Abstract
Background
For hemodialysis (HD) patients, travel to the dialysis facility is an issue that can affect their quality of life (QOL), both physically and mentally. However, evidence on this association of transportation modality with health-related QOL (HRQOL) is scarce.
Methods
We conducted a cohort study among maintenance HD patients participating in the Japanese Dialysis Outcomes and Practice Pattern Study Phase 5. The study included patients who were functionally independent and able to walk. The primary exposure was the means of transportation to the dialysis facility, categorized into three groups, namely transportation by other drivers (Group 1), transportation via self-driving (Group 2) and transportation by bicycle or walking with or without public transportation (Group 3). The primary outcomes were physical and mental health composite scores (PCS and MCS) in the 12-item Short Form at 1 year after study initiation.
Results
Among 1225 eligible patients (Group 1, 34.4%; Group 2, 45.0%; Group 3, 20.7%), 835 were analyzed for the primary outcomes. Linear regression analyses revealed that patients in Groups 2 and 3 had significantly higher PCS and MCS at 1 year than those in Group 1 {adjusted mean differences of PCS 1.42 [95% confidence interval (CI) 0.17–2.68] and 1.94 [95% CI 0.65–3.23], respectively, and adjusted mean differences of MCS 2.53 [95% CI 0.92–4.14] and 2.20 [95% CI 0.45–3.95], respectively}.
Conclusions
Transportation modality was a significant prognostic factor for both PCS and MCS after 1 year in maintenance HD patients.
Keywords: hemodialysis, health-related quality of life, HRQOL, J-DOPPS, quality of life, transportation, transportation modality
INTRODUCTION
Health-related quality of life (HRQOL) is the most important outcome for dialysis patients [1], because they and their caregivers are strongly concerned about day-to-day tasks and the enjoyment of living with their disease. Moreover, HRQOL is a well-known predictor of survival [2–5]. Nevertheless, transportation to dialysis facilities (in-center facilities or satellite clinics) to ensure the regular treatment required to achieve adequate solute and fluid removal also accounts for a nonnegligible part of the patient’s daily life and can therefore be a potential factor affecting HRQOL among maintenance hemodialysis (HD) patients [6]. However, little is known about which components of transportation affect patient HRQOL or how they do so.
Previous studies have suggested associations between a longer transportation time and a decrease in HRQOL and increase in mortality [7, 8] and between a longer transportation distance and an increase in mortality [9]. Apart from moving nearer to the dialysis facility, however, neither length nor time of travel is modifiable. In contrast, the means of transportation might be an important but overlooked component affecting patients’ HRQOL and activities of daily living (ADL), due to the regular and lifelong physical activities involved. In particular, common means of transportation in Japan include walking, bicycling, cars and public transportation. For cars, an important consideration is whether the patient drives him/herself. For patients such as the very elderly and those with multiple co-morbidities, the combination of being picked up at their house, then driven to and dropped off at the dialysis facility by another person can be endured over the long term. Nevertheless, such support might deprive them of the opportunity for physical activity and social connectedness, leading to a decrease in HRQOL and ADL.
To our knowledge, no study has yet longitudinally investigated whether the means of transportation to a dialysis facility affects patient HRQOL. Here we investigated the relationship between means of transportation and HRQOL as measured via validated scales using nationally representative Japanese Dialysis Outcomes and Practice Patterns Study (J-DOPPS) data.
MATERIALS AND METHODS
Design, setting and participants
The DOPPS is an international prospective cohort study of in-center HD patients randomly selected from a representative sample of dialysis facilities. The design of the DOPPS has been detailed elsewhere [10]. Demographic characteristics, comorbidities, laboratory values and drug prescriptions were abstracted from medical records. For the Japanese DOPPS (J-DOPPS), laboratory data, drug information and dialysis conditions were collected every 4 months. Mortality and hospitalization events data were collected during study follow-up. In this study we used J-DOPPS Phase 5, which was conducted between 2012 and 2015.
The target population of this study was adult patients receiving in-center HD who were able to visit the dialysis facility from their home. To be eligible, patients had to be both functionally independent, as determined by the Katz index of independence in ADL [11], and able to walk, as determined based on the patient’s response of ‘yes’ or ‘no’ to the question, ‘Are you able to walk?’ Patients with dementia were excluded.
Study approval was obtained from the central institutional review board of Tokyo Women’s Medical University (approval numbers 709, 1178, 1278, 1527, 1826 and 2143). Additional study approval and written patient consent were obtained as required by national and local ethics committee regulations. This study complied with the Declaration of Helsinki.
Exposure
The main exposure was means of transportation to the dialysis facility at baseline, categorized into three groups according to the dependency of transportation and intensity of physical activity, based on the self-administered patient questionnaire ‘How do you usually get to dialysis?’. Group 1 were dependent on others for transportation (transportation by other drivers, including by taxi or shuttle offered by the dialysis facility), Group 2 were those who were independent but whose transport did not require physical activity (transportation via self-driving) and Group 3 were those who were independent and whose transport required physical activity (transportation by bicycle or walking with or without public transportation).
Outcomes
The primary outcomes were physical and mental health composite scores (PCS and MCS) in the 12-item Short Form (SF-12) at 1 year after study initiation [12]. Secondary outcomes were first hospitalization and any-cause death. Hospitalization events were defined as an inpatient hospitalization with an overnight stay. Time at risk for hospitalization started at study enrollment and ended at the earlier of time of first hospitalization, 7 days after leaving the facility unit because of transfer or change in renal replacement therapy modality, date of transplantation, loss to follow-up or end of DOPPS phase. Time at risk for any-cause death started at study enrollment and ended at the earlier of time of death, 7 days after leaving the facility unit because of transfer or change in renal replacement therapy modality, date of transplantation, loss to follow-up or end of DOPPS phase.
Statistical analysis
Baseline characteristics are presented using standard descriptive statistics: means [standard deviations (SDs)] and medians (25th and 75th percentiles) for continuous variables and percentages for categorical variables. For the primary outcomes, linear regression models were used to estimate the mean differences and 95% confidence intervals (CIs) for PCS and MCS at 1 year after study initiation associated with the difference in transportation modality, with facility clustering accounted for using cluster-robust variance estimation. In multivariable linear regression models, we adjusted for the following clinically important confounding factors measured at study baseline related to both the transportation modality and PCS or MCS at 1 year after study initiation: age; gender; dialysis vintage; body mass index (BMI); primary cause of end-stage kidney disease (ESKD), including diabetes mellitus, chronic glomerulonephritis, hypertension and others; comorbidities, including diabetes mellitus, cardiovascular disease (congestive heart failure and coronary artery disease), pulmonary disease, cerebrovascular disease, malignancy and peripheral vascular disease; levels of hemoglobin, creatinine, albumin and C-reactive protein (CRP); and PCS or MCS. For the secondary outcomes, Cox regression models were used to estimate the associations between the means of transportation and both time to first hospitalization and all-cause mortality accounting for facility clustering, in which hazard ratios (HRs) and 95% CIs were estimated using cluster-robust variance estimation. As a sensitivity analysis, we also evaluated the associations between transportation mode and time to a composite of first hospitalization or any-cause death using a Cox regression model with consideration for facility clustering. In the multivariable Cox regression models, we adjusted for the following clinically important confounding factors measured at study baseline related to both transportation modality and first hospitalization or any-cause death: age; gender; dialysis vintage; BMI; primary renal disease, including diabetes mellitus, chronic glomerulonephritis, hypertension and others; comorbidities, including diabetes mellitus, cardiovascular disease (congestive heart failure and coronary artery disease), pulmonary disease, cerebrovascular disease, malignancy and peripheral vascular disease; and levels of hemoglobin, creatinine, albumin and CRP. Furthermore, we conducted a sensitivity analysis to examine the associations between the means of transportation and the number of hospitalizations and death by estimating incidence rate ratios (IRRs) using a generalized estimating equation with negative binomial distribution, adjusted for the same confounders as mentioned above and clustering by facilities using robust standard estimation.
Patients with complete data on both exposure and (primary and secondary) outcome variables were included in the analyses. Markov chain Monte Carlo methods of multiple imputations were implemented for missing data wherein 20 imputations were performed, assuming that analyzed data were missing at random. To derive effect estimates and 95% CIs from multiple imputed data, the mean value for each of the 20 estimates for coefficients of each model was determined and variances of the 20 estimates were pooled according to Rubin’s rules. P-values <0.05 were considered statistically significant. Statistical analyses were conducted using STATA version 14 (StataCorp, College Station, TX, USA).
RESULTS
Baseline characteristics
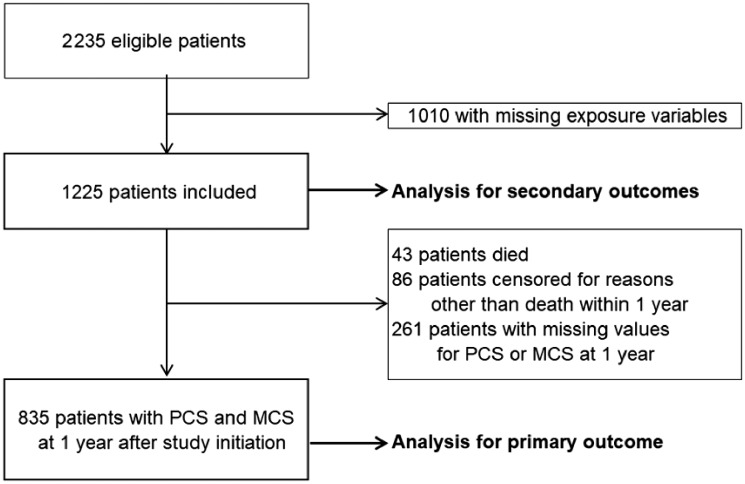
Of the 2235 eligible patients, 1010 with missing exposure variables were excluded, leaving 1225 for analysis of secondary outcomes and 835 for analysis of the primary outcome (Figure 1).
FIGURE 1.
Study flow diagram.
Baseline characteristics of the 1225 subjects are summarized in Table 1. The median age and dialysis vintage were 64 [interquartile range (IQR) 56–72] and 5.5 (IQR 2.2–11.3) years, respectively, and 819 (66.9%) were male. A total of 421 (34.4%) subjects were categorized as Group 1, 551 (45.0%) as Group 2 and 253 (20.7%) as Group 3 according to means of transportation to their dialysis facility. Patients in Group 2 tended to be much younger and much more frequently male and to have a much longer dialysis vintage than those in the other groups. With regard to physical and mental health, patients in Group 1 were more likely to have much lower PCS and MCS than those in the other groups.
Table 1.
Baseline characteristics of the patients
| Characteristics | Total (n = 1225) | Group 1 (n = 421) | Group 2 (n = 551) | Group 3 (n = 253) | Patients with missing data, n |
|---|---|---|---|---|---|
| Age (years)a | 63.7 (12.0); 64 (56–72) | 68.9 (10.3); 70 (63–76) | 60.2 (12.0); 62 (53–68) | 62.8 (11.9); 64 (56–71) | 0 |
| Sex (male), n (%) | 819 (66.9) | 215 (51.1) | 438 (79.5) | 166 (65.6) | 3 |
| Dialysis vintage (years)a | 7.8 (7.4); 5.5 (2.2–11.3) | 7.1 (7.1); 5.1 (2.0–9.5) | 8.7 (8.0); 6.3 (2.4–13.3) | 7.1 (6.6); 5.2 (2.2–10.5) | 6 |
| BMI (kg/m2)a | 21.6 (3.6); 21.2 (19.1–23.6) | 21.3 (3.6); 21.1 (18.6–23.0) | 21.9 (3.7); 21.3 (19.2–24.1) | 21.6 (3.4); 21.1 (19.3–23.6) | 129 |
| Primary cause of ESKD, n (%) | 0 | ||||
| Diabetes | 387 (31.6) | 179 (42.5) | 132 (24.0) | 76 (30.0) | |
| Chronic glomerulonephritis | 483 (39.4) | 130 (30.9) | 269 (48.8) | 84 (33.2) | |
| Hypertension | 72 (5.9) | 31 (7.4) | 24 (4.4) | 17 (6.7) | |
| Others | 283 (23.1) | 81 (19.2) | 126 (22.9) | 76 (30.0) | |
| Comorbidity, n (%) | 0 | ||||
| Diabetes | 440 (35.9) | 199 (47.3) | 155 (28.1) | 86 (34.0) | |
| Cardiovascular disease | 317 (25.9) | 126 (29.9) | 122 (22.1) | 69 (27.3) | |
| Pulmonary disease | 28 (2.3) | 12 (2.9) | 9 (1.6) | 7 (2.8) | |
| Cerebrovascular disease | 73 (6.0) | 36 (8.6) | 26 (4.7) | 11 (4.4) | |
| Peripheral vascular disease | 83 (6.8) | 42 (10.0) | 30 (5.4) | 11 (4.4) | |
| Malignancy | 11.2 (9.1) | 43 (10.2) | 46 (8.3) | 23 (9.1) | |
| Laboratory dataa | |||||
| Hemoglobin (g/dL) | 10.7 (1.1); 10.7 (10.0–11.3) | 10.6 (1.1); 10.6 (10.0–11.2) | 10.8 (1.2); 10.8 (10.1–11.4) | 10.8 (1.0); 10.8 (10.1–11.4) | 9 |
| Creatinine (mg/dL) | 10.8 (2.7); 10.7 (8.9–12.7) | 9.6 (2.3); 9.4 (8.2–11.0) | 11.5 (2.7); 11.5 (9.7–13.3) | 11.1 (2.6); 11.0 (9.2–12.9) | 7 |
| Albumin (g/dL) | 3.7 (0.4); 3.7 (3.5–4.0) | 3.6 (0.4); 3.7 (3.4–3.9) | 3.8 (0.3); 3.8 (3.6–4.0) | 3.7 (0.4); 3.7 (3.5–4.0) | 37 |
| Sodium (mEq/L) | 138.7 (3.2); 139.0 (137.0–141.0) | 138.4 (3.6); 139.0 (136.9–140.2) | 139.0 (2.9); 139.0 (137–141.0) | 138.8 (3.0); 139.0 (137.0–14.01) | 25 |
| Potassium (mEq/L) | 4.8 (0.7); 4.8 (4.3–5.2) | 4.7 (0.7); 4.7 (4.1–5.2) | 4.9 (0.7); 4.8 (4.4–5.3) | 4.8 (0.7); 4.8 (4.3–5.3) | 7 |
| Calcium (mg/dL) | 8.9 (0.8); 8.9 (8.4–9.4) | 8.9 (0.8); 8.9 (8.5–9.3) | 9.0 (0.8); 8.9 (8.4–9.5) | 8.9 (0.7); 9.0 (8.4–9.4) | 147 |
| Phosphorus (mg/dL) | 5.3 (1.4); 5.1 (4.3–6.1) | 5.1 (1.3); 5 (4.2–5.8) | 5.4 (1.4); 5.2 (4.3–6.2) | 5.3 (1.3); 5.2 (4.5–5.9) | 7 |
| CRP (mg/dL) | 0.4 (1.2); 0.1 (0.05–0.3) | 0.6 (1.8); 0.2 (0.1–0.4) | 0.4 (0.9); 0.1 (0.1–0.3) | 0.3 (0.6); 0.1 (0.1–0.2) | 420 |
| SF-12a | |||||
| PCS | 44 (9.0); 45 (37.5–51.9) | 40.5 (9.1); 40.2 (34.1–48.2) | 45.7 (8.5); 47.3 (39.7–52.5) | 45.7 (8.2); 46.9 (39.6–52.4) | 232 |
| MCS | 45.9 (10.4); 45.8 (38.6–54.1) | 44.0 (10.7); 42.9 (36.9–52.1) | 46.9 (10.3); 46.9 (40.1–55.3) | 46.5 (9.7); 46.9 (38.8–54.1) | 232 |
Group 1: patients were driven to their dialysis facility by someone else; Group 2: patients drove to their dialysis facility themselves; Group 3: patients went to their dialysis facility on foot or via public transportation.
Mean (SD); median (IQR).
Baseline characteristics of those eligible patients who were excluded because of missing information on transportation modality are presented in Supplementary data, Table S1. They were older and had a shorter dialysis vintage and a higher likelihood of having diabetes and vascular comorbidities.
Primary outcomes
After excluding 43 (3.5%) of the 1225 eligible study subjects who died, 86 (7.0%) who were censored for reasons other than death within 1 year and 261 (21.3%) with missing values for PCS or MCS at 1 year, 835 (68.2%) were included in the analysis for the primary outcome. Adjusted mean differences in the multivariable linear regression models shown in Table 2 revealed that patients in Group 2 had significantly higher PCS and MCS at 1 year than those in Group 1 [mean difference in PCS 1.42 (95% CI 0.17–2.68) and mean difference in MCS 2.53 (95% CI 0.92–4.14), respectively]. Patients in Group 3 also had significantly higher PCS and MCS at 1 year than those in Group 1 [mean difference in PCS 1.94 (95% CI 0.65–3.23) and mean difference in MCS 2.20 (95% CI 0.45–3.95), respectively]. Age and dialysis vintage were negatively associated with PCS at 1 year but not with MCS at 1 year, whereas the presence of malignancy was negatively associated with MCS at 1 year but not with PCS at 1 year. Moreover, baseline PSC and MCS were positively associated with those at 1 year.
Table 2.
Association of baseline characteristics with PCS and MCS at 1 year after study initiation
| Characteristics | PCS at 1 year |
MCS at 1 year |
||
|---|---|---|---|---|
| Mean difference (95% CI) | P-value | Mean difference (95% CI) | P-value | |
| Group | ||||
| 2 (versus 1) | 1.42 (0.17–2.68) | 0.03 | 2.53 (0.92–4.14) | 0.002 |
| 3 (versus 1) | 1.94 (0.65–3.23) | 0.003 | 2.20 (0.45–3.95) | 0.01 |
| Age per 1 year | −0.09 (−0.14 to −0.04) | <0.001 | 0.02 (−0.05–0.08) | 0.58 |
| Male (versus female) | −0.39 (−1.62–0.83) | 0.53 | −0.29 (−1.82–1.23) | 0.71 |
| Dialysis vintage per 1 year | −0.08 (−0.14 to −0.01) | 0.02 | 0.02 (−0.07–0.10) | 0.73 |
| BMI per 1 kg/m2 | −0.02 (−0.17–0.14) | 0.84 | −0.003 (−0.19–0.18) | 0.97 |
| Primary cause of ESRD | ||||
| Chronic glomerulonephritis (versus diabetes) | 0.20 (−1.87–2.27) | 0.85 | −1.35 (−4.21–1.51) | 0.36 |
| Hypertension (versus diabetes) | 1.81 (−1.24–4.87) | 0.25 | −0.62 (−4.22–2.97) | 0.73 |
| Others (versus diabetes) | 0.42 (−2.08–2.91) | 0.74 | −1.23 (−4.40–1.95) | 0.45 |
| Comorbidity | ||||
| Diabetes yes (versus no) | −0.54 (−2.82–1.73) | 0.64 | −0.19 (−2.87–2.49) | 0.89 |
| Cardiovascular disease yes (versus no) | −0.35 (−1.78–1.08) | 0.63 | −0.55 (−2.17–1.08) | 0.51 |
| Pulmonary disease yes (versus no) | 1.32 (−2.16–4.80) | 0.46 | 1.78 (−2.25–5.81) | 0.39 |
| Cerebrovascular disease yes (versus no) | 1.01 (−1.62–3.64) | 0.45 | 0.31 (−3.00–3.62) | 0.85 |
| Peripheral vascular disease yes (versus no) | −0.98 (−3.16–1.19) | 0.38 | 0.73 (−1.94–3.40) | 0.59 |
| Malignancy yes (versus no) | −0.70 (−2.33–0.93) | 0.4 | −2.83 (−5.21 to −0.45) | 0.02 |
| Laboratory data at baseline | ||||
| Hemoglobin per 1 g/dL | 0.12 (−0.45–0.68) | 0.68 | 0.13 (−0.48–0.73) | 0.68 |
| Creatinine per 1 mg/dL | 0.20 (−0.08–0.47) | 0.17 | 0.12 (−0.21–0.46) | 0.47 |
| Albumin per 1 g/dL | 1.27 (−0.58–3.12) | 0.18 | 1.05 (−1.02–3.11) | 0.32 |
| CRP per 1 mg/dL | −0.41 (−1.03–0.22) | 0.2 | −0.12 (−0.96–0.72) | 0.78 |
| SF-12 score at baseline | ||||
| PCS per 1 | 0.49 (0.42–0.55) | <0.001 | – | |
| MCS per 1 | – | – | 0.54 (0.47–0.62) | <0.001 |
Group 1: patients were driven to their dialysis facility by someone else; Group 2: patients drove to their dialysis facility themselves; Group 3: patients went to their dialysis facility on foot or via public transportation.
P < 0.05 is in bold.
Secondary outcomes
During a median follow-up period of 3 (IQR 2.3–3) years, 746 first hospitalizations and 138 deaths were observed among the 1225 study subjects. Adjusted HRs (aHRs) in the multivariable Cox regression models are shown in Table 3. These revealed that there was no significant difference in either time to first hospitalization or all-cause mortality among the groups [aHRs of Group 2 0.94 (95% CI 0.80–1.10) and 0.75 (95% CI 0.54–1.05), respectively, and of Group 3 0.99 (95% CI 0.81–1.21) and 0.82 (95% CI 0.47–1.43), respectively].
Table 3.
Association of baseline characteristics with first hospitalization and any-cause death
| Characteristics | First hospitalization |
Any-cause death |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Group | ||||
| 2 (versus 1) | 0.94 (0.80–1.10) | 0.42 | 0.75 (0.54–1.05) | 0.09 |
| 3 (versus 1) | 0.99 (0.81–1.21) | 0.94 | 0.82 (0.47–1.43) | 0.48 |
| Age per 1 year | 1.01 (1.00–1.02) | 0.02 | 1.04 (1.02–1.06) | <0.001 |
| Male (versus female) | 1.21 (1.05–1.39) | 0.01 | 2.67 (1.56–4.58) | <0.001 |
| Dialysis vintage per 1 year | 1.01 (1.00–1.02) | 0.14 | 1.03 (1.01–1.06) | 0.01 |
| BMI per 1 kg/m2 | 1.00 (0.98–1.02) | 0.93 | 0.98 (0.91–1.05) | 0.55 |
| Primary cause of ESKD | ||||
| Chronic glomerulonephritis (versus diabetes) | 1.05 (0.74–1.50) | 0.77 | 0.42 (0.18–0.96) | 0.04 |
| Hypertension (versus diabetes) | 1.18 (0.77–1.81) | 0.44 | 0.54 (0.22–1.34) | 0.18 |
| Others (versus diabetes) | 1.10 (0.76–1.60) | 0.61 | 0.59 (0.24–1.46) | 0.25 |
| Comorbidity | ||||
| Diabetes yes (versus no) | 1.27 (0.92–1.76) | 0.14 | 0.77 (0.33–1.79) | 0.54 |
| Cardiovascular disease yes (versus no) | 1.18 (1.02–1.35) | 0.02 | 1.25 (0.89–1.77) | 0.2 |
| Pulmonary disease yes (versus no) | 0.97 (0.55–1.73) | 0.93 | 1.62 (0.65–4.06) | 0.3 |
| Cerebrovascular disease yes (versus no) | 1.21 (0.94–1.58) | 0.15 | 2.20 (1.37–3.53) | 0.001 |
| Peripheral vascular disease yes (versus no) | 1.16 (0.97–1.38) | 0.1 | 0.97 (0.51–1.84) | 0.92 |
| Malignancy yes (versus no) | 0.97 (0.79–1.18) | 0.73 | 1.14 (0.70–1.84) | 0.61 |
| Laboratory data at baseline | ||||
| Hemoglobin per 1 g/dL | 1.01 (0.95–1.08) | 0.72 | 0.97 (0.83–1.13) | 0.69 |
| Creatinine per 1 mg/dL | 0.92 (0.89–0.95) | <0.001 | 0.84 (0.76–0.93) | 0.001 |
| Albumin per 1 g/dL | 0.81 (0.62–1.05) | 0.11 | 0.67 (0.38–1.20) | 0.18 |
| CRP per 1 mg/dL | 1.06 (0.98–1.14) | 0.15 | 1.19 (1.06–1.34) | 0.003 |
Group 1: patients were driven to their dialysis facility by someone else; Group 2: patients drove to their dialysis facility themselves; Group 3: patients went to their dialysis facility on foot or via public transportation.
P < 0.05 is in bold.
Sensitivity analyses
Sensitivity analyses confirmed that means of transportation was not associated with time to first hospitalization or any-cause death [Groups 2 and 3 aHRs 0.98 and 0.97 (95% CIs 0.79–1.22 and 0.74–1.27), respectively]. We also found no association between transportation mode and multiple hospitalization or death [Groups 2 and 3 adjusted IRRs 0.97 and 0.91 (95% CIs 0.86–1.09 and 0.79–1.06), respectively] (Supplementary data, Table S2).
DISCUSSION
To our knowledge, this is the first report to address the relationship between means of transportation and HRQOL among maintenance HD patients. The patients who independently go to the dialysis facility, either by driving a car (Group 2: independent but not active physical activity) or by bicycle or walking with or without public transportation (Group 3: independent and active physical activity) had significantly higher PCS and MCS at 1 year than patients who were dependently transported by other drivers (Group 1: dependent transportation).
The lower MCS and PCS among patients who were transported by other drivers (Group 1) might be explained by the fewer opportunities for social activity as well as the weaker intensity of physical activity compared with those who drove themselves (Group 2). We speculate that patients in Group 1 might have been less likely to engage in physical and social activities during dialysis travel, such as shopping, meeting friends and working, compared with Group 2. This might, in turn, lead to a loss of social connectedness or independence. In addition, the difference in PCS between Groups 1 and 3 was slightly greater than that between Groups 1 and 2. These observations can be explained by a lower intensity of physical activity among Group 2 than Group 3, as driving a car to the dialysis facility is less active than walking or bicycling. Interestingly, the difference in MCS between Groups 1 and 2 was slightly greater than that between Groups 1 and 3. Several previous studies might support the observed relationship between driving a car and MCS. For example, among the elderly general population, both driving a car and having a car itself might be associated with better generic QOL [13, 14].
In contrast, the hard outcomes of mortality and hospitalization were not associated with transport methods among our population. Several explanations are possible. First, Japanese dialysis patients are well known for having a lower mortality rate than their Western counterparts [15]. Second, our patients were relatively younger than average Japanese dialysis patients, who had better HRQOL compared with the prior J-DOPPS cohort [5], and had well-controlled anemia and low CRP, probably because we included only those who could walk alone. Thus our cohort was a relatively low-risk population for mortality.
Our results have several implications for dialysis clinicians and patients. First, means of transportation is commonly a modifiable factor, along with albumin and anemia, in improving HRQOL in ESKD patients [16–20]. Beside the means of transportation, time of transportation was also recognized as a risk factor for worse HRQOL [7–9], and efforts to decrease transportation time were considered to be one possible way of improving HRQOL [8]. However, transportation time is difficult to modify, short of patients moving nearer to their dialysis facility. In contrast, means of transportation is modifiable. For example, replacing dependent transportation with self-driving or walking, bicycling or public transportation may be feasible, and would be expected to improve both MCS and PCS. This notion is partially supported by studies among general populations wherein ‘isotemporal substitution’, namely the replacement of an inactive behavior, such as sedentary behavior, with an active behavior for an equal time can lead to improvements in physical health [21], body weight loss [22] and a reduction in cardiovascular biomarkers [23]. In addition, the relevance of the means of transportation is reflected by the large proportion of patients in Group 1 (34.4%). Indeed, one survey estimated that one in six in-center dialysis patients in Japan used a private pickup transfer service regardless of the patient’s physical independence and transport distance [24]. On the one hand, a private transfer service would ensure a regular HD regimen and consequently reduce unplanned hospitalizations, particularly for patients living in rural areas with limited public transportation systems or with ADL difficulty [6]. On the other hand, given our present results it may be a time for both dialysis facilities and patients to reconsider private transfer services as a ‘double-edged sword’ for selected patients living in urban cities who have the option to visit dialysis facilities by transfer service versus self-driving or walking/bicycling within an acceptable (say, 0.5 h) period.
Second, the observed differences in mean PCS and MCS at 1 year of ∼2–3 points might be clinically relevant. The minimum clinically important difference, the smallest value considered to be worthwhile or important, in the 36-item Short Form Health Survey scales was said to be 3–5 points [25], and expected differences in PCS and MCS among our patients may range from 3 to 5 points, provided that the 2-year observation period is extended and the annual decreases in MCS and PCS remain constant. In addition, our cohort included only Japanese patients, who have generally shown better HRQOL than patients in other DOPPS countries [5], and those who could walk unaided. In this regard, the small difference in HRQOLs seen in our study with only 1 year’s observation is predictive.
This study has several limitations. First, the study cohort was limited to Japanese patients. Thus our results might not be generalizable to other countries, as means of transportation differ among countries, together with differences in health care service, health insurance and transportation system. However, given the emerging worldwide problem of aging societies and rapid increases in the number of elderly dependent dialysis patients, we believe that the means of transportation and potential health consequences presented in this study will serve as a useful reference for other countries expecting to face problems similar to those in Japan in the future. Second, in multivariable analysis, some socioeconomic factors were unavailable and thus not adjusted for. Social support is associated with both HRQOL [5, 26, 27] and selection of the means of transportation. Household income is associated with car ownership and the use of public transportation. Adjustment of these factors might therefore attenuate the strengths of the association between means of transportation and HRQOL. However, we believe that socioeconomic factors such as household income are not directly associated with HRQOL, but rather with ‘general’ QOL [28]. Third, the distance to the dialysis facility, one determinant of the means of transportation, is not considered. For patients living far from their dialysis facilities, switching from dependent transportation to transportation by bicycle or walking is not unrealistic, but is unlikely to occur. Fourth, our results might not be representative of total eligible patients in our study, as we excluded 45% of eligible patients due to missing data on transportation modality. Finally, further studies to better understand the causal relationship between transfer method and HRQOL are warranted, including the new-user approach, in which observation starts at the beginning of use of a particular type of transportation.
In conclusion, this study showed that independent travel to a dialysis facility was associated with significantly better PCS and MCS at 1 year compared with results in those who had someone to drive them. In addition to transportation time and distance, the means of transportation makes an important contribution to HRQOL. Dialysis staff should take these transportation-related issues into account to maintain HRQOL among dialysis patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the Arbor Research Collaborative for Health, Ann Arbor, MI, USA, for administering the J-DOPPS and express our appreciation for the support of Kyowa Hakko Kirin, without restrictions on publication. The DOPPS.org website lists the full details. The authors also thank the study nurses, physicians and medical directors for all the time and attention they devoted to our study.
CONFLICT OF INTEREST STATEMENT
M.Y., K.O., M.I., K.T. and Y.S. have no relevant conflicts of interest. N.K. has been a member of a biostatistics support group for the J-DOPPS program, which is supported by Kyowa Hakko Kirin.
REFERENCES
- 1. Verberne WR, Das-Gupta Z, Allegretti AS. et al. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group. Am J Kidney Dis 2019; 73: 372–384 [DOI] [PubMed] [Google Scholar]
- 2. Kalantar-Zadeh KK, Block G, Humphreys MH.. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol 2001; 12: 2797–2806 [DOI] [PubMed] [Google Scholar]
- 3. Lopes AA, Bragg-Gresham JL, Satayathum S. et al. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2003; 41: 605–615 [DOI] [PubMed] [Google Scholar]
- 4. Mapes DL, Bragg-Gresham JL, Bommer J. et al. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004; 44(Suppl 2): 54–60 [DOI] [PubMed] [Google Scholar]
- 5. Fukuhara S, Lopes AA, Bragg-Gresham JL. et al. Health-related quality of life among dialysis patients on three continents: the dialysis outcomes and practice patterns study. Kidney Int 2003; 64: 1903–1910 [DOI] [PubMed] [Google Scholar]
- 6. Park S, Kear TM.. Current state-of-practice: transportation for patients with end stage renal disease. Nephrol Nurs J 2017; 44: 309–315 [PubMed] [Google Scholar]
- 7. Meers C, Singer MA, Toffelmire EB. et al. Self-delivery of hemodialysis care: a therapy in itself. Am J Kidney Dis 1996; 27: 844–847 [DOI] [PubMed] [Google Scholar]
- 8. Moist LM, Bragg-Gresham JL, Pisoni RL. et al. Travel time to dialysis as a predictor of health-related quality of life, adherence, and mortality: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 51: 641–650 [DOI] [PubMed] [Google Scholar]
- 9. Tonelli M, Manns B, Culleton B. et al. Association between proximity to the attending nephrologist and mortality among patients receiving hemodialysis. CMAJ 2007; 177: 1039–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pisoni RL, Gillespie BW, Dickinson DM. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 2004; 44 (Suppl 2): 7–15 [DOI] [PubMed] [Google Scholar]
- 11. Katz S, Downs TD, Cash HR. et al. Progress in development of the index of ADL. Gerontologist 1970; 10: 20–30 [DOI] [PubMed] [Google Scholar]
- 12. Ware J Jr, Kosinski M, Keller SD.. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233 [DOI] [PubMed] [Google Scholar]
- 13. Wu TY, Chie WC, Liu JP. et al. Association of quality of life with laboratory measurements and lifestyle factors in community dwelling older people in Taiwan. Aging Ment Health 2015; 19: 548–559 [DOI] [PubMed] [Google Scholar]
- 14. Netuveli G, Wiggins RD, Hildon Z. et al. Quality of life at older ages: evidence from the English longitudinal study of aging (wave 1). J Epidemiol Community Health 2006; 60: 357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robinson BM, Zhang J, Morgenstern H. et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 2014; 85: 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kimmel PL, Patel SS.. Quality of life in patients with chronic kidney disease: focus on end-stage renal disease treated with hemodialysis. Semin Nephrol 2006; 26: 68–79 [DOI] [PubMed] [Google Scholar]
- 17. Giannaki CD, Hadjigeorgiou GM, Karatzaferi C. et al. Epidemiology, impact, and treatment options of restless legs syndrome in end-stage renal disease patients: an evidence-based review. Kidney Int 2014; 85: 1275–1282 [DOI] [PubMed] [Google Scholar]
- 18. Kang GW, Lee IH, Ahn KS. et al. Clinical and psychosocial factors predicting health-related quality of life in hemodialysis patients. Hemodial Int 2015; 19: 439–446 [DOI] [PubMed] [Google Scholar]
- 19. Broers NJ, Usvyat LA, Kooman JP. et al. Quality of life in dialysis patients: a retrospective cohort study. Nephron 2015; 130: 105–112 [DOI] [PubMed] [Google Scholar]
- 20. Lacson E Jr, Xu J, Lin SF. et al. Association between achievement of hemodialysis quality-of-care indicators and quality-of-life scores. Am J Kidney Dis 2009; 54: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 21. Buman MP, Hekler EB, Haskell WL. et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol 2010; 172: 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mekary RA, Willett WC, Hu FB. et al. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol 2009; 170: 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buman MP, Winkler EAH, Kurka JM. et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol 2014; 179: 323–334 [DOI] [PubMed] [Google Scholar]
- 24. Hinoshita F, Akiba T, Katsuki T. et al. Survey on the current situation of hemodialysis facilities for aging patients on hemodialysis. J Japan Soc Dial Ther 2015; 48: 341–350 [Google Scholar]
- 25. Hays RD, Woolley JM.. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics 2000; 18: 419–423 [DOI] [PubMed] [Google Scholar]
- 26. Plantinga LC, Fink NE, Harrington-Levey R. et al. Association of social support with outcomes in incident dialysis patients. Clin J Am Soc Nephrol 2010; 5: 1480–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kao TW, Lai MS, Tsai TJ. et al. Economic, social, and psychological factors associated with health-related quality of life of chronic hemodialysis patients in northern Taiwan: a multicenter study. Artif Organs 2009; 33: 61–68 [DOI] [PubMed] [Google Scholar]
- 28. Wilson IB, Cleary PD.. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995; 273: 59–65 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.