Hyperkalaemia [HK; serum potassium (sK) ≥5.5 mEq/L] often occurs due to impaired excretion of potassium in patients with chronic kidney disease (CKD) and other chronic conditions such as heart failure (HF) and/or diabetes mellitus (DM) [1, 2]. At the same time, these patients benefit from renin–angiotensin–aldosterone system inhibitors (RAASis) at maximum tolerated guideline recommended doses, as they can improve cardiovascular and renal outcomes and reduce mortality and hospitalizations [3, 4], particularly in patients with HF with reduced ejection fraction (HFrEF) [1, 5].
However, RAASis and in particular mineralocorticoid-receptor antagonists (MRAs) increase the risk for HK [1], which can have serious clinical consequences, e.g., life-threatening arrhythmias and sudden death, and HK is associated with increased risk of all-cause mortality [1].
HK is often a chronic or recurrent condition requiring long-term management. In a retrospective chart review using a large US patient registry, almost half of the patients with documented HK experienced two or more HK episodes within 1 year [6].
The treatment approach for patients with recurrent HK is undergoing change. Until recently, the recommendations [7] were to restrict the dietary potassium intake; to avoid potassium supplements and drugs affecting renal function; to start a non-potassium-sparing diuretic or to increase the dose if already receiving a diuretic.
We describe the contemporary multidimensional management of recurrent HK and related healthcare resource utilization in routine clinical practice across Europe.
A retrospective chart review was conducted in five European countries including patients not on dialysis with two or more HK episodes (sK ≥5.5 mEq/L) documented within a 12-month observational period. Hospital- and office-based physicians located in France, Germany, Italy, Spain and the UK participated between June and September 2016, if they had at least 3 years of experience in nephrology or cardiology post-residency/fellowship, and if they consulted/visited on average five or more patients with HK per month. The target number of physicians was 50 nephrologists plus 50 cardiologists per country. Each physician included a maximum of three patients. HK treatment and RAASi use were compared between two subsequent HK episodes (HK1 and HK2). Hospitalizations related to HK or comorbidities were documented (expanded methods in Supplementary data).
Out of 825 physicians from France, Germany, Italy, Spain and the UK, 568 physicians were eligible and participated in the study. Two hundred and fifty-three (44.5%) were nephrologists and 315 (55.5%) were cardiologists, and included at least one eligible patient into the study (Supplementary data, Table S1). We studied 1457 patients, mean (SD) age was 66 (12) years. Comorbidities were common: CKD 68%, HF 40%, diabetes 36% and hypertension 72% (Table 1 and Supplementary data, Tables S2 and S3).
Table 1.
Patient demographics and clinical characteristics at the HK1 in all patients and the subgroups with HFrEF or DN
| Parameter | All patients | HFrEF (LVEF ≤40%) | DN |
|---|---|---|---|
| Patients, n | 1457 | 333 | 376 |
| Age; mean (SD) (years) | 66.2 (12. 43) | 68.5 (11.11) | 66.7 (10.92) |
| Male sex, n (%) | 936 (64.2) | 223 (67.0) | 229 (60.9) |
| BMI; mean (SD) (kg/m2) | 27.4 (4.60) | 28.2 (5.10) | 28.9 (4.91) |
| sK; mean (SD)a (mEq/L) | 5.9 (0.78) | 5.9 (0.84) | 5.9 (0.70) |
| Comorbidities | |||
| HF, n (%) | 587 (40.3) | 301 (90.4) | 167 (44.4) |
| Total | 587 (100.0) | 301 (100.0) | 167 (100.0) |
| HF with CKDb | 408 (69.5) | 213 (70.8) | 165 (98.8) |
| HF without CKDb | 179 (30.5) | 88 (29.2) | 2 (1.2) |
| NYHA class, n (%) | |||
| Totalb | 587 (100.0) | 301 (100.0) | 167 (100.0) |
| I | 38 (6.5) | 8 (2.7) | 8 (4.8) |
| II | 315 (53.7) | 149 (49.5) | 91 (54.5) |
| III | 225 (38.3) | 137 (45.5) | 66 (39.5) |
| IV | 9 (1.5) | 7 (2.3) | 2 (1.2) |
| LVEF,bn (%) | |||
| <30 | 74 (5.6) | 74 (22.2) | 21 (6.2) |
| 30–40 | 259 (19.8) | 259 (77.8) | 70 (20.5) |
| 41–49 | 284 (21.7) | 0 | 83 (24.3) |
| 50–75 | 421 (32.1) | 0 | 100 (29.3) |
| >75 | 16 (1.2) | 0 | 1 (0.3) |
| Not done | 257 (19.6) | 0 | 66 (19.4) |
| Not documented | 146 | 0 | 35 |
| DM, n (%) | 520 (35.7) | 125 (37.5) | 345 (91.8) |
| DM type, n (%)b | |||
| Total | 520 (100.0) | 125 (100.0) | 345 (100.0) |
| Type 1 | 29 (5.6) | 8 (6.4) | 19 (5.5) |
| Type 2 | 473 (91.0) | 113 (90.4) | 308 (89.3) |
| HbA1c, n; mean (SD) (%) | 633; 7.0 (1.60) | 142; 7.2 (1.65) | 280; 7.4 (0.95) |
| Hypertension, n (%) | 1047 (71.9) | 256 (76.9) | 300 (79.8) |
| Blood pressure; mean (SD) (mmHg) | |||
| Systolic | 143.2 (18.88) | 141.1 (22.85) | 145.8 (18.78) |
| Diastolic | 83.4 (13.21) | 81.9 (13.89) | 83.4 (11.80) |
| CKD, n (%) | 996 (68.4) | 233 (70.0) | 370 (98.4) |
| CKD stage, n (%)b | |||
| 1 | 63 (6.3) | 14 (6.0) | 16 (4.3) |
| 2 | 237 (23.8) | 65 (27.9) | 79 (21.4) |
| 3 | 445 (44.7) | 110 (47.2) | 178 (48.1) |
| 4 | 216 (21.7) | 40 (17.2) | 90 (24.3) |
| 5 (not on dialysis) | 34 (3.4) | 4 (1.7) | 7 (1.9) |
| Not documented | 1 | 0 | 0 |
| eGFR,cn; mean (SD) (mL/min/1.73 m²) | 757; 44.8 (22.19) | 181; 43.4 (23.56) | 232; 39.0 (15.60) |
| Concomitant medications | |||
| RAASi medication, n (%) | 881 (60.5) | 238 (71.5) | 258 (68.6) |
| ACEis | 593 (40.7) | 180 (54.1) | 174 (46.3) |
| ARBs | 282 (19.4) | 60 (18.0) | 86 (22.9) |
| MRAs | 177 (12.1) | 96 (28.8) | 46 (12.2) |
| Other medications, n (%) | |||
| β-blockers | 515 (35.3) | 201 (60.4) | 157 (41.8) |
| Loop diuretics | 838 (57.5) | 260 (78.1) | 255 (67.8) |
Proportions are given as percent of valid documented cases. Not documented cases are not included in the calculations. They are still presented to see how many values are missing. There were missing values for LVEF in patients without HFrEF (n = 403, 27.65% of total study cohort), for stage of CKD in one individual without CKD (n = 1, 0.06% of total study cohort), for cause of CKD (n = 45, 4.5% of total population) and for microalbuminuria (n = 690, 47.35% of total cohort during HK1, and n = 854, 58.61% of total cohort during HK2).
sK values <1.5 or >8 mEq/L were considered implausible and excluded from the analysis.
The proportions for HF with or without CKD, NYHA class, DM type and CKD stage are based on the number of patients who have documented HF, DM and CKD, respectively (100%). For some patients, the NYHA class, DM type or CKD stage was not documented.
Calculated according to Modification of Diet in Renal Disease formula.
BMI, body mass index; HbA1c, haemoglobin A1c; LVEF, left ventricular ejection fraction; mEq, milliequivalent; NYHA, New York Heart Association.
A RAASi prescription was reported for 60.5% of the patients. Angiotensin-converting enzyme inhibitors (ACEis) were most common (40.7% of all patients), and MRA use was <30% in HFrEF.
A total of 326 hospitalizations were reported in 307 patients, including 112 hospitalizations (36% of all hospitalizations) related directly to HK (Supplementary data, Tables S4 and S5).
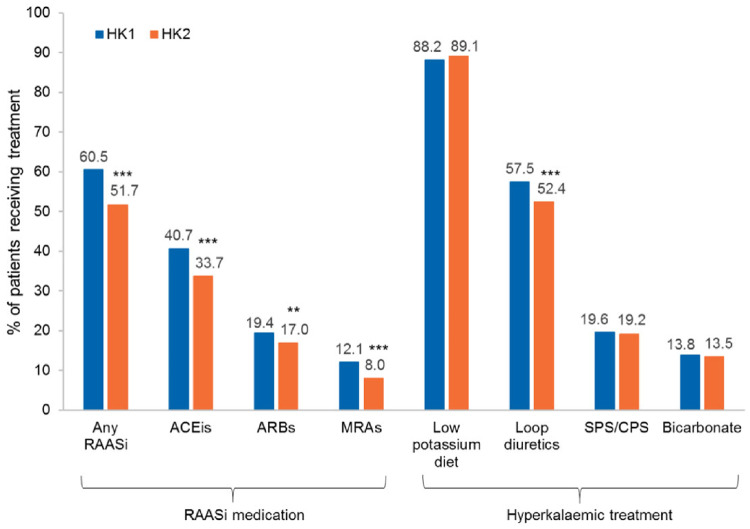
The proportion of patients with RAASi prescription was lower at HK2 than at HK1 for all medication classes. Interventions included low-potassium diet, sodium/calcium polystyrene sulphonate (SPS/CPS) and bicarbonates to similar extents at HK1 (88, 20 and 14%, respectively) and HK2 (89, 19 and 13%, respectively), while loop diuretics were less frequently used at HK2 (52%) versus HK1 (57%; Figure 1). HK treatments varied across countries (Supplementary data, Figures S1 and S2) and by physician specialty (Supplementary data, Table S6).
FIGURE 1.
RAASi use and HK treatment at HK1/HK2 in the 12-month observational period (n = 1457 patients). Significant difference from HK1 McNemar test: **P = 0.006; ***P < 0.001. HK episodes were defined by sK ≥5.5 mEq/L. If the treatment regimen changed during a visit, the new regimen was recorded.
Both specialties reduced RAASi use between HK1 and HK2 (Supplementary data, Table S6). At HK1, nephrologists reported a higher use of SPS/CPS (26.4% versus 13.9% of patients) and bicarbonate (21.7% versus 7.3%) compared with cardiologists. This was also the case at HK2.
The RAASi use at HK1 varied across countries, with higher RAASi use in the UK (78.2%) and Germany (69.7%) but lower use in France (42.4%) than overall (60.5%; Supplementary data, Figure S1). Differences remained at HK2. Use of ACEis, angiotensin receptor blockers (ARBs) and MRAs declined from HK1 to HK2 in all countries. Similarly, HK treatment strategies also varied. At HK1, SPS/CPS was used considerably more in France (45.4%) compared with overall (19.6%). In contrast, loop diuretic use was less common in France (48.8%, overall: 57.5%) and bicarbonate use was more common in Italy (20.1%) than overall (13.8%). Between HK1 and HK2, only small changes were seen for HK management options, with differences between countries remaining at HK2 (Supplementary data, Figure S2).
A logistic regression analysis based on 757–1457 observations showed that the existence of HF, CKD, DM and systolic or diastolic BP above median at baseline were significant independent variables for not receiving RAASi therapy, whereas age, gender, sK+ values and estimated glomerular filtration rate (eGFR) values were not significantly associated with the use of RAASis. Subsequently, in a multiple regression analysis with 11 variables, based on observations with non-missing values in dependent variables (results from 420 observations), only HF at baseline (all HF) remained significant as an independent variable associated with RAASi discontinuation (Supplementary data, Table S7).
SUBGROUP ANALYSES
Patients with HFrEF
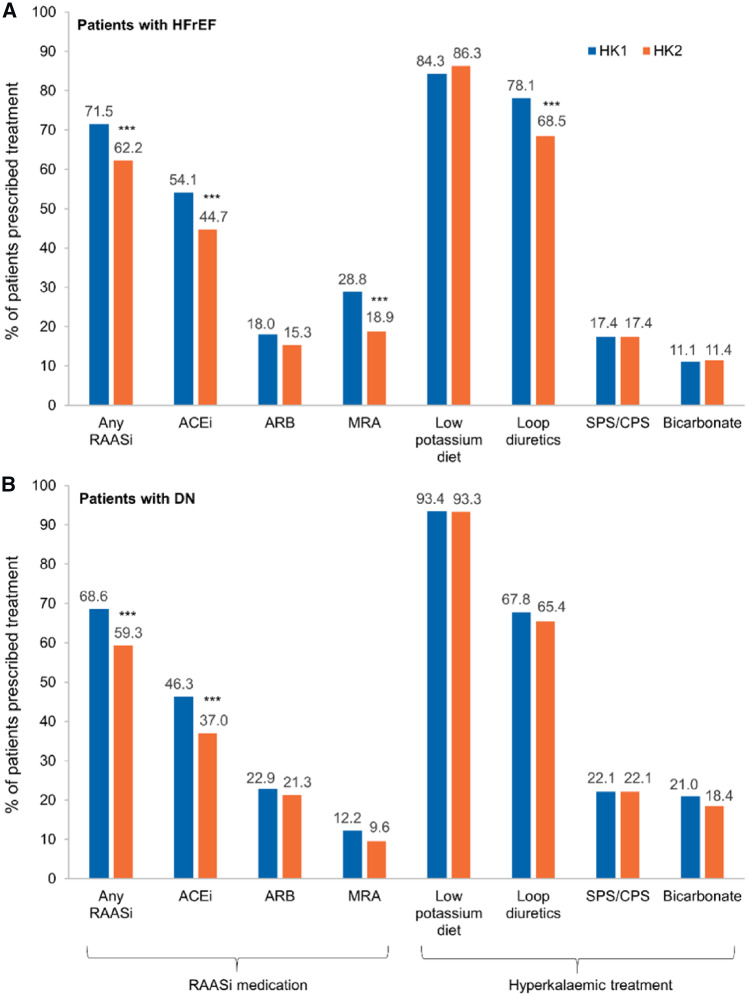
HFrEF was documented in 333 patients (22.8%), mostly with left ventricular ejection fraction of 30–40% (Table 1). The use of RAASis, β-blockers and loop diuretics at HK1 was higher in HFrEF than in the overall population (Table 1). Similar to the overall study population, the RAASi use was lower at HK2 than at HK1 (Figure 2A).
FIGURE 2.
RAASi use and HK treatment at HK1/HK2 in the 12-month observational period in the subgroups of patients with (A) HFrEF (n = 333) or (B) DN (n = 376). Significant difference from HK1, McNemar test: ***P < 0.001.
The prescription rate of ramipril or spironolactone, the most frequently used RAASis, decreased between HK1 and HK2 in patients with HFrEF (Supplementary data, Figure S3A). About half of the patients with a ramipril or spironolactone prescription received the recommended target doses at HK1. For ramipril, this proportion declined at HK2 (Supplementary data, Figure S3B). For the other RAASis, the proportion of patients reaching ≥100% of the recommended target dose at HK1 was 90% for eplerenone, about 50.0% for enalapril and lisinopril, and even lower for valsartan (14.3%) and losartan (6.7%). At HK2, the proportions of patients reaching the target dose declined further, except for eplerenone (data not shown).
Resource utilization, particularly hospitalizations, were more common in the subgroup with HFrEF compared with all other patients (Supplementary data, Table S8).
Patients with CKD and diabetic Nephropathy
Patients with CKD plus HF had substantially higher hospitalization rates compared with HF patients without CKD (76.7% versus 23.3%; Supplementary data, Table S5). The use of RAASis, ACEis, ARBs, MRAs, β-blockers and loop diuretics was similar in patients with CKD (data not shown) and those with diabetic nephropathy (DN) (Table 1).
RAASi use at HK1 was higher in the 376 DN patients (68.6%) than in the overall population (Table 1). Similar to all patients studied, the RAASi use declined in DN between HK1 and HK2, but the change was only significant for ACEis (Figure 2B). The use of loop diuretics and bicarbonates was higher in DN patients compared with the overall population, and hospitalizations were more frequently observed in these patients (Supplementary data, Table S8).
DISCUSSION
To the best of our knowledge, this targeted chart review in cardiologists and nephrologists from five European countries showed for the first time detailed HK treatment patterns in Europe and assessed the number of HK-related hospitalizations in a population with at least two HK episodes within 12 months. RAASi use was lower at HK2 than at HK1. HK treatment options were differently used across the countries and by nephrologists and cardiologists. Hospitalizations related to recurrent HK or comorbidities were documented for 21.1% of the patients (Supplementary data, Table S8). Of these hospitalizations, 36% were directly related to HK, 32% to cardiovascular reasons and 76% concerned CKD patients (Supplementary data, Table S5). This clearly shows the burden of HK in this specific setting and highlights the need for more effective mitigation strategies.
Results for patients with HFrEF or DN were largely consistent with the overall population, but both patient groups had a higher rate of healthcare resource utilization, particularly stationary hospitalizations. Importantly, a multiple regression analysis showed that only HF at baseline remained significant as an independent variable associated with RAASi discontinuation.
Controlling HK in this population is especially important, as recently shown by a Spanish single-centre registry, where potassium normalization after an HK or hypokalaemic episode was associated with lower mortality [8].
The availability of new potassium-binding agents, i.e. patiromer and sodium zirconium cyclosilicate, which are now approved in Europe for the treatment of HK, may change HK treatment patterns and extend the use of RAASis in patients with HF and CKD as already shown with patiromer [2, 9–11]. Whether this translates into better outcomes warrants dedicated clinical trials.
This study has limitations. The questionnaire was not specifically designed to capture changes in RAASi prescription and dose reductions or discontinuation (especially after the first HK episode). The sample of physicians and patients was based on practicability, and hence may not be representative. To minimize the problem, a large number of physicians/centres were included who could enter only up to three patients each. Moreover, the characteristics of HK episodes that led to hospitalizations were not contrasted with those of HK episodes that did not lead to hospitalizations.
There are also strengths to this kind of data collection since chart reviews reflect the real-world situation in routine clinical practice including patients with a wider range of age and co-morbidities than those included in clinical trials.
In conclusion, recurrent HK was associated with RAASi discontinuation and dose lowering. Among patients with recurrent HK, a major cause of hospitalization is HK. More effective HK mitigation strategies thus are needed.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Marian Schmidt (study development, data management), Johannes Stadler (statistical analysis), Dr Maria Niki Aigyptiadou and Bianca Arendt (medical writing) and all Kantar Health GmbH, Munich, Germany, as well as Lorraine Zakin, Vifor Pharma Group, Glattbrugg, Switzerland (input to the data analysis and manuscript), for their services.
FUNDING
This study was developed and funded by the Vifor Pharma, Switzerland.
AUTHORS’ CONTRIBUTIONS
M.P. (Kantar Health GmbH, Germany) developed the conception and design of the study, collaborating with G.C. and T.S. (Vifor), for data analysis and compilation. Academic researchers provided their expertise to independently interpret and report the results. They had unrestricted access to the collected data. P.R. and M.P. helped with the outline of the manuscript and data validation. L.H.L. contributed to data interpretation and critical revision of manuscript. P.R. and L.H.L. provided important guidance for the finalization of the manuscript. All authors contributed to drafting the article or critically revised it, and provided intellectual content of critical importance to the work described. All authors read and approved the final version of the article. D.C.W. helped with data interpretation, revising the manuscript, provided intellectual content and approved the final version.
CONFLICT OF INTEREST STATEMENT
P.R. reports personal fees (consulting) for Idorsia and G3P, honoraria from AstraZeneca, Bayer, CVRx, Fresenius, Grunenthal, Novartis, NovoNordisk, Servier, Stealth Peptides, Ablative Solutions, Corvidia, Relypsa and Vifor Fresenius Medical Care Renal Pharma, outside the submitted work, P.R. is the cofounder of CardioRenal. L.M.R. received consulting and speaker fees from Vifor. A.C. received consulting fees from Vifor Fresenius Medical Care Renal Pharma, Fresenius Kabi, Dr Shar, Shire. M.K. received consulting and lecturing fees from Vifor Fresenius Medical Care Renal Pharma, Vifor Pharma and Fresenius Medical Care. D.C.W. received consultancy fees from Amgen, AstraZeneca, Boehringer Ingelheim, Napp, Vifor Fresenius and GalaxoSmithKline. M.P. is an employee of Kantar Health GmbH, Germany, who conducted the study. G.C. and T.S. are employees of the Vifor Pharma Group. L.H.L. received research grants and consulting fees from AstraZeneca, Vifor Pharma and Relypsa.
REFERENCES
- 1. Collins AJ, Pitt B, Reaven N. et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol 2017; 46: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamargo J, Caballero R, Delpon E.. New therapeutic approaches for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors. Cardiovasc Drugs Ther 2018; 32: 99–119 [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 4.National Institute for Health and Care Excellence (NICE). Chronic Kidney Disease in Adults: Assessment and Management Clinical guideline (CG182) (cited 15 January 2018). https://www.nice.org.uk/guidance/cg182 (12 September 2019, date last accessed)
- 5. The Consensus Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429–1435 [DOI] [PubMed] [Google Scholar]
- 6. Einhorn LM, Zhan M, Hsu VD. et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169: 1156–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med 2015; 128: 1281–1287 [DOI] [PubMed] [Google Scholar]
- 8. Nunez J, Bayes-Genis A, Zannad F. et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation 2018; 137: 1320–1330 [DOI] [PubMed] [Google Scholar]
- 9. Pitt B, Anker SD, Bushinsky DA. et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 2011; 32: 820–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weir MR, Bakris GL, Bushinsky DA. et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372: 211–221 [DOI] [PubMed] [Google Scholar]
- 11. Agarwal R, Rossignol P, Romero A. et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019; doi: 10.1016/S0140-6736(19)32135-X. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.