Abstract
Background
Centers from Europe and United States have reported an exceedingly high number of children with a severe inflammatory syndrome in the setting of coronavirus disease 2019, which has been termed multisystem inflammatory syndrome in children (MIS-C).
Objectives
This study aimed to analyze echocardiographic manifestations in MIS-C.
Methods
A total of 28 MIS-C, 20 healthy control subjects and 20 classic Kawasaki disease (KD) patients were retrospectively reviewed. The study reviewed echocardiographic parameters in the acute phase of the MIS-C and KD groups, and during the subacute period in the MIS-C group (interval 5.2 ± 3 days).
Results
Only 1 case in the MIS-C group (4%) manifested coronary artery dilatation (z score = 3.15) in the acute phase, showing resolution during early follow-up. Left ventricular (LV) systolic and diastolic function measured by deformation parameters were worse in patients with MIS-C compared with KD. Moreover, MIS-C patients with myocardial injury were more affected than those without myocardial injury with respect to all functional parameters. The strongest parameters to predict myocardial injury in MIS-C were global longitudinal strain, global circumferential strain, peak left atrial strain, and peak longitudinal strain of right ventricular free wall (odds ratios: 1.45 [95% confidence interval (CI): 1.08 to 1.95], 1.39 [95% CI: 1.04 to 1.88], 0.84 [95% CI: 0.73 to 0.96], and 1.59 [95% CI: 1.09 to 2.34], respectively). The preserved LV ejection fraction (EF) group in MIS-C showed diastolic dysfunction. During the subacute period, LVEF returned to normal (median from 54% to 64%; p < 0.001) but diastolic dysfunction persisted.
Conclusions
Unlike classic KD, coronary arteries may be spared in early MIS-C; however, myocardial injury is common. Even preserved EF patients showed subtle changes in myocardial deformation, suggesting subclinical myocardial injury. During an abbreviated follow-up, there was good recovery of systolic function but persistence of diastolic dysfunction and no coronary aneurysms.
Key Words: coronary artery abnormality, COVID-19, deformation, echocardiography, multisystem inflammatory syndrome in children (MIS-C), myocarditis
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; EDSR, early diastolic strain rate; EF, ejection fraction; GCS, global circumferential strain; GLS, global longitudinal strain; KD, Kawasaki disease; LAS, left atrial strain; MIS-C, multisystem inflammatory syndrome in children; RVFWLS, longitudinal strain of the right ventricular free wall; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Central Illustration
The novel coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has infected individuals of all ages in nearly every country on Earth. In Europe, Italy was the first country to be affected significantly by the outbreak of COVID-19, which began there in February 2020. From the Bergamo province, the epicenter of the Italian outbreak, Verdoni et al. (1) first reported an exceedingly high number of Kawasaki disease (KD)–like cases starting in March 2020, with a monthly incidence that greatly exceeded the monthly incidence of the previous 5 years. Moreover, there was a clear starting point after the first case of COVID-19 was diagnosed in the Italian epicenter (1).
More recently, several centers in the United States and Europe have reported similar KD-like illness or symptoms of toxic shock syndrome in children who have tested positive for COVID-19 (2,3). These patients may develop a cytokine storm possibly due to activation of macrophages, similar to the secondary hemophagocytic lymphohistiocytosis syndrome triggered by some other viral infections (4). The condition has been termed “multisystem inflammatory syndrome in children (MIS-C)” or “pediatric multisystem inflammatory syndrome (PMIS)” and is characterized by a hyperinflammatory syndrome with multiorgan dysfunction (3,5,6). The disease appears to be a delayed post-infectious response with a lag time of 4 to 6 weeks following mostly asymptomatic exposure to the SARS-CoV-2 (7). This diagnosis has received widespread coverage in the lay press and has been a cause for consternation for both physicians and parents alike, due to a paucity of published research about its cardiac manifestations.
Recent studies have described coronary artery anomalies and conventional echocardiographic parameters like ejection fraction (EF) and annular velocities in MIS-C patients. However, the detailed analysis of cardiac mechanics using deformation parameters are lacking. Deformation parameters are sensitive tools to detect subtle changes in myocardial function and have not been studied in this disease (8). Moreover, comparison of MIS-C with classic KD is sparse in published data and needs to be studied in greater detail.
Our group set out to analyze primarily the cardiac manifestations of MIS-C, following a positive diagnosis of COVID-19. Our aim was to describe anatomic and functional echocardiographic manifestations of this disease in children. We hypothesized that MIS-C may be associated with reduced systolic and diastolic function similar to other forms of viral myocarditis and may produce dilatation of coronary arteries similar to KD.
Methods
Study population
This is a retrospective, single-center study performed at The Children’s Hospital of Philadelphia. We retrospectively reviewed data from patients who were admitted to our institution and its affiliate institutions with the diagnosis of MIS-C over a 2-month period, from April 10, 2020, to June 7, 2020. The clinical diagnosis of MIS-C was made according to the health advisory put forth by the U.S. Centers for Disease Control and Prevention and the World Health Organization (3,5).
All patients were tested for the SARS-CoV-2 virus during the hospital course by nasopharyngeal swab polymerase chain reaction and a chemiluminescent microparticle immunoassay for the qualitative detection of immunoglobulin G antibodies to the nucleocapsid protein of the SARS-CoV-2. The patients were formally diagnosed with COVID-19 infection if any of these tests were positive. We excluded MIS-C patients with underlying cardiac diseases, those with acute respiratory distress syndrome, those treated with extracorporeal membrane oxygenation therapy, as well as those who had undergone chemotherapy in the past. We classified MIS-C patients as “KD-like” or “toxic shock–like” based on their symptoms. Shock-like presentation was characterized by signs of cardiovascular collapse requiring volume resuscitation and vasopressors, and requiring intensive care. Moreover, we divided MIS-C patients into 2 groups: a clinically suspected myocardial injury (+) group versus myocardial injury (−) group. Because none of the patients underwent endomyocardial biopsies or cardiac magnetic resonance imaging (CMR), we defined myocardial injury based on 2 biomarkers, elevated brain natriuretic peptide (BNP) (>500 pg/ml) and/or positive troponin-I (>0.3 ng/ml) (9,10). We further divided MIS-C patients into 2 additional groups: a preserved left ventricle (LV) ejection fraction (EF) group (LVEF ≥55%) versus an impaired LVEF (LVEF <55%) group (11).
As a control group, we included 20 healthy controls among similar age groups with no structural and functional heart defects who visited our hospital before the COVID-19 pandemic for evaluation of heart murmurs, chest pain, or syncope. In addition, we included a second control group of 20 consecutive patients of classic KD from the pre-COVID-19 pandemic era (from January 2019 to December 2019). These classic KD patients had no previous episodes of KD and were studied within the first 7 days of their illness.
We reviewed echocardiographic images in the acute phase in our MIS-C and KD groups. The majority of the initial echocardiographic examination was performed before the administration of intravenous immunoglobulin (IVIG). Follow-up echocardiographic images in subacute period were also reviewed in the MIS-C group.
Echocardiography
Two-dimensional echocardiography was performed by experienced cardiac sonographers using 2 different ultrasound systems. To observe infection control protocols, for some of the MIS-C patients, an Affiniti 70C ultrasound system (Philips Medical Systems, Andover, Massachusetts) was used. For the rest of the COVID-19 patients, all of the KD patients and all normal control subjects, the EPIC CVx ultrasound system (Philips Medical Systems) was used. Standard echocardiographic measurements were made in accordance with American Society of Echocardiography guidelines including LVEF using Simpson’s biplane method, the early and late mitral inflow peak velocities by spectral Doppler, tricuspid annular plane systolic excursion by M-mode, and the early diastolic septal and lateral mitral annular peak velocities and lateral tricuspid annular peak velocity by tissue Doppler. LVEF was confirmed by expert reviewers in all MIS-C patients.
In addition, we paid special attention to evaluate coronary artery abnormalities in both the KD and MIS-C groups in accordance with standard guidelines. Coronary artery z-scores were derived from previously described normative data (Boston z score system) and used to classify coronary artery abnormalities as follows: normal <2, dilatation ≥2 to <2.5, aneurysm ≥2.5. Based on the American Heart Association (AHA) statement paper of the KD committee, we defined ectasia as coronary dilatation without a segmental aneurysm (12). Before listing coronary arteries as dilated or aneurysmal, the measurements were rechecked by 2 investigators (A.B., M.Q.) blinded to clinical data.
Speckle tracking analysis
Myocardial deformation was assessed offline using 2-dimensional speckle tracking vendor-independent software (2D CPA 1.3.0.91, TomTec Imaging Systems, Munich, Germany). The endocardial border in LV was automatically traced after manually setting the timing of end-diastole and -systole in a single-loop. The trace was adjusted manually if needed.
Peak longitudinal strain (LS) and systolic longitudinal strain rate were calculated by the software from 4-, 3-, and 2-chamber images, and averaged for global longitudinal strain (GLS) and global longitudinal strain rate (GLSR). Longitudinal early diastolic strain rate (EDSRL), peak global left atrial strain (LAS), and peak longitudinal strain of the right ventricular free wall (RVFWLS) were measured from 4 chamber images. Peak circumferential strain (CS), systolic circumferential strain rate, circumferential early diastolic strain rate (EDSRC) were measured from midcavity short-axis views. Segments with unreliable tracking on visual inspection were excluded from the analysis.
Statistics
Continuous data were expressed as median and interquartile range (IQR). Differences between an acute and a follow-up study in the MIS-C group were assessed using Wilcoxon signed rank sum test, while the independent 3 groups were assessed using Kruskal-Wallis test followed by Steel-Dwass’s test for pairwise comparisons. For conventional diastolic parameters, we did not perform statistical analysis between the MIS-C and KD groups, due to a big difference in age between the 2 groups. To assess for predictors of myocardial injury in patients presenting with MIS-C, a logistic regression analysis was performed for each echocardiographic measurement of interest adjusted by age and sex. Correlation coefficients between 2 variables were calculated using Spearman’s rank order correlation test. A p value <0.05 was considered as statistical significance. Paired Student’s t-test was performed to analyze the follow-up data. All statistical analyses were performed with STATA 14 (StataCorp, College Station, Texas).
Intraobserver and interobserver variabilities for deformation parameters used in this study have shown a high degree of reproducibility in our laboratory, based on several recent research projects and were not repeated for this study (13, 14, 15).
This study was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia.
Results
Patient characteristics
A total of 28 MIS-C patients were included in this study. Table 1 shows demographic data for our 3 groups of patients, while Supplemental Table 1 shows demographic data for each individual MIS-C patient. Compared with KD patients, MIS-C patients were significantly older (median age: 11.4 years vs. 3.1 years; p < 0.01) and had larger statures (median body mass index: 22.5 kg/m2 vs. 16.0 kg/m2; p < 0.01). In total, 50% of MIS-C patients were considered obese based on the World Health Organization definition. There was no sex difference in MIS-C patients. Our MIS-C population included 5 (18%) with KD-like and 23 (82%) with toxic shock–like symptoms. In 8 patients, polymerase chain reaction was negative but immunoglobulin G testing was positive. Moreover, no other microbial cause of illness was identified in any patient. Medications used included aspirin (86%), IVIG (80%), vasopressors (78%), corticosteroids (96%), and anticoagulants (73%). Respiratory therapy included invasive ventilation (25%), continuous positive airway pressure (7%), and bilevel positive airway pressure (18%).
Table 1.
Demographics in 3 Groups
| MIS-C (Total) (n = 28) | Control (n = 20) | Classic KD (n = 20) | p Value |
|||
|---|---|---|---|---|---|---|
| MIS-C vs. Control | MIS-C vs. KD | Control vs. KD | ||||
| Age, yrs | 11.4 (8.0–13.7) | 9.0 (7.6–10.7) | 3.1 (2.3–4.1) | 0.39 | <0.001 | <0.001 |
| Male | 50 | 55 | 75 | 0.78 | 0.14 | 0.14 |
| Body weight, kg | 47.9 (36.4–65.7) | 31.7 (26.5–43.0) | 16.3 (13.3–17.5) | 0.048 | <0.001 | <0.001 |
| BSA, m2 | 1.43 (1.14–1.73) | 1.09 (1.00–1.35) | 0.64 (0.59–0.71) | 0.07 | <0.001 | <0.001 |
| BMI, kg/m2 | 22.5 (19.4–25.7) | 17.8 (16.7–19.4) | 16.0 (15.3–17.7) | 0.002 | <0.001 | 0.30 |
| Heart rate, beats/min | 116 (89 – 136) | 78 (72–88) | 119 (105–140) | <0.001 | 0.74 | <0.001 |
| Systolic BP, mm Hg | 99 (94–112) | 107 (101–112) | 100 (96–109) | 0.65 | 0.96 | 0.30 |
| Diastolic BP, mm Hg | 56 (51–62) | 59 (56–68) | 55 (50–64) | 0.27 | 0.97 | 0.27 |
| Race/ethnicity | ||||||
| African American | 46 | 20 | 20 | 0.22 | 0.22 | 1.0 |
| Caucasian | 25 | 65 | 40 | 0.02 | 0.41 | 0.41 |
| Hispanic | 14 | 5 | 5 | 1.0 | 1.0 | 1.0 |
| Asian | 4 | 5 | 15 | 1.0 | 0.88 | 1.0 |
| Others/unknown | 11 | 5 | 20 | 1.0 | 1.0 | 1.0 |
Values are median (interquartile range) or %.
BMI = body mass index; BP = blood pressure; BSA = body surface area; KD = Kawasaki disease; MIS-C = multisystem inflammatory syndrome in children.
Laboratory data
All patients presented in a hyperinflammatory state characterized by elevated C-reactive protein, high erythrocyte sedimentation rate, and lymphopenia. Myocarditis was suspected in 17 cases (61%) by elevated BNP (>500 pg/ml) and positive troponin I (>0.3 ng/ml). Additional characteristic findings included other organ dysfunction (renal and/or liver), hypercoagulation state, anemia, and thrombocytopenia. At least 12 cases fulfilled the 2016 classification criteria for macrophage activation syndrome (16). Detailed laboratory data for all 28 patients are depicted in Supplemental Table 2.
Echocardiographic findings
Echocardiographic data for all MIS-C patients are shown in Tables 2, 3, and 4 and Supplemental Table 3. During early follow-up (interval of 5.2 ± 3 days) we evaluated 20 of 28 patients (71%) in MIS-C cohort, because some patients were followed by other institutions.
Table 2.
Results of Conventional Echocardiographic Parameters in 3 Groups
| MIS-C (Total) (n = 28) | Control (n = 20) | Classic KD (n = 20) | p Value |
||
|---|---|---|---|---|---|
| MIS-C vs. Control | MIS-C vs. KD | ||||
| MR > trivial | 13 (46) | 0 (0) | 3 (15) | <0.001 | 0.06 |
| Pericardial effusion | 9 (32) | 0 (0) | 3 (15) | 0.02 | 0.46 |
| Pleural effusion | 11 (39) | 0 (0) | 0 (0) | 0.004 | 0.004 |
| LVEF, % | 57 (48–61) | 64 (61–66) | 66 (59–69) | <0.001 | 0.003 |
| LVFS, % | 32 (26–38) | 38 (35–39) | 36 (32–40) | 0.02 | 0.09 |
| E/A ratio | 1.30 (1.16–2.78) | 2.21 (1.88–2.59) | 1.46 (1.31–2.10) | 0.14 | — |
| Septal mitral eʹ, m/s | 0.13 (0.09–0.13) | 0.12 (0.11–0.13) | 0.11 (0.10–0.11) | 0.97 | — |
| Lateral mitral eʹ, m/s | 0.15 (0.14–0.17) | 0.20 (0.20–0.20) | 0.14 (0.11–0.14) | 0.001 | — |
| Lateral eʹ/septal eʹ ratio | 1.37 (1.24–1.52) | 1.38 (1.26–1.51) | 1.27 (1.10–1.40) | 0.94 | 0.42 |
| Averaged E/eʹ ratio | 8.2 (7.5–10.6) | 6.7 (6.1–8.2) | 8.3 (8.1–9.3) | 0.02 | — |
| TAPSE, cm | 2.0 (1.6–2.1) | 2.1 (1.9–2.4) | 1.9 (1.6–2.0) | 0.01 | 0.99 |
| RVFW eʹ, m/s | 0.15 (0.13–0.18) | 0.14 (0.13–0.15) | 0.14 (0.11–0.16) | 0.47 | — |
Values are n (%) or median (interquartile range).
KD = Kawasaki disease; LVEF = left ventricular ejection fraction; LVFS = left ventricular fractional shortening; MIS-C = multisystem inflammatory syndrome in children; MR = mitral regurgitation; RVFW = right ventricle free wall; TAPSE = tricuspid annular plane systolic excursion.
Table 3.
Results of Coronary Artery Echocardiography in KD and MIS-C Groups
| Acute Phase |
p Value | Follow-Up |
||
|---|---|---|---|---|
| Classic KD (n = 20) | MIS-C (Total) (n = 28) | MIS-C (Total) (n = 20) | ||
| Illness day at echocardiography∗ | 6 (6–8) | 6 (5–7) | 0.20 | 10 (8–14) |
| Coronary dilatation | 2 (10) | 0 (0) | 0.17 | 0 (0) |
| Coronary ectasia | 0 (0) | 1 (4) | 1.0 | 0 (0) |
| Coronary aneurysm | 2 (10) | 0 (0) | 0.17 | 0 (0) |
Values are median (interquartile range) or n (%).
KD = Kawasaki disease; MIS-C = multisystem inflammatory syndrome in children.
Illness day 1 = first day of fever.
Table 4.
Results of Strain Parameters in 3 Groups
| MIS-C (Total) (n = 28) | Control (n = 20) | Classic KD (n = 20) | p Value |
||
|---|---|---|---|---|---|
| MIS-C vs. Control | MIS-C vs. KD | ||||
| GLS, % | −16.2 (−19.5 to −13.6) | −22.5 (−23.7 to −21.1) | −20.1 (−21.8 to −18.1) | <0.001 | 0.02 |
| GLSR, 1/s | −0.84 (−1.05 to −0.71) | −1.15 (−1.24 to −1.08) | −1.18 (−1.30 to −1.05) | <0.001 | <0.001 |
| GCS, % | −18.1 (−21.4 to −16.0) | −23.9 (−26.5 to −22.8) | −21.4 (−25.5 to −19.3) | <0.001 | 0.03 |
| GCSR, 1/s | −0.93 (−1.15 to −0.78) | −1.35 (−1.55 to −1.25) | −1.25 (−1.43 to −1.10) | <0.001 | 0.001 |
| EDSRL, 1/s | 0.91 (0.76 to 1.13) | 1.66 (1.40 to 1.90) | 1.26 (1.05 to 1.48) | <0.001 | 0.003 |
| EDSRC, 1/s | 1.02 (0.90 to 1.15) | 1.77 (1.63 to 2.09) | 1.28 (1.15 to 1.55) | <0.001 | 0.001 |
| LAS, % | 26.5 (20.0 to 33.7) | 40.5 (37.7 to 46.8) | 34.7 (30.4 to 37.0) | <0.001 | 0.04 |
| RVFWLS, % | −18.9 (−24.0 to −14.3) | −28.2 (−30.4 to −24.5) | −24.4 (−27.1 to −21.8) | <0.001 | 0.02 |
Values are median (interquartile range).
EDSRC = circumferential early diastolic strain rate; EDSRL = longitudinal early diastolic strain rate; GCS = global circumferential strain; GCSR = global circumferential strain rate; GLS = global longitudinal strain; GLSR = global longitudinal strain rate; KD = Kawasaki disease; LAS = left atrial strain; MIS-C = multisystem inflammatory syndrome in children; RVFWLS = right ventricular free wall longitudinal strain.
Conventional echocardiographic assessment
Table 2 shows conventional echocardiographic parameters, and Table 3 shows the results of coronary artery echocardiography. The coronary arteries from a typical MIS-C patient are depicted in Figure 1 . It is noteworthy that among 28 MIS-C patients, only 1 case (4%) manifested coronary artery abnormality in our cohort (coronary ectasia; z score: 3.15 in the right coronary artery). No segmental aneurysms were detected. On the other hand, in classic KD, 4 cases (20%) of coronary abnormalities including 2 with aneurysms (max z score: 5.0 in right coronary artery) were detected. During the early follow-up studies, the single MIS-C patient with coronary ectasia showed resolution of the ectasia (z score changed from 3.15 to 1.8 in right coronary artery), and no other progressive coronary lesions were detected in the MIS-C group.
Figure 1.
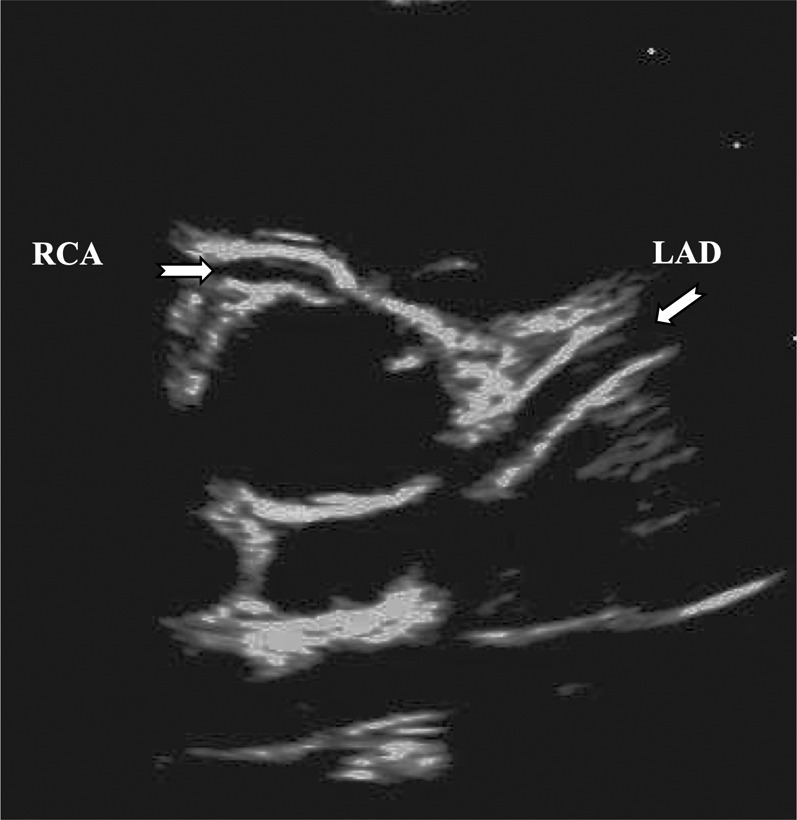
Echocardiographic Image of a Patient With MIS-C With Normal Coronary Arteries
Although the coronary artery may appear prominent, the Z score is 1.7 for left anterior descending artery (LAD) and 1.3 for right coronary artery (RCA).
Compared with the classic KD group, MIS-C patients had more mitral regurgitation (MR) (46% vs. 15%; p = 0.06), more pericardial effusion (32% vs. 15%; p = 0.46), and more pleural effusion (39% vs. 0%; p = 0.004). In addition, MIS-C patients with suspected myocardial injury (+) showed a greater frequency of these anomalies than cases without myocardial injury (−).
Functional echocardiographic assessment
Table 4 shows the results of deformation parameters in 3 groups. Compared with the normal group, MIS-C patients showed both LV systolic and LV diastolic dysfunction. In addition, peak LAS and peak RVFWS were also significantly lower in MIS-C patients.
When compared with classic KD, MIS-C patients demonstrated worse LV systolic and LV diastolic function by all deformation parameters (Figure 2 ). Moreover, MIS-C patients suspected of myocardial injury (+) were more affected than injury (−) MIS-C patients, with respect to all functional parameters (Figure 3, Table 5 ). The strongest predictors associated with myocardial injury in MIS-C patients were GLS, GCS, LAS, and RVFWS (odds ratio: 1.45 [95% confidence interval (CI): 1.08 to 1.95], 1.39 [95% CI: 1.04 to 1.88], 0.84 [95% CI: 0.73 to 0.96], 1.59 [95% CI: 1.09 to 2.34], respectively). Some parameters (GLSR and GCSR) presented overfitted models and the estimates could be misleading; therefore, they are not recognized here (Table 6 ).
Figure 2.
Deformation Parameters in 3 Groups
(A) Global longitudinal strain (GLS). (B) Global longitudinal strain rate (GLSR). (C) Longitudinal early diastolic strain rate (EDSRL). (D) Peak left atrial strain (LAS). Patients with MIS-C were worst affected among 3 groups. KD = Kawasaki disease.
Figure 3.
Strain Curves
Strain curves in a normal (A) and multisystem inflammatory syndrome in children (MIS-C) patient during acute phase (B) for global longitudinal strain (GLS). (C) GLS curves and (D) left atrial strain (LAS) curves for normal, myocardial injury (+), and injury (−) in MIS-C patients. All MIS-C patients show decreased GLS and LAS compared with normal patients. In addition, myocardial injury (+) patients are worse than injury (−) patients. myo. = myocardial.
Table 5.
Results of Echocardiographic Measurements in Myocardial Injury (+) Group and (−) Group in MIS-C
| MIS-C |
Control (n = 20) | p Value |
||||
|---|---|---|---|---|---|---|
| Myocardial Injury (−) (n = 11) | Myocardial Injury (+) (n = 17) | (−) vs. (+) | (−) vs. C | (+) vs. C | ||
| Male | 45 | 53 | 55 | 0.69 | 0.70 | 1 |
| Age, yrs | 11.7 (9.3 to 13.6) | 11.0 (8.1 to 13.5) | 9.0 (7.6 to 10.7) | 0.33 | 0.097 | 0.17 |
| LVSF, % | 38 (36 to 40) | 27 (24 to 30) | 38 (35 to 39) | <0.001 | 0.36 | <0.001 |
| LVEF, % | 62 (60 to 62) | 51 (43 to 58) | 64 (61 to 66) | <0.001 | 0.12 | <0.001 |
| Septal mitral eʹ, m/s | 0.13 (0.12 to 0.13) | 0.11 (0.08 to 0.13) | 0.12 (0.11 to 0.13) | 0.07 | 0.14 | 0.28 |
| Lateral mitral eʹ, m/s | 0.16 (0.14 to 0.18) | 0.15 (0.15 to 0.16) | 0.20 (0.20 to 0.20) | 0.46 | 0.003 | 0.001 |
| Lateral/septal eʹ ratio | 1.24 (1.16 to 1.40) | 1.49 (1.33 to 1.69) | 1.38 (1.25 to 1.50) | 0.04 | 0.09 | 0.23 |
| Averaged E/eʹ ratio | 8.2 (7.4 to 9.0) | 9.3 (7.7 to 10.9) | 6.7 (6.1 to 8.2) | 0.39 | 0.03 | 0.0083 |
| GLS, % | −18.3 (−21.2 to −16.9) | -14.2 (-17.5 to -12.5) | −22.5 (−23.7 to −21.1) | 0.02 | 0.02 | <0.001 |
| GLSR, 1/s | −0.95 (−1.13 to −0.88) | -0.77 (-0.88 to -0.68) | −1.15 (−1.24 to −1.08) | 0.03 | 0.04 | <0.001 |
| GCS, % | −21.2 (−22.0 to −19.1) | -16.4 (-18.7 to -14.0) | −23.9 (−26.5 to −22.8) | 0.03 | 0.005 | <0.001 |
| GCSR, 1/s | −1.15 (−1.23 to −0.98) | -0.85 (-1.05 to -0.65) | −1.35 (−1.55 to −1.25) | 0.03 | 0.004 | <0.001 |
| GLS/GCS ratio | 0.90 (0.84 to 1.02) | 0.95 (0.82 to 1.00) | 0.89 (0.83 to 0.99) | 0.48 | 0.35 | 0.35 |
| EDSRL, 1/s | 0.96 (0.86 to 1.21) | 0.85 (0.72 to 1.08) | 1.66 (1.40 to 1.90) | 0.27 | <0.001 | <0.001 |
| EDSRC, 1/s | 1.08 (1.05 to 1.26) | 0.94 (0.82 to 0.99) | 1.77 (1.63 to 2.09) | 0.056 | 0.0023 | <0.001 |
| LAS, % | 33.3 (26.5 to 39.8) | 21.4 (17.6 to 28.7) | 40.5 (37.7 to 46.8) | 0.009 | 0.02 | <0.001 |
| RVFWLS, % | −24.9 (−26.7 to −23.7) | -16.5 (-19.5 to -13.1) | −28.2 (−30.4 to −24.5) | 0.003 | 0.29 | < 0.001 |
Table 6.
Logistic Regression Analysis for Predictors of Myocardial Injury in Patients Presenting With MIS-C
| MIS-C |
OR (95% CI) | ||
|---|---|---|---|
| Myocardial Injury (−) (n = 11) | Myocardial Injury (+) (n = 17) | ||
| GLS | −18.3 (−21.2 to −16.9) | −14.2 (−17.5 to −12.5) | 1.45 (1.08 to 1.95) |
| GLSR | −0.95 (−1.13 to −0.88) | −0.77 (−0.88 to −0.68) | 109.79 (1.62 to 7,441.82) |
| GCS | −21.2 (−22.0 to −19.1) | −16.4 (−18.7 to −14.0) | 1.39 (1.04 to 1.88) |
| GCSR | −1.15 (−1.23 to −0.98) | −0.85 (−1.05 to −0.65) | 31.43 (0.8 to 1,232.75) |
| EDSRL | 0.96 (0.86 to 1.21) | 0.85 (0.72 to 1.08) | 0.08 (0 to 2.91) |
| EDSRC | 1.08 (1.05 to 1.26) | 0.94 (0.82 to 0.99) | 0.14 (0.01 to 2.49) |
| LAS | 33.3 (26.5 to 39.8) | 21.4 (17.6 to 28.7) | 0.84 (0.73 to 0.96) |
| RVFWLS | −24.9 (−26.7 to −23.7) | −16.5 (−19.5 to −13.1) | 1.59 (1.09 to 2.34) |
Values are median (interquartile range) unless otherwise indicated.
CI = confidence interval; OR = odds ratio; other abbreviations as in Table 4.
Compared with the normal group, the preserved LVEF group in MIS-C had significantly lower GLS (median: −18.7% vs −22.5%; p < 0.01) and lower GCS (median: −19.8% vs. −23.9%; p < 0.001) (Table 7 ). In addition, the preserved LVEF group showed diastolic dysfunction in many echocardiographic parameters.
Table 7.
Comparison Between Preserved and Reduced Ejection Fraction Groups in MIS-C
| MIS-C (LVEF <55%) (n = 12) | MIS-C (LVEF >55%) (n = 16) | Control (n = 20) | p Value |
|||
|---|---|---|---|---|---|---|
| LVEF <55% vs. >55% | LVEF <55% vs. Control | LVEF >55% vs. Control | ||||
| Demographics | ||||||
| Myocarditis | 11 (92) | 6 (38) | 0.006 | — | — | |
| Heart rate, beats/min | 132 (97 to 140) | 110 (89 to 131) | 78 (72 to 88) | 0.65 | <0.001 | 0.001 |
| Systolic BP, mm Hg | 104 (98 to 123) | 98 (91 to 111) | 107 (101 to 112) | 0.71 | 0.99 | 0.39 |
| Diastolic BP, mm Hg | 55 (51 to 65) | 57 (53 to 62) | 59 (56 to 68) | 0.83 | 0.29 | 0.51 |
| Systolic function | ||||||
| LVEF, % | 47 (42 to 51) | 61 (60 to 62) | 64 (61 to 66) | <0.001 | <0.001 | 0.06 |
| LVFS, % | 24 (21 to 28) | 36 (33 to 38) | 38 (35 to 39) | 0.002 | <0.001 | 0.52 |
| GLS, % | −12.8 (−15.1 to −12.3) | −18.7 (−20.8 to −16.5) | −22.5 (−23.7 to −21.1) | <0.001 | <0.001 | 0.005 |
| GCS, % | −15.2 (−16.8 to −13.0) | −19.8 (−21.7 to −18.4) | −23.9 (−26.5 to −22.8) | 0.02 | <0.001 | <0.001 |
| Diastolic function | ||||||
| E/A ratio | 1.8 (1.3 to 3.0) | 1.3 (1.1 to 2.0) | 2.2 (1.9 to 2.6) | 0.87 | 0.88 | 0.04 |
| Septal mitral eʹ, m/s | 0.09 (0.08 to 0.12) | 0.13 (0.12 to 0.13) | 0.12 (0.11 to 0.13) | 0.07 | 0.19 | 0.21 |
| Lateral mitral eʹ, m/s | 0.15 (0.12 to 0.17) | 0.16 (0.15 to 0.17) | 0.20 (0.20 to 0.20) | 0.51 | 0.008 | 0.005 |
| Averaged E/eʹ | 9.9 (7.4 to 11.2) | 8.1 (7.5 to 8.6) | 6.7 (6.1 to 8.2) | 0.74 | 0.051 | 0.12 |
| GLSR, 1/s | −0.68 (−0.80 to −0.64) | −0.93 (−1.13 to −0.86) | −1.15 (−1.24 to −1.08) | 0.009 | <0.001 | 0.03 |
| GCSR, 1/s | −0.78 (−0.90 to −0.59) | −1.08 (−1.16 to −0.89) | −1.35 (−1.55 to −1.25) | 0.04 | <0.001 | <0.001 |
| EDSRL, 1/s | 0.76 (0.63 to 1.07) | 0.96 (0.87 to 1.31) | 1.66 (1.40 to 1.90) | 0.12 | <0.001 | <0.001 |
| EDSRC, 1/s | 0.87 (0.66 to 0.99) | 1.05 (0.99 to 1.26) | 1.77 (1.63 to 2.09) | 0.07 | <0.001 | <0.001 |
| LAS, % | 20.5 (17.8 to 27.7) | 32.8 (24.5 to 37.6) | 40.5 (37.7 to 46.8) | 0.03 | <0.001 | 0.003 |
| Right ventricle | ||||||
| TAPSE, cm | 1.4 (1.3 to 1.6) | 2.1 (2.0 to 2.2) | 2.1 (1.9 to 2.4) | 0.002 | <0.001 | 0.92 |
| RVFW eʹ, m/s | 0.17 (0.15 to 0.19) | 0.14 (0.12 to 0.15) | 0.14 (0.13 to 0.15) | 0.51 | 0.17 | 0.99 |
| RVFWLS, % | −14.3 (−17.7 to −12.4) | −23.9 (−25.2 to −19.9) | −28.2 (−30.4 to −24.5) | 0.004 | <0.001 | 0.011 |
During early follow-up studies (interval of 5.2 ± 3 days), most of the MIS-C patients returned to normal LVEF (median LVEF: from 54% to 64%; p < 0.001) but retained a lower GLS (median: from −15.5% to −18.7%; p < 0.001) (published vendor- and age-specific normal range for GLS is −19.5% to −20.8%, from meta-analysis in children) (17). In addition, diastolic dysfunction persisted (Table 8 , Figures 4 and 5 ). It was especially noticeable that both EDSRL and EDSRC remained low. Moreover, right ventricular (RV) systolic function was still impaired (median RVFWLS: from −17.7% to −22.9%; p < 0.001) (normal range for RVFWLS is −32.9% to −27.2% from meta-analysis in children) (18).
Table 8.
Comparison Between Acute Phase and Early Follow-Up Study in MIS-C Patients
| Acute Phase (n = 20) | Follow-Up Study (n = 20) | p Value | |
|---|---|---|---|
| Demographics | |||
| Heart rate, beats/min | 119 (93 to 136) | 81 (62 to 101) | <0.001 |
| Systolic BP, mm Hg | 101 (92 to 111) | 114 (104 to 116) | <0.001 |
| Diastolic BP, mm Hg | 54 (51 to 61) | 58 (54 to 62) | <0.001 |
| Systolic function | |||
| LVEF, % | 54 (45 to 60) | 64 (59 to 67) | <0.001 |
| LVFS, % | 29 (24 to 35) | 36 (33 to 39) | <0.001 |
| GLS, % | −15.5 (−19.5 to −12.5) | −18.7 (−20.4 to −17.1) | <0.001 |
| GCS, % | −17.3 (−21.2 to −15.3) | −21.5 (−25.5 to −18.8) | <0.001 |
| Diastolic function | |||
| E/A ratio | 1.8 (1.1 to 2.7) | 1.8 (1.4 to 2.3) | <0.001 |
| Septal mitral eʹ, m/s | 0.12 (0.09 to 0.13) | 0.10 (0.09 to 0.12) | 0.02 |
| Lateral mitral eʹ, m/s | 0.15 (0.14 to 0.18) | 0.13 (0.12 to 0.15) | 0.006 |
| Averaged E/eʹ | 8.2 (7.2 to 9.8) | 7.6 (7.2 to 9.1) | <0.001 |
| GLSR, 1/s | −0.78 (−0.99 to −0.68) | −0.93 (−1.05 to −0.81) | <0.001 |
| GCSR, 1/s | −0.88 (−1.11 to −0.69) | −1.05 (−1.33 to −0.93) | <0.001 |
| EDSRL, 1/s | 0.91 (0.70 to 1.22) | 0.94 (0.83 to 1.07) | <0.001 |
| EDSRC, 1/s | 0.97 (0.90 to 1.26) | 1.08 (0.96 to 1.38) | <0.001 |
| LAS, % | 24.8 (18.2 to 32.9) | 37.0 (31.7 to 41.8) | <0.001 |
| Right ventricle | |||
| TAPSE, cm | 1.7 (1.4 to 2.0) | 2.2 (2.0 to 2.5) | <0.001 |
| RVFW e', m/s | 0.14 (0.12 to 0.16) | 0.13 (0.11 to 0.16) | 0.41 |
| RVFWLS, % | −17.7 (−23.1 to −13.2) | −22.9 (−25.1 to −21.3) | <0.001 |
Figure 4.
Early Diastolic Strain Rate Curves
Early diastolic strain rate curves in a normal patient (A), multisystem inflammatory syndrome in children (MIS-C) patient during acute phase (B), and MIS-C patient during early follow-up study (C). Note reduced early diastolic strain rate in the acute phase. During the follow-up study, it still remained lower compared with normal patients. AVC = aortic valve closure; EDSRL = longitudinal early diastolic strain rate; SR = strain rate.
Figure 5.
Deformation Parameters in MIS-C Patients During Acute Stage and Early Follow-Up Period
(A) Global longitudinal strain (GLS). (B) Global longitudinal strain rate (GLSR). (C) Longitudinal early diastolic strain rate (EDSRL). (D) Peak left atrial strain (LAS). During the follow-up study, most MIS-C patients showed recovery of systolic function; however, diastolic function (especially EDSRL and LA strain) still remained low. FU = follow-up.
GLS, GCSR, LAS, and RVFWLS showed significant correlation between both BNP and troponin-I (R2 value for BNP: 0.26, 0.17, −0.38, and 0.29, respectively; R2 value for troponin-I: 0.23, 0.18, −0.31, and 0.46, respectively) (Table 9 ), with LAS and RVFWS showing the highest correlation.
Table 9.
Results of Correlation Coefficients Between Functional Parameters and Biomarkers
| BNP |
Troponin I |
|||
|---|---|---|---|---|
| R2 Value | p Value | R2 Value | p Value | |
| GLS | 0.26 | 0.0098 | 0.23 | 0.01 |
| GLSR | 0.12 | 0.09 | 0.12 | 0.09 |
| EDSRL | −0.02 | 0.50 | −0.01 | 0.67 |
| GCS | 0.14 | 0.06 | 0.14 | 0.06 |
| GCSR | 0.17 | 0.04 | 0.18 | 0.03 |
| EDSRC | −0.14 | 0.07 | −0.12 | 0.09 |
| LAS | −0.38 | 0.001 | −0.31 | 0.003 |
| RVFWLS | 0.29 | 0.01 | 0.46 | <0.001 |
BNP = brain natriuretic peptide; other abbreviations as in Table 4.
Discussion
COVID-19 infection has a myriad of presentations, and in Europe and the United States, children with recent COVID-19 infection have presented with a KD-like or toxic shock–like illness, known as MIS-C. In this pilot study, we have described detailed echocardiographic findings of MIS-C (Central Illustration ). Recent studies from continental Europe, England (19), and the United States (20) have described coronary artery anomalies and conventional echocardiographic parameters in MIS-C patients. However, the detailed analysis of cardiac mechanics using deformation parameters derived not only from the LV, but also from the left atrium (LA) and RV in MIS-C patients, is novel and has not been reported previously in children and sparsely in adults (single study on RV strain in adults) (21). This study demonstrates that the coronary arteries may not be frequently affected in the acute phase and in the early follow-up period of MIS-C. The major finding during the acute phase of MIS-C is a myocarditis-like picture, which may remain subtle and subclinical, particularly in the preserved EF cohort. Even in the presence of normal EF, the latter group showed distinct dysfunction in systolic and diastolic deformation parameters. LA strain, which is an index of atrioventricular coupling and a marker of diastolic dysfunction, was markedly reduced. In addition, RVFWS was significantly decreased in MIS-C patients, and proved to be strongly associated with myocardial injury in MIS-C. During early follow-up, most of the MIS-C patients returned to normal systolic function; however, diastolic dysfunction persisted.
Central Illustration.
Echocardiographic Findings in Multisystem Inflammatory Syndrome in Children
EDSRL = longitudinal early diastolic strain rate; GLS = left ventricular global longitudinal strain; LAS = peak global left atrial strain; RVFWLS = peak longitudinal strain of the right ventricular free wall.
Coronary arteries in MIS-C
The coronary arteries were spared in most MIS-C patients in our cohort of U.S. patients. However, due to the small size of our cohort, no definitive statement can be made. Most patients with MIS-C who had a Kawasaki-like presentation were treated with IVIG in our institution. In this pilot study, due to the small sample size, we cannot comment whether the use of IVIG had any coronary-protective effect. Due to unfamiliarity with MIS-C, physicians in most countries have empirically used all medications, like aspirin, IVIG, and methylprednisolone, that are usually reserved for treatment of KD, as previously described in a smaller case series at our institution (22). Both in the treated and untreated groups, the coronary artery involvement was quite infrequent. This is in line with the findings of the Italian study by Verdoni et al. (1) and the French study by Belhadjer et al. (23). In both these European studies, there was mild dilatation of the coronary arteries in <20% patients, but no discrete, segmental aneurysms were noted in either European study. In our cohort, only 1 patient (4%) had coronary ectasia (z score: +3.15) in the acute phase, which returned to normal during the early follow-up stage. In the brief follow-up period described in our study, no other progressive coronary lesions were noted. However, a longer follow-up period may be needed to fully understand the outcome of this novel illness. The Italian study used the older Japanese Ministry of Health and Welfare criteria for coronary aneurysms (>4 mm in patients ≥5 years of age), whereas the French study used coronary artery z-scores and found 17% of patients with coronary dilatation (z-score >2 but <2.5) and no coronary aneurysms. It is our opinion that established coronary z-scores should be used worldwide for reporting coronary dimensions in children with MIS-C. This would lead to consistency in data analysis across centers worldwide. Some studies in MIS-C have used a z-score of >2 to define coronary artery “aneurysms,” which may lead to an overestimate of true coronary aneurysms. Researchers should take into account the phenomenon of transient coronary dilatation in viral myocarditis in children (24) and in non-KD, febrile illnesses, where the z scores may exceed 2 but virtually never >2.5 (25). Moreover, the term “prominent” coronary arteries should be avoided, lest readers consider it as a true coronary artery abnormality, thereby leading to unnecessary consternation (Figure 1). It has been proposed that transient coronary dilations in the acute phase of KD could reflect a physiological response to increased myocardial oxygen demand caused by fever, tachycardia, myocardial inflammation, local hypoxia, or endothelial dysfunction induced by carditis (24).
The phenomenon of pericoronary brightness, a feature suggesting perivasculitis in KD, has received attention in MIS-C patients. A paper from the United Kingdom showed echo-brightness in most of their MIS-C patients, even with normal coronary dimensions (26). However, it is our opinion that pericoronary brightness may be difficult to assess, due to the subjective nature of this finding, even when 3 experienced observers (D.M., M.D.Q., A.B.) were used in our study. Therefore, although we evaluated it, we have not reported our findings of this phenomenon, as it may not be reliable in the U.S. population (27). It is noteworthy that pericoronary brightness was initially included in the 2004 AHA guidelines for KD, but was removed from the 2017 AHA guidelines for these reasons (12).
Myocardial injury/myocarditis in MIS-C
We observed a higher frequency of cardiogenic shock in MIS-C (85%) than that noted in the classic KD group, where the incidence of shock was 5% (28). Most notably, our study showed a greater degree of overall LV dysfunction in the MIS-C group. Based on our functional observations, we speculate that this dysfunction may be the result of subclinical myocarditis. In the absence of endomyocardial biopsies or CMR, elevated BNP and troponin levels have been used in our study and in other recent studies as biomarkers to diagnose myocardial injury (29). Several indexes used to study cardiac mechanics in our study also pointed to a myocarditis-like picture. Previous studies in viral myocarditis (non-COVID) have shown that tissue deformation indexes, like reduced GLS and EDSR, can independently predict ventricular dysfunction in patients with CMR-proven myocarditis with preserved LVEF, with a high degree of sensitivity and specificity (8). In our series, the majority of patients with MIS-C had evidence of elevated biomarkers and decreased GLS, LAS, and EDSR. The combination of these strain features is strongly suggestive of underlying myocarditis. This is supported by a case series that showed CMR findings in MIS-C (30). In the acute phase of MIS-C, 3 cases with increased BNP and troponin-I levels showed diffuse myocardial hyperemia and edema by CMR without evidence of fibrosis/myocardial necrosis by late gadolinium enhancement, suggesting the presence of myocarditis in this limited number of patients.
The use of LA strain in assessment of diastolic dysfunction is novel in this study, and it proved to be the strongest single index associated with myocardial injury. Peak LAS has not been previously studied in children with myocarditis and may serve as a good index of atrioventricular coupling and LV diastolic dysfunction. Studies in children with enlarged LA from multiple causes have demonstrated decreased peak longitudinal strain (decreased reservoir function) and increased LA stiffness (15). Studies in adults with heart failure with preserved EF have shown that LA strain decreases with worsening diastolic dysfunction in a stepwise fashion and reflects increased LV filling pressures (31). In these publications in adults, there has been a call for incorporating LA strain into the 2016 diastolic function guidelines published by the American Society of Echocardiography. CMR feature tracking analysis of myocarditis in adults confirmed by the Lake Louise Criteria showed significantly lower peak LA strain in myocarditis patients compared with control subjects (32), which is in concordance with our study.
In addition to LV, the RV deformation was decreased in MIS-C patients, a finding that has been reported in a single study on adult patients with COVID-19 from Wuhan, China, but not in children. They showed that an RVFWLS cutoff value of −23% was a significant predictor of mortality in adults age 61 ± 14 years during a median follow-up period of 51 days (21). Our MIS-C patients all survived with good outcome; however, in our study, RVFWLS was strongly associated with myocardial injury.
Cardiac injury associated with COVID-19 in adults versus children
An adult study from the Wuhan cohort concluded that myocardial damage contributed to 40% of SARS-CoV-2 deaths (33). The incidence of myocarditis in 112 COVID-19 patients from Wuhan, China, diagnosed by elevated troponin-I (>0.12 ng/ml), abnormalities on standard echocardiograms, and/or electrocardiograms was 12.5% (34). Despite using a higher troponin-I value (>0.3 ng/ml) to define myocardial injury in our study, the incidence of myocardial injury was much higher (60%) in our MIS-C cohort. This is consistent with a paper by Dufort et al. (29) showing an incidence of myocarditis of 53% in MIS-C patients.
A recent report from New York City, the initial epicenter of COVID-19 in the United States, studied the impact of elevated troponin levels in >2,700 hospitalized adults with COVID-19 infection (35). They showed that even a small increase in troponin I level (0.03 to 0.09 ng/ml) in adults with COVID-19 was significantly associated with higher risk of mortality during hospitalization. In contrast, most of our MIS-C patients, even with high troponin I levels, recovered their systolic function quickly. Therefore, we speculate that the elevation in cardiac troponins may have less dire implications in children so far, likely due to a more transient type of cardiac injury and less comorbidities. Clearly, further studies are needed before a definitive statement can be made. In the largest study in hospitalized children so far, the diagnosis of SARS-CoV-2 infection was confirmed in 95 children. Of these 95 children, fortunately only 2 died (2.1%). This is far less than the death rate in hospitalized adults with COVID-19 with troponin elevation (∼18.5%) (35). Nevertheless, close longitudinal follow-up of these patients is essential.
Clinical implications
MIS-C is a relatively rare disease affecting only 0.6% of children infected by COVID-19 (29). Although MIS-C shares similarities with KD, it is notably distinct in that the coronary arteries may be often spared and there is a higher frequency of LV dysfunction, myocarditis, and shock. However, the similarity of this condition to KD has received widespread attention both in the medical and lay community. The acute echocardiographic findings, which appear to be usually reversible from our study based on a U.S. population, may help U.S. clinicians educate families regarding the acute and short-term status of the heart in MIS-C.
Study limitations
The small sample size of MIS-C patients is an important limitation of this study. MIS-C is a novel disease and is not well studied. With this in mind, our study has enrolled perhaps the maximal achievable number of patients for a single, tertiary-care center collected within a short time frame of 2 months, which is comparable even with multicenter U.S. and European studies (20,23). The short time frame was chosen as it is important to provide detailed cardiac findings about this virtually unknown disease to frontline physicians. During data collection, echocardiography was performed under strict infection-control regulations, where sonographers were required to observe the appropriate protocol for contact minimization and the use of personal protective equipment. Therefore, under such circumstances, the quality of echocardiography may have been affected. In addition, the novel status of this condition and the need to report findings from acute stages, do not allow for prolonged longitudinal follow-up, similar to that performed in KD. Because of this urgency to disseminate our data, we were unable to perform the 2- to 6-week follow-up that is typically performed in KD. A longer follow-up should be the focus of future studies. In this study, our primary focus was to describe echocardiographic findings in MIS-C patients. We have, therefore, not delved into issues related to critical care (e.g., ventilator settings, coagulation status, renal dysfunction, brain-related issues, and so on). Recently published U.S. and European studies have addressed these aspects (20,23).
Conclusions
MIS-C is a novel disease in children with COVID-19 infection. We found that unlike classic KD, coronary arteries may be typically spared in the early stages of the disease; however, cardiac systolic and diastolic dysfunction are more common, likely due to the presence of a myocarditis-like state. Even in patients with preserved ejection fraction, subtle changes in myocardial deformation were detected, suggesting subclinical myocardial dysfunction. During an abbreviated follow-up, there was good functional recovery and no coronary aneurysms.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Blood levels of BNP and troponin-I are commonly elevated in children with the multisystem inflammatory syndrome associated with COVID-19 (MIS-C), consistent with a form of myocarditis. During short-term follow-up, recovery of cardiac function usually occurs without formation of coronary aneurysms.
TRANSLATIONAL OUTLOOK: Longer follow-up of a larger number of patients with MIS-C is needed to better understand the mechanisms responsible for cardiac injury and ascertain late impact on coronary arteries and myocardial structure and function.
Acknowledgment
The authors express their deepest appreciation to the brave sonographers of our institution, who have acquired the images used for this study during this ominous pandemic.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC Health Alert Network Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) https://emergency.cdc.gov/han/2020/han00432.asp Available at:
- 4.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Scientific Brief Multisystem inflammatory syndrome in children and adolescents with COVID-19. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Available at:
- 6.Royal College of Pediatrics and Child Health Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf Available at:
- 7.Belot A., Antona D., Renolleau S. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoo N.S., Smallhorn J.F., Atallah J., Kaneko S., Mackie A.S., Paterson I. Altered left ventricular tissue velocities, deformation and twist in children and young adults with acute myocarditis and normal ejection fraction. J Am Soc Echocardiogr. 2012;25:294–303. doi: 10.1016/j.echo.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Caforio A.L.P., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a–d. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. [Google Scholar]
- 11.Kantor P.F., Lougheed J., Dancea A. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29:1535–1552. doi: 10.1016/j.cjca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 12.McCrindle B.W., Rowley A.H., Newburger J.W. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 13.Yubbu P., Nawaytou H.M., Calderon-Anyosa R., Banerjee A. Diagnostic value of myocardial deformation pattern in children with noncompaction cardiomyopathy. Int J Cardiovas Imaging. 2018;34:1529–1539. doi: 10.1007/s10554-018-1367-4. [DOI] [PubMed] [Google Scholar]
- 14.D'Souza R., Wang Y., Calderon-Anyosa R. Decreased right ventricular longitudinal strain in children with hypoplastic left heart syndrome during staged repair and follow-up: does it have implications in clinically stable patients? Int J Cardiovasc Imaging. 2020 May 3 doi: 10.1007/s10554-020-01870-0. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Hope K.D., Wang Y., Banerjee M.M., Montero A.E., Pandian N.G., Banerjee A. Left atrial mechanics in children: insights from new applications of strain imaging. Int J Cardiovasc Imaging. 2019;35:57–65. doi: 10.1007/s10554-018-1429-7. [DOI] [PubMed] [Google Scholar]
- 16.Ravelli A., Minoia F., Davì S. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. 2016;75:481–489. doi: 10.1136/annrheumdis-2015-208982. [DOI] [PubMed] [Google Scholar]
- 17.Levy P.T., Machefsky A., Sanchez A.A. Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2016;29:209–225. doi: 10.1016/j.echo.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy P.T., Aura A Sanchez A.A., Machefsky A., Fowler S., Holland M.R., Singh G.K. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2014;27:549–560. doi: 10.1016/j.echo.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittaker E., Bamford A., Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020 Jun 8 doi: 10.1001/jama.2020.10369. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushik S., Aydin S.I., Derespina K.R. Multisystem inflammatory syndrome in children (mis-c) associated with SARS-CoV-2 infection: a multi-institutional study from New York City. J Pediatr. 2020 Jun 14 doi: 10.1016/j.jpeds.2020.06.045. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Li H., Zhu S. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. J Am Coll Cardiol Img. 2020 Apr 28 doi: 10.1016/j.jcmg.2020.04.014. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiotos K., Bassiri H., Behrens E.M. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020 May 28 doi: 10.1093/jpids/piaa069. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 May 17 doi: 10.1161/CIRCULATIONAHA.120.048360. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Rached-D'Astous S., Boukas I., Fournier A., Raboisson M.J., Dahdah N. Coronary artery dilatation in viral myocarditis mimics coronary artery findings in Kawasaki disease. Pediatr Cardiol. 2016;37:1148–1152. doi: 10.1007/s00246-016-1411-x. [DOI] [PubMed] [Google Scholar]
- 25.Bratincsak A., Reddy V.D., Purohit P.J. Coronary artery dilation in acute Kawasaki disease and acute illnesses associated with fever. Pediatr Infect Dis J. 2012;31:924–926. doi: 10.1097/INF.0b013e31826252b3. [DOI] [PubMed] [Google Scholar]
- 26.Ramcharan T., Nolan O., Lai C.Y. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020 Jun 12 doi: 10.1007/s00246-020-02391-2. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabinowitz E.J., Rubin L.G., Desai K. Examining the utility of coronary artery lack of tapering and perivascular brightness in incomplete Kawasaki disease. Pediatr Cardiol. 2019;40:147–153. doi: 10.1007/s00246-018-1971-z. [DOI] [PubMed] [Google Scholar]
- 28.Taddio A., Rossi E.D., Monasta L. Describing Kawasaki shock syndrome: results from a retrospective study and literature review. Clin Rheumatol. 2017;36:223–228. doi: 10.1007/s10067-016-3316-8. [DOI] [PubMed] [Google Scholar]
- 29.Dufort E.M., Koumans E.H., Chow E.J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blondiaux E., Parisot P., Redheuil A. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020 Jun 9 doi: 10.1148/radiol.2020202288. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A., Addetia K., Maffessanti F., Mor-Avi V., Lang R.M. LA strain for categorization of LV diastolic dysfunction. J Am Coll Cardiol Img. 2017;10:735–743. doi: 10.1016/j.jcmg.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dick A., Schmidt B., Michels G., Bunck A.C., Maintz D., Baeßler B. Left and right atrial feature tracking in acute myocarditis: a feasibility study. Eur J Radiol. 2017;89:72–80. doi: 10.1016/j.ejrad.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Q., Hu B., Zhang Y. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lala A., Johnson K.W., Januzzi J.L. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








