Abstract
Ifosfamide is a commonly used chemotherapeutic known to have numerous adverse kidney manifestations. In this issue of Clinical Kidney Journal, Ensergueix et al. report a multicentric observational retrospective French study on 34 adult patients with tubular dysfunction and /or kidney dysfunction following ifosfamide treatment. Of these patients, 18% had isolated proximal tubular dysfunction, 14% had isolated acute kidney injury (AKI), 18% had isolated chronic kidney disease (CKD) and 50% had a combination of proximal tubular dysfunction and AKI. Concomitant treatment with cisplatin was identified as a risk factor for the development of AKI, and cisplatin and age were associated with estimated glomerular filtration rate at last follow-up. Interestingly, the cumulative dose of ifosfamide was not associated with renal outcomes. This report highlights the need for additional studies on the prevalence, spectrum and management of ifosfamide-associated nephrotoxicity and clearly demonstrates that patients who received ifosfamide should be followed long term to detect proximal tubular dysfunction and CKD early.
Keywords: adults, Fanconi syndrome, ifosfamide, nephrotoxicity
Ifosfamide is an alkylating agent and a member of the nitrogen mustard family. It is a synthetic analogue of cyclophosphamide that is primarily excreted in the urine (80% of the total dose as unchanged ifosfamide). It is believed to act through interfering with DNA replication and RNA production, and is used to treat different types of cancers in adult and paediatric populations [1, 2]. Common side effects include hair loss, vomiting, nephrotoxicity, neurotoxicity (encephalopathy and peripheral neuropathy) and bone marrow suppression.
Ifosfamide is associated with numerous possible adverse kidney manifestations [3]: acute kidney injury (AKI) due to acute tubular necrosis (ATN) [4], Fanconi syndrome [4, 5], interstitial nephritis [6], glomerular disease [7] and haemorrhagic cystitis [8]. While both ifosfamide and cyclophosphamide can cause haemorrhagic cystitis, only ifosfamide is associated with Fanconi syndrome. The introduction of sodium 2-mercaptoethanesulphonate (mesna) has virtually eliminated haemorrhagic cystitis. However, mesna has no preventive effect on the tubular toxicity of ifosfamide [9, 10]. Ifosfamide-induced tubular toxicity can be associated with metabolic acidosis with a normal anion gap (hyperchloremic acidosis) due to Type 1 (distal) or, less frequently, Type 2 (proximal) RTA. Polyuria due to nephrogenic diabetes insipidus (i.e. resistance to antidiuretic hormone) appears to be relatively rare.
Several risk factors for ifosfamide-induced nephrotoxicity have been identified including pre-existing kidney disease, combination with platinum-based chemotherapy and /or other nephrotoxins, cumulative dose of ifosfamide (>119 g/m2) and renal irradiation [11]. In a Dutch study, it was demonstrated that paediatric patients who received ifosfamide had a lower glomerular filtration rate (GFR) than patients with the same pathologies who did not receive this treatment [12]. The main risk factors for nephrotoxicity in children are a cumulative dose >45 mg/m2, young age (<3 years), previous or concurrent cisplatin treatment, Wilms tumour and unilateral nephrectomy [13]. The incidence of Fanconi syndrome in treated patients has been estimated to be between 1.4% and 5% [9]. Most information on ifosfamide nephrotoxicity comes from studies in children, as its use in paediatric oncology is common [14–16]. In contrast, reports of ifosfamide-related Fanconi syndrome in adult patients are scarce [6, 17]. In a long-term assessment of ifosfamide-related renal toxicity in adult patients, Farry et al. [18] reported a steady decline in the estimated GFR (eGFR), although none of the patients progressed to end-stage renal disease. The mean eGFR fell from 82 to 67 mL/min/1.73 m2 after 5 years; most of this reduction occurred during the course of chemotherapy (likely reflecting AKI), although renal function continued to decline thereafter. So the reduction in kidney function associated with ifosfamide administration occurs in a minority of patients, is permanent and progressive and can also occur long after exposure to ifosfamide [19].
In this issue of Clinical Kidney Journal, Ensergueix et al. [20] report a multicentric observational retrospective study of 34 adult patients from six French nephrology departments with tubular dysfunction and /or kidney dysfunction following ifosfamide treatment. Of these patients, 18% had isolated proximal tubular dysfunction, 14% had isolated AKI, 18% had isolated chronic kidney disease (CKD) and 50% had a combination of proximal tubular dysfunction and AKI. eGFR decreased progressively in 16 of 34 patients, 10 patients progressed to CKD Stage 5, and 6 patients required haemodialysis. Six patients died during follow-up and at the end of follow-up, only 5 of 34 patients were alive without CKD. Concomitant treatment with cisplatin appeared to be a risk factor for the development of AKI, and cisplatin and age were associated with eGFR at last follow-up. Interestingly, the cumulative dose of ifosfamide was not associated with renal outcomes.
Proximal tubular dysfunction is the most common ifosfamide-associated nephrotoxicity. Tubular involvement is generally very prolonged, potentially progressive and may lead to advanced CKD [20, 21]. Ifosfamide-induced proximal tubular toxicity is characterized by aminoaciduria (28%), glucosuria (90%), low molecular weight proteinuria, Fanconi syndrome (1–7%), hypophosphataemia, proximal RTA, hypokalaemia, phosphaturia and, more rarely, calciuria, magnesiuria and natriuria [13]. In the study of Ensergueix et al., the most common finding of proximal tubular dysfunction was hypokalaemia, followed by metabolic acidosis, hypophosphataemia, low molecular weight proteinuria, TmPO42−/eGFR <0.8, hypuricaemia and UAFE >10%. Distal tubular toxicity has also been reported with Type 1 RTA, a defect in urine concentration and nephrogenic diabetes insipidus. Ifosfamide can induce SIADH characterized by hyponatraemia, plasma hypo-osmolality and inadequate urinary osmolality [22, 23]. Rossi et al. [9] performed a follow-up study of 75 patients who had received ifosfamide for various malignancies. Over 31 months of follow-up, five patients developed renal Fanconi syndrome as demonstrated by the presence of hyperaminoaciduria, phosphaturia, glucosuria and low serum bicarbonate [9]. Seven patients developed generalized subclinical tubulopathy, which was defined as an impairment of three or all four parameters of proximal tubular solute transport (amino acids, phosphate, glucose and sodium) in the absence of acidosis or metabolic bone disease [9]. They reported that generalized subclinical tubulopathy occurred before the development of Fanconi syndrome in all five cases, and moderate reduction in creatinine clearance was also reported in them [9].
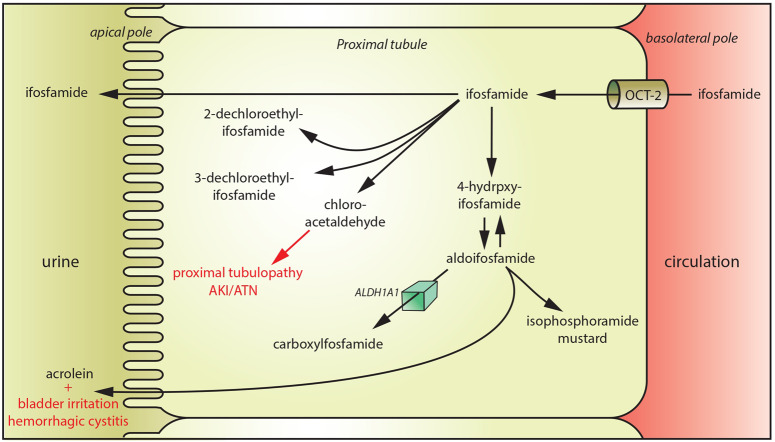
Ifosfamide is more nephrotoxic than cyclophosphamide and this is due to selective uptake of ifosfamide in proximal tubular cells through the organic cation transporter-2 (Figure 1) [24]. Ifosfamide undergoes substantial metabolization with the production of acrolein (responsible for bladder irritation and haemorrhagic cystitis) and chloro-acetaldehyde (responsible for the development of proximal tubulopathy). Studies in rats showed that chloro-acetaldehyde (CAA) causes renal injury by inhibiting nicotinamide adenine dinucleotide (reduced):ubiquinone oxidoreductase (Complex-1; C-I), one of the enzymes in the oxidative phosphorylation pathway [25]. CAA inhibits endocytosis in the rat proximal tubules [26]. This inhibition was attributed to a decrease in adenosine triphosphate (ATP) levels and inhibition of Vacuolar-type H+-ATPase induced by CAA [26]. In the current study, 14 biopsies were available, 3 including electron microscopic evaluation. Histology findings included signs of ATN, vacuolation of epithelial cells and nuclear atypia. Moreover, most biopsies also showed interstitial inflammation and fibrosis. Electron microscopic analysis showed, in addition, evidence of severe mitochondrial abnormalities including irregular mitochondria and attenuation of mitochondrial ridges.
FIGURE 1.
Mechanism of ifosfamide-associated tubular toxicity. Ifosfamide is transported in the proximal tubular cells through organic cation transporter-2. Ifosfamide undergoes substantial metabolization with the production of acrolein (responsible for bladder irritation and haemorrhagic cystitis) and chloro-acetaldehyde (responsible for the development of proximal tubulopathy). ALDH1A1: aldehyde dehydrogenase 1A1.
The limitations of this study are numerous. First of all, only 34 patients are reported in this study. While ifosfamide is a widely used chemotherapeutic and nephrotoxicity is observed in a significant subset of patients, the data remain limited. Secondly, based on the data of Ensergueix et al., it is impossible to establish the prevalence of ifosfamide-associated nephrotoxicity in adults. The study period is very long (1995–2016), and treatment changes have occurred during this time period regarding type and dosing of concomitant chemotherapeutics, which may have impacted the occurrence of nephrotoxicity in these patients. Fifteen patients (44.1%) received cisplatin treatment in addition to ifosfamide chemotherapy. Cisplatin is well-known nephrotoxic drug, making it difficult to attribute kidney dysfunction to ifosfamide in these patients. However, combining chemotherapeutics is a reality in cancer patients, making these data helpful anyway. Finally, only limited histology data are available in this study, with only three patients undergoing electron microscopic evaluation. Although tempting, we need to be very careful regarding the mitochondrial abnormalities observed and putting this forward as the mechanism of action of ifosfamide-associated nephrotoxicity.
In conclusion, Ensergueix et al. report 34 adult patients with ifosfamide-associated nephrotoxicity. The most common forms of ifosfamide-associated nephrotoxicity are proximal tubular dysfunction and AKI. eGFR decreased progressively in 16 of 34 patients, 10 patients developed Stage 5 CKD, 6 required haemodialysis and 6 died. Only five were still alive without CKD at the end of a mean follow-up of 41.8 months. Histologic evaluation in three patients suggests mitochondrial damage as a possible mechanism of ifosfamide-associated nephrotoxicity. Although the data presented in the article do not allow for definite conclusions, ifosfamide was likely an important contributor to the kidney damage in these patients. Patients eligible for ifosfamide treatment should be informed regarding the possibility of irreversible kidney damage. This report highlights the need for additional studies on the prevalence, spectrum and management of ifosfamide-associated nephrotoxicity, and clearly demonstrates that patients who received ifosfamide should be followed long term to detect proximal tubular dysfunction and CKD early. More general, in cancer patients, kidney function should be assessed regularly during but also after treatment, as kidney injury is both common and an important determinant of long-term outcome. The field of onconephrology is relatively new and still developing. Reports like the one of Ensgerueix et al. highlight the importance of a close collaboration between nephrologists and oncologists to improve long-term outcomes in cancer patients.
CONFLICT OF INTEREST STATEMENT
B.S. is a senior clinical investigator of the Research Foundation Flanders (F.W.O.; 1842919N).
REFERENCES
- 1. Voute PA, van den Berg H, Behrendt H. et al. Ifosfamide in the treatment of pediatric malignancies. Semin Oncol 1996; 23: 8–11 [PubMed] [Google Scholar]
- 2. Bokemeyer C, Harstrick A, Beyer J. et al. The use of dose-intensified chemotherapy in the treatment of metastatic nonseminomatous testicular germ cell tumors. German Testicular Cancer Study Group. Semin Oncol 1998; 25: 24–32 [PubMed] [Google Scholar]
- 3. Skinner R. Nephrotoxicity–what do we know and what don’t we know? J Pediatr Hematol Oncol 2011; 33: 128–134 [DOI] [PubMed] [Google Scholar]
- 4. Berns JS, Haghighat A, Staddon A. et al. Severe, irreversible renal failure after ifosfamide treatment. A clinicopathologic report of two patients. Cancer 1995; 76: 497–500 [DOI] [PubMed] [Google Scholar]
- 5. Hall AM, Bass P, Unwin RJ.. Drug-induced renal Fanconi syndrome. QJM 2014; 107: 261–269 [DOI] [PubMed] [Google Scholar]
- 6. Negro A, Regolisti G, Perazzoli F. et al. Ifosfamide-induced renal Fanconi syndrome with associated nephrogenic diabetes insipidus in an adult patient. Nephrol Dial Transplant 1998; 13: 1547–1549 [DOI] [PubMed] [Google Scholar]
- 7. Prasad VK, Lewis IJ, Aparicio SR. et al. Progressive glomerular toxicity of ifosfamide in children. Med Pediatr Oncol 1996; 27: 149–155 [DOI] [PubMed] [Google Scholar]
- 8. Wiltshaw E, Westbury G, Harmer C. et al. Ifosfamide plus mesna with and without adriamycin in soft tissue sarcoma. Cancer Chemother Pharmacol 1986; 18: S10–S12 [DOI] [PubMed] [Google Scholar]
- 9. Rossi R, Pleyer J, Schäfers P. et al. Development of ifosfamide-induced nephrotoxicity: prospective follow-up in 75 patients. Med Pediatr Oncol 1999; 32: 177–182 [DOI] [PubMed] [Google Scholar]
- 10. Yaseen Z, Michoudet C, Baverel G. et al. In vivo mesna and amifostine do not prevent chloroacetaldehyde nephrotoxicity in vitro. Pediatr Nephrol 2008; 23: 611–618 [DOI] [PubMed] [Google Scholar]
- 11. Skinner R, Cotterill SJ, Stevens MC.. Risk factors for nephrotoxicity after ifosfamide treatment in children: a UKCCSG Late Effects Group study. United Kingdom Children’s Cancer Study Group. Br J Cancer 2000; 82: 1636–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dekkers IA, Blijdorp K, Cransberg K. et al. Long-term nephrotoxicity in adult survivors of childhood cancer. Clin J Am Soc Nephrol 2013; 8: 922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujieda M, Matsunaga A, Hayashi A. et al. Children’s toxicology from bench to bed–drug-induced renal injury (2): nephrotoxicity induced by cisplatin and ifosfamide in children. J Toxicol Sci 2009; 34 (Suppl 2): 251–257 [DOI] [PubMed] [Google Scholar]
- 14. Loebstein R, Atanackovic G, Bishai R. et al. Risk factors for long-term outcome of ifosfamide-induced nephrotoxicity in children. J Clin Pharmacol 1999; 39: 454–461 [PubMed] [Google Scholar]
- 15. Oberlin O, Fawaz O, Rey A. et al. Long-term evaluation of Ifosfamide-related nephrotoxicity in children. J Clin Onchol 2009; 27: 5350–5355 [DOI] [PubMed] [Google Scholar]
- 16. Stohr W, Paulides M, Bielack S. et al. Ifosfamide-induced nephrotoxicity in 593 sarcoma patients: a report from the Late Effects Surveillance System. Pediatr Blood Cancer 2007; 48: 447–452 [DOI] [PubMed] [Google Scholar]
- 17. Beckwith C, Flaharty KK, Cheung AK. et al. Fanconi’s syndrome due to ifosfamide. Bone Marrow Transplant 1993; 11: 71–73 [PubMed] [Google Scholar]
- 18. Farry JK, Flombaum CD, Latcha S.. Long term renal toxicity of ifosfamide in adult patients–5 year data. Eur J Cancer 2012; 48: 1326–1331 [DOI] [PubMed] [Google Scholar]
- 19. Akilesh S, Juaire N, Duffield JS. et al. Chronic Ifosfamide toxicity: kidney pathology and pathophysiology. Am J Kidney Dis 2014; 63: 843–850 [DOI] [PubMed] [Google Scholar]
- 20. Skinner R. Chronic ifosfamide nephrotoxicity in children. Med Pediatr Oncol 2003; 41: 190–197 [DOI] [PubMed] [Google Scholar]
- 21. Friedlaender MM, Haviv YS, Rosenmann E. et al. End-stage renal interstitial fibrosis in an adult ten years after ifosfamide therapy. Am J Nephrol 1998; 18: 131–133 [DOI] [PubMed] [Google Scholar]
- 22. Culine S, Ghosn M, Droz JP.. Inappropriate antidiuretic hormone secretion induced by ifosfamide. Eur J Cancer 1990; 26: 922. [DOI] [PubMed] [Google Scholar]
- 23. Kirch C, Gachot B, Germann N. et al. Recurrent ifosfamide-induced hyponatraemia. Eur J Cancer 1997; 33: 2438–2439 [DOI] [PubMed] [Google Scholar]
- 24. Ciarimboli G, Holle SK, Vollenbrocker B. et al. New clues for nephrotoxicity induced by ifosfamide: preferential renal uptake via the human organic cation transporter 2. Mol Pharm 2011; 8: 270–279 [DOI] [PubMed] [Google Scholar]
- 25. Nissim I, Horyn O, Daikhin Y. et al. Ifosfamide-induced nephrotoxicity: mechanism and prevention. Cancer Res 2006; 66: 7824–7831 [DOI] [PubMed] [Google Scholar]
- 26. Yaseen Z, Michoudet C, Baverel G. et al. Mechanisms of the ifosfamide-induced inhibition of endocytosis in the rat proximal kidney tubule. Arch Toxicol 2008; 82: 607–614 [DOI] [PubMed] [Google Scholar]