Abstract
Patient: Male, 15-year-old
Final Diagnosis: COVID-19 • COVID-19 skin lesions • taste disorder
Symptoms: Sore throat
Medication: —
Clinical Procedure: —
Specialty: Otolaryngology
Objective:
Unusual clinical course
Background:
The coronavirus disease 2019 (COVID-19) pandemic that spread from China is caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2). The head and neck region can be variably affected in adult patients, and taste and smell disorders are typical manifestations. However, pediatric clinical signs are less severe, making the onset diagnosis challenging to interpret. The variability of nasal olfactory symptoms in children and adolescents is intertwined with possible warning signs, including gastrointestinal, ocular, or dermato-logical symptoms. We present a case involving a 15-year-old boy with clinically confirmed COVID-19 who had late-onset rash and transient taste and smell disorders.
Case Report:
The boy’s clinical history revealed that a family member was positive for SARS-CoV-2. In the preceding 3 days, the boy’s eating habits had changed; he perceived a metallic taste while eating and had a loss of appetite. He also had erythematous skin lesions on the lower limbs for the 2 previous days. A sore throat, nasal congestion, and a runny nose were reported on head and neck examination. A real-time polymerase chain reaction test was positive, confirming the initial diagnostic hypothesis.
Conclusions:
SARS-CoV-2 virus infection in children and adolescents can be asymptomatic, but it can also occur with fever, dry cough, fatigue, and gastrointestinal symptoms. Due to the unique immune characteristics of pediatric and adolescent patients, the correct interpretation of the gustatory and skin symptoms associated with specific laboratory tests for SARS-CoV-2 infection can lead to the most appropriate management and supportive care.
MeSH Keywords: Adult Children, COVID-19, Dysgeusia, SARS Virus, Taste
Background
The coronavirus disease 2019 (COVID-19) pandemic that spread from China is caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) [1]. The associated symptom-atology can be extremely variable, with presentation ranging from nonspecific symptoms to severe pneumonia and multiple organ dysfunction [1–3].
The head and neck region (including the ear, nose, and throat [ENT]) can be variably affected, and a typical manifestation of COVID-19 described in the literature is the disturbance of smell and taste. However, this symptom assumes variable relevance in children and adolescents [3]. It is also known that certain comorbidities, such as diabetes, lung disease, or asthma, negatively affect patients’ prognosis [4].
Pediatric clinical manifestations have been reported as being less severe, probably due to a lower expression of angiotensin-converting enzyme 2 (ACE2), which is physiologically involved in the maturation of angiotensin II and can bind the spike proteins of SARS-CoV-2 [5]. Incomplete immunological development of children and adolescents may also play an important role.
Although data regarding SARS-CoV-2 infection in children and adolescents are currently incomplete, it appears that infection can be asymptomatic in some individuals, while others may experience fever, dry cough, and fatigue, as well as gastrointestinal symptoms such as nausea, vomiting, abdominal pain, and diarrhea [6–11].
To the best of our knowledge, transient, isolated disorders of taste and skin manifestations of SARS-CoV-2 infections in children have not yet been described. Our clinical case shows a correlation between a variable and nuanced taste symptom-atology preceding late-onset COVID-19 skin manifestation in an adolescent patient with a clinical history of family infection.
Case Report
A 15-year-old boy was sent to our ENT unit after pediatric consultation that revealed the presence of erythematous skin lesions on the lower limbs for 2 days and asthenia. No lesions were present in the head and neck region (Figures 1, 2).
Figure 1.
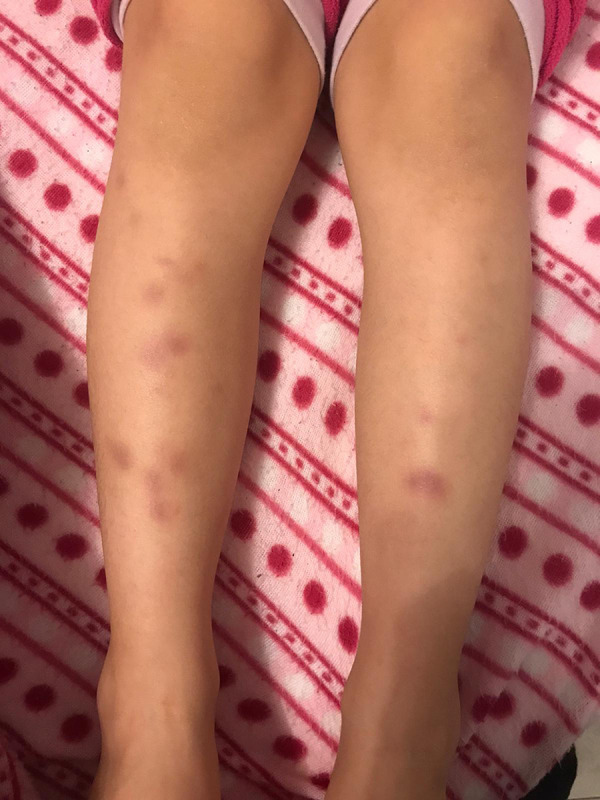
Multiple patchy erythematous lesions spreading on both lower limbs of the patient.
Figure 2.
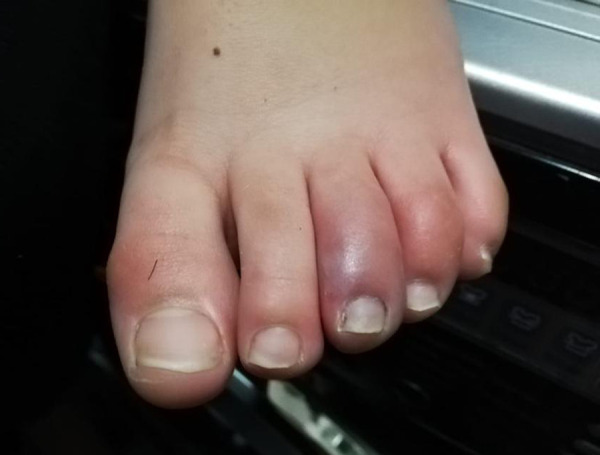
Erythematous and edematous lesions of the toes with associated pain and itching. Characteristic purple erythematous manifestations located on the extremity of the third toe.
The patient’s medical history included probable exposure to SARS-CoV-2 due to another family member who had tested positive for the virus and was symptomatic. The boy’s mother had experienced a mild respiratory disorder in the absence of respiratory distress or desaturations, which only required symptomatic home therapy and preventive quarantine for 2 weeks. The boy’s parents reported that his eating habits had changed in the preceding 3 days in association with a loss of appetite and the emergence of smell-related disorders.
On clinical examination, the patient had a mild fever (37.7°C), and the ENT specialist found that the patient had a sore throat, nasal congestion, and a runny nose. No neurological symptoms were reported upon specialist examination.
Routine medical laboratory tests were performed to assess inflammation indices, the possibility of cholestasis, and renal function (Table 1). The patient also underwent community COVID-19 screening, which involved testing for SARS-Cov-2 nucleic acid on nasopharyngeal and oropharyngeal swabs according to the guidelines provided by the manufacturer of the Cepheid/GenXpert system.
Table 1.
Hematic, blood chemistry and organ function laboratory tests performed at the clinical presentation.
| Test type | Values (range) |
|---|---|
| White blood cell count, ×109/L | 10.33 (3.5–9.5) |
| Lymphocyte count, ×109/L | 5.21 (1.1–3.2) |
| Neutrophil count, ×109/L | 3.99 (1.8–6.3) |
| Platelet count, ×109/L | 315 (125–350) |
| C-reactive protein (CRP), mg/L | 8.3 (0–10) |
| Alanine aminotransferase (ALT), U/L | 21 (9–50) |
| Aspartate aminotransferase (AST), U/L | 27 (15–40) |
| Urea, mmol/L | 6.81 (3.1–8.0) |
| Creatinine, umol/L | 49.2 (57–97) |
Within the next 48 hours, the real-time polymerase chain reaction (PCR) test was positive, confirming the initial diagnostic hypothesis. The laboratory tests were repeated every 48 hours to monitor the inflammation indices and white blood cell count.
Four days after the initial tests were performed, the patient exhibited a new-onset cough, a higher fever (38.5°C), and mild respiratory symptoms. Considering the definite SARS-CoV-2 diagnosis obtained with PCR testing, we treated symptoms with acetaminophen and azithromycin by oral suspension. We only clinically observed the patient due to his continuing hospitalization. Throughout the patient’s stay, he had a lack of appetite and transiently reported a metallic taste while eating. The respiratory symptoms regressed 7 days after the initial onset, and the boy started eating normally without impaired taste 12 days later. The skin manifestations disappeared 16 days from the first detection.
The follow-up at day 21 revealed a complete absence of the clinical-instrumental relevant data, which was further confirmed by a negative mucosal swab and ENT examination. Currently, the patient does not present any signs of pathology or other noteworthy disorders.
Discussion
The SARS-CoV-2 pandemic dates back to December 2019 when patients with pneumonia of unknown etiology were described in the city of Wuhan, China [12]. As proved by Xu et al. [13], ACE2 acts as a doorway for infection by being the functional receptor of SARS-CoV-2.
Currently, although the pathology is infectious, it is often difficult to identify the disease in the adult population, even in the presence of mild pneumonia, because symptoms can be masked. The symptoms of infection may only be a fever, asthenia, or disorientation, which delays diagnosis and potentially leads to a worse prognosis [14,15].
The heterogeneous presentation can involve several body systems other than the respiratory system. The most frequent nonrespiratory signs include gastrointestinal symptoms in up to 40% of patients, with diarrhea in particular being an initial warning sign for SARS-CoV-2 infection [14–17] .Some patients can experience neurological disorders, including headache, dizziness, or altered mental state, as well as more severe issues such as ischemic or hemorrhagic stroke [18].
In addition, owing to the presence of ACE2 receptors in the cardiovascular system, possible implications of COVID-19 range from variable minor myocardial lesions to impaired cardiac function due to myocarditis and myopericarditis [19,20]. Of particular interest is a recent pediatric finding that showed slight changes in troponin and creatine kinase [21]; however, our patient did not show any altered indices. Furthermore, a recent study reporting a case of ocular involvement discussed the role of ACE2 expression in the cornea and conjunctiva with regard to ocular surface tissues being a potential target for SARS-CoV-2 infection [21].
Recently, a multicenter European study investigated the occurrence of ENT symptoms, particularly olfactory and gustatory dysfunction in patients with laboratory-confirmed COVID-19 [3]. Although face pain and nasal obstruction were the most frequent disease-related ENT manifestations, the authors found that 85.6% and 88.0% of patients reported olfactory and gustatory dysfunction, respectively. Notably, taste and smell disorders were present before other symptoms in 11.8% of the patients.
Previously, Temmel et al. [22] observed olfactory loss in 39% of patients with infectious diseases affecting the upper respiratory tract. These authors also recognized various categories for prognostic characteristics. In particular, they highlighted that the recovery rate from infectious olfactory loss was higher in young people in whom a smell disorder induced more frequent alimentary problems, especially in men and in hyposmic patients compared with anosmic ones.
More recently, Dong et al. [23] analyzed the effect of age in 2135 pediatric patients with COVID-19 and found that children of all ages appeared susceptible to infection (mean onset age 7 years), without any significant difference between age groups. Furthermore, over 90% of patients were asymptomatic to moderately symptomatic, and the most vulnerable were infants [23].
Taste or olfactory disorders were noted in up to 53% of the cases in a small cohort from Italy, and anosmia was proposed as a new criterion for diagnosis, especially in young people with few other symptoms [24,25]. Additional ENT symptoms may present as a runny nose or nasal obstruction, postnasal drip, sore throat, ear pain, or dysphagia [3].
On physical examination, our patient manifested mild fever, a runny nose, and sore throat. However, based on the clinical history reported by his parents, a transient taste disturbance was supposed. Of particular interest is the finding that the patient’s isolated taste dysfunction began about 3 days before the rhinitis appeared and was probably a sentinel symptom.
The average latent period between dysfunction of the sense of smell and taste and the onset of respiratory symptoms has been reported to be 1 to 4 days [26], as was found in our patient (3 days). Furthermore, although the average duration of sensory symptoms described is about 16.1 days, our patient experienced a better regression time (12 days). A probable explanation for the olfactory and taste involvement widely described in the literature is the expression of the receptor for SARS-CoV-2 on the epithelial cells of the mucous membrane in the oral and nasal cavity [27].
As Mizumoto et al. [28] demonstrated with temporal data and a model for estimating the symptomless proportion of a population, the risk of transmission by asymptomatic people or those with minor symptoms such as a runny nose or sore throat remains to be quantified, although it is suspected that it could be high.
The characteristic clinical course of our patient was complicated by the subsequent presence of late-onset skin lesions on the lower limbs and asthenia in the absence of further skin localization in the head and neck region. Several studies in the literature have correlated SARS-CoV-2 infection to cutaneous manifestations, mostly chilblain-like lesions [29–33]. In a recent preliminary study on 63 adolescent patients, Piccolo et al. [33] reported various phenotypes of skin lesions in adolescents, with the most common types being edematous, erythematous, painful, or itchy lesions on the fingers or soles of the feet.
Moreover, Docherty et al. [34] observed different clusters of symptoms upon patients’ hospital admission, including clusters characterized by musculoskeletal, enteric, and, less commonly, mucocutaneous symptoms.
Laboratory tests of pediatric patients have shown regular or reduced white blood cell counts with probable neutrophilia and thrombocytopenia. Although C-reactive protein and procalcitonin levels may be normal, liver enzymes and lactic dehydrogenase have been found to be altered in up to 30% of cases [35].
In our case, laboratory tests showed that the patient’s lymphocyte count was slightly higher than normal; the opposite trend is often reported in adults [7]. Therefore, considering the variability in the existing data, an analysis of the phenomenon with a large COVID-19 pediatric sample divided by age is undoubtedly necessary.
This need is underscored by reports that COVID-19 could alter the immune system’s response in children and adolescents and lead to severe pathology such as Kawasaki-type disease [36,37]. Further, the pathological process of COVID-19 appears to induce dysregulation of the immune response, with roles played by neutrophils, CD4+ T cells, and B cells [38].
The process underlying these finding is conceivable given the different physiological functions of CD8 T cells, which commonly participate in antiviral immunity in children. CD8 T cells have been shown to be more susceptible to age-related changes for several functional subgroups that have a more pronounced capacity than naive cells and functional memory subsets. Indeed, Qin et al. [39] observed patients from the epidemic in Wuhan who presented a reduced T-cell number due to probable dys-regulation of the immune response of T lymphocytes.
The diagnosis of COVID-19 might be particularly complicated in specific populations; in particular, children frequently experience milder disease than adults, with few or no symptoms [39–44]. As reported in a review by Zimmermann et al. [43], children and adolescents have the same probability of contracting the virus as adults, and typical epidemiological and clinical features include cough (48%; range, 19–100%), fever (42%; 11–100%), and pharyngitis (30%; 11–100%), as well as nasal congestion, rhinorrhea, tachypnea, wheezing, diarrhea, vomiting, headache, and fatigue. However, Qiu et al. [45] noted that asymptomatic COVID-19 children and adolescents is difficult to identify, which represents a danger for communities.
Diagnostic imaging in children can provide essential information for clinical and therapeutic orientation [40]. For example, a chest x-ray can often show alterations in peripheral lung consolidations or frosted-glass-like opacities. In addition, computed tomography of the chest can provide even more detailed information with the identification of subpleural involvement [41–42].
Treatment is based on providing adequate fluid and calorie support and supplying oxygen to preserve respiratory function and global status. Moreover, anti-inflammatory drugs and prophylactic antibiotic therapy can avoid the onset of secondary infectious complications [46].
Conclusions
The diagnosis of the SARS-CoV-2 patient is often difficult, especially in the initial disease stages in which the patient can be paucisymptomatic or present a nonspecific phenotype without respiratory distress. Due to the singular immune features of pediatric and adolescent patients, diagnosis is even more complicated in these populations, and ENT symptoms paired with abnormal laboratory test results can direct the clinician toward a different differential diagnosis.
Our report of late-onset rash and transient taste disorders associated with COVID-19 in a 15-year-old boy shows that children and adolescents might initially present with no or ambiguous symptoms. However, the correct interpretation of gustatory and skin symptoms associated with specific laboratory testing for SARS-CoV-2 infection can lead to the most appropriate management and supportive care.
Footnotes
Conflict of interest
None.
References:
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–73. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen K, Yang Y, Wang T, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: Experts’ consensus statement. World J Pediatr. 2020;16:223–31. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–61. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–69. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristiani L, Mancino E, Matera L, et al. Will children reveal their secret? The coronavirus dilemma. Eur Respir J. 2020;55(4):2000749. doi: 10.1183/13993003.00749-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavone P, Giallongo A, La Rocca A, et al. Recent COVID-19 outbreak: Effects in childhood. Infect Dis Trop Med. 2020;6:e594. [Google Scholar]
- 7.Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong H, Wang Y, Chung HT, et al. Clinical characteristics of novel corona-virus disease 2019 (COVID-19) in newborns, infants and children. Pediatr Neonatol. 2020;61:131–32. doi: 10.1016/j.pedneo.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavone P, Ceccarelli M, Taibi R, et al. Outbreak of COVID-19 infection in children: fear and serenity. Eur Rev Med Pharmacol Sci. 2020;24:4572–75. doi: 10.26355/eurrev_202004_21043. [DOI] [PubMed] [Google Scholar]
- 10.Zheng F, Liao C, Fan QH, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40:275–80. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020 doi: 10.1016/S2352-4642(20)30177-2. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modelling of its spike protein for risk of human transmission. Sci China Life. 2020;63:457–60. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan. Allergy. 2020;75:1730–41. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y, Liu P, Shi XL, et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69:1143–44. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 17.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–55. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247–50. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu P, Liang L, Chen C, Nie S. A child confirmed COVID-19 with only symptoms of conjunctivitis and eyelid dermatitis. Graefes Arch Clin Exp Ophthalmol. 2020;258:1565–66. doi: 10.1007/s00417-020-04708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Temmel AF, Quint C, Schickinger-Fischer B, et al. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002;128:635–41. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 23.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 24.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study. Clin Infect Dis. 2020;71:889–90. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089–90. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelardi M, Trecca E, Cassano M, Ciprandi G. Smell and taste dysfunction during the COVID-19 outbreak: A preliminary report. Acta Biomed. 2020;91:230–31. doi: 10.23750/abm.v91i2.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (covid-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guarneri C, Rullo EV, Pavone P, et al. Silent COVID-19: What your skin can reveal. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30402-3. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. 2020;98(2):75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recalcati S. Cutaneous manifestations in COVID-19: A first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212–13. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 33.Piccolo V, Neri I, Filippeschi C, et al. Chilblain-like lesions during COVID-19 epidemic: A preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34(7):e291–93. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang XF, Yuan J, Zheng YJ, et al. [Retracted: Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen.] Zhonghua Er Ke Za Zhi. 2020 doi: 10.3760/cma.j.issn.0578-1310.2020.0008. [Online ahead of print] [in Chinese] [DOI] [PubMed] [Google Scholar]
- 36.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: Prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet. 2020;395(10239):1771–78. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3(6):e2010895. doi: 10.1001/jamanetworkopen.2020.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–68. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babyn PS, Chu WC, Tsou IY, et al. Severe acute respiratory syndrome (SARS): Chest radiographic features in children. Pediatr Radiol. 2004;34:47–58. doi: 10.1007/s00247-003-1081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng K, Yun YX, Wang XF, et al. [Analysis of CT features of 15 children with 2019 novel coronavirus infection.] Zhonghua Er Ke Za Zhi. 2020 doi: 10.3760/cma.j.cn112140-20200210-00071. [Online ahead of print] [in Chinese] [DOI] [PubMed] [Google Scholar]
- 42.Li AM, Ng PC. Severe acute respiratory syndrome (SARS) in neonates and children. Arch Dis Child Fetal Neonatal Ed. 2005;90:F461–65. doi: 10.1136/adc.2005.075309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: A review of epidemiologic and clinical features. Pediatr Infect Dis J. 2020;39:469–77. doi: 10.1097/INF.0000000000002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.2430. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect Dis. 2020;20(6):689–96. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020;16:240–46. doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


