Abstract
The hippocampus plays a critical role in episodic memory, among other cognitive functions. However, few tools exist to causally manipulate hippocampal function in healthy human participants. Recent work has targeted hippocampal–cortical networks by performing TMS to a region interconnected with the hippocampus, posterior inferior parietal cortex (pIPC). Such hippocampal-targeted TMS enhances associative memory and influences hippocampal functional connectivity. However, it is currently unknown which stages of mnemonic processing (encoding or retrieval) are affected by hippocampal-targeted TMS. Here, we examined whether hippocampal-targeted TMS influences the initial encoding of associations (vs. items) into memory. To selectively influence encoding and not retrieval, we performed continuous theta-burst TMS before participants encoded object–location associations and assessed memory after the direct effect of stimulation dissipated. Relative to control TMS and baseline memory, pIPC TMS enhanced associative memory success and confidence. Item memory was unaffected, demonstrating a selective influence on associative versus item memory. The strength of hippocampal–pIPC functional connectivity predicted TMS-related memory benefits, which was mediated by parahippocampal and retrosplenial cortices. Our findings indicate that hippocampal-targeted TMS can specifically modulate the encoding of new associations into memory without directly influencing retrieval processes and suggest that the ability to influence associative memory may be related to the fidelity of hippocampal TMS targeting. Our results support the notion that pIPC TMS may serve as a potential tool for manipulating hippocampal function in healthy participants. Nonetheless, future work combining hippocampal-targeted continuous theta-burst TMS with neuroimaging is needed to better understand the neural basis of TMS-induced memory changes.
INTRODUCTION
Medial temporal lobe (MTL) structures, including the hippocampus and surrounding parahippocampal cortex (PHC), play an essential role in long-term episodic memory formation (Squire, 1992; Scoville & Milner, 1957). A major function of the hippocampus is to form associations between individual elements of experience, both in the service of episodic memory and other cognitive processes (Yonelinas, 2013; Olsen, Moses, Riggs, & Ryan, 2012). The convergence of contextual information from PHC and retrosplenial cortex (RSC) and item representations via perirhinal cortex allows the hippocampus to form item–context associations that form the basis of episodic memory (Ranganath & Ritchey, 2012; Davachi, 2006).
Much work has used functional neuroimaging to assess how hippocampal–cortical networks are related to long-term memory and other cognitive functions (e.g., Staresina, Cooper, & Henson, 2013; Andrews-Hanna, Reidler, Huang, & Buckner, 2010). However, beyond correlational measures that can be assessed using neuroimaging, few approaches exist to causally target and manipulate hippocampal–cortical networks and their associated functions in healthy human participants. Such manipulations not only would constitute a useful tool in cognitive neuroscience, allowing researchers to examine the direct contribution hippocampal–cortical networks to aspects of cognition, but also could potentially be applied in clinical settings to modulate hippocampal network dysfunction (LaJoie et al., 2014; Chen & Etkin, 2013).
TMS is a powerful tool for performing noninvasive brain stimulation in healthy human participants. Although TMS is typically used to investigate the contribution of individual brain regions to particular cognitive processes, TMS can also be used to modify neural activity beyond the direct site of stimulation. Considerable evidence indicates that TMS influences distal brain regions and networks connected with stimulation sites (Romei et al., 2016; Halko, Farzan, Eldaief, Schmahmann, & Pascual-Leone, 2014; Fox, Halko, Eldaief, & Pascual-Leone, 2012; Eldaief, Halko, Buckner, & Pascual-Leone, 2011; Ruff, Driver, & Bestmann, 2009). For example, stimulation to superficial cortical sites causes neurotransmitter release in distally connected subcortical and cortical regions (Cho & Strafella, 2009; Strafella, Paus, Fraraccio, & Dagher, 2003; Strafella, Paus, Barrett, & Dagher, 2001). In addition, TMS applied to locations in the cerebellum that are linked with distinct networks in the cerebral cortex selectively influences connectivity in these distal networks and their associated behaviors (Esterman et al., 2017; Halko et al., 2014). Thus, TMS can serve as a tool for modulating neural function at the network level.
Prior work has employed network-targeted TMS approaches to modulate hippocampal–cortical networks. Although MTL structures cannot be directly accessed with standard noninvasive stimulation approaches, TMS has been used to target the hippocampus by leveraging its robust functional connectivity with posterior inferior parietal cortex (pIPC; Wang et al., 2014; Kahn, Andrews-Hanna, Vincent, Snyder, & Buckner, 2008; Vincent et al., 2006). For example, recent work employing this strategy has targeted a left pIPC site showing maximal functional connectivity with the hippocampus (Wang et al., 2014). Such hippocampal-targeted TMS applied to left pIPC over the course of 5 days modulates large-scale patterns of resting hippocampal functional connectivity (Wang et al., 2014), enhances associative memory (Nilakantan, Bridge, Gagnon, VanHaerents, & Voss, 2017; Wang et al., 2014), and modifies parietal cortex activity during associative retrieval (Nilakantan et al., 2017). However, the temporally extended 5-day TMS protocol used in this work leaves open the question of which mnemonic processes were influenced by stimulation (i.e., processes mediating encoding, retrieval, or both phases of memory). It is thus currently unknown whether hippocampal-targeted TMS to pIPC is capable of influencing the initial encoding of new associations into memory or whether pIPC TMS modulates access to previously encoded episodic details via a generalized influence on episodic retrieval processes. Given the extensive evidence that successful associative retrieval or recollection engages left pIPC (Rugg & King, 2017; Sestieri, Shulman, & Corbetta, 2017; Vilberg & Rugg, 2008; Wagner, Shannon, Kahn, & Buckner, 2005) and that recollection-related activity in left parietal cortex was influenced by left pIPC TMS (Nilakantan et al., 2017), it is possible that the previously reported changes in associative memory after hippocampal-targeted TMS were due to an influence on retrieval-related processes in left pIPC (as opposed to hippocampal processes per se).
To understand the basic memory processes that can be influenced by hippocampal-targeted TMS, here, we asked whether hippocampal-targeted TMS to right pIPC modulates the initial encoding of associations into memory without a direct influence of TMS during retrieval. To do so, we utilized continuous theta-burst TMS (cTBS), a TMS protocol that influences neural function in a time-limited manner, for approximately 50 min after stimulation (Wischnewski & Schutter, 2015; Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005). Specifically, cTBS influences cortical excitability and BOLD responses for up 50 min after stimulation, with these measures returning to pre-cTBS baseline levels 1 hr after stimulation (Wischnewski & Schutter, 2015; Hubl et al., 2008; Huang et al., 2005). Similar to prior work (Nilakantan et al., 2017; Wang et al., 2014), the site of stimulation in pIPC was chosen to maximize each participant’s functional connectivity with the hippocampus (Figure 1). To selectively influence memory encoding and not retrieval, we applied time-limited cTBS to right pIPC immediately before participants encoded item–context (object–location) associations and tested their memory several hours later, after the effect of cTBS dissipated (Wischnewski & Schutter, 2015; Huang et al., 2005).
Figure 1.
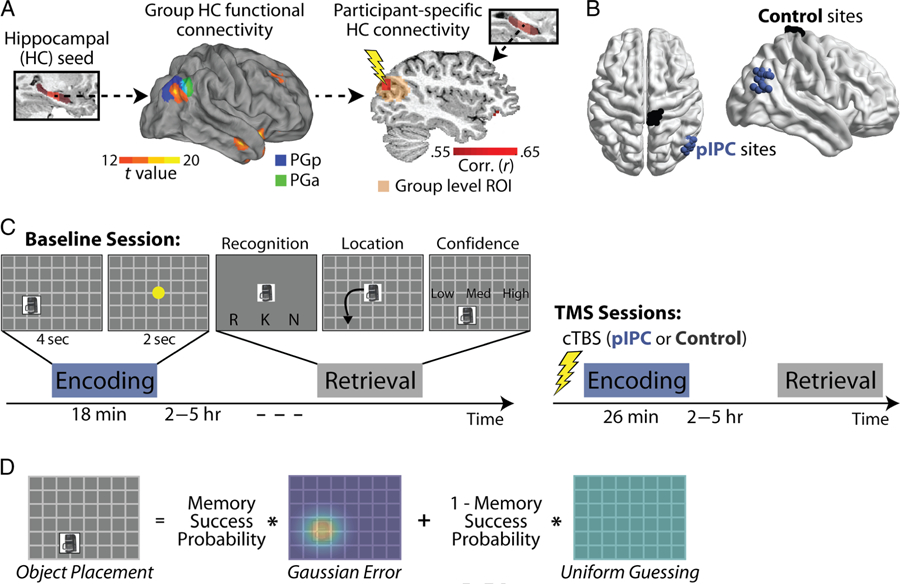
TMS targeting and experimental design. (A) A group level map of hippocampal functional connectivity (center) was created using the right middle hippocampus as a seed region (left) from the fMRI study. The connectivity map is overlaid on regions of the angular gyrus defined based on cytoarchitectonic features, posterior PG (PGp, in blue) and anterior PG (PGa, in green; Caspers et al., 2010). A group level ROI (15-mm radius) was created around the location of peak hippocampal connectivity in pIPC. To define hippocampal-targeted pIPC TMS sites (right), participant-specific hippocampal functional connectivity maps were created (red correlation map) and overlaid on the group level ROI (transformed into each participant’s native space; orange shaded region). The TMS site was chosen as the location of each participant’s maximal hippocampal functional connectivity within the group level ROI. All group level maps are available on neurovault.org (neurovault.org/collections/AELKYBHZ/). (B) TMS sites displayed for all participants as spheres (pIPC in blue; control in black). (C) The TMS study consisted of three sessions across 3 consecutive weeks (in addition to a baseline MRI/TMS session). Each week comprised encoding and retrieval sessions, separated by 2–5 hr. During encoding, participants intentionally encoded unique objects and their associated spatial locations and performed an unrelated color discrimination task during the ITI. During retrieval, participants performed a remember/know/new decision for each object, then placed objects labeled as old in their associated spatial location, and rated the confidence of their placement. Behavioral sessions were performed during the baseline (first) session (left). cTBS was performed immediately before encoding during subsequent sessions to either pIPC or the control site (right), with the order of TMS sites counterbalanced across participants. (D) Object placement was modeled as a mixture of memory success (represented as a Gaussian distribution around each object’s location during encoding) and guessing behavior (represented as a uniform distribution across the screen), with the probability of memory success representing the proportion of trials belonging to the memory success distribution.
We specifically examined object-location memory in the current study as it is impaired by right hippocampal damage and successful object–location binding elicits hippocampal activation (Baumann, Chan, & Mattingley, 2010; Hannula & Ranganath, 2008; Piekema, Kessels, Mars, Petersson, & Fernández, 2006; Holdstock, Mayes, Gong, Roberts, & Kapur, 2005; Sommer, Rose, Gläscher, Wolbers, & Büchel, 2005; Stepankova, Fenton, Pastalkova, Kalina, & Bohbot, 2004; Nunn, Graydon, Polkey, & Morris, 1999; Baxendale, Thompson, & Van Paesschen, 1998; Bohbot et al., 1998; Smith & Milner, 1981, 1989). In contrast, object-location memory is not reliably influenced by lesions to right pIPC, and successful encoding does not elicit activation in right pIPC (Zimmermann & Eschen, 2017; Baumann et al., 2010; van Asselen et al., 2009; Sommer, Rose, Gläscher, et al., 2005; Cansino, Maquet, Dolan, & Rugg, 2002). More generally, pIPC has not been implicated in successful item–context memory encoding in prior meta-analyses (Kim, 2011; Uncapher & Wagner, 2009). Thus, although the aim of the current study was behavioral in nature (to assess whether hippocampal-targeted TMS applied selectively during encoding can influence subsequent memory), demonstrating such an influence would add indirect support to the notion that hippocampal-targeted TMS can influence memory via a remote impact on hippocampal networks rather than on retrieval processes that have been strongly linked with pIPC. Object-location memory is additionally advantageous as it provides a continuous measure of associative memory (error in object placement). This graded memory assessment can be used to estimate the overall probability of successful retrieval as well as the precision of retrieved information using mixture models (Harlow & Yonelinas, 2016; Richter, Cooper, Bays, & Simons, 2016), allowing for a more detailed understanding of how memory formation may be influenced by hippocampal-targeted stimulation.
We also examined the specificity of TMS on memory formation. A large body of evidence indicates that associative memory is supported by the hippocampus whereas memory for individual items can be supported by extrahippocampal structures (Diana, Yonelinas, & Ranganath, 2007; Mayes, Montaldi, & Migo, 2007; Davachi, 2006; Ranganath et al., 2004). We thus tested the prediction that hippocampal-targeted TMS would preferentially influence associative versus item memory. As a final aim, we sought to probe potential networks underlying the influence of TMS by examining whether baseline levels of intrinsic functional connectivity were predictive of TMS-related changes in memory. We first tested the prediction that, if an influence of pIPC TMS on associative memory is due to a distal impact on hippocampal function, then participant-specific levels of intrinsic hippocampal–pIPC functional connectivity should predict TMS-related changes in associative memory across individuals. To probe potential networks underlying pIPC–hippocampal connectivity, we asked which brain regions statistically mediate relationships between baseline pIPC–hippocampal functional connectivity and TMS-related changes in associative memory.
METHODS
Participants
Two groups of participants were used. We first localized a group level region in pIPC showing reliable functional connectivity with the hippocampus from one set of participants (fMRI study), which was then used to constrain the definition of participant-specific TMS sites in the main TMS study. The TMS study was performed on a second group of participants (with N = 4 overlapping participants across groups). Informed consent was obtained from all participants in accordance with the Committee for Protection of Human Subjects at the University of California, Berkeley.
fMRI Study
Data from 57 participants (27 men and 30 women, mean age = 21 years, range = 18–27 years) were analyzed. Participants were recruited as part of a separate study.
TMS Study
Data from 25 participants were collected. Three participants were excluded, two with close-to-chance item memory performance during the baseline session (before TMS) and one because of improper placement of the TMS coil. The average age of the remaining 22 participants was 20.7 years (range = 18–28 years), which included five male and 17 female participants.
Experimental Design
fMRI Study
Rest scans were used to isolate the general location within pIPC that showed reliable functional connectivity with the hippocampus at the group level. Isolation of a group level ROI within pIPC served to broadly constrain the location of pIPC TMS sites, allowing us to define each participant’s pIPC TMS site (in the TMS study) as their location of maximal hippocampal functional connectivity within the group-level pIPC ROI (Figure 1). Rest scans were also used to perform mediation analyses. These scans were acquired as part of a separate study and were acquired first in the scanning session.
TMS Study
A within-participant design was used to assess the influence of pIPC TMS on memory formation. Participants first completed a baseline MRI and TMS motor thresholding session and then performed encoding and memory testing sessions. The baseline MRI and TMS session was used to localize participant-specific TMS sites (based on each participant’s anatomical scan and resting hippocampal functional connectivity) and measure each participant’s active motor threshold (AMT). The MRI session consisted of a high-resolution anatomical scan followed by a rest scan. AMT was measured after MRI scanning (see details below).
Encoding and retrieval sessions were performed across 3 consecutive weeks (Figure 1C), with 1 week in between sessions and 2–5 hr between encoding and retrieval. All sessions occurred at the same time across weeks, ensuring that the delay between encoding and retrieval was fixed for each participant (e.g., encoding at 11 a.m. and memory testing at 3 p.m.). The study-test delay was permitted to vary between participants to accommodate participants with different schedules (note that all comparisons of interest are within-participant). cTBS influences neural function for approximately 50 min after stimulation, with levels of cortical excitability and BOLD responses returning to pre-cTBS baseline levels after 1 hr and beyond (Wischnewski & Schutter, 2015; Hubl et al., 2008; Huang et al., 2005). Thus, the 2- to 5-hr delay (mean = 3.4 ± 0.9 hr) between encoding and retrieval allowed us to selectively influence memory encoding (and potentially an initial consolidation phase) without explicitly influencing retrieval processes. Participants left the laboratory and went about normal activities during the study-test delay. To ensure that the variable study-test delay did not influence our results, we examined relationships between delay and pIPC TMS-related changes in associative memory; no reliable relationships were found.
During the first (baseline) session, participants performed encoding and memory testing and no TMS was administered. During Sessions 2 and 3, participants performed encoding and retrieval, with cTBS administered immediately before encoding (pIPC or a control site, primary somatosensory cortex, S1). The order of TMS sites was counterbalanced across participants. An active rather than a sham TMS condition was used to ensure that any influence of pIPC TMS was not due to nonspecific effects of stimulation or potential changes induced by the acoustic or tactile scalp sensations associated with TMS. As in prior work (Nee & D’Esposito, 2017; Rahnev, Nee, Riddle, Larson, & D’Esposito, 2016; Gratton, Lee, Nomura, & D’Esposito, 2013; Lee & D’Esposito, 2012), medial S1 was chosen as the control site because it demonstrates relatively local connectivity within somatomotor cortex (Yeo, Krienen, Chee, & Buckner, 2014), making it unlikely that S1 cTBS would influence MTL function. We did not expect the medial S1 control site to play an active role in associative memory formation as it corresponds to the leg somatosensory representation, does not play a known role in item–context memory encoding (see Kim, 2011, for a meta-analysis), and is not activated during successful object-location memory formation (Sommer, Rose, Weiller, & Büchel, 2005; Cansino et al., 2002). In summary, this design allowed us to examine how pIPC cTBS influenced subsequent memory performance relative to both control TMS and a baseline memory assessment that was not influenced by TMS.
Stimuli
Seven hundred eighty unique object stimuli were used across the three sessions. Each object spanned 194 × 194 pixels and was presented on a gray grid spanning the screen (1680 × 1050 pixels). Each object was only seen once by a participant, with each encoding and retrieval session composed of unique stimuli (no stimuli repeated across weeks). For each participant, objects were randomly assigned to be seen during encoding or to serve as new stimuli during memory testing.
Encoding Sessions
Before each session, participants were instructed to try to memorize both the details of each object and its spatial location on the grid (Figure 1C); participants were informed that their memory for both individual objects and their spatial locations would be tested later in the day. Participants underwent three encoding sessions. The first week consisted of 136 associations, whereas Weeks 2 and 3 contained 192 associations. All trials began with a red cross in the center of the grid for 1 sec. For half of the encoding trials, each object was shown in its associated spatial location on the grid for 4 sec (no delay trials). For the other half of the trials (delay trials), the spatial location was first indicated by a cue (black frame shown for 750 msec), followed by a brief delay in which the grid remained on the screen (250 msec), with the object then presented in the middle of the screen (3 sec). Participants were instructed to memorize the spatial location associated with the initial cue during delay trials. Spatial locations were uniformly distributed across the screen in a pseudorandom fashion, with an equal number of objects assigned to each quadrant of the screen (for each delay/no-delay condition).
A color discrimination task was performed during the intertrial interval (ITI). A blue or yellow dot was presented in the center of the grid for 2 sec (Figure 1C). Participants were instructed to indicate the color of the dot via keypress (“j” for yellow or “k” for blue).
Memory Testing
All memory testing was self-paced. Each trial began with the presentation of an object in the center of the screen (Figure 1C). Participants first responded remember, know (indicated by familiar), or new. If an object was labeled as “old” (remember or know response), the spatial grid appeared and participants were instructed to drag the object to its spatial location seen during encoding. After each object was placed, participants rated their confidence in object placement (high, medium, or low).
Each memory test consisted of all objects studied during encoding (for that week) and half the number of new stimuli. This resulted in 204 stimuli for the first memory test (during Week 1) and 288 stimuli for the memory tests on Weeks 2 and 3.
Before each memory test, participants were trained on remember/know judgments. After reading detailed instructions, participants explained the meaning of remember and know in their own words. The memory test was administered once the participants had correctly understood the instructions; that is, they judged an object as “remembered” when it brought back to mind a specific detail from the episodic context in which the object had been experienced, such as a sensory detail, a thought, or a feeling.
TMS Sites
Control and pIPC stimulation sites were defined for each participant (Figure 1B). pIPC TMS sites were defined using a two-step procedure; we first defined the general location of reliable hippocampal function connectivity within pIPC at the group level (across participants in our fMRI study), resulting in a group level ROI specifying a broad region in pIPC (colored sphere in Figure 1A). Participant-specific pIPC TMS sites were then isolated by finding each participant’s location of maximal hippocampal functional connectivity within the broader group-level ROI. To define the group level ROI, we performed a hippocampal connectivity analysis (with the right middle hippocampus as a seed region) using data from the fMRI study (see fMRI Connectivity and Mediation Analyses) and created a 15-mm-radius sphere around the location of peak connectivity in right pIPC (Montreal Neurological Institute [MNI] coordinates: +43, −67, +28). The right hippocampus was specifically targeted (used as a seed region) as object-location associative memory is most impaired by right compared with left hippocampal damage (Stepankova et al., 2004; Nunn et al., 1999; Baxendale et al., 1998; Bohbot et al., 1998; Smith & Milner, 1981). Using the right hippocampus as a seed region, functional connectivity with pIPC was stronger (higher correlation values) and more reliable (higher T statistics) on the ipsilateral rather than contralateral side. We thus choose to perform TMS to right rather than left pIPC to best target right hippocampal function. Although retrieval success effects in pIPC tend to be left lateralized (Kim, 2013; Wagner et al., 2005), this did not have strong implications for our design, as we sought to use TMS to influence encoding and not retrieval.
To define TMS sites in individual participants, the group level ROI isolated from the fMRI study was projected into each TMS participant’s native anatomical space (using advanced normalization tools [ANTs]; Avants, Epstein, Grossman, & Gee, 2008). A conjunction analysis was performed between the group level ROI, each participant’s hippocampal functional connectivity map (constructed from their rest scan obtained during the baseline MRI session), and each participant’s GM probability map (thresholded at .75). The pIPC TMS target was defined by placing a 3-mm sphere centered on the GM voxel with maximal hippocampal functional connectivity (within the group level ROI). If this voxel was not superficial (within approximately 10 mm of the surface), we lowered the threshold of the correlation map and repeated the procedure. The control site (medial S1) was defined anatomically (the most superior portion of the right postcentral gyrus that fell within a GM mask), as in prior work (Nee & D’Esposito, 2017; Rahnev et al., 2016; Cameron, Riddle, & D’Esposito, 2015; Lee & D’Esposito, 2012).
TMS PROCEDURES
Participants completed three TMS sessions. TMS was applied via a MagStim Super Rapid 2 stimulator using a figure-eight double air film coil with a 70-mm diameter. AMT was measured during the first TMS session, which occurred on a separate day as the behavioral sessions. EMG was recorded using electrodes placed on the first dorsal interosseus muscle on the left hand. To localize the hand area of right motor cortex, single TMS pulses were delivered to find the region that elicited a visible finger twitch when the participant’s hand was at rest. AMT was then defined as the minimum intensity required to produce a motor-evoked potential on 5 of 10 trials while the participant maintained voluntary contraction of the first dorsal interosseus muscle. Visual feedback of the EMG signal was presented to the participant to aid in the maintenance of muscle contraction.
cTBS was applied before encoding during sessions on Weeks 2 and 3 (to either pIPC or the control site). TMS targets were localized using a computerized frameless stereotaxic system (Brainsight, Rogue Research). cTBS was applied at 80% of AMT and composed of 50-Hz triplets (three single pulses separated by 20 msec) repeated at a frequency of 5 Hz (every 200 msec) for a duration of 40 sec or 600 pulses (using parameters from Huang et al., 2005). This TMS protocol is considered to be inhibitory, as it reduces cortical excitability (Wischnewski & Schutter, 2015; Huang et al., 2005) and BOLD responses for up to an hour after application (Hubl et al., 2008), such that measures of neural function return to pre-TMS baseline levels when measured 1–2 hr after stimulation. Thus, the delay between encoding and retrieval (mean delay = 3.4 ± 0.9 hr) allowed us to selectively influence memory encoding (and potentially an initial consolidation phase) without affecting retrieval processes.
MRI Data Acquisition
Scanning was performed using a 3-T Siemens Tim/Trio MRI system with a 12-channel whole-head coil located at the Henry H. Wheeler Jr. Brain Imaging Center at the University of California, Berkeley. Functional T2*-weighted data were collected using a gradient EPI sequence (repetition time = 2 sec, echo time = 28 msec, field of view = 210 mm, 32 slices, 3 × 3 × 3 mm voxel size, 0.6-mm interslice gap, flip angle = 78°). A single rest scan lasting 9 min contained 270 volumes, after discarding the first five volumes to allow for T1 equilibration. Participants were instructed to close their eyes and simply rest and think about anything that they wanted to while remaining awake. High-resolution T1-weighted (magnetization prepared rapid-acquisition gradient echo) images were acquired before each rest scan (240 × 256 × 160 matrix of 1-mm isotropic voxels, repetition time = 2300 msec, echo time = 2.98 msec, flip angle = 9°).
MRI Preprocessing
The imaging data were preprocessed using SPM8 (Wellcome Department of Cognitive Neuroscience, University College London). Functional data were first corrected for differences in slice timing acquisition followed by motion correction across all runs and removal of low-frequency trends (<0.009 Hz). Each participant’s functional data were coregistered to their own T1-weighted anatomical image and spatially smoothed with a 6-mm FWHM isotropic Gaussian kernel. A group-level anatomical template was created using ANTs based on the T1-weighted images of the 57 participants in the fMRI study.
Noise corrections were applied to rest scans using a modified version of aCompCor (Behzadi, Restom, Liau, & Liu, 2007), as previously implemented (Tambini & Davachi, 2013). The top principal components (PCs) from white matter and cerebrospinal fluid masks, in addition to six motion parameters and their first temporal derivatives, were removed from the BOLD signal in a voxel-wise manner using linear regression. As in Behzadi et al. (2007), a variable number of white matter/cerebrospinal fluid PCs were used for each participant based on null simulations. A low-pass filter was applied (<0.1 Hz, fourth-order Butterworth filter) after nuisance signals were removed. For analyses comparing connectivity values across participants, a fixed number of PCs (5) were regressed out of the data to avoid differentially biasing overall connectivity levels across participants (Muschelli et al., 2014; Chai, Castañón, Ongür, & Whitfield-Gabrieli, 2012).
FMRIB Software Library’s FIRST segmentation was used to define the hippocampus, based on each participant’s T1-weighted scan. All ROIs were manually inspected and edited when appropriate to ensure proper definitions according to Pruessner et al. (2000). The hippocampus was then divided into anterior, middle, and posterior portions by splitting the coronal sections into thirds along the anterior–posterior axis.
Behavioral Analysis
Object-location memory was assessed by computing the Euclidean distance between each object’s location during encoding and its placed location during memory testing (for correctly recognized old objects). Probabilistic mixture models were used to model spatial error as a combination of a guessing process (resulting in uniformly distributed placement across the screen) and successful memory retrieval (resulting in object placement centered around the location seen during encoding; see Figure 1D for an example). This procedure was used as it allows us to understand whether TMS influenced the probability of successful associative retrieval versus an influence on the quality of retrieved representations (cf. Nilakantan et al., 2017), which both influence overall spatial error. In addition, unlike average error, it is less susceptible to the influence of outlier trials with excessively high spatial error (which would be classified as a guess trial using a mixture model).
Similar to prior approaches (Schneegans & Bays, 2016), three free parameters were estimated from the distribution of spatial error: the probability of retrieval success that determined the mixing of the memory success and guessing distributions (Figure 1D) and the standard deviation (width and height) of the 2-D Gaussian memory distribution. Guessing was modeled as a uniform distribution as locations assigned during encoding were uniformly distributed across the screen, and visual inspection of the data did not reveal strong nonuniform biases in object placement. The height of the guessing distribution was set to the guess rate (1/the screen size or 1/1050 * 1680 pixels). The retrieval success distribution was modeled using mvnpdf in MATLAB. Free parameters were estimated using a maximum likelihood approach, such that the negative log likelihood of the mixture model was minimized using a constrained search via the fmincon MATLAB function. A constrained search was used to obtain plausible estimates of the free parameters (the retrieval success parameter was set to be between 0 and 1, and the standard deviations were constrained to 1–800 pixels). Separate models were fit for each session in each participant.
Object-location memory confidence was assessed by the proportion of medium and high confident responses. The same pattern of results was found when averaging confidence scores across all trials (values of 1, 2, and 3 were assigned to “low,” “medium,” and “high” confident responses, respectively).
Object-location associative memory was also assessed for distinct subsets of trials (remember vs. know trials and delay or no-delay trials). To estimate memory success for these trial types, we did not fit separate mixture models for distinct trial subtypes as smaller trial counts for these conditions resulted in poor model fits. We instead estimated the probability of memory success for distinct trial types based on the fitted Gaussian memory distribution (using parameters fit for that session and participant) and compared this with the probability of belonging to the uniform guessing distribution. Each trial was labeled as representing memory success if the Gaussian probability was greater than the guessing probability, and the probability of successful memory was averaged across trials. Two participants who had very low numbers of know trials (fewer than five) during one TMS session were excluded from the analysis of object-location memory based on remember/know responses.
Item memory was assessed by examining proportions of object hits and false alarms. Overall memory accuracy was assessed by hit and false alarm rates collapsed across remember and know responses. Recollection and familiarity were subsequently examined separately. Recollection was measured as the proportion of remember hits minus remember false alarms. Independent estimates of corrected familiarity were measured according to prior definitions (Koen & Yonelinas, 2016; Yonelinas & Jacoby, 1995). Specifically, familiarity for old items was estimated as the proportion of old items labeled “familiar” divided by 1 minus the proportion of old items labeled “remember.” Familiarity for new items was measured in the same manner (proportion of new items labeled “familiar” divided by 1 minus the proportion of new items associated with remember responses). Corrected familiarity was then estimated as familiarity for old items minus familiarity for new items (Koen & Yonelinas, 2016). Separate ANOVAs, as described above, were performed each measure of object memory (total hits, hits minus false alarms, recollection, and familiarity).
To assess whether TMS influenced behavioral performance during encoding, we measured average RT and accuracy during the color judgment task performed during the ITI. Robust regression was used to assess correlations between TMS-related changes in performance on the color discrimination task and associative memory success (pIPC minus control TMS).
Changes in all behavioral measures across the 3 weeks of the experiment were assessed using mixed-effects ANOVAs (implemented in R) with a within-participant factor of Session (baseline, control TMS, and pIPC TMS) and a between-participant factor of TMS site order. Differences in memory between individual sessions, for example, based on TMS site, were assessed using two-way ANOVAs (within-participant factor of TMS site, pIPC and control, and a between-participant factor of TMS site order). Object-location memory precision (standard deviation of memory distributions) was assessed using the same mixed-effects ANOVAs specified above, but an additional within-participant factor of Space was included (width and height of the standard deviation). Differential changes in object-location memory across trial types were assessed using the same mixed-effects ANOVA, but a within-participant factor of Trial type was added (e.g., remember/know trails or no-delay/delay conditions). The differential influence of TMS on item versus associative memory was primarily assessed using mixed-effects ANOVAs with within-participant factors of Session or TMS site and Memory type (item and associative memory) and a between-participant factor of TMS site order. To ensure that any differential influence of TMS on associative versus item memory was not due to differences in the overall scale of these memory measures, we also computed a normalized measure of the percent change in memory (100 × pIPC TMS memory minus control TMS memory, divided by average memory across sessions). We then compared these normalized measures of memory change across item and associative memory.
fMRI Connectivity and Mediation Analyses
Rest scans were used to estimate hippocampal functional connectivity maps (for TMS site localization) and to probe potential pathways underlying hippocampal–pIPC connectivity. Hippocampal functional connectivity maps were computed using the middle portion of the right hippocampus as a seed (see Hippocampus ROI Definition), and Pearson correlations were computed in a voxel-wise fashion with the seed time series. The middle portion of the hippocampus was used as it yielded the most reliable and robust connectivity with pIPC in our fMRI study and in other data sets explored during pilot testing. To generate the group-level hippocampal functional connectivity map (shown in Figure 1A), Pearson correlation values were Fisher Z-transformed, and the resulting map in each participant’s native space was transformed to the group template using the transformation parameters generated from ANTs. To assess the reliability of functional connectivity across participants and find the site of maximal hippocampal connectivity within pIPC, a one-sample t test was performed comparing the Z-transformed correlation value of all 57 participants with zero. All group-level statistical maps can be found on neurovault.org (neurovault.org/collections/AELKYBHZ/; Gorgolewski et al., 2015).
We also examined whether the strength of individual participants’ baseline or intrinsic levels of hippocampal–pIPC functional connectivity was predictive of TMS-related changes in associative memory. To do so, we used the rest scan for each TMS participant (acquired in the baseline MRI session) to measure participant-specific levels of pIPC TMS-site–hippocampal functional connectivity for all individuals in the TMS study. Specifically, we computed the correlation between the time series of each participant’s right hippocampus ROI and the pIPC TMS ROI during rest (hippocampal–pIPC functional connectivity) and assessed the correlation between this measure and TMS-related associative memory changes (difference in the probability of associative memory success for pIPC compared with control TMS). Robust regression (robust-fit in MATLAB) was used for all across-participant correlations. To ensure that this relationship was not driven by global or nonspecific BOLD fluctuations (Power, Plitt, Laumann, & Martin, 2017), the global signal was included as a regressor of no interest when estimating relationships between functional connectivity and TMS-related memory changes (although excluding this regressor did not alter the results).
Mediation analyses were used to examine potential pathways underlying hippocampal–pIPC functional connectivity. We first searched for brain regions that most strongly mediated the relationship between the hippocampal seed and pIPC time series during rest. Specifically, using the participants in the fMRI study who did not participate in the TMS study (N = 53 participants), we conducted a mediation analysis using the time series of the right middle hippocampus as the independent variable and the IPC time series as the dependent variable. The pIPC time series was extracted from a 2-mm sphere around the group-level peak location of hippocampal functional connectivity. This ROI was used as participant-specific pIPC ROIs were only defined in the TMS study and not the fMRI study. We estimated the total or full effect of the hippocampal time series on the pIPC time series for each participant in the fMRI study (beta weight estimated using linear regression) and, in a voxel-wise fashion, assessed the direct path or effect between the hippocampus and pIPC controlling for the time series of that particular voxel for each participant (beta weight representing the relationship between hippocampus and pIPC when each voxel was also included as a predictor). To control for brain-wide or nonspecific connectivity, the global brain signal was included as a covariate of no interest when estimating the full and direct effects (Power et al., 2017). Mediation of hippocampal–pIPC connectivity was tested by performing a paired voxel-wise t test between the full and direct effects. We initially performed a strict correction for multiple comparisons across voxels (Bonferroni correction) to isolate the most statistically robust mediators of hippocampal–pIPC function connectivity, as we reasoned that the strongest regions to emerge from this analysis would be most likely to explain variance in TMS-related memory changes. However, we also used a less conservative threshold to ensure that we did not miss potentially meaningful mediators of hippocampal–pIPC connectivity (cluster-based thresholding using FWE correction with a cluster forming threshold of p < .0001, implemented in FMRIB Software Library’s randomise). Spherical ROIs (with a 2-mm radius) were defined around peak voxels that survived correction (listed in Table 1).
Table 1.
Statistical Mediators of pIPC–hippocampal Functional Connectivity Identified from the fMRI Study
| x | y | z | t Value | Region | Replicate Mediation Effect in TMS Group | Correlation after Controlling for Region |
|---|---|---|---|---|---|---|
| 9 | −52 | 13 | 9.21 | R RSC | t = 8.3 (p = 5 × 10−8) | r = .27 (p = .22) |
| 31 | −32 | −15 | 8.34 | R PHC | t = 2.9 (p = .008) | r = .09 (p = .70) |
| −25 | −36 | −12 | 8.1 | L PHC | t = 4.30 (p = .0003) | r = .12 (p = .59) |
| 59 | −4 | −18 | 6.46 | R anterior temporal cortex | t = 7.9 (p = 1 × 10−7) | r = .48 (p = .023) |
| 42 | −55 | 31 | 6.3 | R IPC | t = 8.0 (p = 9 × 10−8) | - |
| −48 | −70 | 32 | 6.11 | L IPC | t = 6.5 (p = 2 × 10−6) | - |
| 24 | 32 | 49 | 6.02 | R superior frontal sulcus | t = 5.5 (p = 2 × 10−5) | r = .64 (p = .001) |
| −14 | −69 | 23 | 5.67 | L parieto-occipital sulcus | t = 5.4 (p = 2 × 10−5) | r = .45 (p = .037) |
| 7 | 62 | 12 | 5.63 | R medial pFC | t = 4.6 (p = .00015) | r = .46 (p = .031) |
| −19 | 24 | 50 | 5.42 | L superior frontal sulcus | t = 4.7 (p = .00011) | r = .56 (p = .007) |
| −33 | 31 | 1 | 5.26 | L inferior frontal sulcus | t = 1.7 (p = .10) | r = .45 (p = .037) |
| 35 | 47 | 27 | 5.1 | R MFG | t = 1.8 (p = .085) | r = .40 (p = .064) |
| 56 | 14 | −2 | 4.9 | R frontal operculum | t = 0.32 (p = .76) | - |
| 6 | 31 | −11 | 4.61 | R ventromedial pFC | t = 5.16 (p = 4 × 10−5) | r = .51 (p = .015) |
| −63 | −1 | −16 | 4.6 | L anterior temporal cortex | t = 5.2 (p = 4 × 10−5) | r = .56 (p = .006) |
Peak coordinates are in MNI space. The “Replicate Mediation Effect in TMS Group” column presents results of the statistical mediation analysis of hippocampal–pIPC TMS site functional connectivity in TMS participants. Right column indicates the correlation between TMS-related associative memory changes and hippocampal–pIPC connectivity, after controlling for each region when estimating hippocampal–pIPC connectivity.
After isolating regions that statistically mediate hippocampal–pIPC functional connectivity from the fMRI study, we tested (1) whether these effects replicate in our TMS group (the regions are reliable mediators of hippocampal connectivity with pIPC TMS sites) and (2) whether controlling for these regions decreased the relationship between baseline hippocampal–pIPC functional connectivity and TMS-related changes in associative memory. To assess whether these regions mediate hippocampal–pIPC functional connectivity in our TMS group, we performed the mediation analysis outlined above using each participant’s hippocampal ROI, pIPC TMS ROI, and each of the mediating brain regions (after transforming the group-level mediation ROIs into each participant’s native space). We then asked whether controlling for each of the mediating brain regions reduced the correlation between hippocampal–pIPC connectivity and TMS-related associative memory benefits. To do so, we measured the direct path between the hippocampal ROI and each participant’s IPC TMS ROI when controlling for the BOLD signal in each mediating brain region and the global signal. This estimation of the direct path was performed after z scoring each time series, such that resulting beta weights are directly comparable with correlation coefficients. We measured the correlation between the direct hippocampal–IPC path when controlling for each mediating brain region and TMS-related changes in associative memory. To evaluate whether the relationship between TMS-related changes in associative memory was significantly reduced by controlling for each mediating brain region, we first assessed the true difference in correlation values between the full hippocampal–pIPC path and memory changes versus the direct hippocampal–pIPC path (when controlling for a mediating brain region) and memory changes. Nonparametric permutation tests were then used to assess the reliability of this correlation difference. Null simulations (10,000) were performed in which participant labels were permuted before estimating relationships between connectivity and memory changes, and the difference in correlation values was computed. The true difference in correlation values for each mediating brain region was compared with this null distribution to test for significance.
In addition to examining statistical mediators of hippocampal–pIPC connectivity, as a control, we also examined connectivity of regions in pFC (the medial frontal gyrus, MFG), superior parietal cortex (SPC), and “default network” regions. To examine connectivity with pFC, we defined a region in the right MFG that has been implicated in successful object-location encoding in prior work (Hales & Brewer, 2013; MNI coordinates: 34, 35, 16). Specifically, this region was revealed from a subsequent associative memory contrast and showed greater activation during encoding for associative hits (when viewing objects for which the object and its location were subsequently remembered) compared with associative misses (when viewing objects in which the object was remembered but the location was forgotten). To examine regions in SPC, we defined regions in right SPC and posterior inferior parietal sulcus (IPS) from prior work (Sestieri, Shulman, & Corbetta, 2010; MNI coordinates: SPC = 24, −60, 64; IPS = 21, −77, 40). These regions correspond to group level peaks that showed a greater activity during attentional search versus memory retrieval, which have been proposed to antagonistically interact with IPC (Sestieri et al., 2017). “Core” default network regions were defined as those regions in “Default Network A” in the right hemisphere from the work of Schaefer et al. (2017), which is very similar to the core default network defined by Yeo et al. (2011; see also Andrews-Hanna, Smallwood, & Spreng, 2014; Andrews-Hanna, Reidler, Huang, et al., 2010). All regions in MNI space (MFG, SPC, and core default network ROIs) were reverse normalized into individual participant space, and the same analyses performed for the mediators of hippocampal–pIPC functional connectivity were performed for these regions. When examining functional connectivity of the core default regions, we averaged correlation values across all pairs of core default network regions.
Finally, in an exploratory analysis, we examined whether baseline pIPC (TMS site) functional connectivity with any other region was correlated with TMS-related changes in associative memory. No regions survived FWE correction (using permutation testing at various cluster-forming thresholds).
RESULTS
Hippocampal Functional Connectivity
To define participant-specific hippocampal-targeted TMS sites, we first isolated the location within pIPC showing reliable functional connectivity with the right hippocampus across participants in our fMRI study (see Methods), resulting in a group level ROI. This group level ROI was then used to constrain the location of participant-specific TMS sites (Figure 1A). The right hippocampus was specifically targeted as object-location associative memory is most impaired by right compared with left hippocampal damage (Stepankova et al., 2004; Nunn et al., 1999; Baxendale et al., 1998; Bohbot et al., 1998; Smith & Milner, 1981). Figure 1A shows group-level resting functional connectivity with the middle portion of the right hippocampus. This analysis of connectivity with the right hippocampus yielded stronger and more reliable ipsilateral than contralateral connectivity in pIPC; thus, we choose to stimulate right rather than left pIPC. A clear peak can be seen in the posterior portion of the right angular gyrus (MNI coordinates: +43, −67, +28), overlapping with the posterior portion of region PG (shown in blue in Figure 1A), as in prior work (Uddin et al., 2010, see also Wang, Ritchey, Libby, & Ranganath, 2016; Wang et al., 2014; Kahn et al., 2008; Vincent et al., 2006). A group level ROI was defined by placing a 15-mm sphere around this location of peak hippocampal connectivity across participants. We then isolated participant-specific pIPC TMS sites by finding each TMS participant’s location of maximal resting functional connectivity with their hippocampal seed region within this broader group-level ROI (see Methods; Figure 1B). In this way, the group level analysis served to constrain the location of participant-specific hippocampal-targeted TMS sites within pIPC.
Influence of TMS on Subsequent Associative Memory
Object-location associative memory was assessed across three sessions: a baseline session (without TMS) and two TMS sessions, during which cTBS was applied to pIPC or a control site immediately before encoding (Figure 1C; the order of TMS sites was counterbalanced across participants). Object-location memory was analyzed using mixture models to separately estimate the overall probability of successful associative retrieval from the precision of recalled information (Figure 1D). The probability of object-location memory varied in a nearly significant manner across all sessions (main effect of Session: F(2, 40) = 3.09, p = .056, = .14). As shown in Figure 2A, the probability of memory success was significantly greater for hippocampal-targeted pIPC TMS relative to control TMS, F(1, 20) = 6.82, p = .017, = .25, and baseline memory testing, F(1, 20) = 4.96, p = .038, = .20. Object-location memory success did not differ between baseline and control TMS sessions, F(1, 20) = 0.045, p = .83. These results suggest that pIPC cTBS applied before encoding enhanced the probability of successful object-location memory formation, as indicated by greater object-location memory hours later, after the influence of TMS dissipated.
Figure 2.
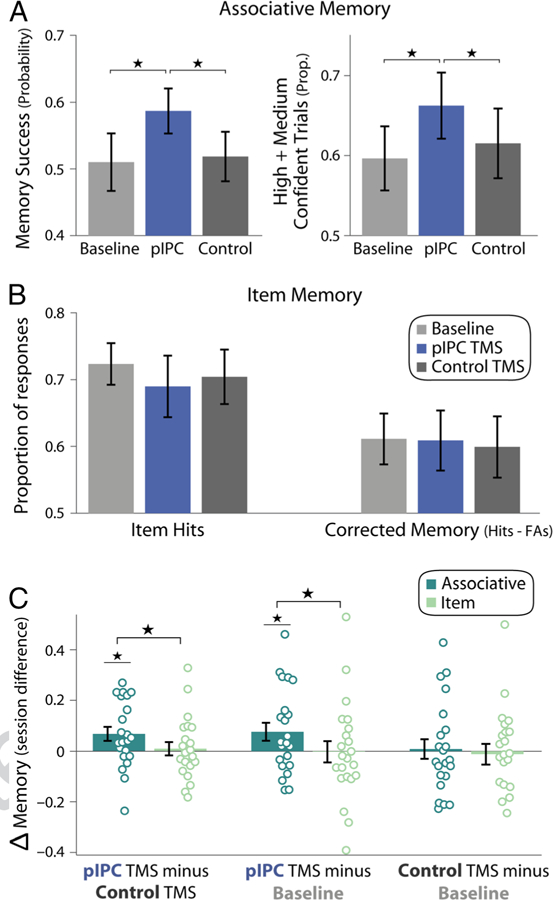
Associative and item memory across sessions. (A) Associative (object–location) memory for each session (baseline memory testing, pIPC TMS, and control TMS). The probability of object-location memory success is shown in the left plot, and the proportion of high and medium confident trials is shown in the right plot. (B) Item (object) memory for each session. Left bars show the proportion of item hits, and right bars show corrected item memory (proportion of hits minus false alarms). (C) Between-session changes in associative memory (probability of object-location memory success; dark green) and corrected item memory (light green). All error bars show standard deviation of the mean across participants, and individual circles correspond to data points for individual participants. *p < .05.
A similar influence of pIPC TMS on subsequent associative memory was found when examining continuous object-location error. As in the prior analysis, graded levels of object-location error varied across all sessions (main effect of Session: F(2, 40) = 4.45, p = .018, = .25). A decrease in object-location error, or more accurate object placement, was found for pIPC TMS relative to control TMS (mean pIPC TMS error = 325 ± 18 pixels, mean control TMS error = 352 ± 19 pixels, average benefit of 27 ± 11 pixels; F(1, 20) = 6.57, p = .019, = .25). Object-location error was also lower for pIPC TMS relative to baseline testing (mean baseline error = 372 ± 20 pixels, average benefit of 47 ± 16 pixels; F(1, 20) = 8.62, p = .008, = .30). Graded object-location error did not differ between control TMS and baseline memory (average benefit of control TMS relative to baseline testing = 19 ± 19 pixels; F(1, 20) = 0.98, p = .33).
In addition to objective object-location memory, subjective confidence in object placement was enhanced by pIPC TMS. The proportion of high and medium confident associations varied across sessions (Figure 2A, right bars; F(2, 40) = 4.54, p = .017, = .19), which was driven by a greater proportion of high and medium confident responses for pIPC TMS versus control TMS, F(1, 20) = 5.25, p = .033, = .21, and compared with baseline memory testing, F(1, 20) = 6.39, p = .02, = .25. Again, no difference in the proportion of associations rated as high or medium confident was found between control TMS and baseline memory testing, F(1, 20) = 0.84, p = .37.
The above results suggest that hippocampal-targeted TMS to pIPC enhanced both the probability of subsequent object-location memory and subjective confidence in object placement. We also assessed memory precision by considering the spread or standard deviation of fitted memory distributions (see Methods; Figure 1D). The standard deviation did not reliably differ between sessions (main effect of session: F(2, 40) = 2.12, p = .13) or TMS sites (standard deviation width: F(1, 20) = 1.74, p = .20; standard deviation height: F(1, 20) = 0.76, p = .39). These results indicate that hippocampal-targeted pIPC TMS did not reliably influence the fine-scale precision of subsequent object-location memory but instead enhanced the overall probability of associative memory success.
The influence of TMS on object-location associative memory success was consistent across no-delay and delay trial types present during encoding. No reliable Trial Type × Session, F(2, 40) = 0.27, p = .76, or TMS Site, F(1, 20) = 0.54, p = .47, interactions were found. Consistent with our prior analysis, a main effect of TMS site was present, F(1, 20) = 5.1, p = .035, as well as a trend level effect of Session, F(2, 40) = 2.70, p = .08, suggesting a similar influence of TMS on associative memory success across trial types. However, object-location memory was reduced for delay versus no-delay trials (main effect of trial type: F(1, 20) = 46.2, p < 10−5, = .70). Thus, it appears that pIPC TMS similarly influenced object-location memory across all trial types, although it is unclear if differences in overall associative memory across these conditions may have precluded the ability of TMS to differentially influence these trial types.
Influence of TMS on Subsequent Object Memory
In contrast to changes in object-location associative memory, item (object) memory was not consistently influenced by TMS. Overall item memory (hits) and corrected memory accuracy (hits minus false alarms) did not reliably differ between sessions (Figure 2B; main effect of Session, hits: F(2, 40) = 1.06, p = .36; corrected memory: F(2, 40) = 0.06, p = .94) or TMS sites (hits: F(1, 20) = 0.64, p = .43; corrected memory: F(1, 20) = 0.13, p = .72). Similar to item memory, estimates of subjective recollection and familiarity did not differ between sessions (main effect of Session, remember hits minus false alarms: F(2, 40) = 0.47, p = .63; familiarity: F(2, 40) = 0.92, p = .41) or TMS sites (remember hits minus false alarms: F(1, 20) = 0.23, p = .63; familiarity: F(1, 20) = 0.04, p = .85).
The prior results indicate that hippocampal-targeted pIPC TMS influenced object-location associative memory, but not item (object) memory. We formally tested whether TMS differentially influenced associative versus item memory. Direct comparisons of memory across TMS sites revealed significant interactions between associative versus item memory and TMS site, both when comparing the probability of object-location memory success with corrected item memory (Figure 2C, left bars), F(1, 20) = 6.17, p = .022, = .24, and item hits, F(1, 20) = 12.3, p = .002, = .38. Differential changes in associative versus item memory were also found when examining normalized percent changes across sessions, with a 14.5 ± 7% increase in the probability of associative memory success for pIPC relative to control TMS, which was greater than changes in corrected item memory (0 ± 7%), t(21) = 2.13, p = .046, and item hits (−5.7 ± 5%), t(21) = 2.52, p = .02. A specific influence of pIPC TMS on associative versus item memory was also found when comparing pIPC TMS with baseline memory (Figure 2C, middle bars; corrected item memory: F(1, 20) = 4.37, p = .049, = .18; item hits: F(1, 20) = 10.35, p = .004, = .34). This differential influence of pIPC TMS relative to baseline memory testing was also confirmed when assessing normalized between-session memory changes, with a 20 ± 9% increase in the probability of associative memory, which was greater than normalized changes in corrected item memory (−5 ± 10%), t(21) = 2.57, p = .018, and item hits (−10 ± 7%), t(21) = 2.98, p = .007. These results indicate that hippocampal-targeted pIPC TMS specifically influenced subsequent object-location memory, but not item (object) memory, suggesting a selective influence on associative memory formation.
Relationships between Object-location Memory and Recollection
We sought to understand why pIPC TMS influenced object-location associative memory (Figure 2A), but not subjective recollection, because both aspects of memory are dependent on hippocampal function (Eichenbaum, Yonelinas, & Ranganath, 2007). As a manipulation check, we ensured that remember versus know responses were accompanied by a higher probability of object-location memory (ps < 10−7 for all sessions) and that fewer remember versus know false alarms were present (ps < .031 for all sessions). In addition, a consistent influence of TMS on the probability of object-location memory was found for objects labeled as remember versus know; no Trial Type (remember/know) × TMS Site interaction, F(1, 18) = 0.02, p = .89, was present.
Although no group level changes in subjective recollection were found, we asked whether participants showing the greatest TMS-related benefit in object-location memory also showed an increase in subjective recollection. Positive relationships were found between TMS-related benefits in the probability of object-location memory and recollection across participants (Figure 3), r = .59, t(20) = 3.29, p = .0037. In contrast, TMS-related benefits in the probability of object-location memory were not related to changes in familiarity (Figure 3), r = .26, t(20) = 1.18, p = .25, and the difference between these correlations approached significance (permutation test: p = .06). These results suggest that participants showing the greatest influence of hippocampal-targeted pIPC TMS on object-location memory may also show enhanced levels of subjective recollection.
Figure 3.
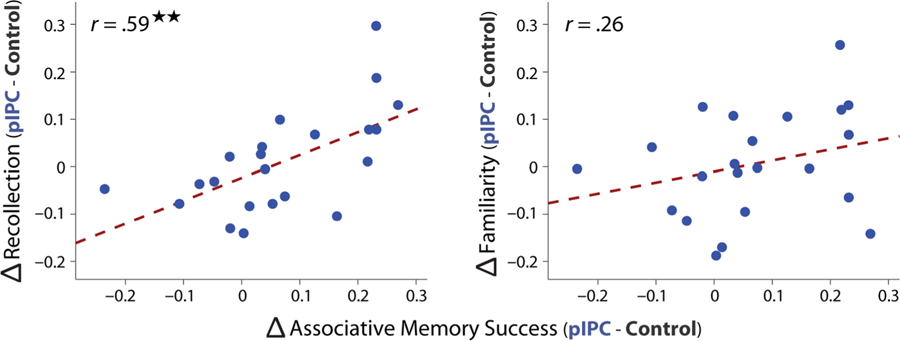
Individual differences in memory changes across TMS sessions. TMS-related changes in associative (object–location) memory success were correlated with changes in recollection as a function of hippocampal-targeted TMS (pIPC minus control TMS; left plot) across participants. Differences in familiarity based on TMS site were not reliably related to changes in associative memory success (right plot). Robust regression was used to assess all across-participant correlations.
Influence of TMS on Performance during Encoding
In addition to examining the effect of pIPC TMS on memory hours later, a period outside the direct influence of stimulation, we assessed whether TMS influenced behavioral responses during encoding (an unrelated color discrimination performed during the ITI; Figure 1C). No differences across sessions or TMS sites were found on the color discrimination task, for both RT (main effect of Session: F(2, 40) = 0.56, p = .57; main effect of TMS site: F(1, 20) = 0.21, p = .64) and accuracy (main effect of Session: F(2, 40) = 0.70, p = .50; main effect of TMS site: F(1, 20) = 0.27, p = .61). Although accuracy was high and relatively close to ceiling (mean across sessions = 91.3 ± 1.8), possibly precluding an influence of TMS, RT was not close to floor (mean = 0.84 ± 0.03 sec). In addition, TMS-related benefits in the probability of object-location associative memory were not predicted by changes in accuracy or RT across TMS sessions (RT: r = .15, t(20) = 0.66, p = .51; accuracy: r = .21, t(20) = 0.89, p = .38). These findings suggest that the influence of pIPC on subsequent associative memory was not driven by large shifts in attentional engagement or task-orientated processing during encoding.
Intrinsic Connectivity Is Related to Associative Memory Benefits of TMS
We tested the prediction that, if pIPC TMS is capable of modulating associative memory encoding via a distal influence on hippocampal function, then levels of intrinsic connectivity between the pIPC stimulation site and the hippocampus, estimated for each TMS participant, should predict the extent of changes in associative memory after pIPC stimulation. Indeed, a positive relationship was found between participant-specific levels of hippocampal–pIPC functional connectivity and the TMS-related associative memory benefit (increased probability of object-location memory for pIPC relative to control TMS) across participants (Figure 4A), r = .44, t(20) = 2. 19, p = .04. In contrast, hippocampal functional connectivity with the control TMS site did not predict TMS-related associative memory changes (Figure 4B), r = −.12, t(20) = −0.54, p = .59, and hippocampal–pIPC connectivity was significantly more related to the TMS-related benefit than hippocampal connectivity with the control site (p = .018, permutation test). These results provide a link between the potential effectiveness of hippocampal targeting via stimulation to pIPC, as assessed via levels of hippocampal–pIPC functional connectivity, and TMS-related changes in associative memory.
Figure 4.
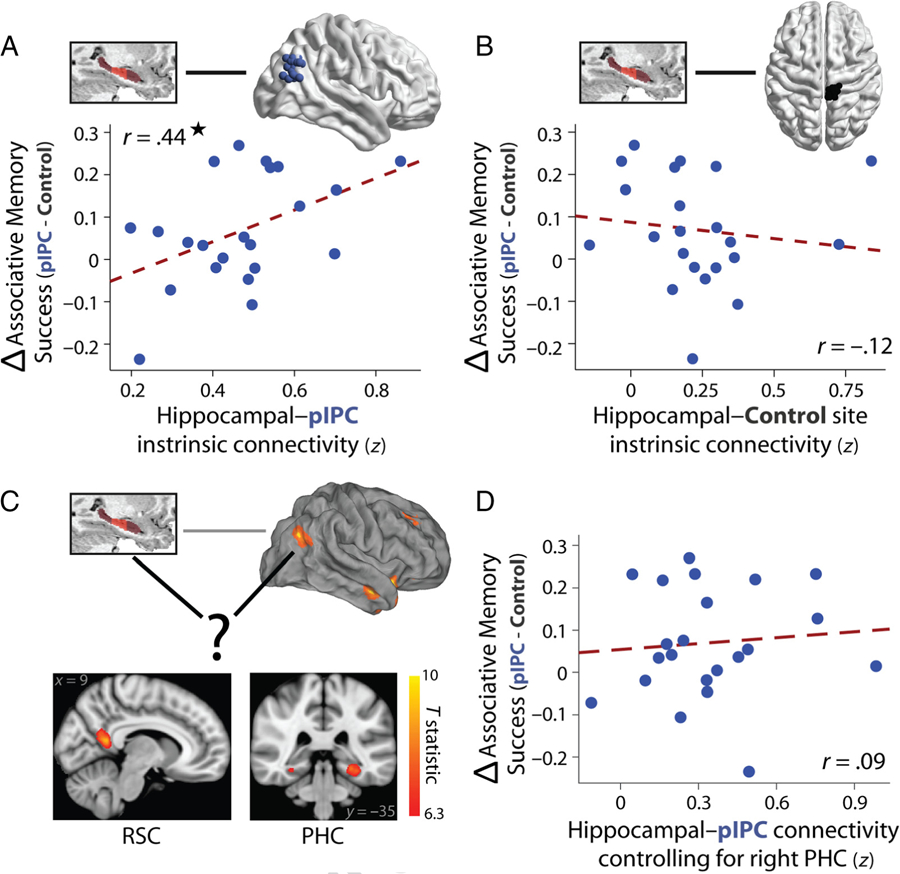
Intrinsic levels of hippocampal–pIPC functional connectivity, TMS-related benefits in associative memory, and potential mediators of hippocampal–pIPC functional connectivity. (A) Intrinsic functional connectivity between the hippocampus and pIPC TMS site was related to changes in the probability of associative memory success for pIPC versus control TMS. (B) Functional connectivity between the hippocampus and the control TMS site was not related to changes in associative memory success between TMS sites. (C) The strongest mediators of hippocampal–pIPC functional connectivity were identified from the fMRI study (excluding overlapping participants with the TMS study) and included RSC (left) and PHC (right). The statistical map is available on neurovault.org. (D) Intrinsic hippocampal–pIPC functional connectivity no longer predicted TMS-related benefits in associative memory success when controlling for the BOLD signal in right PHC. This relationship was significantly weaker than the correlation in A.
We next considered what pathways may mediate pIPC–hippocampal interactions and their relationship with associative memory benefits after TMS. One possibility is that direct connections from pIPC to the hippocampus could be responsible for the influence of hippocampal-targeted pIPC TMS (Ding, Van Hoesen, & Rockland, 2000; Rockland & Van Hoesen, 1999; Andersen, Asanuma, Essick, & Siegel, 1990; Selemon & Goldman-Rakic, 1988; Seltzer & Pandya, 1984; Seltzer & Van Hoesen, 1979). However, PG/7a in macaques strongly projects to both PHC and RSC, which in turn project to the hippocampus and entorhinal cortex (Kobayashi & Amaral, 2003, 2007; Suzuki & Amaral, 1994; Andersen et al., 1990; Cavada & Goldman-Rakic, 1989; Seltzer & Pandya, 1984), suggesting that PHC and RSC may play a role in mediating the influence of pIPC TMS on associative memory (although the homology between macaque and human angular gyrus is unclear; cf. Seghier, 2013; Husain & Nachev, 2007).
To address this question, we asked whether any brain regions statistically mediate functional connectivity between the hippocampus and pIPC (Figure 4C) from our fMRI study cohort (excluding participants also in the TMS study) and then examined their functional relevance in the TMS study. We initially used strict multiple comparison correction for identifying mediators of hippocampal–pIPC connectivity to reveal the regions that most robustly statistically mediate functional connectivity and thus would be most likely to explain variance in our TMS results. The strongest mediator was in RSC (bilaterally, peak t statistic = 9.2), followed by right and left PHC (peak t statistic = 8.34 and 8.1, respectively; Figure 7B). The only other regions that survived strict multiple comparison correction (Bonferroni correction) were the right anterior temporal cortex (middle temporal gyrus/STS, peak t statistic = 6.46) and a region in pIPC close to stimulation site (the map can be found on neurovault.org). To assess the functional relevance of these regions, we asked whether they mediate the relationship between hippocampal–pIPC connectivity and TMS-related benefits in associative memory. Hippocampal-pIPC functional connectivity no longer predicted TMS-related changes in the probability of object-location associative memory when controlling for the BOLD signal in PHC (Figure 4D; right: r = .09, t(20) = 0.38, p = .70; left: r = .12, t(20) = 0.54, p = .59) or RSC, r = .27, t(20) = 1.27, p = .22. The relationship between intrinsic hippocampal–IPC connectivity and the TMS-related benefit in associative memory success was significantly reduced after taking into account the BOLD signal in PHC (permutation test: ps = .036 and .03 for right and left PHC, respectively), with a trend observed for RSC (p = .095). Connectivity of these regions when controlling for the hippocampus was not related to TMS-related enhancements in associative memory (all ps > .30). Critically, not all regions that statistically mediated hippocampal–pIPC functional connectivity demonstrated behavioral relevance. Hippocampal–pIPC functional connectivity was still significantly related to TMS-related benefits in associative memory when controlling for the BOLD signal in anterior temporal cortex, r = .48, t(20) = 2.46, p = .023, and thus was not significantly altered (p = .61). Collectively, these results indicate that hippocampal–pIPC functional connectivity, potentially mediated by PHC and RSC, was related to changes in associative memory after hippocampal-targeted pIPC TMS.
We initially used a relatively conservative criterion to identify the strongest statistical mediators of hippocampal–pIPC functional connectivity, as we reasoned that they would be the most likely to functionally mediate relationships between hippocampal–pIPC connectivity and TMS-related memory changes and because the resulting regions strongly aligned with predictions based on anatomical connectivity. Nonetheless, to ensure that this approach did not miss other potential mediators, we isolated all regions that survived a more liberal threshold (see Methods; Table 1). Before assessing their relevance in mediating the behavioral influence of TMS, we tested whether basic mediation effects replicated in our TMS group. As shown in Table 1, most of these regions reliably mediated hippocampal–pIPC TMS site functional connectivity in our TMS group, with the exception of one region (right frontal operculum). However, none of these additional regions showed evidence of mediating the relationship between hippocampal–pIPC connectivity and TMS-related changes in associative memory (see right column in Table 1); correlations between TMS-related associative memory changes and hippocampal–pIPC functional connectivity remained significant or nearly significant when controlling for the BOLD signal in each of these regions. These results demonstrate that not all regions that statistically mediated hippocampal–pIPC functional connectivity showed evidence of mediating TMS-related memory changes. In doing so, these findings highlight the specificity of PHC and RSC in mediating the relationship between pIPC–hippocampal connectivity and associative memory benefits seen after pIPC TMS.
We further asked whether relationships between intrinsic connectivity and TMS-related associative memory changes were specific to hippocampal–pIPC interactions. As noted above, hippocampal functional connectivity with the control TMS site was not related to TMS-related associative memory benefits (Figure 4B). We considered whether baseline functional connectivity between the pIPC stimulation site and the middle frontal gyrus (MFG), which has been implicated in object-location associative encoding (Hales & Brewer, 2013), similarly predicts TMS-related associative memory benefits. MFG–pIPC functional connectivity was not related to the influence of TMS on associative memory, r = .04, t(20) = 0.16, p = .88, nor was hippocampal–MFG connectivity, r = .12, t(20) = 0.54, p = .59. These findings suggest that baseline levels of pIPC interactions with other regions involved in associative encoding were not related to the influence of pIPC TMS on associative memory.
We also considered a potential influence of pIPC TMS on nearby regions in SPC. A recent model of parietal cortex highlights the role of competitive interactions between IPC and SPC (Sestieri et al., 2017), such that cTBS applied to IPC could indirectly enhance attentional and perceptual processes implemented in SPC (Sestieri, Capotosto, Tosoni, Romani, & Corbetta, 2013), which could in turn enhance memory encoding and subsequent memory performance. To address this possibility, we examined whether hippocampal and pIPC connectivity with SPC and the IPS also predicted TMS-related changes in object-location memory. No reliable correlations were found between TMS-related changes in the probability of associative memory and hippocampal–SPC connectivity, r = .05, t(20) = 0.22, p = .83; hippocampal–IPS connectivity, r = .04, t(20) = 0.20, p = .84; IPC–SPC connectivity, r = .27, t(20) = 1.27, p = .22; and IPC–IPS connectivity, r = .22, t(20) = 1.00, p = .33. Furthermore, the relationship between hippocampal–pIPC connectivity and TMS-related memory benefits remained reliable when controlling for the BOLD signal in these regions (SPC: r = .42, t(20) = 2.09, p = .048; IPS: r = .36, t(20) = 1.73, p = .098). Finally, because the pIPC and hippocampus are often associated with the default network, as an additional control, we examined whether TMS-related changes in associative memory were related to connectivity within “core” regions of the default network (Schaefer et al., 2017; Andrews-Hanna et al., 2014; see Methods). TMS-related associative memory benefits were not related to average functional connectivity within the core default network, r = .12, t(20) = 0.52, p = .61; IPC–default-network connectivity, r = −.17, t(20) = −0.76, p = .46; or hippocampal–default-network connectivity, r = −.02, t(20) = −0.10, p = .92. These results highlight the specificity of hippocampal–pIPC connectivity in predicting TMS-related associative memory benefits, which was not seen for regions in SPC or pFC or within core regions of the default network.
DISCUSSION
The hippocampus plays a critical role in associative memory formation. Here, we examined whether this behavioral signature of hippocampal function, associative memory formation, can be influenced by TMS to pIPC, a region chosen based on showing robust functional connectivity with the hippocampus. We administered time-limited cTBS before memory encoding and examined the influence of TMS on associative and item memory performance hours later, after the direct influence of TMS dissipated. IPC stimulation enhanced subsequent memory for object-location associations, both in the probability of memory success and confidence ratings. This memory benefit was specific to associative (object–location) versus item (object) memory, which is consistent with a particular influence on hippocampal function. In addition, the strength of intrinsic functional connectivity between the pIPC TMS site and the hippocampus predicted TMS-related changes in associative memory across participants, consistent with the notion that the influence of pIPC TMS may be related to the ability to distally target the hippocampus through pIPC stimulation. This relationship was specific, as connectivity with other brain regions and networks did not predict TMS-related associative memory benefits.
Recent work has been shown that TMS to left pIPC over a duration of 5 days enhances associative memory (Nilakantan et al., 2017; Wang et al., 2014). Our findings complement this work by demonstrating a similar memory enhancement after a single session of cTBS targeting right pIPC. However, our study differs in several ways. First, our TMS protocol was brief (40 sec in duration), which has the practical advantage of minimal side effects compared with other TMS protocols (Oberman, Edwards, Eldaief, & Pascual-Leone, 2011). Second, because of the use of an extended, 5-day TMS protocol in prior work, it is unknown which phase(s) of memory (encoding, consolidation, or retrieval) were influenced by stimulation and thus responsible for the observed memory enhancement. The cTBS protocol used in our study impacts neural function for approximately 50 min after stimulation, with levels of cortical excitability (Wischnewski & Schutter, 2015; Huang et al., 2005) and BOLD responses (Hubl et al., 2008) returning to pre-cTBS baseline levels 1 hr after stimulation. Thus, by applying cTBS before encoding and separating encoding and retrieval sessions by an average of 3.4 hr, we could use TMS to selectively modulate memory encoding (and possibly an initial consolidation phase) without directly influencing retrieval processes. This selective targeting of memory encoding is notable, as a large body of fMRI and TMS evidence suggests that the direct area of stimulation, pIPC, plays a role in associative retrieval (Rugg & King, 2017; Thakral, Madore, & Schacter, 2017; Yazar, Bergström, & Simons, 2014, 2017; Kim, 2013; Hutchinson, Uncapher, & Wagner, 2009; Vilberg & Rugg, 2008; Wagner et al., 2005). This leaves open the possibility that hippocampal-targeted stimulation in prior work may enhance associative memory via a direct influence on retrieval processes supported by left pIPC, as suggested by modified retrieval-related activity in parietal cortex after the 5-day TMS protocol (Nilakantan et al., 2017). Our findings are distinct from this work as they indicate that hippocampal-targeted TMS can specifically influence the encoding of novel associations into memory. This result has practical implications, suggesting that this protocol may provide an opportunity for enhanced encoding of specific information into long-term memory and could potentially be used in patient populations to improve deficits in associative encoding. It will be critical to examine whether the memory enhancement observed here is maintained at longer study-test delays when considering its potential use in applied settings.
Our findings provide additional specificity regarding the influence of hippocampal-targeted TMS on subsequent memory. To understand the nature of memory processes affected by stimulation, we examined the influence of TMS on subsequent item and associative information as well as on associative memory success and precision. A robust body of evidence indicates that the hippocampus supports the encoding of associative or relational information whereas item memory can be supported by extrahippocampal structures (Diana et al., 2007; Mayes et al., 2007; Davachi, 2006; Smith & Milner, 1981, 1989). We thus tested the prediction that, if hippocampal-targeted stimulation particularly influences hippocampal function, it should preferentially modulate associative versus item memory. Indeed, a selective influence was found on object-location associative memory compared with item or object memory. This result importantly extends prior work by providing evidence that hippocampal-targeted TMS specifically influences memory processes linked with hippocampal function.
In the current study, hippocampal-targeted cTBS enhanced the probability of subsequent object-location memory success, with no influence on memory precision. In contrast, Nilakantan and colleagues (2017) found that hippocampal-targeted TMS to pIPC enhanced memory precision and did not alter overall retrieval success. There are several possible explanations for these differences across studies. First, in our study, participants encoded nearly 200 object–location associations in each session with a study-test delay of several hours, whereas in the study by Nilakantan and colleagues, each session contained 24 associations with a shorter delay of 90 sec before memory testing. An influence on memory precision may be favored in the case of a reduced memory demand and brief retention interval (in which overall retrieval success may be close to ceiling levels), whereas an increase in precision may be less likely with a longer study-test delay and a larger encoding set, leading to a higher level of interference (Sun et al., 2017). Other methodological differences across studies could have been responsible for producing different results (e.g., the use of distinct TMS protocols influencing different memory phases, as discussed above, or differing methods for estimating memory precision and success). Another possibility is that the diverging behavioral effects may be driven by a different locus of TMS effects across studies. A recent study found that distinct brain regions track long-term memory precision versus success (Richter et al., 2016), with pIPC activation reflecting the precision of retrieved content and the hippocampus tracking overall memory success. This suggests the possibility that different brain regions may be influenced by the distinct TMS approaches employed across studies. Nonetheless, further work is needed to understand the conditions under which hippocampal-targeted TMS modulates memory success versus precision.
Our results indicate that continuous theta-burst stimulation, cTBS, performed to pIPC enhanced a form of hippocampal-dependent memory, associative memory formation. It is important to consider how cTBS to pIPC may have enhanced associative memory, via a potential influence either on the hippocampus or on other brain regions. First, as we did not collect fMRI data after TMS, we cannot definitively conclude that our protocol influenced behavior via an impact on hippocampal or MTL mechanisms supporting memory encoding, nor can we conclusively rule out an influence on other brain regions that may have driven our behavioral findings. Nonetheless, we think that our results are parsimoniously explained by a potential influence on the hippocampus given the selective change in associative versus item memory after TMS and because baseline levels of pIPC–hippocampal functional connectivity predicted TMS-related associative memory benefits, which was not seen for several other brain regions or networks that we examined. In contrast, it appears unlikely that the observed behavioral effects were due to a local influence on encoding-related processes implemented in pIPC for several reasons. First, although pIPC plays a clear role in associative retrieval (Rugg & King, 2017; Hutchinson et al., 2009), the stimulation site in right pIPC has not been implicated in object-location encoding (Sommer, Rose, Gläscher, et al., 2005; Sommer, Rose, Weiller, et al., 2005; Cansino et al., 2002) or successful item–context encoding across many prior studies (summarized in meta-analyses: Kim, 2011; Uncapher & Wagner, 2009). Greater activity associated with memory formation, perceptual, and attentional processes is typically found in SPC (Sestieri et al., 2017; Hales & Brewer, 2013; Uncapher & Wagner, 2009). It is also important to highlight that, if processes or representations in right pIPC did support successful associative encoding in our paradigm (Lee, Chun, & Kuhl, 2017), the application of inhibitory cTBS would predict a reduction in subsequent associative memory. Indeed, cTBS and other inhibitory TMS protocols applied to pIPC before retrieval impair aspects of associative memory (Thakral et al., 2017; Yazar et al., 2014, 2017; Sestieri et al., 2013). Instead, we observed an enhancement in subsequent associative memory after cTBS to pIPC (see below for a discussion of the directionality of the observed effects). Thus, given that right pIPC is not typically implicated in object-location or associative encoding, as well as the inhibitory nature of cTBS, it appears unlikely that the influence of TMS in the present work was due to a direct impact on associative encoding processes supported by pIPC. However, future work combining TMS with neuroimaging is needed to directly examine which memory-related processes were modified by hippocampal-targeted cTBS and whether altered encoding-related processes in pIPC may drive the observed memory enhancements.
Beyond a potential role of pIPC in supporting memory mechanisms per se, pIPC is a node within the default mode network, which is typically deactivated during externally oriented task performance (Sestieri et al., 2010; Buckner, Andrews-Hanna, & Schacter, 2008; Fox et al., 2005; Raichle et al., 2001). Default network activity has been related to internally or self-oriented aspects of cognition, such as thinking about future or past experiences, memory retrieval, and self-referential mentation (Sestieri, Corbetta, Romani, & Shulman, 2011; Andrews-Hanna, Reidler, Huang, et al., 2010; Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Mason et al., 2007). The presence of internally oriented or task-unrelated mentation can impair memory formation, likely by reducing attention to external stimuli (Smallwood, Baracaia, Lowe, & Obonsawin, 2003). Given the inhibitory nature of cTBS (Huang et al., 2005), it is possible that disruption of pIPC function may have reduced internally oriented cognition, resulting in greater task-related attentional focus and improved memory encoding. In addition, proposed antagonistic interactions between IPC and SPC predict that inhibition of IPC could result in enhanced attention and perceptual processes in SPC (Sestieri et al., 2017), leading to better encoding. However, we think that these explanations do not provide the most parsimonious explanation of our findings. First, in contrast to hippocampal–pIPC connectivity, connectivity of regions in SPC with pIPC or the hippocampus did not predict TMS-related memory benefits, nor did connectivity of core default network regions. Second, RT and performance on the discrimination task performed during encoding were not affected by pIPC TMS, and individual differences in these measures did not predict TMS-related benefits in associative memory. This suggests that large shifts in externally oriented attention during encoding were not likely driving the observed memory enhancement, as measured by performance on this externally oriented task. However, we acknowledge that this control task lacked challenging RT demands, so it is possible that an influence on externally oriented attention may have been revealed using a task with greater attention demands. Finally, if pIPC TMS did enhance overall task or externally oriented attention, this would predict a general boost in the encoding of both objects and object–location associations. Yet, a specific enhancement in associative versus item memory was observed, consistent with an influence on hippocampal function rather than a generalized attentional influence. For these reasons, we think it is unlikely that the enhancement of associative memory formation seen after pIPC TMS was due to a generalized influence on attentional processes or antagonistic interactions between IPC and SPC. Nonetheless, further work is needed to more carefully rule out all of these alternative possible explanations.
Regarding the direction of memory changes seen after TMS, we observed an enhancement in object-location memory, paralleling associative memory improvements seen in prior work employing hippocampal-targeted TMS (Nilakantan et al., 2017; Wang & Voss, 2015; Wang et al., 2014). The cTBS protocol used here decreases cortical excitability and is considered to have an inhibitory effect on local processing (Suppa et al., 2016; Hoogendam, Ramakers, & Di Lazzaro, 2010; Stagg et al., 2009; Huang et al., 2005). Although it may seem counterintuitive that the application of an inhibitory protocol could potentially enhance encoding-related mechanisms in interconnected brain regions, a growing body of work indicates that repetitive inhibitory TMS protocols enhance the excitability, activity, and connectivity of brain regions that are interconnected with the direct stimulation site (Cocchi et al., 2015; Valchev et al., 2015; Capotosto, Babiloni, Romani, & Corbetta, 2014; Watanabe et al., 2014; Andoh & Zatorre, 2013; Gratton et al., 2013; Eldaief et al., 2011; Vercammen, Knegtering, Liemburg, den Boer, & Aleman, 2010; Suppa et al., 2008). Complementary decreases in excitability, activity, and connectivity have been observed after excitatory repetitive TMS (Watanabe et al., 2014; Eldaief et al., 2011; Suppa et al., 2008). Moreover, local decreases in cerebral blood flow under the direct site of stimulation have been associated with increases in functional network connectivity, providing a possible link between greater local inhibitory influences of TMS and distal increases in function (Gratton, Lee, Nomura, & D’Esposito, 2014). It is thus possible to speculate that inhibition of one region within a larger network may result in a compensatory increase in activity in interconnected regions, such that inhibition of pIPC may result in increased activity in the hippocampus and interconnected regions. Although speculative, this notion is consistent with patient data showing that MTL damage can enhance functional connectivity of the default network outside the MTL, including pIPC (Hayes, Salat, & Verfaellie, 2012). Combining cTBS and fMRI in future studies will be beneficial to understand how this inhibitory TMS protocol influences encoding-related activity and enhances associative memory.
In addition to examining the influence of pIPC TMS on subsequent memory, we used mediation analyses to probe potential pathways that may underlie TMS-related memory enhancements. We found that baseline levels of hippocampal functional connectivity with the pIPC TMS site were related to enhanced associative memory after pIPC TMS. However, we note that our sample size is relatively low (N = 22), raising the possibility that the effect size of this correlation was overestimated (Button et al., 2013); it will be important to replicate this relationship in future work using larger sample sizes. Nonetheless, this finding suggests that the behavioral benefit of pIPC on associative memory may be related to our ability to effectively target or influence hippocampal function via pIPC stimulation. It is possible that direct connectivity from pIPC to the hippocampus, reported in macaques (Ding et al., 2000; Rockland & Van Hoesen, 1999), may underlie our observed effects. Considering indirect pathways, we found that RSC, PHC, and anterior temporal cortex most strongly mediated hippocampal–pIPC functional connectivity. Controlling for the BOLD signal in PHC and RSC reduced the relationship between hippocampal–IPC functional connectivity and TMS-related associative memory benefits, suggesting that these regions may play a functional role in mediating the influence of pIPC TMS. These findings are consistent with prior evidence that these regions cluster together to comprise a functional network (Maguire & Mullally, 2013; Vann, Aggleton, & Maguire, 2009), corresponding to the proposed posterior–medial memory network (Ritchey, Yonelinas, & Ranganath, 2014; Ranganath & Ritchey, 2012) and the “medial temporal” subsystem of the default network identified in resting functional connectivity (Schaefer et al., 2017; Andrews-Hanna et al., 2014; Yeo et al., 2011; Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010). It is possible that the influence of hippocampal-targeted TMS in prior work may also be related to baseline functional connectivity in this network (Nilakantan et al., 2017; Wang et al., 2014).
In summary, this work indicates that a brief session of hippocampal-targeted cTBS applied to pIPC selectively enhances the encoding of new associations versus individual items into memory. This adds to a growing body of work suggesting that TMS can be used to target and potentially manipulate MTL function (Nilakantan et al., 2017; Wang et al., 2014). However, as noted above, future investigations combining hippocampal-targeted cTBS with fMRI, additional behavioral measures, and sources of variability that influence the efficacy of TMS (Jannati, Block, Oberman, Rotenberg, & Pascual-Leone, 2017) will be critical to better understand how this protocol influences memory and neural function.
Acknowledgments
This work was supported by the National Institutes of Health (NIH MH63901, MH106280, and NS0802069). We thank Jeffrey Crawford, Matthew Bergendahl, Jenna Collins, Chelsea Harmon, and Alyson Seah for assistance with data collection and Regina Lapate and Liang-Tien (Frank) Hsieh for helpful comments and discussions.
REFERENCES
- Andersen RA, Asanuma C, Essick G, & Siegel RM (1990). Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobe. Journal of Comparative Neurology, 296, 65–113. [DOI] [PubMed] [Google Scholar]
- Andoh J, & Zatorre RJ (2013). Mapping interhemispheric connectivity using functional MRI after transcranial magnetic stimulation on the human auditory cortex. Neuroimage, 79, 162–171. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, & Buckner RL (2010). Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology, 104, 322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional–anatomic fractionation of the brain’s default network. Neuron, 65, 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, & Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Chan E, & Mattingley JB (2010). Dissociable neural circuits for encoding and retrieval of object locations during active navigation in humans. Neuroimage, 49, 2816–2825. [DOI] [PubMed] [Google Scholar]
- Baxendale SA, Thompson PJ, & Van Paesschen W (1998). A test of spatial memory and its clinical utility in the pre-surgical investigation of temporal lobe epilepsy patients. Neuropsychologia, 36, 591–602. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, & Nadel L (1998). Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia, 36, 1217–1238. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14, 365–376. [DOI] [PubMed] [Google Scholar]
- Cameron IGM, Riddle JM, & D’Esposito M (2015). Dissociable roles of dorsolateral prefrontal cortex and frontal eye fields during saccadic eye movements. Frontiers in Human Neuroscience, 9, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, & Rugg MD (2002). Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex, 12, 1048–1056. [DOI] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, & Corbetta M (2014). Resting-state modulation of alpha rhythms by interference with angular gyrus activity. Journal of Cognitive Neuroscience, 26, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, & Goldman-Rakic PS (1989). Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. Journal of Comparative Neurology, 287, 393–421. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Ongür D, & Whitfield-Gabrieli S (2012). Anticorrelations in resting state networks without global signal regression. Neuroimage, 59, 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, & Etkin A (2013). Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology, 38, 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, & Strafella AP (2009). rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One, 4, e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, U.S.A, 106, 8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Sale MV, Lord A, Zalesky A, Breakspear M, & Mattingley JB (2015). Dissociable effects of local inhibitory and excitatory theta-burst stimulation on large-scale brain dynamics. Journal of Neurophysiology, 113, 3375–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2007). Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences, 11, 379–386. [DOI] [PubMed] [Google Scholar]
- Ding S-L, Van Hoesen GW, & Rockland KS (2000). Inferior parietal lobule projections to the presubiculum and neighboring ventromedial temporal cortical areas. Journal of Comparative Neurology, 425, 510–530. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, & Ranganath C (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, & Pascual-Leone A (2011). Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences, U.S.A, 108, 21229–21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Thai M, Okabe H, DeGutis J, Saad E, Laganiere SE, et al. (2017). Network-targeted cerebellar transcranial magnetic stimulation improves attentional control. Neuroimage, 156, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, & Pascual-Leone A (2012). Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage, 62, 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, U.S.A, 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski KJ, Varoquaux G, Rivera G, Schwarz Y, Ghosh SS, Maumet C, et al. (2015). NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Lee TG, Nomura EM, & D’Esposito M (2013). The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Frontiers in Systems Neuroscience, 7, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Lee TG, Nomura EM, & D’Esposito M (2014). Perfusion MRI indexes variability in the functional brain effects of theta-burst transcranial magnetic stimulation. PLoS One, 9, e101430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales JB, & Brewer JB (2013). Parietal and frontal contributions to episodic encoding of location. Behavioural Brain Research, 243, 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, & Pascual-Leone A (2014). Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. Journal of Neuroscience, 34, 12049–12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, & Ranganath C (2008). Medial temporal lobe activity predicts successful relational memory binding. Journal of Neuroscience, 28, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow IM, & Yonelinas AP (2016). Distinguishing between the success and precision of recollection. Memory, 24, 114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Salat DH, & Verfaellie M (2012). Default network connectivity in medial temporal lobe amnesia. Journal of Neuroscience, 32, 14622–14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Gong QY, Roberts N, & Kapur N (2005). Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus, 15, 203–215. [DOI] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GMJ, & Di Lazzaro V (2010). Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimulation, 3, 95–118. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, & Rothwell JC (2005). Theta burst stimulation of the human motor cortex. Neuron, 45, 201–206. [DOI] [PubMed] [Google Scholar]
- Hubl D, Nyffeler T, Wurtz P, Chaves S, Pflugshaupt T, Lüthi M, et al. (2008). Time course of blood oxygenation level-dependent signal response after theta burst transcranial magnetic stimulation of the frontal eye field. Neuroscience, 151, 921–928. [DOI] [PubMed] [Google Scholar]
- Husain M, & Nachev P (2007). Space and the parietal cortex. Trends in Cognitive Sciences, 11, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, & Wagner AD (2009). Posterior parietal cortex and episodic retrieval: Convergent and divergent effects of attention and memory. Learning & Memory, 16, 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannati A, Block G, Oberman LM, Rotenberg A, & Pascual-Leone A (2017). Interindividual variability in response to continuous theta-burst stimulation (cTBS) in healthy adults. Clinical Neurophysiology, 128, 2268–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, & Buckner RL (2008). Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2011). Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage, 54, 2446–2461. [DOI] [PubMed] [Google Scholar]
- Kim H (2013). Differential neural activity in the recognition of old versus new events: An activation likelihood estimation meta-analysis. Human Brain Mapping, 34, 814–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, & Amaral DG (2003). Macaque monkey retrosplenial cortex: II. Cortical afferents. Journal of Comparative Neurology, 466, 48–79. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, & Amaral DG (2007). Macaque monkey retrosplenial cortex: III. Cortical efferents. Journal of Comparative Neurology, 502, 810–833. [DOI] [PubMed] [Google Scholar]
- Koen JD, & Yonelinas AP (2016). Recollection, not familiarity, decreases in healthy aging: Converging evidence from four estimation methods. Memory, 24, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJoie R, Landeau B, Perrotin A, Bejanin A, Egret S, Pélerin A, et al. (2014). Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer’s and semantic dementia-targeted networks. Neuron, 81, 1417–1428. [DOI] [PubMed] [Google Scholar]
- Lee H, Chun MM, & Kuhl BA (2017). Lower parietal encoding activation is associated with sharper information and better memory. Cerebral Cortex, 27, 2486–2499. [DOI] [PubMed] [Google Scholar]
- Lee TG, & D’Esposito M (2012). The dynamic nature of top–down signals originating from prefrontal cortex: A combined fMRI-TMS study. Journal of Neuroscience, 32, 15458–15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, & Mullally SL (2013). The hippocampus: A manifesto for change. Journal of Experimental Psychology: General, 142, 1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, & Macrae CN (2007). Wandering minds: The default network and stimulus-independent thought. Science, 315, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, & Migo E (2007). Associative memory and the medial temporal lobes. Trends in Cognitive Sciences, 11, 126–135. [DOI] [PubMed] [Google Scholar]
- Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, & Mostofsky SH (2014). Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage, 96, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, & D’Esposito M (2017). Causal evidence for lateral prefrontal cortex dynamics supporting cognitive control. eLife, 6, e28040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilakantan AS, Bridge DJ, Gagnon EP, VanHaerents SA, & Voss JL (2017). Stimulation of the posterior cortical–hippocampal network enhances precision of memory recollection. Current Biology, 27, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn JA, Graydon FJX, Polkey CE, & Morris RGM (1999). Differential spatial memory impairment after right temporal lobectomy demonstrated using temporal titration. Brain, 122, 47–59. [DOI] [PubMed] [Google Scholar]
- Oberman L, Edwards D, Eldaief M, & Pascual-Leone A (2011). Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. Journal of Clinical Neurophysiology, 28, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RK, Moses SN, Riggs L, & Ryan JD (2012). The hippocampus supports multiple cognitive processes through relational binding and comparison. Frontiers in Human Neuroscience, 6, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekema C, Kessels RPC, Mars RB, Petersson KM, & Fernández G (2006). The right hippocampus participates in short-term memory maintenance of object–location associations. Neuroimage, 33, 374–382. [DOI] [PubMed] [Google Scholar]
- Power JD, Plitt M, Laumann TO, & Martin A (2017). Sources and implications of whole-brain fMRI signals in humans. Neuroimage, 146, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. (2000). Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: Minimizing the discrepancies between laboratories. Cerebral Cortex, 10, 433–442. [DOI] [PubMed] [Google Scholar]
- Rahnev D, Nee DE, Riddle J, Larson AS, & D’Esposito M (2016). Causal evidence for frontal cortex organization for perceptual decision making. Proceedings of the National Academy of Sciences, U.S.A, 113, 6059–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, U.S.A, 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, & Ritchey M (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience, 13, 713–726. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, & D’Esposito M (2004). Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia, 42, 2–13. [DOI] [PubMed] [Google Scholar]
- Richter FR, Cooper RA, Bays PM, & Simons JS (2016). Distinct neural mechanisms underlie the success, precision, and vividness of episodic memory. eLife, 5, e18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Yonelinas AP, & Ranganath C (2014). Functional connectivity relationships predict similarities in task activation and pattern information during associative memory encoding. Journal of Cognitive Neuroscience, 26, 1085–1099. [DOI] [PubMed] [Google Scholar]
- Rockland KS, & Van Hoesen GW (1999). Some temporal and parietal cortical connections converge in CA1 of the primate hippocampus. Cerebral Cortex, 9, 232–237. [DOI] [PubMed] [Google Scholar]
- Romei V, Bauer M, Brooks JL, Economides M, Penny W, Thut G, et al. (2016). Causal evidence that intrinsic beta-frequency is relevant for enhanced signal propagation in the motor system as shown through rhythmic TMS. Neuroimage, 126, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Driver J, & Bestmann S (2009). Combining TMS and fMRI: From “virtual lesions” to functional-network accounts of cognition. Cortex, 45, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, & King DR (2017). Ventral lateral parietal cortex and episodic memory retrieval. Cortex, 1–13. [DOI] [PMC free article] [PubMed]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, et al. (2017). Local–global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebral Cortex. [DOI] [PMC free article] [PubMed]
- Schneegans S, & Bays PM (2016). No fixed item limit in visuospatial working memory. Cortex, 83, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry, 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML (2013). The angular gyrus: Multiple functions and multiple subdivisions. The Neuroscientist, 19, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, & Goldman-Rakic PS (1988). Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. Journal of Neuroscience, 8, 4049–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, & Pandya DN (1984). Further observations on parieto-temporal connections in the rhesus monkey. Experimental Brain Research, 55, 301–312. [DOI] [PubMed] [Google Scholar]
- Seltzer B, & Van Hoesen GW (1979). A direct inferior parietal lobule projection to the presubiculum in the rhesus monkey. Brain Research, 179, 157–161. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Capotosto P, Tosoni A, Romani GL, & Corbetta M (2013). Interference with episodic memory retrieval following transcranial stimulation of the inferior but not the superior parietal lobule. Neuropsychologia, 51, 900–906. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, & Shulman GL (2011). Episodic memory retrieval, parietal cortex, and the default mode network: Functional and topographic analyses. Journal of Neuroscience, 31, 4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, & Corbetta M (2010). Attention to memory and the environment: Functional specialization and dynamic competition in human posterior parietal cortex. Journal of Neuroscience, 30, 8445–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, & Corbetta M (2017). The contribution of the human posterior parietal cortex to episodic memory. Nature Reviews Neuroscience, 18, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Baracaia SF, Lowe M, & Obonsawin M (2003). Task unrelated thought whilst encoding information. Consciousness and Cognition, 12, 452–484. [DOI] [PubMed] [Google Scholar]
- Smith ML, & Milner B (1981). The role of the right hippocampus in the recall of spatial location. Neuropsychologia, 19, 781–793. [DOI] [PubMed] [Google Scholar]
- Smith ML, & Milner B (1989). Right hippocampal impairment in the recall of spatial location: Encoding deficit or rapid forgetting? Neuropsychologia, 27, 71–81. [DOI] [PubMed] [Google Scholar]
- Sommer T, Rose M, Gläscher J, Wolbers T, & Büchel C (2005). Dissociable contributions within the medial temporal lobe to encoding of object-location associations. Learning & Memory, 12, 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Rose M, Weiller C, & Büchel C (2005). Contributions of occipital, parietal and parahippocampal cortex to encoding of object–location associations. Neuropsychologia, 43, 732–743. [DOI] [PubMed] [Google Scholar]
- Squire LR (1992). Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review, 99, 195–231. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, et al. (2009). Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. Journal of Neurophysiology, 101, 2872–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Cooper E, & Henson RN (2013). Reversible information flow across the medial temporal lobe: The hippocampus links cortical modules during memory retrieval. Journal of Neuroscience, 33, 14184–14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepankova K, Fenton AA, Pastalkova E, Kalina M, & Bohbot VD (2004). Object–location memory impairment in patients with thermal lesions to the right or left hippocampus. Neuropsychologia, 42, 1017–1028. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, & Dagher A (2001). Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. Journal of Neuroscience, 21, RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, & Dagher A (2003). Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain, 126, 2609–2615. [DOI] [PubMed] [Google Scholar]
- Sun SZ, Fidalgo C, Barense MD, Lee ACH, Cant JS, & Ferber S (2017). Erasing and blurring memories: The differential impact of interference on separate aspects of forgetting. Journal of Experimental Psychology: General, 146, 1606–1630. [DOI] [PubMed] [Google Scholar]
- Suppa A, Huang Y-Z, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, et al. (2016). Ten years of theta burst stimulation in humans: Established knowledge, unknowns and prospects. Brain Stimulation, 9, 323–335. [DOI] [PubMed] [Google Scholar]
- Suppa A, Ortu E, Zafar N, Deriu F, Paulus W, Berardelli A, et al. (2008). Theta burst stimulation induces after-effects on contralateral primary motor cortex excitability in humans. Journal of Physiology, 586, 4489–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, & Amaral DG (1994). Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. Journal of Comparative Neurology, 350, 497–533. [DOI] [PubMed] [Google Scholar]
- Tambini A, & Davachi L (2013). Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proceedings of the National Academy of Sciences, U.S.A, 110, 19591–19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, & Schacter DL (2017). A role for the left angular gyrus in episodic simulation and memory. Journal of Neuroscience, 37, 8142–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, et al. (2010). Dissociable connectivity within human angular gyrus and intraparietal sulcus: Evidence from functional and structural connectivity. Cerebral Cortex, 20, 2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, & Wagner AD (2009). Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual-attention theory. Neurobiology of Learning and Memory, 91, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valchev N, Ćurčić-Blake B, Renken RJ, Avenanti A, Keysers C, Gazzola V, et al. (2015). cTBS delivered to the left somatosensory cortex changes its functional connectivity during rest. Neuroimage, 114, 386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asselen M, Kessels RPC, Frijns CJM, Kappelle LJ, Neggers SFW, & Postma A (2009). Object-location memory: A lesion–behavior mapping study in stroke patients. Brain and Cognition, 71, 287–294. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, & Maguire EA (2009). What does the retrosplenial cortex do? Nature Reviews Neuroscience, 10, 792–802. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, & Aleman A (2010). Functional connectivity of the temporo-parietal region in schizophrenia: Effects of rTMS treatment of auditory hallucinations. Journal of Psychiatric Research, 44, 725–731. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, & Rugg MD (2008). Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia, 46, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. (2006). Coherent spontaneous activity identifies a hippocampal–parietal memory network. Journal of Neurophysiology, 96, 3517–3531. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, & Buckner RL (2005). Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences, 9, 445–453. [DOI] [PubMed] [Google Scholar]
- Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, et al. (2014). Targeted enhancement of cortical–hippocampal brain networks and associative memory. Science, 345, 1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, & Voss JL (2015). Long-lasting enhancements of memory and hippocampal–cortical functional connectivity following multiple-day targeted noninvasive stimulation. Hippocampus, 25, 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-F, Ritchey M, Libby L, & Ranganath C (2016). Functional connectivity based parcellation of the human medial temporal lobe. Neurobiology of Learning and Memory, 134, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Ohminami S, Tsutsumi R, Terao Y, et al. (2014). Bidirectional effects on interhemispheric resting-state functional connectivity induced by excitatory and inhibitory repetitive transcranial magnetic stimulation. Human Brain Mapping, 35, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischnewski M, & Schutter DJLG (2015). Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimulation, 8, 685–692. [DOI] [PubMed] [Google Scholar]
- Yazar Y, Bergström ZM, & Simons JS (2014). Continuous theta burst stimulation of angular gyrus reduces subjective recollection. PLoS One, 9, e110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazar Y, Bergström ZM, & Simons JS (2017). Reduced multimodal integration of memory features following continuous theta burst stimulation of angular gyrus. Brain Stimulation, 10, 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Chee MWL, & Buckner RL (2014). Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage, 88, 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (2013). The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioural Brain Research, 254, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, & Jacoby LL (1995). The relation between remembering and knowing as bases for recognition: Effects of size congruency. Journal of Memory and Language, 34, 622–643. [Google Scholar]
- Zimmermann K, & Eschen A (2017). Brain regions involved in subprocesses of small-space episodic object-location memory: A systematic review of lesion and functional neuroimaging studies. Memory, 25, 487–519. [DOI] [PubMed] [Google Scholar]


