Abstract
KRAS, one of the most prevalent oncogenes and sought-after anticancer targets, has eluded chemists for decades until an irreversible covalent strategy targeting a specific mutation (G12C) paved the way for the first KRAS inhibitors to reach the clinic. MRTX849 is one such clinical candidate with promising initial results in patients harboring the mutation. The impressive optimization story of MRTX849 highlights challenges and solutions in the development of covalent drugs, including the use of an α-fluoroacrylamide electrophile.
KRAS is one of the most prevalent oncogenes and is mutated in a large fraction of lethal cancers including pancreatic, colorectal, and lung cancer. The crucial importance of constitutively active KRAS signaling in these cancers has turned it into a “holy grail” for cancer drug discovery for several decades. However, KRAS turned out to be a notoriously difficult target for inhibitors, due to the supposed lack of pockets for small molecule binders, the extremely high affinity toward its natural ligands GTP and GDP, and the relatively minor structural differences between the wild type and mutants, which complicates mutant selective inhibition. The KRASG12C mutation, which is found in a large proportion of KRAS driven lung cancers and also in additional cancers, offered an opportunity for the development of potent and selective inhibitors. Groundbreaking work by Shokat and colleagues1 demonstrated the first covalent KRASG12C inhibitors that targeted a hydrophobic pocket below the switch-II loop and locked the protein in its inactive GDP bound state. This finding launched a race for the development of covalent inhibitors with improved potency and pharmacological properties, culminating in several compounds reaching the clinic including ARS-3248, AMG-510,2 and MRTX849.3 In this issue, Fell et al. report the development and optimization story of MRTX849.4 Starting from a potent but metabolically unstable lead, the authors performed a detailed metabolites characterization and used structure-based design to improve the potency and pharmacological properties of the compound. MRTX849 inhibits KRASG12C with nM potency, engages KRAS potently and selectively in vivo, exhibits potent anticancer activity in mice, and shows promising initial results in patients.
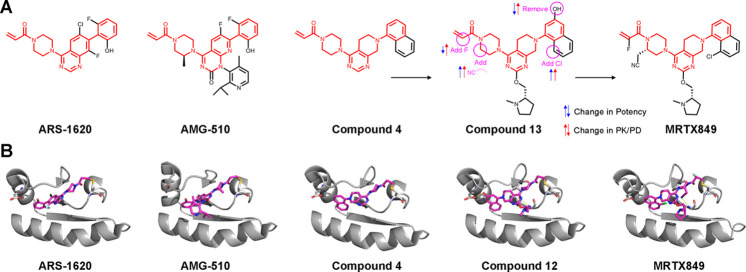
Fell et al. previously reported performing a covalent fragment screen of the Array BioPharma collection,5 which ultimately led to compound 4 (Figure 1A), which despite originating from an independent fragment screen shows marked similarity to the previously reported KRASG12C acrylamide inhibitors ARS-1620 and AMG-510 (Figure 1A). Using structure-based design, they optimized compound 4 to compound 13 that was the starting point of the current effort. While compound 13 inhibited KRASG12C potently in animal models and led to tumor regression, it suffered from rapid clearance and very low oral bioavailability.
Figure 1.
Structures and optimization of clinical covalent KRASG12C inhibitors. (A) Chemical structures of advanced KRASG12C inhibitors. Highlighted in red is the almost identical scaffold shared by all three major series. Compound 4 previously reported by Fell et al.5 was progressed to compound 13 that suffered from clearance problems. Now, compound 13 was optimized to the clinical compound MRTX849. Red and blue arrows indicate improvement or deterioration in potency and PK/PD, respectively, for each modification introduced. (B) Cocrystal structures of KRASG12C in complex with the various binders show the highly similar binding modes of these compounds. No structure is available for compound 13; however a very close analog (compound 12(5)) illustrates the changes in interactions with the protein achieved by the introduced modifications. PDB codes from left to right are the following: 5V9U, 6OIM, 6N2J, 6N2K, 6UT0.
The authors first carefully characterized the metabolites formed by compound 13 in order to identify the most metabolically sensitive positions in the molecule. These tests clearly identified the naphthol group and the acrylamide as the most sensitive positions. Removing the hydroxyl from the naphthol group resulted in improved stability and permeability but loss of potency due to the loss of a hydrogen bond with Asp69 (Figure 1B). To regain potency, they added substituents both to the piperazine ring, which displaced a water molecule that was hydrogen bonded to Gly10 and Thr58, and to position 8 of the naphthalene group to occupy a vacant hydrophobic pocket. This resulted in dramatic increase in potency and to further improvement in pharmacological properties.
The resulting compound showed marked antitumor activity in mice but rapid clearance and low bioavailability in dogs. The authors found that the acrylamide group is metabolized primarily by GST-mediated reaction with glutathione. To mitigate this problem, they explored substituted acrylamides with diminished reactivity toward glutathione. This led to the α-fluoro substituted MRTX849, which had slightly lower potency but much better stability and bioavailability. Such α-fluoroacrylamides were previously reported for EGFR covalent inhibitors6 and are conceptually similar to cyanoacrylamides, suggesting a possible role for reversibility of the covalent bond formation. These electrophiles should be further investigated, and undoubtedly such a successful example will spur much interest in their future incorporation in targeted covalent inhibitors.
MRTX849 displayed excellent antitumor activity in mice that lasted beyond cessation of treatment, efficient KRAS engagement in tumors, and a good pharmacokinetic profile. Proteomics studies with a thiol-reactive probe indicated high selectivity with only one significant off-target. A crystal structure (Figure 1B) validated the design hypotheses and showed that MRTX849 binds the switch-II pocket in KRASG12C with key hydrogen bonds formed by the cyanomethyl group and a salt bridge between the pyrrolidine and Glu62.
MRTX849 and previous KRASG12C inhibitors highlight the potential of covalent fragment screens to enable lead discovery for challenging targets. The progression from the first reported covalent fragments1 in late 2013 to the clinic took less than six years. The MRTX849 development story exemplifies how such leads can be optimized all the way to the clinic while taking into consideration the unique challenges of covalent inhibitors such as inactivation by glutathione.
MRTX849 and additional G12C inhibitors are undergoing clinical trials and may dramatically improve the outcomes of cancer patients with this mutation. However, other mutants of KRAS, such as the more prevalent G12D mutation, are significantly more challenging to target and still lack inhibitors. Addressing these mutants will require highly potent noncovalent KRAS binders. Targeting the G12D mutation in particular may require the development of new carboxyl-reactive electrophiles. These should overcome both the low nucleophilicity of carboxylic acids and the much higher abundance of glutamate and aspartate in the proteome, compared to cysteine, that may pose significant selectivity problems. Nevertheless, it is a worthy challenge and perhaps the next “summit” now that one KRAS mutation may have already been conquered.
The authors declare no competing financial interest.
References
- Ostrem J. M.; Peters U.; Sos M. L.; Wells J. A.; Shokat K. M. K. Ras(G12C) Inhibitors Allosterically Control GTP Affinity and Effector Interactions. Nature 2013, 503, 548–551. 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canon J.; Rex K.; Saiki A. Y.; Mohr C.; Cooke K.; Bagal D.; Gaida K.; Holt T.; Knutson C. G.; Koppada N.; Lanman B. A.; Werner J.; Rapaport A. S.; San Miguel T.; Ortiz R.; Osgood T.; Sun J.-R.; Zhu X.; McCarter J. D.; Volak L. P.; Houk B. E.; Fakih M. G.; O’Neil B. H.; Price T. J.; Falchook G. S.; Desai J.; Kuo J.; Govindan R.; Hong D. S.; Ouyang W.; Henary H.; Arvedson T.; Cee V. J.; Lipford J. R. The Clinical KRAS(G12C) Inhibitor AMG 510 Drives Anti-Tumour Immunity. Nature 2019, 575, 217–223. 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- Hallin J.; Engstrom L. D.; Hargis L.; Calinisan A.; Aranda R.; Briere D. M.; Sudhakar N.; Bowcut V.; Baer B. R.; Ballard J. A.; Burkard M. R.; Fell J. B.; Fischer J. P.; Vigers G. P.; Xue Y.; Gatto S.; Fernandez-Banet J.; Pavlicek A.; Velastagui K.; Chao R. C.; Barton J.; Pierobon M.; Baldelli E.; Patricoin E. F. 3rd; Cassidy D. P.; Marx M. A.; Rybkin I. I.; Johnson M. L.; Ou S.-H. I.; Lito P.; Papadopoulos K. P.; Jänne P. A.; Olson P.; Christensen J. G. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discovery 2020, 10, 54–71. 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J. B.; Fischer J. P.; Baer B. R.; Blake J. F.; Bouhana K.; Briere D. M.; Brown K. D.; Burgess L. E.; Burns A. C.; Burkard M. R.; Chiang H.; Chicarelli M. J.; Cook A. W.; Gaudino J. J.; Hallin J.; Hanson L.; Hartley D. P.; Hicken E. J.; Hingorani G. P.; Hinklin R. J.; Mejia M. J.; Olson P.; Otten J. N.; Rhodes S. P.; Rodriguez M. E.; Savechenkov P.; Smith D. J.; Sudhakar N.; Sullivan F. X.; Tang T. P.; Vigers G. P.; Wollenberg L.; Christensen J. G.; Marx M. A. Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of Cancer. J. Med. Chem. 2020, 10.1021/acs.jmedchem.9b02052. [DOI] [PubMed] [Google Scholar]
- Fell J. B.; Fischer J. P.; Baer B. R.; Ballard J.; Blake J. F.; Bouhana K.; Brandhuber B. J.; Briere D. M.; Burgess L. E.; Burkard M. R.; Chiang H.; Chicarelli M. J.; Davidson K.; Gaudino J. J.; Hallin J.; Hanson L.; Hee K.; Hicken E. J.; Hinklin R. J.; Marx M. A.; Mejia M. J.; Olson P.; Savechenkov P.; Sudhakar N.; Tang T. P.; Vigers G. P.; Zecca H.; Christensen J. G. Discovery of Tetrahydropyridopyrimidines as Irreversible Covalent Inhibitors of KRAS-G12C with In Vivo Activity. ACS Med. Chem. Lett. 2018, 9, 1230–1234. 10.1021/acsmedchemlett.8b00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G.; Chen W.; Zhang J.; Shao J.; Zhang Y.; Huang W.; Zhang L.; Qi W.; Sun X.; Li B.; Xiang Z.; Ma C.; Xu J.; Deng H.; Li Y.; Li P.; Miao H.; Han J.; Liu Y.; Shen J.; Yu Y. A Chemical Tuned Strategy to Develop Novel Irreversible EGFR-TK Inhibitors with Improved Safety and Pharmacokinetic Profiles. J. Med. Chem. 2014, 57, 9889–9900. 10.1021/jm5014659. [DOI] [PubMed] [Google Scholar]