 |
| Recurrent cellulitis/gangrene | 11 | 5 | 6 | 6 | 0 | 2 | 1 | 2 | – | – | – | – | 9 | 5 | 3 | 5 |
Values are represented as mean (SD) or prevalence.
Includes vascular nephropathy in France.
Includes patients who received anti-hypertensive treatment in France.
Monitoring of hemoglobin and iron indices
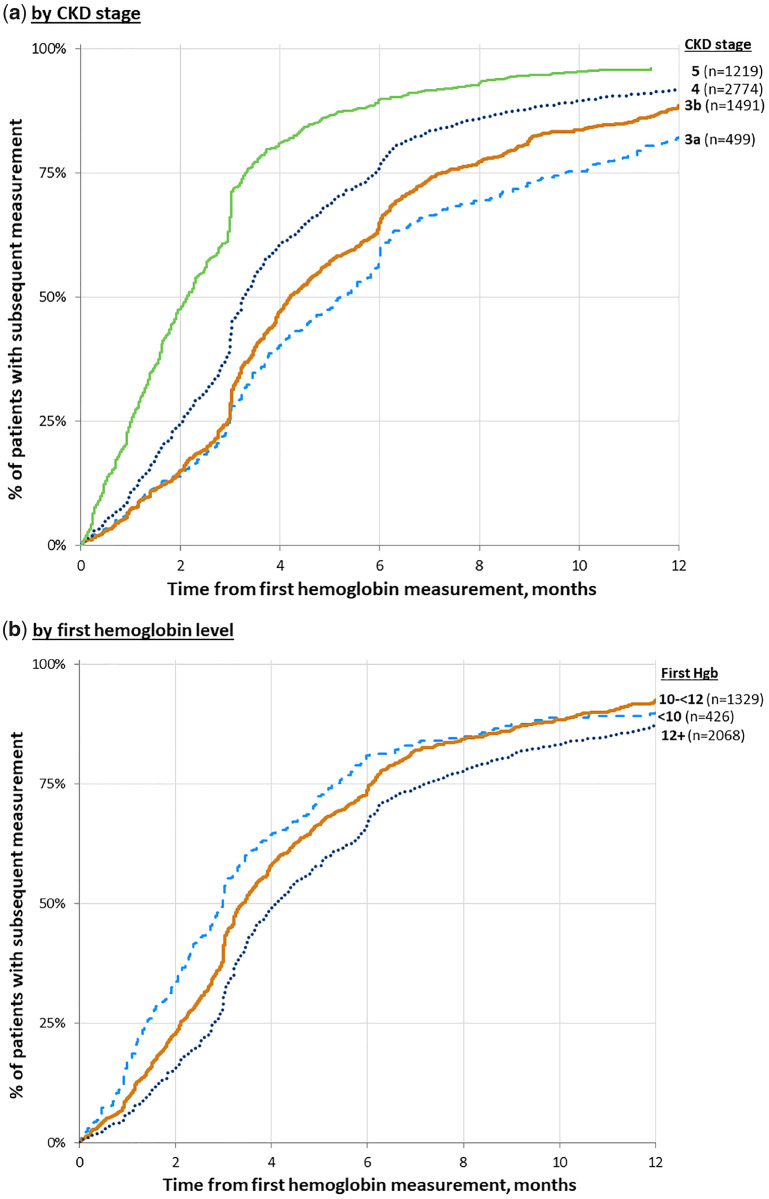
Figure 1 shows the cumulative incidence of a subsequent hemoglobin measurement over 1 year, by CKD stage and index hemoglobin level. Hemoglobin was measured more frequently with advancing CKD, with 60% of patients having their next hemoglobin measurement in 6, 4 and 3 months, respectively, for CKD Stages 3, 4 and 5ND patients. Similarly, hemoglobin was measured more frequently among patients with lower index hemoglobin levels, with 60% having their next hemoglobin level measured in 5, 4 and 3 months, respectively, for patients with index hemoglobin levels of ≥12, 10 to <12 and <10 g/dL. Cumulative incidence of hemoglobin measurements differed little across countries. By comparison, almost all patients had repeat serum creatinine measurements after study entry, with median times until the next creatinine measurement of approximately 4 and 2 months, respectively, for CKD Stages 3a and 5ND patients.
FIGURE 1.
Cumulative incidence of hemoglobin measurement. (a) By CKD stage. (b) By hemoglobin level. Graph by CKD stage allows patients to contribute once for each stage. French data are excluded from the graph as the laboratory measurements are collected according to a predefined study protocol. Time 0 is the first hemoglobin (Hgb) measurement. KDIGO 2012 anemia guidelines [21]: (i) for nondialysis CKD patients with anemia not being treated with an ESA, hemoglobin should be measured when clinically indicated and at least every 3 months in patients with CKD Stages 3–5ND; (ii) for nondialysis CKD patients being treated with an ESA, hemoglobin should be measured at least monthly during the initiation phase of ESA therapy, whereas hemoglobin should be measured at least every 3 months during the maintenance phase of ESA therapy.
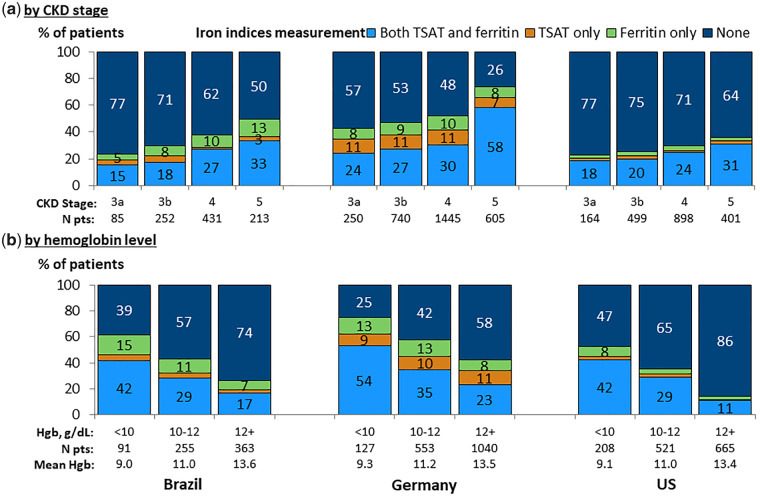
Figure 2 shows measurement of TSAT and serum ferritin within ±3 months of the index hemoglobin measurement. Iron indices were measured more commonly in patients having lower hemoglobin levels and more advanced CKD. Measurement rates were highest in Germany, with approximately 50% of CKD Stages 3b and 4 patients and 75% of CKD Stage 5ND patients having a measured TSAT or ferritin. In Brazil and the USA, TSAT or ferritin was measured for 23–50% of patients by CKD stage. Among patients with hemoglobin <10 g/dL, measurement of TSAT or ferritin was least common in the USA (53%), versus 75% in Germany and 61% in Brazil. Ferritin and TSAT were usually measured together. Similar results were seen in a sensitivity analysis of iron status measurement excluding CKD Stage 3a patients.
FIGURE 2.
Measurement of iron parameters, by country. (a) By CKD stage. (b) By hemoglobin level. Patients could contribute once for each CKD stage experienced during the study for (a). Measurement within ±3 months from hemoglobin (Hgb) value. French data are excluded from the graph as the laboratory measurements are collected according to a predefined study protocol.
Prevalence of anemia and iron deficiency
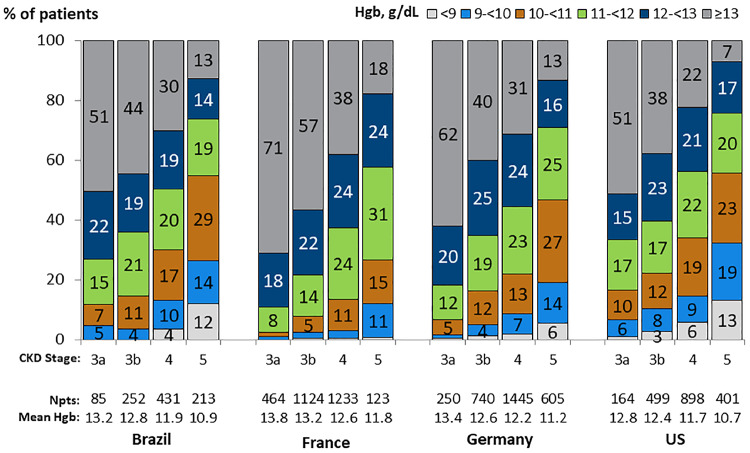
Within all CKD stages, mean hemoglobin was highest in France, intermediate in Brazil and Germany, and lowest in the USA (Table 1;Figure 3). In all countries, the prevalence of anemia was higher among patients with lower eGFR and in females than males, though this difference was less pronounced at higher CKD stages (Figure 3; Supplementary data, Figure S2). The prevalence of low hemoglobin levels was substantial in advanced CKD, particularly for females: 37% of the USA and 17–26% of French, German and Brazilian CKD Stage 5ND females had index hemoglobin levels <10 g/dL (Supplementary data, Figure S2).
FIGURE 3.
Hemoglobin distribution, by country and CKD stage. Patients could contribute once for each CKD stage experienced during the study for (a). First hemoglobin (Hgb) for each stage was taken if there were multiple measures.
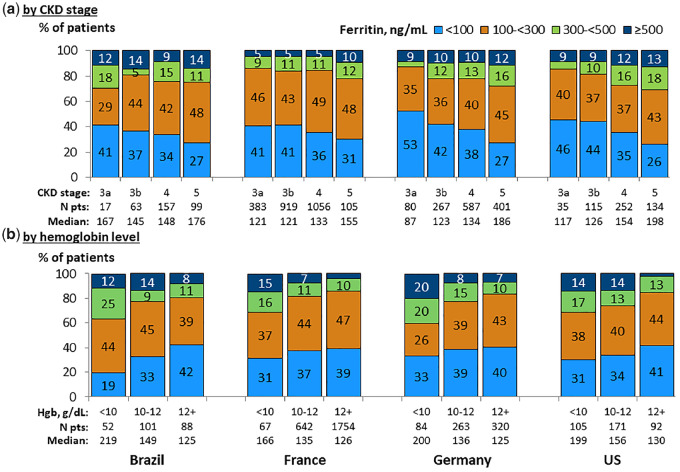
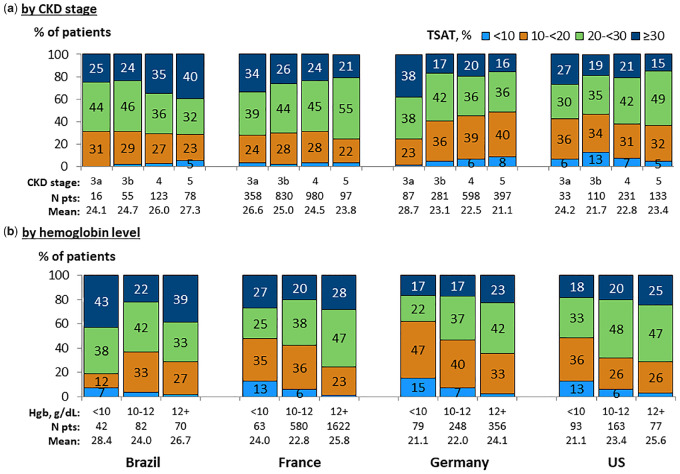
Median serum ferritin levels were higher with advancing CKD and with lower hemoglobin levels across all four countries (Figure 4). By contrast, TSAT did not vary with CKD stage in a consistent pattern across the four CKDopps countries (Figure 5). However, low TSAT levels (<20%) were 1.5- to 2-fold more common at low (<10 g/dL) versus higher (≥12 g/dL) hemoglobin levels, with 48–62% of patients having TSAT <20% among patients with hemoglobin <10 g/dL in France, Germany and the USA. TSAT levels by hemoglobin level were generally higher in Brazil compared with all other countries.
FIGURE 4.
Serum ferritin distribution, by country. (a) By CKD stage. (b) By hemoglobin level. Patients could contribute once for each CKD stage experienced during the study for (a). Ferritin values within ±3 months from hemoglobin (Hgb) value.
FIGURE 5.
TSAT distribution, by country. (a) By CKD stage. (b) By hemoglobin level. Patients could contribute once for each CKD stage experienced during the study for (a). TSAT values within ±3 months from hemoglobin (Hgb) value.
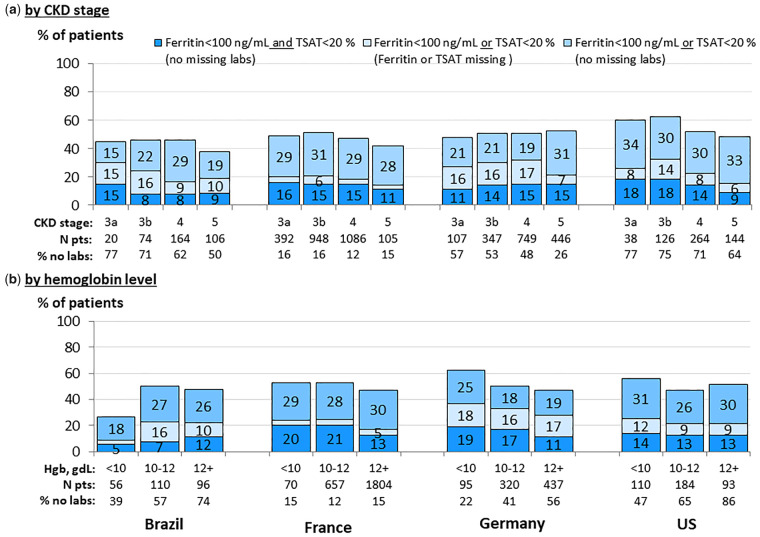
Prevalence of iron deficiency was assessed among patients with at least one available iron lab parameter (Figure 6). Across all CKD stages, 8–18% of patients had absolute iron deficiency (both TSAT <20% and ferritin <100 ng/mL), while 15–34% of patients had incongruent iron parameters [either TSAT <20% and ferritin >100 ng/mL (functional iron deficiency) or TSAT >20% and ferritin <100 ng/mL]. Approximately 3–17% of patients across CKD stages had either TSAT <20% (with no ferritin available) or ferritin <100 ng/mL (with no TSAT available).
FIGURE 6.
Iron (Fe) status, by country. (a) By CKD stage. (b) By hemoglobin level. Patients could contribute once for each CKD stage experienced during the study for (a). Ferritin and TSAT values within ±3 months from hemoglobin value in (b).
Prescription of iron and ESAs
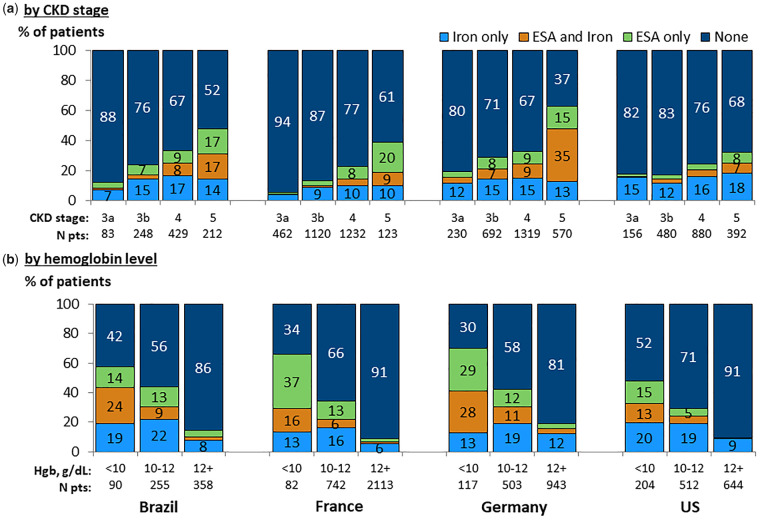
Prescription of iron supplementation and ESAs was more common among patients with lower eGFR (Figure 7a) and lower hemoglobin levels (Figure 7b). Among patients with hemoglobin <10 g/dL, 48% in the USA, 58% in Brazil, 66% in France and 70% in Germany were prescribed an ESA or iron in the 3 months following hemoglobin measurement. Among patients prescribed anemia treatment: (i) for patients having hemoglobin <10 g/dL, an ESA, either alone or in combination with iron, was the most common therapy; and (ii) for patients with hemoglobin 10–12 g/dL, approximately 68% in the USA and 45–50% in all other countries were prescribed supplemental iron alone without an ESA. The most commonly prescribed ESA was epoetin alfa in Brazil and Germany, and darbepoetin alfa in France and the USA. Among patients with ferritin <100 ng/mL or TSAT <20%, 27–44% of patients who also had hemoglobin <12 g/dL were prescribed iron supplementation, compared with 40–50% of patients with hemoglobin <10 g/dL. In all countries, prescription of iron was higher for patients having TSAT <20% or ferritin <100 ng/mL, versus having TSAT ≥20% and ferritin ≥100 ng/mL (Supplementary data, Table S2). The opposite was seen regarding ESA prescription, with ESA prescription typically being higher among patients with TSAT ≥20% and ferritin ≥100 ng/mL versus having TSAT < 20% or ferritin <100 ng/mL. Among patients prescribed iron, intravenous (IV) iron prescription was greater among patients with lower hemoglobin concentrations, ranging across hemoglobin categories from 5% to 18% IV iron prescription in the USA, 6% to 21% in France, 8% to 41% in Brazil and 30% to 54% in Germany (Supplementary data, Table S3). Similar results for TSAT and ferritin distributions and ESA/iron prescriptions were obtained in sensitivity analyses excluding CKD Stage 3a patients.
FIGURE 7.
Prescription of ESAs and iron (oral or IV), by country. (a) By CKD stage. (b) By hemoglobin level. Patients could contribute once for each CKD stage experienced during the study for (a). Prescription within 3 months subsequent to hemoglobin measurement. Graph by CKD stage allows patients to contribute once for each stage. ESA = Erythropoiesis-stimulating agent.
Overall, ESA use was more common in Brazil (16%) and Germany (16%) than in France (9%) and the USA (8%). Among patients with hemoglobin <10 g/dL, the percentage of patients receiving ESA was 28% in the USA, 39% in Brazil, 52% in France and 57% in Germany (Supplementary data, Table S3).
A subgroup analysis demonstrated that 86% of patients with index hemoglobin <10 mg/dL but not treated with iron or ESA within 3 months after the index hemoglobin had a subsequent hemoglobin measurement during follow-up, of which 62% had hemoglobin <10 mg/dL on repeat measurement. Twelve percent of patients with persistent hemoglobin <10 mg/dl on two consecutive measurements were treated with either iron or ESA within 3 months after the second hemoglobin <10 mg/dL (9% in Brazil, 13% in Germany and 12% in the USA) (Supplementary data, Table S4).
Between-clinic variation in anemia measures and treatment
Large variability was seen across CKD clinics in each country in measurement of anemia parameters, prevalence of anemia, iron status, and prescription of iron and ESAs, among patients with CKD Stages 3b–4 (Supplementary data, Figure S3). Germany had the highest use of ESA and iron treatments, and highest prevalence of ferritin and TSAT measurement, as well as the greatest inter-clinic variability in monitoring and anemia treatment practices. Higher inter-clinic variability in anemia prevalence and iron status was observed in Brazil compared with other countries.
DISCUSSION
Our study demonstrates variations in the evaluation and prevalence of anemia and iron deficiency, and related treatment of CKD patients. Hemoglobin levels were lowest in the USA and highest in France. A high proportion of patients received no recorded treatment for anemia.
The frequency of hemoglobin measurement was similar among Brazil, Germany and the USA. However, measurement of iron parameters was much higher in Germany than in Brazil and the USA. The KDIGO guidelines provide clear recommendations for frequency of hemoglobin measurement based on anemia status and ESA therapy, while specific recommendations for iron status monitoring are provided only in the context of ESA therapy, and clinical discretion is recommended in other circumstances [22]. Nevertheless, the high proportion of patients without iron indices measured in this analysis highlights an area for improvement in care.
Our findings indicate a high prevalence of iron deficiency, as well as iron deficiency anemia (IDA), corroborating publications using different methods and study samples [6, 31]. Among these, a study assessing bone marrow iron stores found nearly half of CKD 3–5ND patients with hemoglobin <11 g/dL were iron-depleted [32]. Data from the general population sample in National Health and Nutrition Examination Survey (NHANES) indicated that approximately 60% of men and 70% of women with creatinine clearance <60 mL/min had ferritin <100 ng/mL or TSAT <20% [33].
Median ferritin levels varied inversely with hemoglobin levels, and were higher in CKD Stage 5ND despite higher anemia prevalence with CKD progression. In contrast, no consistent pattern of TSAT distribution by CKD stage was discernable across countries, whereas TSAT <20% was more common at lower hemoglobin levels. Ferritin and TSAT demonstrated opposite associations with hemoglobin levels, thereby reinforcing the central role of inflammation in the pathogenesis of anemia of CKD. These findings are supportive of previous studies suggesting that TSAT <20% is more sensitive than ferritin <100 ng/mL for iron deficiency in advanced CKD [33, 34]. While low ferritin (<100 ng/mL) is specific for iron deficiency, elevated ferritin levels may be seen in iron-deficient patients due to inflammation, malnutrition, infection or malignancy.
We observed that a high proportion of patients with anemia and either ferritin <100 ng/mL or TSAT <20% were not treated with iron, even among those with persistent hemoglobin <10 g/dL on two consecutive measurements. There is likely high variability in nephrologists’ thresholds for treatment in nondialysis CKD patients, perhaps because guidelines reflect uncertainty about optimal care. For example, the KDIGO anemia guidelines contain a Grade 2C suggestion (low-quality evidence) to consider iron therapy in anemic patients with TSAT <30% and ferritin <500 ng/mL, indicating a very wide threshold [22]. Following this recommendation, data from our study indicate that approximately 75% of anemic patients merit consideration for iron therapy. Conversely, the ERBP position statement suggests iron therapy be initiated if TSAT is <25% and ferritin is <200 ng/mL, or if there is absolute iron deficiency (TSAT <20% and ferritin <100 ng/mL). Among patients with hemoglobin <12 g/dL plus either TSAT <20% or ferritin <100 ng/mL, treatment with iron therapy was more prevalent in Germany, Brazil and the USA (43–44%), compared with France (27%) (Supplementary data, Table S2). While this variation may partially reflect differences in guidelines commonly utilized in each region (ERBP in European countries versus KDIGO in USA and Brazil), the differences in treatment between Germany and France suggest that additional factors are at play. For example, the lower severity of anemia observed among patients in France likely contributed to their lower rate of iron therapy prescription.
There is no current consensus on the optimal administration route for iron in nondialysis CKD patients. In this analysis, oral iron was far more commonly prescribed than IV iron. In addition to its gastrointestinal side effects, oral iron may not be effectively absorbed in the setting of high hepcidin levels, which are associated with inflammation [35]. Clinical trials comparing oral with IV iron in patients with CKD and IDA demonstrated inconsistent findings regarding cardiovascular and infection outcomes [36, 37]. This analysis did not assess use of new iron-based phosphate binders such as ferric citrate [38, 39].
The international PRE‐dialysis Survey on Anemia Management [40] and a recent US study of Medicare and commercially insured patients report that 13 and 7% of older CKD patients were treated with ESAs and IV iron, respectively [41]. That study, in contrast to ours, relied on diagnosis codes, had no data on oral iron prescription, and did not report whether patients were followed in nephrology clinics.
The KDIGO guidelines recommend that ESAs generally not be initiated when hemoglobin is ≥10.0 g/dL, but also indicate that individualization of therapy is reasonable [22]. ERBP offers a similar stance, suggesting ESA initiation at hemoglobin values between 9 and 10 g/dL, with consideration of initiation at higher hemoglobin levels in patients with worsening ischemic symptoms associated with anemia [25]. Preliminary data from the CKDopps nephrologist practice survey indicate that the most common hemoglobin threshold for prescribing ESA is ≤9 g/dL in the USA, ≤9.5 g/dL in France and ≤10 g/dL in Brazil [42]. The lower hemoglobin threshold for ESA initiation favored among US nephrologists may explain the relatively lower ESA prescription rate in the USA compared with other CKDopps countries. Differences in guideline use, reimbursement programs, medication availability and use of protocols for anemia management not captured in this analysis presumably also explain some of the between-country variation in iron and ESA prescription, as well as the dramatic clinic-level variation we report within countries. Also of note, hemoglobin levels were generally highest in France, within each CKD stage and among both males and females, despite a comparable proportion treated for anemia (by hemoglobin category) as in Brazil and the USA, and a lower proportion than in Germany. Explanations for this observation beyond differences in guidelines and anemia management practices merit investigation, and likely include differences in patient characteristics between countries, such as CKD etiology (lower prevalence of diabetic kidney disease in France), and the percentage of black patients who displayed lower hemoglobin levels than other patients—consistent with the findings of Matos et al. [43] in Brazil. Variations in use of medications affecting hemoglobin levels such as RAS inhibitors may also contribute to differences in mean hemoglobin levels between countries.
Major clinical trials of ESA use in CKD have demonstrated either no benefit or greater harm with normalizing hemoglobin, compared with lower targets, in outcomes including mortality, cardiovascular events and time to dialysis [44–46]. As a result, most guidelines recommend a hemoglobin target range of 10–12 g/dL [47]. However, our results demonstrate undertreatment to this target range. Further, published data indicate that hemoglobin levels are lower at dialysis initiation than in our sample of patients with early CKD Stage 5ND. It is probable that earlier treatment would limit the rapid worsening of anemia seen in the period prior to dialysis start, and plausible that this may be one part of a multifaceted strategy to lower the excessively high mortality that characterizes the early dialysis period [48, 49].
With respect to patient-reported outcomes (PROs), improved quality of life and fatigue scores were observed in the higher hemoglobin target group in the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta Trial (CREATE) [44] and Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) trials [45], respectively, but not in the Correction of Hemogloblin and Outcomes in Renal Insufficiency (CHOIR) trial [46]. To date, there has been insufficient research on the impact of CKD-related anemia and its treatment on PROs, such as functional status, activities of daily living or ability to work. The question of whether anemia treatment in advanced CKD can help to maintain patients’ lifestyles or even delay the start of kidney replacement therapy by potentially limiting symptoms remains of high importance to patients and health-care providers.
While the cross-sectional design of this study limits causal inference, the design was appropriate to address the study’s goal to describe anemia management practices among CKD patients. Another limitation is that this analysis is restricted to nephrology practices participating in CKDopps in four countries, and therefore is not generalizable to other settings. We were unable to differentiate missing data from true clinical variation in laboratory monitoring practices. However, laboratory data collection was fairly complete, as other laboratory data such as creatinine were collected more frequently than anemia parameters. We could not assess routine laboratory monitoring practices in France, where the study has a mandated laboratory collection protocol. The analysis of iron deficiency was limited by the large number of patients with no iron measurements in Brazil, Germany and the USA. There may have been underreporting of IV iron and ESA therapy due to their intermittent administration, and this analysis did not assess rates of blood transfusions.
In summary, this international analysis of anemia management in patients with nondialysis CKD treated in nephrology clinics identified substantial clinic- and country-level variation in prevalence of anemia and iron deficiency, frequency of anemia monitoring and treatment. We identified a high proportion of patients without measurement of iron parameters, and also patients with IDA who are not treated with iron or ESA, especially in the USA and Brazil. Anemia monitoring and treatment are a ripe area for quality improvement in CKD care. Further research is needed to gain understanding of determinants of anemia in CKD, as well as the effect of its treatment on PROs and clinical outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
Acknowledgements for France: (i) Country Investigators: Christian Combe, Christian Jacquelinet, Z.A.M., B.S., Carole Ayav, Serge Briançon, Denis Fouque, Luc Frimat and Maurice Laville (Christophe Pascal and Yves-Edouard Herpe). (ii) Coordinating center: Céline Lange, Karine Legrans, Sophie Liabeuf, Marie Metzger and Elodie Speyer.
The authors wish to express thanks to Ms Jennifer McCready-Maynes for editorial assistance in preparing this article. We acknowledge and thank the following individuals for their contributions. CKDopps Steering Committee members and Country Investigators: A.A.L. and R.P.-F. (Brazil); Christian Combe, Christian Jacquelinet, Z.A.M. and B.S. (France); Johannes Duttlinger, Danilo Fliser, Gerhard Lonnemann and H.R. (Germany); Takashi Wada and Kunihiro Yamagata (Japan); R.L.P. and B.M.R. (USA). Additional CKDopps Research Group members: Viviane Calice da Silva and Ricardo Sesso (Brazil); Elodie Speyer (France); Koichi Asahi, Junichi Hoshino and I.N. (Japan); R.L.P., F.K.P., N.S., M.M.Y.W., Eric Young and Jarcy Zee (USA).
FUNDING
Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. This manuscript was directly supported by Vifor and Keryx. For details see https://www.dopps.org/AboutUs/Support.aspx. All grants were made to Arbor Research Collaborative for Health and not to coauthors directly.
The French CKD-Renal Epidemiology and Information Network (REIN) is funded by the Agence Nationale de la Recherche through the 2010 ‘Cohortes-Investissements d’Avenir’ program and by the 2010 national Programme Hospitalier de Recherche Clinique. CKD-REIN is also supported through a public–private partnership with Amgen, Fresenius Medical Care, GlaxoSmithKline (GSK), since 2012, Lilly France since 2013, and Otsuka Pharmaceutical since 2015, Baxter and Merck Sharp & Dohme-Chibret (MSD France) from 2012 to 2017 and Sanofi-Genzyme from 2012 to 2015.
In Germany, funding support for participation of German CKD clinics in CKDopps is provided by WiNe (Wissenschaftliches Institut für Nephrologie) of the Verband Deutsche Nierenzentren.
In the USA and Brazil, support for the CKDopps Coordinating Center has been provided by Keryx.
In Japan, CKDopps is part of the REACH-J-CKD Cohort, which is supported in part by a Grant-in-Aid for Research on Advanced CKD, Practical Research Project for Renal Diseases from Japan Agency for Medical Research and Development (AMED).
CONFLICT OF INTEREST STATEMENT
C.T., R.L.P. and B.M.R. are employees of Arbor Research Collaborative for Health. M.M.Y.W. was previously a consultant for Arbor Research Collaborative for Health. R.L.P., Y.L., N.S., A.A.L. and F.K.P. declare that they have no relevant financial interests. R.P.-F. consulted for Fresenius Medical Care, Astra Zeneca, Novo Nordisc, Akebia, Janssen and received payment or reimbursement of travel/accommodation expenses for expert testimony or lectures (including service on speakers’ bureaus) for Astra Zeneca, Novo Nordisc, Akebia Janssen. Z.A.M. received grants for CKD REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp and Dohme-Chibret, Sanofi-Genzyme, Lilly, Otsuka and the French government, as well as fees and grants to charities from Amgen, Bayer and Sanofi-Genzyme. H.R. has received honoraria and consulting fees from Amgen, Teva and Hexal. B.S. has received payment or reimbursement of travel/accommodation expenses for expert testimony or lectures (including service on speakers’ bureaus)/MSD, Lilly. I.N. has received donations for education and research from Kyowa-Hakko-Kirin and Chugai Pharmaceutical.
The results presented in this article have not been published previously in whole or part, except in abstract format.
Contributor Information
the CKDopps Investigators:
Christian Combe, Christian Jacquelinet, Carole Ayav, Serge Briançon, Denis Fouque, Luc Frimat, Maurice Laville, Christophe Pascal, Yves-Edouard Herpe, Céline Lange, Karine Legrans, Sophie Liabeuf, Marie Metzger, and Elodie Speyer
REFERENCES
- 1. Moranne O, Froissart M, Rossert J. et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 2009; 20:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Astor BC, Muntner P, Levin A. et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988-1994). Arch Intern Med 2002; 162: 1401–1408 [DOI] [PubMed] [Google Scholar]
- 3. McClellan W, Aronoff SL, Bolton WK. et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin 2004; 20:1501–1510 [DOI] [PubMed] [Google Scholar]
- 4. Astor BC, Coresh J, Heiss G. et al. Kidney function and anemia as risk factors for coronary heart disease and mortality: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2006; 151: 492–50 [DOI] [PubMed] [Google Scholar]
- 5. Babitt JL, Lin HY.. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012; 23: 1631–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iimori S, Naito S, Noda Y. et al. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: the CKD‐ROUTE study. Nephrology 2015; 20:601–608. [DOI] [PubMed] [Google Scholar]
- 7. McGonigle RJ, Wallin JD, Shadduck RK. et al. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int 1984; 25: 437–444 [DOI] [PubMed] [Google Scholar]
- 8. Sato Y, Mizuguchi T, Shigenaga S. et al. Shortened red blood cell lifespan is related to the dose of erythropoiesis‐stimulating agents requirement in patients on hemodialysis. Ther Apher Dial 2012; 16: 522–528. [DOI] [PubMed] [Google Scholar]
- 9. Babitt JL, Lin HY.. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis 2010; 55: 726–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rambod M, Kovesdy CP, Kalantar-Zadeh K.. Combined high serum ferritin and low iron saturation in hemodialysis patients: the role of inflammation. Clin J Am Soc Nephrol 2008; 3: 1691–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tran L, Batech M, Rhee CM. et al. Serum phosphorus and association with anemia among a large diverse population with and without chronic kidney disease. Nephrol Dial Transplant 2015; 31: 636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bogin E, Massry SG, Levi J. et al. Effect of parathyroid hormone on osmotic fragility of human erythrocytes. J Clin Invest 1982; 69: 1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao DS, Shih M-S, Mohini R.. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med 1993; 328: 171–175 [DOI] [PubMed] [Google Scholar]
- 14. Meytes D, Bogin E, Ma A. et al. Effect of parathyroid hormone on erythropoiesis. J Clin Invest 1981; 67: 1263–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Icardi A, Paoletti E, De Nicola L. et al. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrol Dial Transplant 2013; 28: 1672–1679 [DOI] [PubMed] [Google Scholar]
- 16. Patel NM, Gutiérrez OM, Andress DL. et al. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int 2010; 77: 715–720 [DOI] [PubMed] [Google Scholar]
- 17. Morrone LF, Di Paolo S, Logoluso F. et al. Interference of angiotensin-converting enzyme inhibitors on erythropoiesis in kidney transplant recipients: role of growth factors and cytokines1 . Transplant 1997; 64: 913–918 [DOI] [PubMed] [Google Scholar]
- 18. Pratt M, Lewis‐Barned N, Walker R. et al. Effect of angiotensin converting enzyme inhibitors on erythropoietin concentrations in healthy volunteers. Br J Clin Pharmacol 1992; 34: 363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mrug M, Stopka T, Julian BA. et al. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest 1997; 100: 2310–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Meer P, Lipsic E, Westenbrink BD. et al. Levels of hematopoiesis inhibitor N-acetyl-seryl-aspartyl-lysyl-proline partially explain the occurrence of anemia in heart failure. Circulation 2005; 112: 1743–1747 [DOI] [PubMed] [Google Scholar]
- 21. Azizi M, Rousseau A, Ezan E. et al. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest 1996; 97: 839–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 23.National Institute for Health and Care Excellence. Chronic Kidney Disease: Managing Anemia. Published June 2015. https://nice.org.uk/guidance/ng8 (11 July 2019, date last accessed).
- 24. Yamamoto H, Nishi S, Tomo T. et al. 2015 Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Renal Replacement Therapy 2017; 3: 36 [Google Scholar]
- 25. Locatelli F, Bárány P, Covic A. et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant 2013; 28;1346–1359 [DOI] [PubMed] [Google Scholar]
- 26. Kliger AS, Foley RN, Goldfarb DS. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 2013; 62: 849–859 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. In: de Benoist B, McLean E, Egli I. et al (eds). Geneva: WHO Press, World Health Organization, 2008
- 28. https://www.fda.gov/Drugs/DrugSafety/ucm259639.htm (21 September 2018, date last accessed)
- 29. Mariani L, Stengel B, Combe C. et al. The CKD outcomes and practice patterns study (CKDopps): rationale and methods. Am J Kidney Dis 2016; 68: 402–413 [DOI] [PubMed] [Google Scholar]
- 30. Stengel B, Combe C, Jacquelinet C. et al. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study. Nephrol Dial Transplant 2014; 29: 1500–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mercadal L, Metzger M, Haymann JP. et al. A 3-marker index improves the identification of iron disorders in CKD anaemia. PloS One 2014; 9: e84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stancu S, Stanciu A, Zugravu A. et al. Bone marrow iron, iron indices, and the response to intravenous iron in patients with non–dialysis-dependent CKD. Am J Kidney Dis 2010; 55: 639–647 [DOI] [PubMed] [Google Scholar]
- 33. Fishbane S, Pollack S, Feldman HI. et al. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol 2009; 4: 57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH.. The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 2006; 1: S9–S18 [DOI] [PubMed] [Google Scholar]
- 35. Macdougall IC, Bircher AJ, Eckardt K-U. et al. Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2016; 89: 28–39 [DOI] [PubMed] [Google Scholar]
- 36. Macdougall IC, Bock AH, Carrera F. et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 2014; 29: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agarwal R, Kusek JW, Pappas MK.. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 2015; 88: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Umanath K, Jalal DI, Greco BA. et al. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol 2015; 26: 2578–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Negri AL, Ureña Torres PA.. Iron-based phosphate binders: do they offer advantages over currently available phosphate binders? Clin Kidney 2014; J8: 161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valderrábano F, Hörl WH, Macdougall IC. et al. PRE‐dialysis survey on anaemia management. Nephrol Dial Transplant 2003; 18: 89–100. [DOI] [PubMed] [Google Scholar]
- 41. St. Peter WL, Guo H, Kabadi S. et al. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non-dialysis dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol 2018; 19: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wong MM, Tu C, Zepel L. et al. Anemia prevalence and treatment among patients with chronic kidney disease stage 3-5: data from the chronic kidney disease outcomes and practice patterns study (CKDOPPS). Nephrol Dialysis Transplant 2016; 31: i16–i17 [Google Scholar]
- 43. Matos CM, Silva LF, D'Ávila Melo NA. et al. Prevalence and management of anemia in hemodialysis patients in a Brazilian population of predominantly African descent. Int J Artif Organs 2013; 36: 640–649 [DOI] [PubMed] [Google Scholar]
- 44. Drueke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 45. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 46. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 47. Locatelli F, Mazzaferro S, Yee J.. Iron therapy challenges for the treatment of nondialysis CKD patients. Clin J Am Soc Nephrol 2016; 11: 1269–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.United States Renal Data System. 2018USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018
- 49. Robinson BM, Zhang J, Morgenstern H. et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 2014; 85: 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.