Abstract
Background
Renal biopsies are essential in nephrology but they are invasive and complications can occur. The aim of this study was to explore clinical parameters that can be used as predictors for biopsy complications.
Methods
Clinical parameters such as demographics, biopsy indications, serology, comorbidities and clinical chemistry were retrieved from a regional biopsy registry between 2006 and 2015 and from a nationwide registry between 2015 and 2017. Clinical data before biopsy were compared with data on major biopsy complications. Fisher’s exact and χ2 tests were used and odds ratios (ORs) with 95% confidence intervals (CIs) were presented. Univariate and multiple binary logistic regression analyses were performed with complications as outcome. A two-sided P-value <0.05 was considered significant.
Results
In total, 2835 consecutive native kidney biopsies were analysed (39% women and 61% men, median age 57 years). No death and nephrectomy due to biopsy complications were registered. The frequency of major biopsy complications was 5.65%. In the multiple logistic regression, the risk for complications increased in women [OR 1.51 (95% CI 1.08–2.11)] and decreased with age: 45–64 years age group [OR 0.66 (95% CI 0.44–0.99)] and >74 years age group [OR 0.51 (95% CI 0.27–0.96)]. Among comorbidities, patients with diabetes mellitus type 2 [OR 2.07 (95% CI 1.15–3.72)] and non-ischaemic heart disease [OR 3.20 (95% CI 1.64–6.25)] had a higher risk for major biopsy complications.
Conclusions
Female gender, younger age (≤44 years), diabetes mellitus type 2 and non-ischaemic heart disease were found as risk factors for major biopsy complications.
Keywords: biopsy complications, clinical parameters, major complications, native kidney biopsy, risk factors
INTRODUCTION
Kidney biopsies are essential tools in nephrology to ensure a correct diagnosis, predict prognosis and enable optimal treatment for patients [1]. Renal biopsies are invasive procedures and complications can occur. The rate of catastrophic biopsy complications such as death has decreased from 0.12% to 0.02% after the introduction of techniques like ultrasound guidance and automated spring-loaded devices [2, 3]. Important contraindications for renal biopsy are bleeding diathesis and uncontrolled hypertension [4]. However, when observing these contraindications in clinical practice, the remaining risk attributed to common clinical parameters are incompletely known. There are conflicting results in the literature regarding the influence of age; both young age [5–8] and old age [9, 10] have been reported as risk factors. There are also studies indicating female gender [5, 6], higher blood pressure [10, 11], impaired kidney function [7, 9, 11–13] and the size of the biopsy needles [13, 14] are associated with a higher rate of complications. We have previously reported, using a regional registry, that female gender, younger age and lower body mass index (BMI) are risk factors for major biopsy complications in native kidney biopsies [5; Supplementary Table 1].
The aim of this study was to analyse the risk of major biopsy complications and explore clinical parameters predicting complications by extended register data and statistical analyses.
MATERIALS AND METHODS
The regional registry has previously been described in detail [5]. In short, this registry from western and northern Sweden was designed in 2006 for the prospective registration of biopsy complications.
The national Swedish biopsy registry was launched in 2015 and is an integrated part of the Swedish Renal Registry. The coverage of the registry is growing and we estimate that during the study period it captured ∼40% of all native kidney biopsies in Sweden. Clinical parameters in the registry include age, sex, length, weight, blood pressure, kidney function, clinical chemistry, serology, biopsy indication, certain medications and comorbidities.
Only adverse events considered to be major biopsy complications are entered into the national registry. These are defined as complications requiring documented action from the hospital staff [15]. Macroscopic haematuria is not listed as a major complication if did not result in blood transfusion or prolonged hospital stay. Complications are listed as present/non-present in the following categories: death, nephrectomy, blood transfusion, obstruction of the urinary tract, clinically significant haematoma, extended hospital care because of complications, infection requiring antibiotic treatment, decrease in blood pressure requiring treatment, damage to other organs during the procedure, invasive interventions related to complications and readmission because of complications.
The data collection started on 1 January 2006 and ended on 31 December 2017. The Regional Ethical Review Board in Gothenburg, Sweden approved the study.
All biopsies were performed by real-time ultrasound guidance and with an automated spring-loaded biopsy device. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula [16].
For the statistical analysis, SPSS Statistics 22 (IBM, Armonk, NY, USA) was used. Fisher’s exact test and χ2 analyses were used and risk comparisons were presented as odds ratios (ORs) with 95% confidence intervals (CIs). Univariate and multiple binary logistic regression analyses using the Enter method were used with biopsy complications as the outcome. In the multiple binary logistic regression analyses, only variables with a P-value <0.1 in the univariate analyses were included. A two-sided P-value <0.05 was considered significant.
In detail, we started out by selecting all variables that are captured in both registries and that we considered as potential predictors of complications (Table 1). In the first step, we performed univariate analyses with these variables. In the second step, in the multiple binary logistic regression analyses, only variables with a P-value <0.1 in the univariate analyses were included. As a third step, we selected a new set of variables present only in the national biopsy registry (Table 1). We performed univariate analyses with these variables. We then, as Step 4, subjected variables revealed in Step 3 to multiple logistic regression together with the variables identified in Step 1.
Table 1.
Variables that are captured in the registries
| Variables | Regional biopsy registry (1256 biopsies) | National biopsy registry (1579 biopsies) |
|---|---|---|
| Length (cm) | Yes | Yes |
| Weight (kg) | Yes | Yes |
| Systolic blood pressure (mmHg) | Yes | Yes |
| Diastolic blood pressure (mmHg) | Yes | Yes |
| Serum creatinine (µmol/L) | Yes | Yes |
| eGFR MDRD (mL/min/1.73 m2) | Yes | Yes |
| Number of glomeruli per biopsy | Yes | Yes |
| MAP (mmHg) | Yes | Yes |
| Age (years) | Yes | Yes |
| BMI (kg/m2) | Yes | Yes |
| Plasma albumin (g/L) | No | Yes |
| Plasma CRP (mg/L) | No | Yes |
| Urine albumin/creatinine (mg/mmol) | No | Yes |
| Urine albumin per day (mg/day) | No | Yes |
| Serum haemoglobin (g/L) | No | Yes |
| Plasma cystatin C (mg/L) | No | Yes |
| Cystatin C GFR (mL/min/1.73 m2) | No | Yes |
| Grade of haematuria | No | Yes |
| Smoking | No | Yes |
| Comorbidity | No | Yes |
| Immunological blood samples | No | Yes |
| Virus serology | No | Yes |
| Indications for biopsies | No | Yes |
| Biopsy needle size (gauge) | Yes | No |
| Number of passes per biopsy | Yes | No |
| Specialty of biopsy performer | Yes | No |
| Minor biopsy complications | Yes | No |
CRP, C-reactive protein; MAP, mean arterial pressure.
RESULTS
In total, 2835 consecutive native kidney biopsies (1111 women and 1724 men) were registered. Data were merged for native kidney biopsies using both registries (the regional biopsy registry with 1256 biopsies and the Swedish national biopsy registry with 1579 biopsies). The patients’ median age was 57 years (range 16–90). The baseline data of the registered clinical variables are shown in Table 2.
Table 2.
Baseline data of kidney biopsy patients
| Variables | Mean | Median | SD | Range | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Length (cm) | 173 | 173 | 10 | 64 | 143 | 207 |
| Weight (kg) | 82 | 81 | 19 | 144 | 35 | 179 |
| Systolic blood pressure (mmHg) | 135 | 135 | 17 | 146 | 70 | 216 |
| Diastolic blood pressure (mmHg) | 78 | 80 | 11 | 92 | 41 | 133 |
| Serum creatinine (µmol/L) | 205 | 145 | 190 | 1919 | 29 | 1948 |
| Plasma albumin (g/L) | 31 | 32 | 8 | 47 | 5 | 52 |
| Plasma CRP (mg/L) | 14 | 5 | 27 | 254 | 0.2 | 254 |
| Urine albumin/creatinine (mg/mmol) | 260 | 151 | 300 | 2000 | 0.1 | 2000 |
| Urine albumin per day (mg/day) | 2352 | 1300 | 3255 | 21 000 | 0 | 21 000 |
| Serum haemoglobin (g/L) | 123 | 123 | 21 | 119 | 71 | 190 |
| Plasma cystatin C (mg/L) | 2 | 2 | 1 | 6 | 0.6 | 7 |
| eGFR MDRD (mL/min/1.73 m2) | 48 | 40 | 33 | 208 | 2 | 210 |
| Cystatin C GFR (mL/min/1.73 m2) | 41 | 34 | 24 | 117 | 3 | 120 |
| Number of glomeruli per biopsy | 22 | 19 | 13 | 100 | 0 | 100 |
| MAP (mmHg) | 96 | 97 | 11 | 77 | 60 | 137 |
| Age (years) | 54 | 57 | 18 | 74 | 16 | 90 |
| BMI (kg/m2) | 27 | 27 | 6 | 49 | 13 | 61 |
CRP, C-reactive protein.
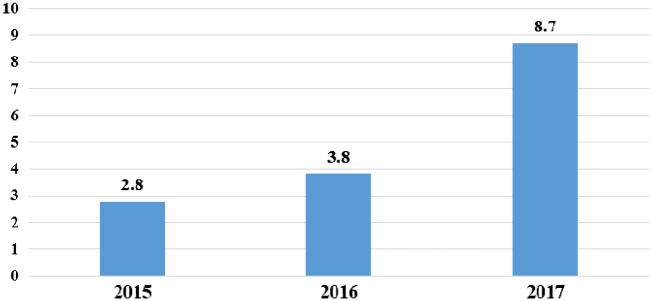
The frequency for major biopsy complications was 5.65% (6.3% in the regional and 5% in the national biopsy registry). The types and numbers of major biopsy complications are described in Table 3. No death or nephrectomy due to biopsy complications was registered. The biopsies were performed at an inpatient ward in 97% of patients and at an outpatient clinic in 3%. The percentage of outpatient procedures per year is shown in Figure 1. Two of the outpatient clinic biopsies [82 patients (2.4%)] compared with 156 patients of the inpatient clinic biopsies [2749 patients (5.7%)] developed major complications. This difference was not significant. An inpatient procedure was defined as the patient was admitted to a hospital ward for overnight observation after the biopsy. Typically the patient comes to the hospital the day before the procedure and leaves the hospital the day after.
Table 3.
Types and numbers of major biopsy complications
| Type of complication | n (%) |
|---|---|
| Bleeding complications | |
| Bleeding requiring blood transfusion | 37 (23.42) |
| Obstruction of the urinary tract | 28 (17.72) |
| Clinical significant haematoma | 62 (39.25) |
| Extended hospital care because of gross haematuria | 4 (2.53) |
| Other | |
| Infection requiring antibiotic treatment | 20 (12.66) |
| Fall in blood pressure requiring treatment | 5 (3.16) |
| Perforation of the small intestine with peritonitis, requiring blood transfusion and surgery | 1 (0.63) |
| Extended care because of fever after biopsy | 1 (0.63) |
| Total number | 158 |
Invasive interventions related to complications (n = 2) and readmission because of complications are included in the total number of complications above.
FIGURE 1.
The percentage of outpatient procedures per year. No outpatient procedures were performed between the years 2006 and 2014.
When univariate analyses with potential risk factors for major biopsy complications were performed (Table 4), a significantly higher risk was found for women compared with men [OR 1.51 (95% CI 1.10–2.10)]. Patients >44 years of age had a lower risk of major biopsy complications when compared with younger patients [45–64 years, OR 0.62 (95% CI 0.42–0.91); 65–74 years, OR 0.62 (95% CI 0.40–0.96); >74 years, OR 0.48 (95% CI 0.26–0.89)]. Furthermore, patients with a BMI ≥30 had a lower frequency of major biopsy complications [OR 0.56 (95% CI 0.36–0.89)].
Table 4.
Univariate analysis of major complications
| Factors | Major complications, % (n) | Univariate model |
|
|---|---|---|---|
| OR (95% CI) | P-value | ||
| Sex | |||
| Men (n = 1721) | 4.7 (81) | Reference | – |
| Women (n = 1110) | 6.9 (77) | 1.51 (1.10–2.10) | 0.015 |
| Age category (years) | |||
| ≤44 (n = 899) | 7.6 (68) | Reference | – |
| 45–64 (n = 951) | 4.8 (46) | 0.62 (0.42–0.91) | 0.016 |
| 65–74 (n = 662) | 4.8 (32) | 0.62 (0.40–0.96) | 0.031 |
| >74 (n = 319) | 3.8 (12) | 0.48 (0.26–0.89) | 0.021 |
| BMI category (kg/m2) | |||
| <24 (n = 715) | 7.6 (54) | Reference | – |
| 24–27 (n = 686) | 5.5 (38) | 0.72 (0.47–1.10) | 0.130 |
| 28–29 (n = 513) | 5.3 (27) | 0.68 (0.42–1.10) | 0.113 |
| ≥30 (n = 706) | 4.4 (31) | 0.56 (0.36–0.89) | 0.013 |
A multiple logistic regression with major complications as the outcome was performed (Table 5). After including BMI, age and sex in the model, the risk for major complications remained higher for women [OR 1.51 (95% CI 1.08–2.11)] and for younger age. The risk seemed to decrease with age: 45–64 years, OR 0.66 (95% CI 0.44–0.99); >74 years, OR 0.51 (95% CI 0.27–0.96).
Table 5.
Multiple logistic regression analysis of major complications
| P-value | OR | 95% CI for OR |
||
|---|---|---|---|---|
| Variables | Lower | Upper | ||
| Female | 0.016 | 1.510 | 1.078 | 2.113 |
| Age (years) | ||||
| ≤44 | – | Reference | – | – |
| 45–64 | 0.046 | 0.661 | 0.440 | 0.993 |
| 65–74 | 0.082 | 0.674 | 0.432 | 1.052 |
| >74 | 0.038 | 0.511 | 0.272 | 0.963 |
| BMI | ||||
| <24 | – | Reference | – | – |
| 24–27 | 0.358 | 0.815 | 0.527 | 1.260 |
| 28–29 | 0.359 | 0.797 | 0.490 | 1.295 |
| ≥30 | 0.057 | 0.638 | 0.402 | 1.013 |
| Constant | 0.000 | 0.082 | – | – |
Only the national registry included data on comorbidities. Patients with diabetes mellitus type 2 (302 patients), but not type 1 (54 patients), had a higher risk for biopsy complications [OR 2.12 (95% CI 1.29–3.48); P = 0.004] in univariate analysis. There were 25 major biopsy complications in the patients with diabetes mellitus type 2 and no biopsy complications in the group of patients with diabetes mellitus type 1. When performing a multiple logistic regression, the risk for biopsy complications remained higher in patients with diabetes mellitus type 2 [OR 2.07 (95% CI 1.15–3.72); P = 0.015] after adjusting for BMI, age, eGFR, sex and mean arterial pressure (MAP; Table 6).
Table 6.
Multiple logistic regression analysis of major complications and patients with diabetes mellitus type 2
| P-value | OR | 95% CI for OR |
||
|---|---|---|---|---|
| Variables | Lower | Upper | ||
| BMI | 0.530 | 0.986 | 0.942 | 1.031 |
| Age | 0.553 | 0.995 | 0.979 | 1.011 |
| eGFR MDRD | 0.580 | 1.002 | 0.994 | 1.010 |
| Sex (men) | 0.286 | 0.764 | 0.465 | 1.253 |
| Diabetes mellitus type 2 | 0.015 | 2.069 | 1.152 | 3.715 |
| MAP | 0.024 | 1.025 | 1.003 | 1.047 |
| Constant | 0.000 | 0.008 | – | – |
Patients with non-ischaemic heart disease, but not ischaemic heart disease, also had a higher risk for biopsy complications compared with patients without such disease [OR 2.81 (95% CI 1.57–5.02); P = 0.002] in univariate analysis. After performing a multiple logistic regression, the risk for biopsy complications remained higher in patients with non-ischaemic heart disease [OR 3.20 (95% CI 1.64–6.25); P = 0.001] after adjusting for diabetes mellitus type 2, BMI, MAP, age, sex and eGFR (Table 7).
Table 7.
Multiple logistic regression analysis of major complications and patients with non-ischaemic heart disease
| P-value | OR | 95% CI for OR |
||
|---|---|---|---|---|
| Variables | Lower | Upper | ||
| BMI | 0.386 | 0.980 | 0.935 | 1.026 |
| MAP | 0.012 | 1.028 | 1.006 | 1.050 |
| Age | 0.208 | 0.989 | 0.973 | 1.006 |
| Diabetes mellitus type 2 | 0.045 | 1.841 | 1.013 | 3.347 |
| Sex (men) | 0.204 | 0.722 | 0.437 | 1.193 |
| eGFR MDRD | 0.538 | 1.002 | 0.995 | 1.010 |
| Non-ischaemic heart disease | 0.001 | 3.198 | 1.638 | 6.245 |
| Constant | 0.000 | 0.009 | – | – |
Other clinical variables as described in Table 2, such as high blood pressure and impaired kidney function (eGFR MDRD), were not found to be risk factors for major biopsy complications. Additional factors, registered only in the national biopsy registry (Supplementary Table 2), like grade of haematuria before biopsy, other comorbidities (hypertension, malignancy, ischaemic heart disease, cerebrovascular disease and peripheral vascular disease), smoking (ongoing and former), immunological blood samples [proteinase 3 or myeloperoxidase anti-neutrophil cytoplasmic antibodies, anti-nuclear antibodies, anti-double-stranded DNA (anti-dsDNA), anti-glomerular basement membrane antibodies, anti-phospholipid antibodies, anti-phospholipase A2 receptor anti-bodies, complement factors (C3, C4, C1q) or virus serology (hepatitis B and C, human immunodeficiency virus)] were not found to be risk factors for major biopsy complications. In addition, the biopsy indications (nephrotic or nephritic syndrome, all stages of chronic kidney disease and other acute kidney injury) (Supplementary Table 3), known heredity of kidney diseases and M component in serum or urine were not risk factors for biopsy complications. Furthermore, neither treatment with antihypertensive drugs nor steroids was a risk factor for biopsy complications. The use of platelet aggregation inhibitors (including aspirin), warfarin, new oral anticoagulants and heparin is captured by the national biopsy registry. The use of such drugs should be checked even when they are temporarily paused, which is the regular practice in our country. The use of these drugs is included in the univariate analysis and was not found to be a risk factor for major biopsy complications.
DISCUSSION
The aim of this study was to identify risk factors that could be clinically useful for the prediction of complications after native kidney biopsies. We found female sex, lower age, diabetes mellitus type 2 and non-ischaemic heart disease to be independent risk factors for major complications.
Female sex has previously been reported as a risk factor for major complications after native kidney biopsies by Manno et al. [6], in a meta-analysis by Corapi et al. [13] and in our previous publication for the regional registry [5]. This finding is difficult to explain; some authors have explained that women with the same serum creatinine have lower GFRs [13]. However, impaired kidney function was not found to be a risk factor for biopsy complications in our as well in other studies [6, 17]. Another possibility is that smaller kidneys are more vulnerable; that there is an increased risk that the needle transverses the parenchyma and hits larger vessels if the absolute kidney size is small.
Another finding in our study, that younger age is a risk factor for biopsy complications after native kidney biopsies, has been described in earlier studies [5–8, 18] as well. However, it was not noted in the studies by Tondel et al. [9] and Eiro et al. [10]. The finding that patients with younger age have a higher risk for biopsy complications can possibly be explained by the fact that they are more mobile and active in the period after the biopsies are performed [5]. Other possible explanations are related to renal blood flow and renal mobility.
Comorbidities are not always included in the analysis of complication risks. To our knowledge, we are the first to report that patients with diabetes mellitus type 2 and patients with non-ischaemic heart disease have a higher risk for major complications after native kidney biopsies. As we found the risk only for patients with diabetes mellitus type 2 and not type 1, it is more likely related to metabolic syndrome than to diabetic microangiopathy. Increased risk of bleeding has also been found in diabetes patients with pulmonary embolism receiving anticoagulant therapy [19]. We found an increased risk of complications in patients with non-ischaemic heart disease but not among those with ischaemic heart disease. Non-ischaemic heart disease is a broad category including entities such as atrial fibrillation, pacemaker treatment, heart failure, malformations and valvular disease. It is possible that our threshold for listing a complication as ‘major’, i.e. the need for intervention, is easier to achieve in a patient with a severe comorbidity.
Hypertension has previously been reported as a risk factor for biopsy complications [10, 11]. We found no correlation between complications and blood pressure or with the presence of hypertensive disease as indicated by ongoing hypertensive therapy. This may well be due to more restrictive routines, with blood pressure levels >160/95 mmHg being considered as a contraindication at most centres in our country.
Knowledge of risk factors for biopsy complications may have direct implications on clinical practice. Previous studies have indicated a possible benefit of giving desmopressin as prophylaxis immediately prior to biopsies [20, 21]. If the results of this study can be confirmed, that for instance patients with type 2 diabetes mellitus are at greater risk, the use of desmopressin prophylaxis could be considered in such patients.
Another way the results of this and similar studies can be utilized is to select patients who need more intensive observation after biopsy. In our country, there is a growing interest in performing biopsies as outpatient procedures. It is prudent that patients with risk factors have their biopsies performed at an inpatient ward.
This study has several important limitations that have to be kept in mind when interpreting the results. First, we have merged data from two separate registries capturing different data sets. Even though the national registry was based on the experiences from the regional registry, there are some differences in the definitions of complications. The national registry only captures events that render an active response from the medical staff as a complication, thus minor complications are not listed. Second, we do not have complete coverage of all native kidney biopsies in the region/country. The regional registry was, at the beginning of the study period, in the build-up phase and the same is true for the national registry from 2015. We cannot exclude that centres more interested in renal biopsies were prone to start registering earlier and that they have a somewhat different complication panorama. A third limitation is that the data are not validated. Other parts of the Swedish Renal Registry have been validated and shown to possess a high degree of accuracy, but this has not yet been done in the renal biopsy part. International normalized ratio, platelet count and activated partial thromboplastin time are not recorded in the two registries. These blood tests are performed on a regular basis in our country prior to biopsy. Abnormal values are considered as a contraindication to biopsy. This study also has many merits. Capturing data from the entire country, we avoid selection bias when limited to university hospitals. We also present a large data set collected in a relatively short time period, thus our data tend to reflect the current praxis.
In conclusion, we found female gender, younger age (≤44 years), diabetes mellitus type 2 and non-ischaemic heart disease to be independent risk factors for developing major complications after native kidney biopsies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to the patients and the medical staffs at all the centres who participated in our study. The authors also gratefully acknowledge funding from the Research Fund (FoU) at Skaraborg Hospital, Skövde, Sweden and the Healthcare Committee, Region Västra Götaland, Sweden. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee where the studies were conducted (Institutional Review Board approval 701-08 and T626-18) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Hogan JJ, Mocanu M, Berns JS.. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol 2016; 11: 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Korbet SM, Volpini KC, Whittier WL.. Percutaneous renal biopsy of native kidneys: a single-center experience of 1,055 biopsies. Am J Nephrol 2014; 39: 153–162 [DOI] [PubMed] [Google Scholar]
- 3. Whittier WL, Gashti C, Saltzberg S. et al. Comparison of native and transplant kidney biopsies: diagnostic yield and complications. Clin Kidney J 2018; 11: 616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whittier WL, Korbet SM.. Renal biopsy: update. Curr Opin Nephrol Hypertens 2004; 13: 661–665 [DOI] [PubMed] [Google Scholar]
- 5. Peters B, Andersson Y, Stegmayr B. et al. A study of clinical complications and risk factors in 1001 native and transplant kidney biopsies in Sweden. Acta Radiol 2014; 55: 890–896 [DOI] [PubMed] [Google Scholar]
- 6. Manno C, Strippoli GF, Arnesano L. et al. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int 2004; 66: 1570–1577 [DOI] [PubMed] [Google Scholar]
- 7. Diaz-Buxo JA, Donadio JV Jr. Complications of percutaneous renal biopsy: an analysis of 1,000 consecutive biopsies. Clin Nephrol 1975; 4: 223–227 [PubMed] [Google Scholar]
- 8. Christensen J, Lindequist S, Knudsen DU. et al. Ultrasound-guided renal biopsy with biopsy gun technique–efficacy and complications. Acta Radiol 1995; 36: 276–279 [PubMed] [Google Scholar]
- 9. Tondel C, Vikse BE, Bostad L. et al. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol 2012; 7: 1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eiro M, Katoh T, Watanabe T.. Risk factors for bleeding complications in percutaneous renal biopsy. Clin Exp Nephrol 2005; 9: 40–45 [DOI] [PubMed] [Google Scholar]
- 11. Shidham GB, Siddiqi N, Beres JA. et al. Clinical risk factors associated with bleeding after native kidney biopsy. Nephrology (Carlton) 2005; 10: 305–310 [DOI] [PubMed] [Google Scholar]
- 12. Whittier WL, Korbet SM.. Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol 2004; 15: 142–147 [DOI] [PubMed] [Google Scholar]
- 13. Corapi KM, Chen JL, Balk EM. et al. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis 2012; 60: 62–73 [DOI] [PubMed] [Google Scholar]
- 14. Arora K, Punia RS, D'Cruz S.. Comparison of diagnostic quality of kidney biopsy obtained using 16G and 18G needles in patients with diffuse renal disease. Saudi J Kidney Dis Transpl 2012; 23: 88–92 [PubMed] [Google Scholar]
- 15. Whittier WL. Complications of the percutaneous kidney biopsy. Adv Chronic Kidney Dis 2012; 19: 179–187 [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Coresh J, Greene T. et al. Collaboration CKDE: expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53: 766–772 [DOI] [PubMed] [Google Scholar]
- 17. Mackinnon B, Fraser E, Simpson K. et al. Is it necessary to stop antiplatelet agents before a native renal biopsy? Nephrol Dial Transplant 2008; 23: 3566–3570 [DOI] [PubMed] [Google Scholar]
- 18. Harmankaya O, Okuturlar Y, Kocoglu H. et al. Renal biopsy in the elderly: a single-center experience. Int Urol Nephrol 2015; 47: 1397–1401 [DOI] [PubMed] [Google Scholar]
- 19. Zhang Z, Zhai Z, Yang Y. et al. Diabetes mellitus is associated with increased bleeding in pulmonary embolism receiving conventional anticoagulant therapy: findings from a “real-world” study. J Thromb Thrombolysis 2017; 43: 540–549 [DOI] [PubMed] [Google Scholar]
- 20. Manno C, Bonifati C, Torres DD. et al. Desmopressin acetate in percutaneous ultrasound-guided kidney biopsy: a randomized controlled trial. Am J Kidney Dis 2011; 57: 850–855 [DOI] [PubMed] [Google Scholar]
- 21. Peters B, Hadimeri H, Molne J. et al. Desmopressin (Octostim®) before a native kidney biopsy can reduce the risk for biopsy complications in patients with impaired renal function: a pilot study. Nephrology (Carlton) 2018; 23: 366–370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.