Abstract
Biotin (vitamin B7) is a dietary supplement that can lead to falsely abnormal endocrine function tests. The impact of biotin on both 25-hydroxyvitamin D [25(OH)D] and intact parathyroid hormone (iPTH) have not been previously described in end-stage renal disease (ESRD). A woman with ESRD on hemodialysis taking biotin 10 mg daily had a 25(OH)D spike from 25 to >100 ng/mL and an iPTH decrease from 966 to 63 pg/mL. After discontinuation of biotin, her 25(OH)D and iPTH returned to baseline. Biotin can cause erroneous 25(OH)D and iPTH results in ESRD that could adversely affect patient care.
Keywords: biotin, dialysis, end-stage renal disease, parathyroid hormone, 25-hydroxyvitamin D
BACKGROUND
Biotin (vitamin B7) is a water-soluble vitamin found in multivitamins and dietary supplements that are marketed for hair, skin and nail growth. Over the last few years there has been increasing awareness regarding the potential impact of biotin on the validity of hormone immunoassays. The Food and Drug Administration issued a safety communication that biotin can cause falsely high or low test results, leading to misdiagnosis and incorrect patient management [1]. Recommendations on when labs should be drawn in relation to the consumption of biotin in the end-stage renal disease (ESRD) patient population are lacking, as current pharmacokinetic studies do not include this population [2]. We present a case of erroneous 25-hydroxyvitamin D [25(OH)D] and intact parathyroid hormone (iPTH) results due to biotin interference in an ESRD patient on maintenance dialysis.
CASE REPORT
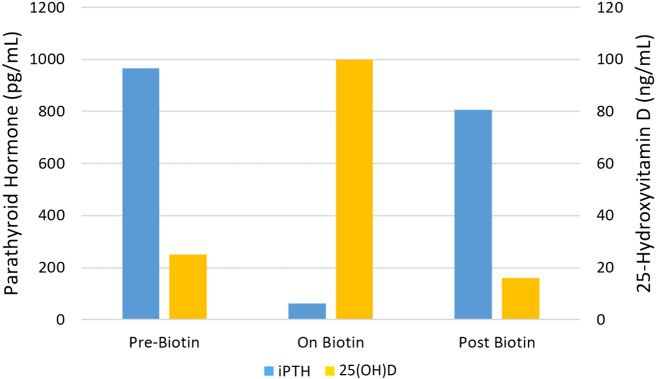
A 58-year-old female with ESRD on maintenance hemodialysis was being routinely managed for renal osteodystrophy when a marked decrease in her serum iPTH from 966 to 63 pg/mL (reference range 15–65 pg/mL) and a significant increase in her 25(OH)D level from 25 to >100 ng/mL (reference range 29–100 ng/mL) was observed within a 6-month time period. Her alkaline phosphatase was 148 U/L (reference range 35–104 U/L). Her serum calcium levels ranged from 7.9 to 8.9 mg/dL and phosphorous levels ranged from 3.7 to 5.4 mg/dL. She was on a stable medical regimen that included calcium acetate 667 mg, three tabs orally with meals, calcium carbonate 1000 mg between meals and calcitriol 0.5 μg on dialysis days and 0.25 μg on nondialysis days with no additional 25(OH)D supplementation prescribed.
Given the concern for oversuppression of her iPTH and risk for adynamic bone disease, combined with the discrepancy in her unusually high 25(OH)D levels, a detailed review of her use of over-the-counter (OTC) medications was conducted. The patient reported taking OTC biotin supplement 10 mg daily, and recognition of its impact on endocrine function tests brought into question the validity of the reported levels. A serum biotin level was noted to be elevated at 5.05 ng/mL (reference range 0.05–0.83 ng/mL), although it was drawn 1 week after discontinuation of the supplement. Our center uses an immunoassay with a streptavidin–biotin detection system (Roche Cobas 8000 immunoassay analyzer) for both 25(OH)D and iPTH, with the manufacturer recommendation that patients have an 8-h biotin washout period before blood samples are taken to prevent interference. No medication changes were made and 7 weeks after stopping biotin, her iPTH level was found to be substantially higher (806 pg/mL) and 25(OH)D was lower (16 ng/mL) compared with levels while taking OTC biotin (see Figure 1).
FIGURE 1.
iPTH and 25(OH)D levels by biotin use.
DISCUSSION
This case highlights the need for increased awareness of biotin as a potential source of falsely increased or decreased endocrine function tests in order to properly manage bone and mineral disorders in the ESRD population. There are multiple published reports on biotin interference of endocrine function test immunoassays with streptavidin–biotin detection systems, leading to incorrect biochemical diagnoses [3]. Li et al. [4] assessed the performance of biotinylated immunoassays in six healthy adults taking biotin 10 mg/day for 7 days and demonstrated assay interference [falsely high 25(OH)D and falsely low iPTH] in some but not all assays. Meany et al. [5] reported a falsely low iPTH level in an ESRD patient treated with biotin 10 mg/day. To our knowledge, there are no published reports on the effect of biotin supplements on both 25(OH)D and iPTH assay results in ESRD.
In a pharmacokinetic study, Grimsey et al. [2] showed in vitro that serum biotin levels <30 ng/mL did not interfere with immunoassays. For biotin regimens up to 1000 μg/day (as found in daily multivitamins such as Nephrocaps, which contain 150 μg), the serum levels fell below the threshold within 2 h, suggesting minimal interference, if any, at these doses. At higher biotin doses of 5000–10 000 μg/day, the serum levels were below this threshold after 8 h of drug discontinuation, supporting an 8-h washout period. However, patients with renal insufficiency were not included in this study. Although our patient was below the reported interference threshold, the level was still 5 times our labs’ reference range after hemodialysis and 1 week after discontinuation. Therefore patients on maintenance hemodialysis may need an extended washout period for higher doses.
Our case illustrates the importance of inquiring about biotin supplement use among ESRD patients and holding exogenous biotin before performing 25(OH)D and iPTH immunoassays. The popularity and increasing use of biotin as a supplement could be a significant contributor to misdiagnosis and erroneous management of bone and mineral disorders in the ESRD population.
ACKNOWLEDGEMENTS
The views expressed in this article are those of the authors and do not reflect the official policy of the US Army, Navy and Air Force, Department of Defense or US government.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.U.S. Food and Drug Administration. The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication, 2017. https://www.fda.gov/medical-devices/safety-communications/fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication (5 August 2019, date last accessed)
- 2. Grimsey P, Frey N, Bendig G. et al. Population pharmacokinetics of exogenous biotin and the relationship between biotin serum levels and in vitro immunoassay interference. Int J Pharmacokinet 2017; 2: 247–256 [Google Scholar]
- 3. Piketty ML, Polak M, Flechtner I. et al. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med 2017; 55: 780–788 [DOI] [PubMed] [Google Scholar]
- 4. Li D, Radulescu A, Shrestha RT. et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA 2017; 318: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meany DL, Jan de Beur SM, Bill MJ. et al. A case of renal osteodystrophy with unexpected serum intact parathyroid hormone concentrations. Clin Chem 2009; 55: 1737–1739 [DOI] [PubMed] [Google Scholar]