Abstract
In the 2017 Annual Report of the ERA-EDTA Registry, hypertension continues to be the second or third most common cause of renal replacement therapy (RRT) in Europe, tied with glomerulonephritis. There is, however, one little issue: hypertension-induced end-stage renal disease (ESRD) might not exist at all as currently understood, that is, as hypertensive nephrosclerosis. In this regard, the incidence of RRT due to hypertensive nephropathy is related to the incidence of other causes of ESRD but not to the burden of hypertension per country. The current definition of hypertensive nephropathy is non-specific, outdated and only allows a delayed diagnosis by exclusion. It is not helpful that 80% of chronic kidney disease patients develop hypertension and kidney biopsy has no findings specific for hypertensive nephropathy. There is an urgent need to redefine the concept of hypertensive nephropathy with a clear and comprehensive set of criteria that at least should indicate how other nephropathies, including familial nephropathies, should be excluded. Correct causality assessment and aetiology-based therapy is a key to the progress of nephrology and it should no longer be accepted that ‘hypertensive nephropathy’ serves to disguise a suboptimal diagnostic workup. A diagnosis of nephropathy of unknown cause would be more honest when the full range of alternative aetiological diagnoses is not explored.
Keywords: Alport, autosomal dominant tubulointerstitial disease, cause, glomerulonephritis, hypertension, nephrosclerosis, registry, renal replacement therapy
HYPERTENSIVE KIDNEY DISEASE AS THE SECOND MOST COMMON NEPHROPATHY REQUIRING RRT: CAN THIS STATEMENT BE MAINTAINED IN THE 21ST CENTURY?
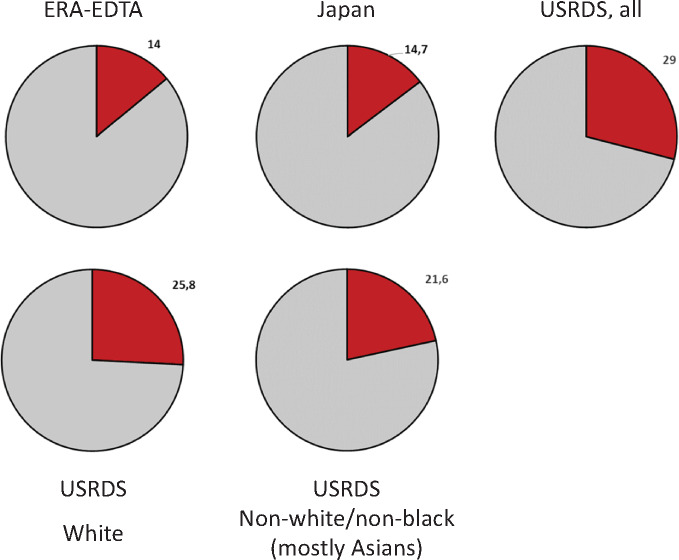
This issue of Clinical Kidney Journal contains the summary of the 2017 Annual Report of the ERA-EDTA Registry [1]. In recent years, hypertension has been the second or third most common cause of renal replacement therapy (RRT) in Europe, tied with glomerulonephritis [1–3]. Hypertensive nephrosclerosis is also the second most frequent cause of RRT in the USA and the third in Japan [4, 5] (Figure 1). However, hypertensive nephrosclerosis remains a diagnosis of exclusion [6], which, in practical terms, means that the lower the quality of the aetiologic diagnostic workup, the higher the chances of being diagnosed as hypertensive nephrosclerosis. This is contrary to the spirit of aetiological diagnosis. Additionally, since the two key diagnostic requirements are hypertension and chronic kidney disease (CKD) and >80% of CKD patients develop hypertension, CKD patients with hypertension will fulfil diagnostic criteria for hypertensive nephropathy, especially when no diagnostic workup is made. This means that a diagnosis of hypertensive nephropathy essentially means CKD of unknown origin in a patient with hypertension, thus potentially relegating a diagnosis of CKD of unknown origin to the scarce CKD patients that do not have hypertension. The fact that we can escape so easily from recognizing that we do not know what caused CKD in the patient sitting in front of us will contribute to delay progress in aetiological diagnosis and personalized medicine in nephrology.
FIGURE 1.
Percentage of incident patients starting RRT because of hypertensive nephropathy in the 2017 ERA-EDTA, Japanese and US Renal Data System registries [4–6]. Note the more frequent diagnosis of hypertensive nephropathy in the USA. This more frequent diagnosis is only partially accounted for by African-American carriers of the APOL1 risk variant, as the frequency of hypertensive nephropathy is also higher in US whites and others (mostly Asians) than in Europe and Japan. Results presented as percentages.
THE CONTRIBUTION OF HYPERTENSIVE NEPHROPATHY TO RRT IS NOT RELATED TO THE BURDEN OF HYPERTENSION
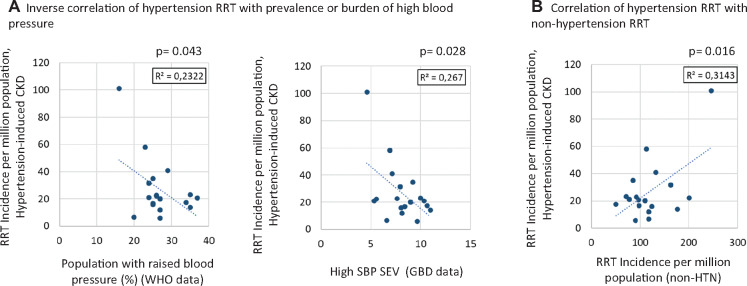
If indeed, hypertension was such an aetiological contributor to CKD requiring RRT, we would expect a relationship between the burden of hypertension in different countries and the contribution of hypertensive nephropathy to RRT in the same country. However, when plotting burden of hypertension, as estimated by the World Health Organization (WHO) [7] or the Global Burden of Disease (GBD) Study [8] against hypertensive nephropathy as a cause of RRT in the USA and Europe, no positive relationship is found. Indeed, there was an inverse relationship!! Thus countries with a higher percentage of the population with elevated blood pressure according to the WHO or higher systolic blood pressure (SBP) summary exposure values according to the GBD had a lower impact of hypertensive nephropathy on RRT (Figure 2A). In contrast, there was a direct relationship between the incidence of hypertensive nephropathy and the incidence of other nephropathies needing RRT (Figure 2B). This epidemiological evidence questions the aetiological role of hypertension in nephropathy ascribed to hypertension and suggests that in countries with a higher incidence of RRT, a fixed percentage of those patients are (randomly?) diagnosed as hypertensive nephropathy. There are, however, several potential modifiers that should be considered, such as the competing risk of cardiovascular death, differences in access to quality healthcare or even RRT entry criteria. Thus the higher negative impact of hypertension despite its lower prevalence in the USA than in Europe may relate to compromised access to hypertension care and treatments in the USA. However, there is further evidence supporting that the current concept of hypertensive nephropathy should be thoroughly revised.
FIGURE 2.
Relationship between different measures of hypertension burden in the community and the diagnosis of hypertensive nephropathy in incident RRT patients in Europe and the USA. (A) There was an inverse correlation between measures of hypertension burden and hypertensive nephropathy in incident RRT patients in the ERA-EDTA and US Renal Data System registries [5, 9]. Data were obtained from the WHO for the population with elevated blood pressure (BP) [7] and from the GBD study for high systolic BP summary exposure values [8]. (B) In contrast, there was a direct relationship between the incidence of hypertensive nephropathy and the incidence of other (non-hypertensive: non-HTN) nephropathies needing RRT, suggesting that patients on RRT are being diagnosed as hypertensive nephropathy in a random manner. Results shown are for Pearson correlation (r and P values). Represented countries are those with information available for 2017 in the ERA-EDTA Registry and US data in the 2019 report [5, 9].
THERE IS A HUGE VARIABILITY IN THE PERCENTAGE OF RRT PATIENTS DIAGNOSED AS HYPERTENSIVE NEPHROPATHY
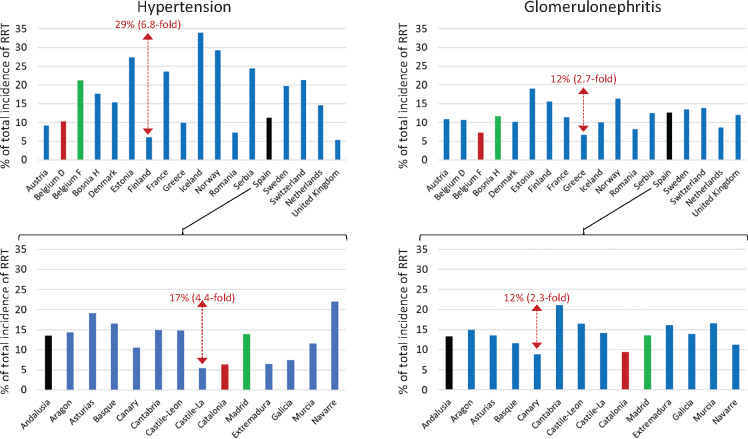
A further aspect that questions whether hypertensive nephropathy is being diagnosed in the same manner by different nephrologists is the high variability of the diagnosis of hypertensive nephropathy among patients starting RRT in different countries (Figure 3). There was an almost 7-fold difference in the contribution of hypertensive nephropathy to RRT in different European countries and large differences exist even between different regions (>4-fold) of the same country. In contrast, the difference for a nephropathy with better established diagnostic criteria, such as glomerulonephritis, was <3-fold between European countries or regions. Some surprising data emerge for populations with similar environmental, cultural and genetic backgrounds. For example, the percentage contribution of hypertensive nephropathy to RRT was more than double in one Belgium region than in another, and this was also the case for two of the most populated Spanish regions (Madrid and Catalonia). These data clearly suggest that in different countries or regions, different sets of patients are diagnosed as having hypertensive nephropathy.
FIGURE 3.
Frequency of hypertensive nephropathy and glomerulonephritis as a cause for RRT in diverse European countries and regions. Data from the 2017 ERA-EDTA Registry ([9]: Table B.2.6 Incidence per million population by primary renal disease, adjusted at Day 1 and adjusted for age and sex). Hypertension and glomerulonephritis vie for the second position as a cause for RRT in Europe. Note a 6.8-fold difference in the frequency of hypertensive nephropathy as a cause of RRT in different countries (in absolute values, 29 percentage points, when in some countries hypertension accounts for just 6% of patients on dialysis). In contrast, the largest difference in glomerulonephritis as a cause of RRT is 2.7-fold (a difference of 12 percentage points). These differences are also observed within countries. In Spain, there was a 4.4-fold difference in the frequency of hypertensive nephropathy (17 percentage points) between regions (e.g. it was more than double in Madrid than in Catalonia, both identified as colored bars, as is Andalucía, which completes the trio of more populated regions), while the difference for glomerulonephritis was 2.3-fold (12 percentage points). A regional government official in the Madrid Registry told one of the authors that he would change the underlying nephropathy to hypertensive nephropathy in centres reporting too many unknown causes, as ‘we all know that hypertension is the second most common cause of ESRD’, thus supporting a self-fulfilling prophecy. Belgium French speaking and Belgium Dutch speaking are clearly identified by different colours, as in French-speaking Belgium, hypertensive nephropathy is 2-fold more frequent that in Dutch-speaking Belgium, while this is not the case for glomerulonephritis. The most likely explanation is that a different concept of hypertensive nephropathy exists in the two regions.
THE CURRENT CONCEPT OF HYPERTENSIVE NEPHROSCLEROSIS IS OUTDATED
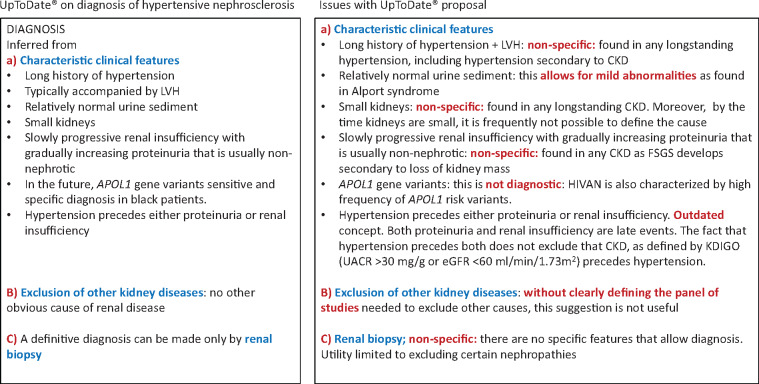
If there is epidemiological evidence of a disconnect between the burden of hypertension and the incidence of RRT due to hypertension, could this be explained by the criteria used to diagnose hypertensive nephropathy? As indicated above, the diagnosis of hypertensive nephropathy remains one of exclusion and, furthermore, the diagnostic criteria are non-specific [6] and have been rendered obsolete since the publication of the consensus criteria to diagnose CKD by the Kidney Disease: Improving Global Outcomes (KDIGO) organization [10] (Figure 4). Thus the diagnostic criteria in some popular textbooks such as UpToDate should be viewed with the 21st century prism of the KDIGO and through this prism they do not make the same sense they may have made in the 20th century [6]. UpToDate states that the diagnosis of hypertensive nephropathy is based on characteristic clinical features, exclusion of other kidney diseases and eventually on kidney biopsy features [6].
FIGURE 4.
Current hypertensive nephrosclerosis concept as per UpToDate and issues with the concept [6]. LVH: left ventricular hypertrophy; HIVAN: human immunodeficiency virus-associated nephropathy; UACR: urine albumin:creatinine ratio; FSGS: focal segmental glomerulosclerosis.
However, the characteristic clinical features are totally non-specific and may be found in any form of CKD: long history of hypertension, left ventricular hypertrophy, small kidneys, relatively normal urine sediment (the addition of the term ‘relatively’ opens the door to diagnosing Alport syndrome as hypertensive nephropathy) and slowly progressive renal insufficiency with gradually increasing proteinuria that is usually non-nephrotic (evidence of focal segmental glomerulosclerosis secondary to loss of kidney mass). A key potential characteristic (hypertension precedes either proteinuria or renal insufficiency) is an outdated concept. Both proteinuria and renal insufficiency are late events. The fact that hypertension precedes both proteinuria or renal insufficiency does not exclude that CKD, as defined by KDIGO criteria [urine albumin:creatinine ratio >30 mg/g or estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 for >3 months], precedes hypertension. Indeed, while not formally recognized as CKD until eGFR decreases to <60 mL/min/1.73 m2, there is evidence (e.g. urinary proteomics or imaging for certain aetiologies) that the CKD process starts well before >50% of the functional renal mass is lost, at which point, eGFR decreases to <60 mL/min/1.73 m2 and CKD is formally diagnosed [11, 12]. This is the so-called blind spot of the CKD process [12].
Furthermore, there is no well-defined panel of diagnostic procedures that allow excluding other causes. Among them, genetic causes of CKD are likely the elephant in the room, as discussed below. What would be the diagnosis of a nephropathy that is already present in the newborn, results in hypertension at ~30 years of age and leads to RRT at ~60 years of age if kidney imaging did not exist? Surely we would be diagnosing polycystic kidney disease patients as hypertensive nephropathy. Well for many genetic kidney diseases there is no imaging that allows diagnosing CKD before hypertension develops. In any case, some of the diagnostic criteria for hypertensive nephropathy (long-lasting hypertension and small kidneys) suggest that by the time a diagnosis of hypertensive nephropathy is made, the kidney disease is so advanced that it is no longer possible to diagnose the cause.
Finally, contrary to statements in some popular textbooks, such as UpToDate, the diagnosis of hypertensive nephropathy cannot be confirmed by renal biopsy since there are no specific features [6]. The value of renal biopsy lies in excluding certain nephropathies, not in providing positive evidence of hypertensive nephropathy. Thus the features described as characteristic of hypertensive nephropathy can be found in any long-standing CKD of any aetiology: intimal thickening and luminal narrowing of the large and small renal arteries and the glomerular arterioles, medial hypertrophy and fibroblastic intimal thickening, deposition of hyaline-like material into arteriolar walls, focal global or segmental sclerosis, glomerular enlargement, interstitial fibrosis and atrophy, as listed in UpToDate [6].
HYPERTENSION-ASSOCIATED KIDNEY DISEASE: PERHAPS NO MORE
This is the title of an editorial comment published in 2008 that could have been the epitaph of hypertensive nephropathy [13]. While the reason to claim the end of the concept of hypertension-associated kidney disease was partially wrong (the high incidence of CKD in African-Americans had been linked to MYH9 variants, but while MYH9 variants may cause kidney disease, they are not the cause of the increased risk of CKD in African-Americans [14]), it did point in the right direction: 2 years later, in 2010, the high incidence of CKD (and of hypertensive CKD) in African-Americans was pinpointed to risk variants in apolipoprotein 1 (APOL1) [15, 16]. Indeed, APOL1 risk variants underlie the high risk of African-Americans for human immunodeficiency virus–associated nephropathy and other nephropathies [17]. Our interpretation is that APOL1 nephropathy is a distinct familial kidney disease of variable penetrance whose severity may be influenced by environmental factors and eventually therapy targeting the molecular defect may be developed. Thus we do not concur with statements such as ‘recognition of the variants on the APOL1 gene will likely provide a sensitive and specific diagnostic tool (for hypertensive nephropathy) in black patients’. Indeed, in our view, the presence of such APOL1 variants should prevent the diagnosis of hypertensive nephropathy since an alternative cause for the kidney disease has been found.
GENETIC KIDNEY DISEASE: THE ELEPHANT IN THE ROOM
Recent advances in genetics have identified a higher than expected prevalence of diseases such as Alport syndrome, autosomal dominant tubulointerstitial disease (ADTKD) and even nephronophthisis among adult patients with CKD or on RRT [18–22]. Hypertensive nephropathy was a pre-existent diagnosis found in genetic diseases that were later diagnosed as such through exome sequencing, including autosomal Alport syndrome (5–10% of these patients were diagnosed of hypertensive nephropathy), ADTKD (25% of these patients) and other genetic diseases [22]. Autosomal dominant Alport syndrome is now thought to be as frequent as autosomal dominant polycystic kidney disease; the latter being a difficult-to-miss diagnosis that accounts for 5–10% of patients on RRT. ADTKD patients will need RRT at ages ranging from 30 to 70 years, and hypertension was present in >60% and proteinuria in up to 25% at diagnosis [19–20]. Even nephronophthisis, usually thought of as a cause of end-stage renal disease (ESRD) in children or young persons, may be more frequent than expected. Homozygous NPHP1 deletions may account for 0.5% of dialysis patients and lead to ESRD at ages 18–61 years: 90% of patients were undiagnosed before genetic studies, having a range of diagnoses that included hypertensive nephropathy [21]. If a single genetic variant in a single gene of the at least 20 genes that can cause the obscure, ‘rare’ cause of CKD nephronophthisis may already account for 0.5% of patients on RRT, what is the potential for all the different genetic variants of the >625 nephropathy-associated genes?
These data clearly show that before entertaining a diagnosis of ‘hypertensive nephrosclerosis’, a thorough genetic study should be performed to exclude genetic nephropathies. Thorough means that it should expand beyond next-generation sequencing (NGS), as NGS cannot diagnose certain common MUC1 variants causing ADTKD. In a series in which MUC1 gene variants were specifically assessed, they were the most common cause of ADTKD [19, 20]. Thus a comprehensive exclusion of other nephropathies should include both NGS and search for specific genetic variants known to be relatively common and to be missed by NGS. Only when genetic nephropathies have been excluded can a diagnosis of hypertensive nephropathy be considered. While we agree that such an extensive diagnostic workup may be beyond the means and interest of routine nephrology, there is already a term available for cases in which the cause is not found after a limited aetiological workup: kidney disease of unknown cause. This would be the correct term in the absence of an extensive diagnostic workup, never hypertensive nephropathy.
THE DIAGNOSIS OF HYPERTENSIVE NEPHROPATHY AS A REMORA FOR THE ADVANCE OF NEPHROLOGY
There are objective reasons to believe that hypertensive nephropathy is overdiagnosed. This is a major issue that goes well beyond the choice of therapy for a different cause of progressive CKD. It is often argued that given the paucity of therapeutic options in nephrology, a diagnosis of hypertensive nephropathy will not greatly change the therapeutic approach; once immune-mediated glomerulonephritis has been reasonably excluded on clinical grounds, therapy consists basically of optimized renin–angiotensin system blockade for most nephropathies. However, the fact that most nephropathies get a label (e.g. hypertensive nephropathy) is likely hindering research efforts to develop tools that allow diagnosis of nephropathies currently without an aetiological diagnosis. And in the absence of tools to diagnose a disease, there will not be advances in understanding the pathogenesis or developing therapies. In the absence of a diagnosis of Alport syndrome, for example, patients cannot be enrolled in ongoing Alport syndrome clinical trials. Thus, if the complexity in the aetiological diagnosis of CKD is not embraced, nephrology will miss in the precision medicine revolution.
CONCLUSION
Hypertensive nephropathy remains a diagnosis of exclusion that in practice means CKD of unknown cause in a hypertensive patient. It is time to get rid of the term or define strict diagnostic criteria that allow diagnosis in early stages of the disease. A correct causality assessment and aetiology-based therapy are key to the progress of nephrology and it should no longer be accepted that ‘hypertensive nephropathy’ serves to disguise an insufficient diagnostic workup. A diagnosis of nephropathy of unknown cause would be more honest and useful to the nephrology community. In this regard, the aetiological workup to exclude other nephropathies should, in the 21st century, include a genetic panel for familial kidney disease as well as specific assessment of genetic variants like MUC1 that cannot be diagnosed by NGS. While a thorough aetiological diagnostic workup is costly and currently not possible for all patients in a routine clinical setting, nor will it have a major impact on therapy for most patients, pilot experiences are needed that challenge the widespread use of the term hypertensive nephropathy as a synonym for an insufficient diagnostic workup. We should remove the stigma assigned to a diagnosis of CKD of unknown cause. CKD of unknown cause should be considered a precise description of the diagnostic status whenever the full range of diagnostic tests has not been employed. Advances in the science of nephrology require a detailed understanding of the causes of kidney disease and precise diagnostic criteria that allow us to explore pathogenic mechanisms that may be specific for different nephropathies: it is the first required step for precision nephrology.
FUNDING
Sources of support include FIS/Fondos FEDER PI17/00257, PI18/01386, PI19/00588, PI19/00815, DTS18/00032, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/0009), Sociedad Española de Nefrología, FRIAT, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM.
CONFLICT OF INTEREST STATEMENT
No conflict of interest.
REFERENCES
- 1. Kramer A, Boenink R, Noordzij M. et al. The ERA–EDTA Registry Annual Report 2017: a summary. Clin Kidney J 2020; 13: 693–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary. Clin Kidney J 2019; 12: 702–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: a summary. Clin Kidney J 2018; 11: 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nitta K, Masakane I, Hanafusa N et al.. Annual dialysis data report 2017. Ren Replace Ther 2019; 5: 53. [Google Scholar]
- 5.United States Renal Data System. 2019 ADR Reference Tables. Volume 2 ESRD incidence. https://usrds.org/reference.aspx (9 July 2020, date last accessed)
- 6. Mann JFE, Hilgers KF.. Clinical Features, Diagnosis, and Treatment of Hypertensive Nephrosclerosis. Literature Review Current Through Jun 2020, Topic Last Updated: 15 July 2019 UpToDate; www.uptodate.com (9 July 2020, date last accessed)
- 7.World Health Organization. Noncommunicable Diseases Country Profiles 2018 https://apps.who.int/iris/handle/10665/274512 (9 July 2020, date last accessed)
- 8.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1923–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ERA-EDTA Registry. ERA-EDTA Registry Annual report 2017. Department of Medical Informatics, Amsterdam UMC, location AMC, Amsterdam, the Netherlands, 2019. https://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2017.pdf (9 July 2020, date last accessed)
- 10. Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez E. et al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019; 12: 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodríguez-Ortiz ME, Pontillo C, Rodríguez M. et al. Novel urinary biomarkers for improved prediction of progressive eGFR loss in early chronic kidney disease stages and in high risk individuals without chronic kidney disease. Sci Rep 2018; 8: 15940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanchez-Niño MD, Sanz AB, Ramos AM. et al. Clinical proteomics in kidney disease as an exponential technology: heading towards the disruptive phase. Clin Kidney J 2017; 10: 188–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freedman BI, Sedor JR.. Hypertension-associated kidney disease: perhaps no more. J Am Soc Nephrol 2008; 19: 2047–2051 [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Prado R, Carriazo-Julio SM, Torra R. et al. MYH9-related disease: it does exist, may be more frequent than you think and requires specific therapy. Clin Kidney J 2019; 12: 488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tzur S, Rosset S, Shemer R. et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 2010; 128: 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Genovese G, Friedman DJ, Ross MD. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kopp JB, Nelson GW, Sampath K. et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011; 22: 2129–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bullich G, Domingo-Gallego A, Vargas I. et al. A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int 2018; 94: 363–371 [DOI] [PubMed] [Google Scholar]
- 19. Ayasreh N, Bullich G, Miquel R. et al. Autosomal dominant tubulointerstitial kidney disease: clinical presentation of patients with ADTKD-UMOD and ADTKD-MUC1. Am J Kidney Dis 2018; 72: 411–418 [DOI] [PubMed] [Google Scholar]
- 20. Olinger E, Hofmann P, Kidd K. et al. Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease due to mutations in UMOD and MUC1 Kidney Int 2020; doi: 10.1016/j.kint.2020.04.038] [DOI] [PubMed] [Google Scholar]
- 21. Snoek R, van Setten J, Keating BJ. et al. NPHP1 (nephrocystin-1) gene deletions cause adult-onset ESRD. J Am Soc Nephrol 2018; 29: 1772–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groopman EE, Marasa M, Cameron-Christie S. et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 2019; 380: 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]