Abstract
Background
Snakebite is a common occupational hazard in tropical countries. To date, the literature on snakebite-related acute kidney injury (AKI) has been limited by retrospective study designs, lack of uniformity in case definitions of AKI and limited follow-up. This study aims to identify the in-hospital outcomes and long-term changes in kidney function that follow haemotoxic envenomation.
Methods
All adult patients admitted with AKI following haemotoxic envenomation from January 2016 to June 2017 were recruited and followed up until July 2018. Predictors of in-hospital mortality was assessed. Long-term follow-up data on kidney function were collected from survivors.
Results
In total, 184 patients with haemotoxic envenomation and AKI were recruited. The mean age of the subjects was 42.2 years [95% confidence interval (CI) 40.3–44.7]. The majority were male (71.2%). The mortality of patients with haemotoxic envenomation was 21.5%. The mortality was considerably higher in patients with Kidney Disease: Improving Global Outcomes (KDIGO) Stage 3 AKI [relative risk (RR) 4.45 (95% CI 1.14–17.42)] and those who met KDIGO urine output criteria [RR 20.45 (95% CI 2.84–147.23)]. A Cox regression model identified mechanical ventilation [odds ratio (OR) 5.59 (95% CI 2.90–10.81)], hypotension [OR 2.48 (95% CI 1.31–4.72)] and capillary leak syndrome [OR 2.02 (95% CI 1.05–3.88)] as independent predictors of mortality. Long-term follow-up data were available for 73 patients. A total of 21 patients (28.7%) developed adverse renal outcomes (glomerular filtration rate <60 mL/min/1.73 m2, urine albumin excretion >30 mg/g and new-onset hypertension or prehypertension).
Conclusions
AKI resulting from snake envenomation is associated with considerable risk of mortality. The greater the AKI stage the greater the likelihood of mortality. One-third of patients with AKI developed long-term complications like chronic kidney disease, prehypertension and hypertension over the follow-up period.
Keywords: acute kidney injury, capillary leak syndrome, long-term renal outcomes, prognosis, snake envenomation
INTRODUCTION
Snakebite is a common occupational hazard in southeast Asia. The World Health Organization estimates that snakebites account for ∼138 000 deaths per annum, while direct estimates have shown that envenomation accounts for 0.5% of all deaths in India each year [1]. Daboia russelii (Russell’s viper) and Echis carinatus (saw-scaled viper) are the predominant species responsible for haemotoxic envenomation in India. Envenomation is a common cause of community-acquired acute kidney injury (AKI) in tropical and subtropical areas, contributing to significant morbidity and mortality [2, 3]. Even though AKI is a recognized complication of snakebite, the existing data concerning outcomes of AKI resulting from haemotoxic envenomation have been limited by retrospective study designs, a lack of uniformity in case definitions of AKI and limited follow-up [4–8]. The recent Kidney Disease: Improving Global Outcomes (KDIGO) definition and staging criteria have facilitated the early diagnosis of AKI, but the prognostic utility of these classification schemas in snakebite-related AKI is not known. The majority of outcome data are limited to in-hospital outcomes. Capillary leak syndrome (CLS), characterized by generalized vascular leak and significant fluid accumulation in the third space, is a unique complication following D. russelii envenomation that contributes to mortality [9–11]. However, very few reports on the impact of CLS on outcomes have been published to date and there is limited literature regarding the predictors of progression to chronic kidney disease (CKD) following envenomation-related AKI [12, 13]. This study aims to identify the in-hospital outcomes and long-term changes in kidney function that follow haemotoxic envenomation.
MATERIALS AND METHODS
All adult patients admitted with AKI following haemotoxic envenomation from January 2016 to June 2017 were recruited. A diagnosis of snakebite was made from a clinical history of snakebite with the presence of characteristic fang marks. Snake species were identified as per patient description or by an expert (if the dead snake was brought to the hospital). Patients with a prior history of CKD [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, urine albumin/creatinine ratio > 30 mg/g, and abnormal urine sediment or contracted kidneys on ultrasound] were excluded. In patients without previous documented renal function, abdomen ultrasound was performed to assess kidney size prior to recruitment. Those with a bipolar length of kidneys <9 cm or evidence of stones, cysts or hydronephrosis were excluded. Informed consent was obtained from patients or a legally accepted relative for patients who were unable to give consent. All patients initially received 7–10 vials of polyvalent anti-snake venom [ASV; neutralizing Naja naja (cobra), E. carinatus, Bungarus caeruleus (common Krait), and D. russelii] on admission. Additional doses of 7–10 vials were given if the coagulopathy persisted beyond 6 h. Demographic, clinical and laboratory details were collected during hospital admission. The subjects were classified into two groups based on outcomes, survival and non-survival, and the characteristics were compared. A first follow-up visit was scheduled 2 weeks after discharge from the hospital. Subsequent follow-up visits were scheduled at 3 months, 6 months and a final visit in June–July 2018. Blood pressure, serum creatinine and urine albumin excretion were checked at follow-up visits. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation (2009). The protocol was approved by our institutional ethics committee (JIP/IEC/2015/21/751).
Case definitions
Systemic haemotoxicity was defined as a history of snakebite with any one of the following symptoms: new-onset bleeding tendencies following snakebite, a whole-blood clotting time >20 min or a platelet count <100 000 cells/mm3. AKI was defined according to the KDIGO criteria [14]. Disseminated intravascular coagulation was defined as a prolonged prothrombin time/international normalized ratio (>1.3) and platelet counts <100 000 cell/mm3, with the presence of bleeding. CLS was defined as the presence of chemosis, periorbital oedema and bilateral parotid swelling with any two of the following: systolic blood pressure <90 mmHg, increase in haematocrit by >20% from baseline, serum albumin <3.0 g/dL and evidence of fluid in the third space on imaging. Bite-to-needle time was defined as the time interval between snakebite and the administration of the first dose of ASV. Adverse renal outcomes were defined as the presence of any one of the following: eGFR <60 mL/min/1.73 m2, urine albumin creatinine ratio >30 mg/g, and new onset hypertension or prehypertension. Systemic hypertension was defined as systolic blood pressure >140/90 mmHg and prehypertension was defined as systolic blood pressure 120–139 mmHg and diastolic blood pressure 80–89 mmHg on two or more office visits.
Statistical analysis
All categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as mean with 95% confidence intervals or median with interquartile range (IQR) based on the normality of the data. All categorical variables were compared by chi-square test. All continuous variables were compared by Student’s t-test or Mann–Whitney U test according to the distribution. Mortality between groups was expressed as relative risk (RR) with confidence intervals (CIs). A Cox regression analysis was done to assess the independent predictors of in-hospital mortality. The changes in eGFR between groups over the follow-up period were compared by linear mixed models. A P-value <0.05 was taken as significant. The data was analysed using the statistical software SPSS version 19.0 (IBM, Armonk, NY, USA).
RESULTS
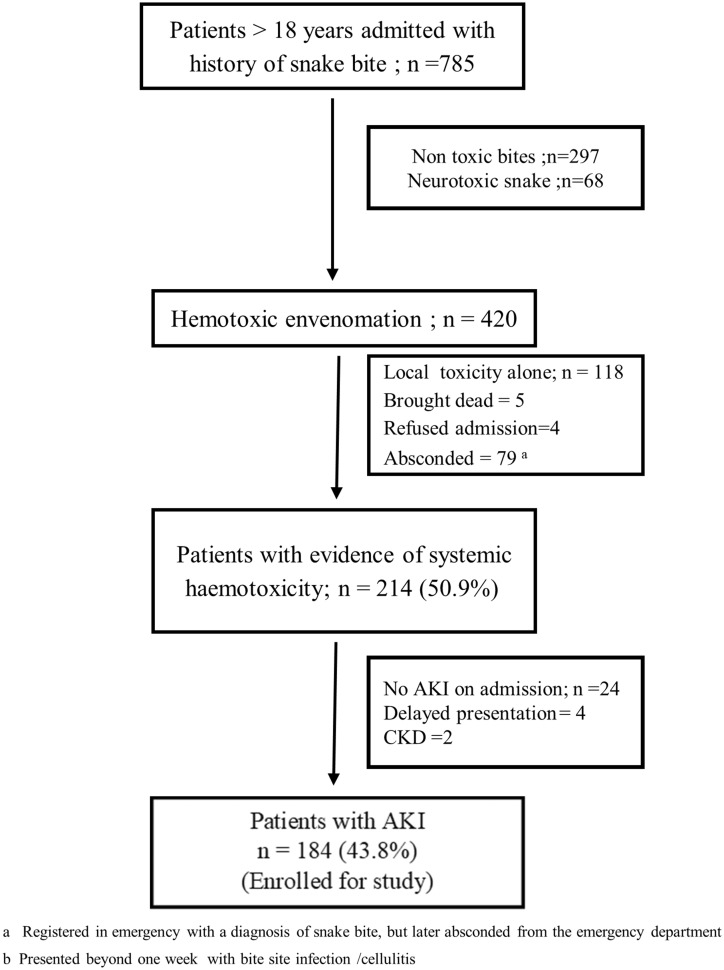
In total, 420 patients with haemotoxic snakebites were assessed over the study period. Of these, 214 (50.9%) had systemic haemotoxicity and 184 patients (43.8%) satisfied the KDIGO criteria for AKI (Figure 1). A total of 164 patients had AKI on admission [AKI 1−14 (7.6%), AKI 2–35 (19%) and AKI 3–115 (62.5%)] and 20 (10.8%) patients developed AKI within 48 h of admission. KDIGO urine output and creatinine criteria were met in 106 (57.6%) patients, while 45 (24.5%) patients met only creatinine criteria and 33 (17.9%) met only urine output criteria. Daboia russelii accounted for 22.2% (n = 41) of bites and E. carinata was responsible for 14.1% (n = 26). The species of snake was not identified in 117 patients (63.6%). Bite-to-needle time was ≤2 h in 113 (61.4%) patients, but was >4 h in 34 patients (18.5%). Comorbidities present in the population were systemic hypertension (n = 7) and diabetes mellitus (n = 2). Four patients had neurological manifestations in addition to haemotoxic manifestations. Eighteen patients (9.8%) had sought traditional remedies before seeking medical attention. CLS was present in 26 (14.1%) patients, among which 11 patients had hypotension requiring inotropic support, 12 needed mechanical ventilation and 21 needed dialysis.
FIGURE 1.
Flow chart showing patients admitted with snake envenomation during the study period.
All patients requiring renal replacement therapy were given haemodialysis or sustained low-efficiency dialysis. The mortality for patients with haemotoxic envenomation was 21.5% (n = 46/214; AKI = 44, no AKI = 2). Among the 44 AKI deaths, 19 (10.3%) occurred in the first 48 h, 12 (06.5%) occurred between 48 h and 7 days, 7 (3 .8%) occurred in the second week and 6 (3.2%) occurred >2 weeks after snakebite. The characteristics of survivors and non-survivors with AKI are given in Table 1. Mortality was considerably higher in patients with Stage 3 AKI and those who only met the KDIGO urine output criteria (Table 2). A Cox regression analysis identified the need for mechanical ventilation, hypotension and CLS as independent predictors of mortality (Table 3).
Table 1.
Demographic and clinical characteristics of survival and non-survival groups (n = 184)
| Parameter | Total (n = 184) | Survivors (n = 140) | Non-survivors (n = 44) | P |
|---|---|---|---|---|
| Age (years)a | 42.2 (40.3–44.1) | 42.3 (40.1–44.4) | 41.9 (37.5–46.3) | 0.888 |
| Male gender, n (%) | 131 (71.2) | 99 (70.7) | 32 (72.7) | 0.797 |
| Requirement of inotropes, n (%) | 32 (17.4) | 12 (8.6) | 20 (45.5) | 0.000 |
| Mechanical ventilation, n (%) | 33 (17.9) | 6 (4.3) | 27 (61.4) | 0.000 |
| Renal replacement therapy, n (%) | 114 (61.9) | 79 (56.4) | 35 (79.5) | 0.007 |
| CLS, n (%) | 26 (14.1) | 9 (6.4) | 17 (38.6) | 0.000 |
| Time from snakebite to ASV administration (h)b | 2.00 (1.5–4) | 2.00 (1.62–4.00) | 2.00 (1.00–4.00) | 0.461 |
| Total dose of ASV received (vials)b | 18.33 (16.8–19.9) | 17.6 (15.9–19.3) | 20.8 (17.2–24.3) | 0.081 |
| Duration of hospitalization (days)a | 12.3 (10.9–13.7) | 14.3 (12.6–15.6) | 6.8 (4.1–9.6) | 0.000 |
| Urine output (mL)b | 500 (200–1000) | 550 (200–1100) | 300 (200–575) | 0.033 |
| Urea (mg/dL)b | 70 (37.5–100) | 66 (36–95.2) | 81 (38–114) | 0.124 |
| Serum creatinine (mg/dL)a | 3.1 (2.7, 3.4) | 3.2 (2.4, 3.9) | 3 (2.6, 3.4) | 0.657 |
| Haemoglobin (g/dL)a | 11.9 (11.4–12.3) | 11.8 (11.3–12.3) | 11. 7 (10.8–12.9) | 0.971 |
| Total leucocyte count (cells/mm3)b | 16 280 (12 130–24 850) | 15 800 (11 925–22360) | 21905 (12 222–29 390) | 0.027 |
| Platelet count (cells/mm3)b | 45 000 (20 000–75 000) | 48 500 (21 000–77 250) | 30 140 (16 000–65 000) | 0.117 |
| Prothrombin time (s)b | 21.9 (16.9–38.8) | 21.05 (16.27–34.5) | 32.7 (21.7–44.0) | 0.002 |
| Total bilirubin (mg/dL)b | 1.25 (0.7–2.0) | 1.15 (0.70–1.97) | 1.3 (0.07–2.70) | 0.250 |
| Aspartate aminotransferase (IU/L)b | 101 (57–184) | 86 (51.25–145) | 180.5 (74.5–298) | 0.000 |
| Alanine aminotransferase (IU/L)b | 60 (32.25–93) | 49 (32–88.5) | 87 (43–119) | 0.008 |
| Alkaline phosphatise (IU/L)b | 149.5 (102–196) | 142 (98.5–193.5) | 179 (127.5–216) | 0.017 |
| Serum albumin (g/dL)a | 3.0 (2.9–3.1) | 3.1 (2.9–3.2) | 2.8 (2.4–3.1) | 0.025 |
Mean with CIs.
Median with IQR.
P< 0.05 taken as significant.
Table 2.
RR of mortality according KDIGO AKI stage and diagnostic criteria
| Haemotoxic envenomation (n = 214) | Death (n = 46) | Survival (n = 168) | RR (95% CI) |
|---|---|---|---|
| Haemotoxic snakebite without AKI (n = 30) | 2 | 28 | Reference |
| AKI Stage 1 (n = 22) | 0 | 22 | 0.27 (0.01–5.35) |
| AKI Stage 2 (n = 24) | 03 | 21 | 1.87 (0.34–10.34) |
| AKI Stage 3 (n = 138) | 41 | 97 | 4.45 (1.14–17.42) |
|
| |||
| Haemotoxic envenomation with AKI (n = 184) | Death (n = 44) | Survival (n = 168) | RR (95% CI) |
|
| |||
| Creatinine criteria (n = 45) | 1 | 44 | Reference |
| Urine output criteria (n = 33) | 15 | 18 | 20.45 (2.84–147.23) |
| Both (n = 106) | 28 | 78 | 11. 89 (1.67–84.72) |
Table 3.
Cox regression analysis for independent predictors of mortality (N = 184)
| Parameter | OR (95% CI) | P |
|---|---|---|
| Mechanical ventilation | 5.59 (2.90–10.81) | 0.000 |
| Hypotension | 2.48 (1.31–4.72) | 0.005 |
| CLS | 2.02 (1.05–3.88) | 0.036 |
| AKI Stage 3 | 1.83 (0.50–6.64) | 0.370 |
| Renal replacement therapy | 1.45 (0.64–3.25) | 0.362 |
Recovery and follow-up
Among survivors (n = 140), at the time of discharge 44 patients (31.4%) had an eGFR level >60 mL/min/1.73 m2. Among the 69 patients who attended their first follow-up appointments scheduled ∼2 weeks post-discharge, 23 patients had eGFR values <60 mL/min/1.73 m2 (33.3%).
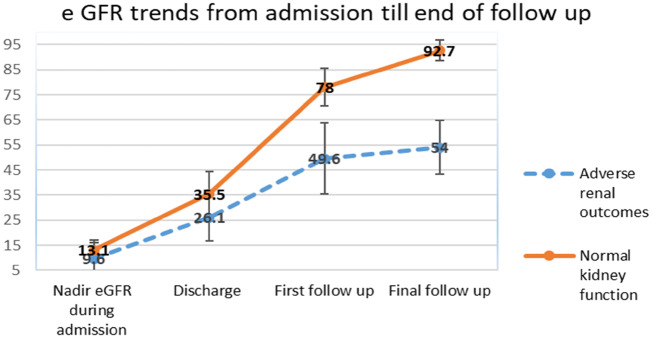
Follow-up data beyond 3 months were available for 73 patients. Median follow-up duration was 15.5 months (IQR 6–21). Two patients had hypertension and one had diabetes mellitus. Mean eGFR at the end of follow-up was 80.9 mL/min/1.73 m2 (95% CI 74.8–87). A total of 21 patients (28.7%) developed adverse renal outcomes (Table 4). Among the 14 patients with an eGFR <60 mL/min/1.73 m2, 2 were CKD Stage 4, 5 were CKD Stage 3b and 6 patients were CKD Stage 3a. One patient did not recover from the AKI and remained dialysis-dependent; renal biopsy showed thrombotic microangiopathy. Kidney biopsy was performed in two more patients with CKD, which showed chronic thrombotic microangiopathy in one and persistent acute tubular necrosis in the other. The characteristics of patients who developed adverse renal outcomes are shown in Table 5. Patients with adverse renal outcomes had lower GFRs from the point of hospitalization (P ≤ 0.001, Figure 2).
Table 4.
Adverse renal outcomes on follow-up (n = 73)
| Patients with adverse renal outcomesa | 21 (28.7) |
| GFR <60 mL/min/1.73 m2 | 14 (19.2 %) |
| Urine albumin/creatinine ratio >30 mg/g | 5 (06.8%) |
| Systemic hypertension | 4 (05.5%) |
| Prehypertension | 1 (01.4%) |
Some patients have more than one adverse renal outcomes. Total numbers might exceed 21.
Table 5.
Characteristics at the time of admission for patients with and without adverse renal outcomes
| Parameter | Adverse renal outcomes (n = 21) | Normal kidney function (n = 52) | P |
|---|---|---|---|
| Age (years) | 48.5 (44.8–53.1) | 39.3 (36.08–42.42) | 0.002 |
| Haemoglobin at admission (g/dL)a | 10.5 (8.7–12.1) | 12.3 (11.4–13.2) | 0.034 |
| Stage 3 AKI, n (%) | 17 (81.0) | 44 (84.6) | 0.734 |
| Need for dialysis, n (%) | 16 (76.2) | 37 (71.2) | 0.777 |
| Bite-to-needle time (h)b | 02 (01–3) | 02 (01–04) | 0.621 |
| Total ASV received (vials)b | 14 (10–23) | 16 (10–23) | 0.807 |
| Serum creatinine (at admission) (mg/dL)b | 3.7 (2.0–4.5) | 2.9 (1.1–4.4) | 0.158 |
| Serum albumin (at admission) (g/dL)a | 3.2 (2.9–3.4) | 3.0 (2.9–3.2) | 0.397 |
| Duration of hospitalization (days)b | 19 (9.5–28) | 12.5 (9–18) | 0.040 |
| eGFR 2 weeks post-dischargec | 49.6 (35.6–63.6) | 78 (70.1–85.6) | 0.001 |
Mean with CIs.
Median with IQR.
The median duration was 30 days (IQR 24–35) since the envenomation.
FIGURE 2.
Trends in eGFR from admission until the end of the follow-up period.
DISCUSSION
Snake envenomation is a common cause of AKI in tropical countries and predominantly affects young individuals engaged in agriculture-related activities. In this study, we observed that 44% of patients with poisonous snakebites developed AKI. The reported prevalence rates of AKI following haemotoxic envenomation vary from 14 to 44% [4–8, 15]. Relatively higher AKI prevalence rates have been reported by studies that have employed both urine output and creatinine-based criteria for the diagnosis of AKI, compared with studies that have employed creatinine-based criteria alone [5, 8, 16].
The bite-to-needle time is considered to be the most important determinant of AKI following envenomation. The venom should be neutralized as early as possible to prevent complications and mortality; however, the optimum bite-to-needle time to prevent complications has not yet been defined. Dharod et al. observed a mean bite-to-needle time of 7.6 h in patients with AKI as opposed to 20 h in those who did not develop AKI [15], while Athappan et al. reported that a bite-to-needle time >2 h is associated with a higher chance of kidney failure [17]. On the other hand, Paul and Dasgupta reported much shorter bite-to-needle times (66 min) in patients who developed AKI [18]. These reported variations might be secondary to the varied definitions of AKI as well the effect of ease of access to health care. In this study, 42/44 patients who succumbed received ASV within 2 h of sustaining the bite. We did not find any evidence that early administration of ASV protected patients from AKI or mortality.
One-half of the deaths in this study happened in the initial 48 h after snakebite, and resulted from cardiorespiratory failure and CLS. The contribution of CLS towards mortality in snakebite is often overlooked. CLS commonly occurs following D. russelii envenomation and results from widespread endothelial injury. The pathogenesis of CLS is poorly understood. One mechanism that has been proposed is endothelial apoptosis secondary to vascular apoptosis-inducing proteins (VAP 1 and VAP 2) and l-amino oxidase present in the snake venom. Other suggested mechanisms include phospholipase A2-mediated activation of cytokines and direct vascular toxicity by zinc metalloproteinases present in the venom [11]. CLS is associated with increased risk of hypotension, respiratory failure and death [9–11]. It is believed that ASV available in India is ineffective in preventing CLS [11]. The features of CLS start to appear 12–24 h after envenomation, with the development of the full-blown syndrome by 48–72 h. There is high variability in the reported prevalence and outcomes of CLS. It is believed that CLS resulting from envenomation is subject to significant regional variations. The majority of CLS cases following envenomation are reported in the state of Kerala in Southern India [11] and the reported mortality rates range from 43 to 67%. A lack of uniformity in case definitions and non-recognition of milder variants of CLS might be responsible for these differences. Moreover, the presence of elevated haematocrit, a cardinal feature of CLS, might be masked due to the presence of venom-induced haemolysis.
The current polyvalent ASV available in India neutralizes the venom of N. naja, D. russelii, E. carinatus and B. caeruleus. There is increasing evidence that other viper species, like hump-nosed pit vipers (Hypnale hypnale), might be responsible for lethal envenomations in southeast Asia [11, 19]. Pit vipers can be easily mistaken for saw-scaled vipers and were reported to account for one-third of envenomations in a case series from Sri Lanka [20]. Minimal data on the prevalence of AKI and mortality following envenomation by pit vipers are available from India. A study from Kerala, India reported a high incidence of kidney failure among patients who sustained pit viper bites [21]. On the other hand, a study from Brazil reported a lower prevalence of AKI (15%) and no mortality following envenomation predominantly by Bothrops species, which represent a type of pit viper [22]. It should be recalled that, in this study, species identification was not possible in more than two-thirds of all cases. To date, no published data are available regarding the diversity of the reptile population in the geographical area where the study was conducted. Another potential contributory factor might be regional variations in snake venom observed across different parts of India, which might influence the toxicity profile as well as the neutralizing capacity of ASV [23].
AKI is an established risk factor for the development of CKD in the long-term and limited data exist regarding the long-term outcomes of patients who develop AKI following envenomation. We observed that one-third of our patients developed adverse renal outcomes on long-term follow-up. Herath et al. [13] reported that 37% of patients who sustain AKI following envenomation develop CKD by the end of 1 year. However, the patients were a decade older than the participants in our study, and the majority had comorbidities like hypertension and diabetes, which are independent risk factors for CKD. Waikhom et al. [12] reported that 41% of patients who sustain envenomation develop persistent renal abnormalities in the long-term. The patients who developed adverse renal outcomes in this study were older and had a lower GFR at the time of hospital discharge. Advanced age and severe renal failure are established risk factors for CKD progression [24], However, in our study, we did not observe any differences in the dialysis requirements or severity of AKI among patients who developed adverse renal outcomes. This might be due to the considerable attrition on follow-up. Most of the Stage 1 and 2 AKI cases were lost to follow-up, resulting in selective inclusion of patients with more severe renal failure.
In this study, the severity of the envenomation did not appear to be a major determinant of future adverse renal events. There was no relationship between ASV dosage, bite-to-needle time, or serum albumin levels and long-term renal damage. Similar findings were reported by Waikhom et al. [12]. On the other hand, it appeared that a longer duration of renal failure was associated with a higher risk of CKD. Patients who developed adverse renal outcomes had lower nadir eGFRs as well as longer recovery times. The tendency to have a lower GFR was evident at the time of discharge as well as at the first follow-up appointment. In addition to the severity of AKI, the duration of AKI is also an important prognostic determinant of long-term outcome [25, 26]. Early recovery from AKI, especially with in first 7 days, is reported to be an associated with better long-term prognosis [27]. Lower haemoglobin levels in patients who develop adverse renal outcomes might be secondary to the venom-induced haemolysis.
To the best of our knowledge, this study is the largest series on AKI following envenomation to date. The data were collected prospectively from the time of hospitalization. The limitations of the study include considerable loss of follow-up, especially for patients with milder degrees of AKI. Follow-up data were available for only half of the patients who survived envenomation. As most of the patients were manual labourers, logistical issues stood in the way of periodic follow-up. Even though the study protocol included a follow-up visit at 3 and 6 months, the majority of the patients could not attend their scheduled appointments. Baseline eGFRs taken prior to the illness were not available for any of the recruited patients. In a developing country like India, apparently healthy individuals, especially belonging to low-income groups, seldom undergo any periodic health check-ups. Abdomen ultrasound was performed for all patients to rule out any abnormalities in kidney size. However, it is still possible that some of the patients had milder renal dysfunction with normal kidney size.
CONCLUSIONS
AKI resulting from snake envenomation is accompanied by considerable risk of mortality. The greater the stage number of AKI, the poorer the outcome. The presence of CLS, hypotension and respiratory failure are independent predictors of a mortality. One-third of patients with AKI develop long-term complications like CKD, prehypertension and hypertension on follow-up. Early recovery from AKI is associated with better preservation of GFR in the long-term.
FUNDING
The work was funded by Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry 605006, India.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.World Health Organization. Snakebite envenoming http://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming. (14 November 2018, date last accessed)
- 2. Mohapatra B, Warrell DA, Suraweera W. et al. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis 2011; 5: e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burdmann EA, Jha V.. Acute kidney injury due to tropical infectious diseases and animal venoms. A tale of 2 continents. Kidney Int 2017; 91: 1033–1046 [DOI] [PubMed] [Google Scholar]
- 4. Vikrant S, Jaryal A, Parashar A.. Clinicopathological spectrum of snake bite-induced acute kidney injury from India. World J Nephrol 2017; 6: 150–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukhopadhyay P, Mishra R, Mukherjee D.. Snakebite mediated acute kidney injury, prognostic predictors, oxidative and carbonyl stress: a prospective study. Indian J Nephrol 2016; 26: 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harshavardhan L, Lokesh AJ, Tejeshwari HL. et al. A study on the acute kidney injury in snake bite victims in a tertiary care centre. J Clin Diagn Res 2013; 7: 853–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pulimaddi R, Parveda AR, Brahmanpally B. et al. Incidence & prognosis of acute kidney injury in individuals of snakebite in a tertiary care hospital in India. Indian J Med Res 2017; 146: 754–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh RR, Uraiya D, Kumar A.. Early demographic and clinical predictors of developing acute kidney injury in snake bite patients: A retrospective controlled study from an Indian tertiary care hospital in North Eastern Uttar Pradesh India. Indian J Crit Care Med 2016; 20: 404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kendre PP, Jose MP, Varghese AM. et al. Capillary leak syndrome in Daboia russelii bite-a complication associated with poor outcome. Trans R Soc Trop Med Hyg 2018; 112: 88–93 [DOI] [PubMed] [Google Scholar]
- 10. Jayakrishnan MP, Geeta MG, Krishnakumar P.. Snake bite mortality in children: beyond bite to needle time. Arch Dis Child 2017; 102: 445–449 [DOI] [PubMed] [Google Scholar]
- 11. Udayabhaskaran V, Thomas ET, Shaji B.. Capillary leak syndrome following snake bite envenomation. Indian J Crit Care Med 2017; 21: 698–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waikhom R, Sircar D, Patil K.. Long-term renal outcome of snake bite and acute kidney injury: a single-center experience. Ren Fail 2012; 34: 271–274 [DOI] [PubMed] [Google Scholar]
- 13. Herath HM, Wazil AW, Abeysekara DT.. Chronic kidney disease in snake envenomed patients with acute kidney injury in Sri Lanka: a descriptive study. Postgrad Med J 2012; 88: 138–142 [DOI] [PubMed] [Google Scholar]
- 14.KDIGO. KDIGO Clinical Practice Guideline for Acute Kidney Injury . Kidney Int Suppl 2012; 2: 19–36 [Google Scholar]
- 15. Dharod MV, Patil TB, Deshpande AS.. Clinical predictors of acute kidney injury following snake bite envenomation. N Am J Med Sci 2013; 5: 594–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aye KP, Thanachartwet V, Soe C.. Clinical and laboratory parameters associated with acute kidney injury in patients with snakebite envenomation: a prospective observational study from Myanmar. BMC Nephrol 2017; 18: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Athappan G, Balaji MV, Navaneethan U. et al. Acute renal failure in snake envenomation: a large prospective study. Saudi J Kidney Dis Transpl 2008; 19: 404–410 [PubMed] [Google Scholar]
- 18. Paul J, Dasgupta S.. Early prediction of acute kidney injury by clinical features of snakebite patients at the time of hospital admission. N Am J Med Sci 2012; 4: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simpson ID, Norris RL.. Snakes of medical importance in India: is the concept of the “Big 4” still relevant and useful? Wilderness Environ Med 2007; 18: 2–9 [DOI] [PubMed] [Google Scholar]
- 20. Ariaratnam CA, Thuraisingam V, Kularatne SA. et al. Frequent and potentially fatal envenoming by hump-nosed pit vipers (Hypnale hypnale and H. nepa) in Sri Lanka: lack of effective antivenom. Trans R Soc Trop Med Hyg 2008; 102: 1120–1126 [DOI] [PubMed] [Google Scholar]
- 21. Arun Thomas ET, Bhagya S, Udayabhaskaran V. et al. Is the concept of “big 4” still relevant in India? Comparison of envenomation by hump-nosed pit viper with Russell’s viper. Eur J Pharm Med Res 2018; 3: 306–310 [Google Scholar]
- 22. Albuquerque PLMM, Silva Junior GB, Jacinto CN. et al. Acute kidney injury after snakebite accident treated in a Brazilian tertiary care centre. Nephrology (Carlton) 2014; 19: 764–770 [DOI] [PubMed] [Google Scholar]
- 23. Sharma M, Gogoi N, Dhananjaya BL.. Geographical variation of Indian Russell’s viper venom and neutralization of its coagulopathy by polyvalent antivenom. Toxin Rev 2014; 33: 7–15 [Google Scholar]
- 24. Lo LJ, Go AS, Chertow GM. et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 2009; 76: 893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horne KL, Packington R, Monaghan J.. The effects of acute kidney injury on long-term renal function and proteinuria in a general hospitalised population. Nephron Clin Pract 2014; 128: 192–200 [DOI] [PubMed] [Google Scholar]
- 26. Mehta S, Chauhan K, Patel A. et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol 2018; 19: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ronco C, Ferrari F, Ricci Z.. Recovery after acute kidney injury: a new prognostic dimension of the syndrome. Am J Respir Crit Care Med 2017; 195: 711–714 [DOI] [PubMed] [Google Scholar]