Abstract
Purpose:
The aims of this study were to report on our initial experience using 18F-fluciclovine PET/CT to detect recurrent prostate carcinoma in patients with low serum prostate-specific antigen (PSA) after definitive treatment of primary disease and to conduct a preliminary investigation for factors associated with positive scan findings.
Patients and Methods:
In this retrospective study, 18F-fluciclovine PET/CT scans from 28 men with suspected recurrence of prostate carcinoma and PSAvalues of 1 ng/mL or less were examined to identify the site(s) of disease recurrence. Differences in detection rate for Gleason scores of 7 and greater than 7, T2 and T3 disease, negative and positive surgical margins, and negative and positive seminal vesicle invasion were compared using the Fisher exact test. Mean PSA and mean PSA doubling time of patients with positive scans and negative scans were compared using the independent 2-group t test.
Results:
At least one site of disease recurrence was identified in 13 (46.4%) of 28 patients. Disease detection rate was significantly higher in patients with history of Gleason score greater than 7 (Fisher exact test, P = 0.004). Mean PSA and PSA doubling time were not significantly different between patients with positive and negative 18F-fluciclovine PET/CT scans (P = 0.29 and 0.70, respectively).
Conclusions:
Detection of recurrent prostate cancer using 18F-fluciclovine PET/CT is possible in patients with low but rising PSA levels of 1 ng/mL or less. In such patients, local and nodal recurrences are more common than distant metastasis, and Gleason score greater than 7 is associated with positive scan results.
Keywords: prostate, cancer, fluciclovine, PET/CT, biochemical, recurrence, PSA
Biochemical recurrence (BCR) occurs in 20% to 50% of men who have undergone primary definitive therapy for localized prostate carcinoma, and approximately 25% of these cases eventually develop metastatic disease.1 Management strategies for patients with BCR include active surveillance, systemic androgen deprivation therapy (ADT), and local salvage therapy (radiation therapy or surgery).2 However, of these treatments, only local salvage therapy may be potentially curative, and there has been increasing use of local salvage therapy in recent years, prompting a need for better diagnostic imaging.3 Patients with unidentified distant disease are destined to recur after local salvage therapy, and optimal patient selection and treatment planning require accurate determination of the exact sites of disease recurrence. Historically, this has been difficult to achieve because standard-of-care imaging modalities such as contrast-enhanced CT, MRI, and 99mTc-MDP bone scintigraphy exhibit suboptimal sensitivity in detecting early sites of disease recurrence and are often negative at prostate-specific antigen (PSA) levels less than 10 ng/mL.4 Recently, 18F-fluciclovine, a synthetic amino acid PET radiotracer, has been approved for imaging evaluation of patients with BCR of prostate cancer.5–7
Initial studies of 18F-fluciclovine PET/CT imaging of biochemically recurrent prostate carcinoma reported detection rates of 40.0% to 77.4% across a spectrum of PSA levels.1,8,9 However, detection rates are lower in patients with low PSA levels less than or equal to 1 ng/mL and have ranged from 21.0% to 41.4%.1,8,9 These patients continue to pose a dilemma. Evidence suggests that early administration of salvage therapy at low PSA levels (<1 ng/mL) leads to better outcomes, but the lower detection rate in these patients raises questions about the optimum timing of imaging.4 The identification of clinical factors predictive of positive 18F-fluciclovine PET/CT imaging findings in patients with low PSA levels could aid in the selection of patients most likely to benefit from this imaging modality.
The purpose of this study was to evaluate 18F-fluciclovine PET/CT detection of prostate carcinoma recurrence in men with low but increasing PSA values (less than or equal to 1 ng/mL) at our institution and to conduct an investigation for relevant clinical factors associated with positive scan findings.
PATIENTS AND METHODS
This study was performed in accordance with the Declaration of Helsinki and was approved by our institutional review board. Adult participant consent was not required because the research involved no more than minimal risk to the subjects.
Patient Selection
Data for this retrospective study was obtained from our electronic medical record. A search was performed to identify all patients who underwent 18F-fluciclovine PET/CT between July 27, 2017, and May 31, 2018. Patients were included if the indication for the examination was BCR. Patients were excluded if (1) there was an existing diagnosis of recurrence diagnosed on a previous imaging examination, (2) no PSA level was available, or (3) PSA level was greater than 1 ng/mL. Our search returned 86 patients who underwent 18F-fluciclovine PET/CT for BCR. Of these, 25 patients were excluded because there was known recurrent disease on prior imaging, 1 patient was excluded because no PSA level was available in the electronic medical record, and 32 patients were excluded because the most recent PSA level was greater than 1 ng/mL. Patient characteristics are depicted in Table 1.
TABLE 1.
Patient Characteristics
| Positive Scan | Negative Scan | Overall | |
|---|---|---|---|
| Patients, n (%) | 13 (46.4) | 15 (53.6) | 28 (100) |
| Age, y | |||
| Mean | 67.5 | 66.8 | 67.1 |
| Range | 57–77 | 53–77 | 53–77 |
| Primary therapy, n (%) | |||
| RP alone | 8 (61.5) | 14 (93.3) | 22 (78.6) |
| RP + EBRT | 2 (15.4) | 1 (6.7) | 3 (10.7) |
| RP + EBRT + ADT | 1 (7.7) | 0 | 1 (3.6) |
| EBRT + ADT | 2 (15.4) | 0 | 2 (7.1) |
| Salvage therapy, n (%) | |||
| RT alone | 3 (23.1) | 3 (20.0) | 6 (21.4) |
| ADT alone | 1 (7.7) | 0 | 1 (3.6) |
| RT + ADT | 1 (7.7) | 0 | 1 (3.6) |
| LND | 0 | 1 (6.7) | 1 (3.6) |
| Gleason score, n (%) | |||
| 7 | 5 (38.5) | 14 (93.3) | 19 (67.9) |
| 8 | 3 (23.1) | 0 | 3 (10.7) |
| 9 | 5 (38.5) | 1 (6.7) | 6 (21.4) |
| T classification, n (%) | |||
| T2 | 2 (15.4) | 7 (46.7) | 9 (32.1) |
| T3 | 9 (69.2) | 7 (46.7) | 16 (57.1) |
| Unknown | 0 | 1 (6.7) | 1 (3.6) |
| No surgery | 2 (15.4) | 0 | 2 (7.1) |
| N classification, n (%) | |||
| NX | 1 (7.7) | 3 (20.0) | 4 (14.3) |
| N0 | 9 (69.2) | 10 (66.7) | 19 (67.9) |
| N1 | 1 (7.7) | 1 (6.7) | 2 (7.1) |
| Unknown | 0 | 1 (6.7) | 1 (3.6) |
| No surgery | 2 (15.4) | 0 | 2 (7.1) |
| Surgical margin, n (%) | |||
| Positive | 4 (30.8) | 3 (20.0) | 7 (25.0) |
| Negative | 7 (53.8) | 12 (80.0) | 19 (67.9) |
| No surgery | 2 (15.4) | 0 | 2 (7.1) |
| Seminal vesicle invasion, n (%) | |||
| Positive | 4 (30.8) | 4 (26.6) | 8 (28.6) |
| Negative | 7 (53.8) | 10 (66.7) | 17 (60.7) |
| Unknown | 0 | 1 (6.7) | 1 (3.6) |
| No surgery | 2 (15.4) | 0 | 2 (7.1) |
| PSA, ng/mL | |||
| Mean | 0.50 | 0.38 | 0.44 |
| Range | 0.11–1.00 | 0.10–0.88 | 0.1–1.00 |
| PSA doubling time, mo | |||
| Mean | 6.9 | 7.61 | 6.38 |
| Range | 2.4–16.8 | 1.6–16.7 | 1.6–16.8 |
EBRT, external beam radiation therapy.
18F-Fluciclovine PET/CT Protocol
18F-Fluciclovine (Axumin; Blue Earth Diagnostics, Burlington, MA) was obtained from PETNET Solutions (Culver City, CA). Patients were instructed to fast for 6 hours before examination. Approximately 3 to 5 minutes after IV administration of 10 mCi 18F-fluciclovine, PET/CT was performed on a Siemens Biograph TruePoint PET/CT scanner (Siemens, Munich, Germany). Single time point PET data were obtained using 4-minute static acquisitions in 5 bed positions starting with the pelvis, and images were reconstructed using the TrueX reconstruction algorithm. Low-dose CT images were obtained over the same anatomic range and reconstructed at 3-mm slice thickness.
Image Interpretation
Images were reviewed by a board-certified nuclear medicine physician on a diagnostic workstation using MIM software version 6.7.9 (MIM Software Inc, Beachwood, OH). Image interpretation was guided by consensus criteria developed by the Society of Nuclear Medicine and Molecular Imaging Clinical Trials Network.10 Foci of radiotracer activity in lymph nodes or the prostate gland/prostatectomy bed were considered positive for recurrent disease if greater than 1 cm in maximal dimension and exhibiting activity higher than mean bone marrow activity (Fig. 1) or if less than 1 cm and exhibiting activity higher than mean blood pool activity. Foci of uptake in bone were considered positive for metastatic disease if clearly visualized on MIP or PET-only images. Mild-to-moderate, symmetric radiotracer activity in otherwise normal-appearing hilar, axillary, or inguinal lymph nodes was considered physiologic.5,6
FIGURE 1.
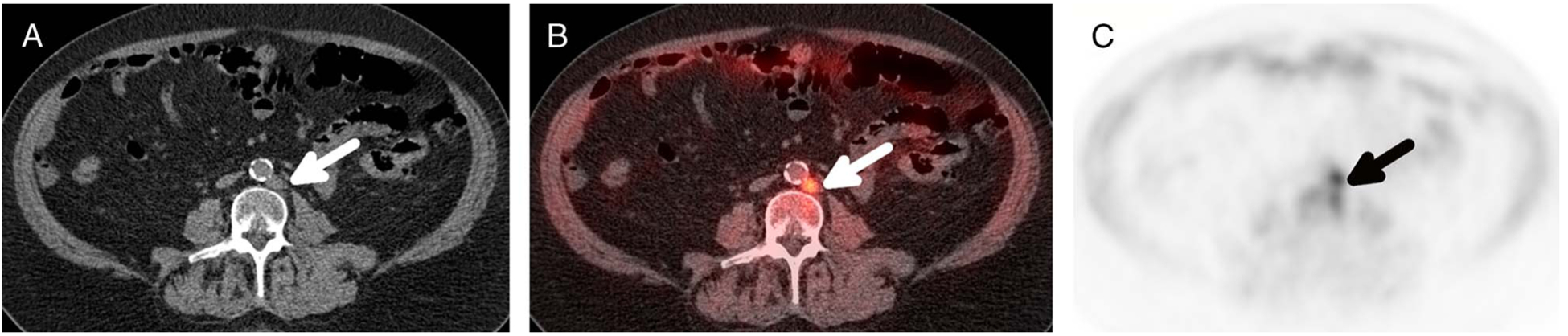
Positive left para-aortic lymph node on 18F-fluciclovine PET/CT scan in a 72-year-old man with history of prostate carcinoma originally treated with RP who presented with BCR, PSA from 0.05 to 0.13 ng/mL in a 6-month period. Axial CT (A), PET/CT fusion (B), and PET (C) images show an intensely avid, 10-mm left para-aortic lymph node (arrows) that exhibits activity above bone marrow both visually and quantitatively, SUVmax of 5.6 compared with bone marrow SUVmean of 3.6.
Statistical Analysis
Statistical analysis was performed using the R programming language and statistical computing environment version 3.4.3.11 Differences in detection rate, defined as percent of patients with a positive finding on 18F-fluciclovine PET/CT, were compared using the Fisher exact test for the following groups: Gleason score sum of 7 and greater than 7 for all 28 patients, T2 and T3 disease for the 25 patients who underwent radical prostatectomy (RP) with known pathologic T classification, negative and positive surgical margins for the 26 patients who underwent RP, and negative and positive seminal vesicle invasion (SVI) for the 25 patients who underwent RP with known SVI status. Differences in detection rate between different primary therapy groups and node-positive and node-negative groups were not compared because only 2 patients underwent nonsurgical treatment (both with positive scan findings) and only 2 patients were node positive at surgery. Mean PSA and mean PSA doubling time of patients with positive scans (ie, any findings on 18F-fluciclovine PET/CT consistent with recurrent disease) and patients with negative scans were compared using the independent 2-group t test with unequal variance. PSA doubling times were calculated by least square regression without logarithmic transformation using 3 values for 18 patients and 2 values for 10 patients.12,13 Mean time between 18F-fluciclovine PET/CTexamination and the most recent PSA measurement was 45.4 days (range, 0–182).
RESULTS
Sites of Detected Recurrent Disease
Of 13 patients with positive 18F-fluciclovine PET/CT scan findings, 10/13 patients (76.9%) had pelvic or retroperitoneal lymph node recurrence (6/10 with nodal recurrence only, 4/10 with nodal and local recurrence), 7/13 (53.8%) had local recurrence in the prostate gland or prostatectomy site (3/7 with local recurrence only, 4/10 with local and nodal recurrence), and 1/13 patient (7.6%) had a distant osseous metastatic lesion in addition to local and nodal recurrence.
Clinical Factors
Detection rate comparison by clinical factor is depicted in Figure 2. Detection rate was significantly higher for patients with Gleason score greater than 7 than those with score of 7 (88.8% vs 26.3%; odds ratio [OR], 19.6; P = 0.004). Detection rate was not significantly different between patients with T2 and T3 disease (22.2% vs 56.3%; OR, 4.2; P = 0.21), positive and negative surgical margins at RP (57.1% vs 36.8%; OR, 2.2; P = 0.41), or positive and negative SVI at RP (50.0% vs 41.2%; OR, 1.4; P = 1.0).
FIGURE 2.
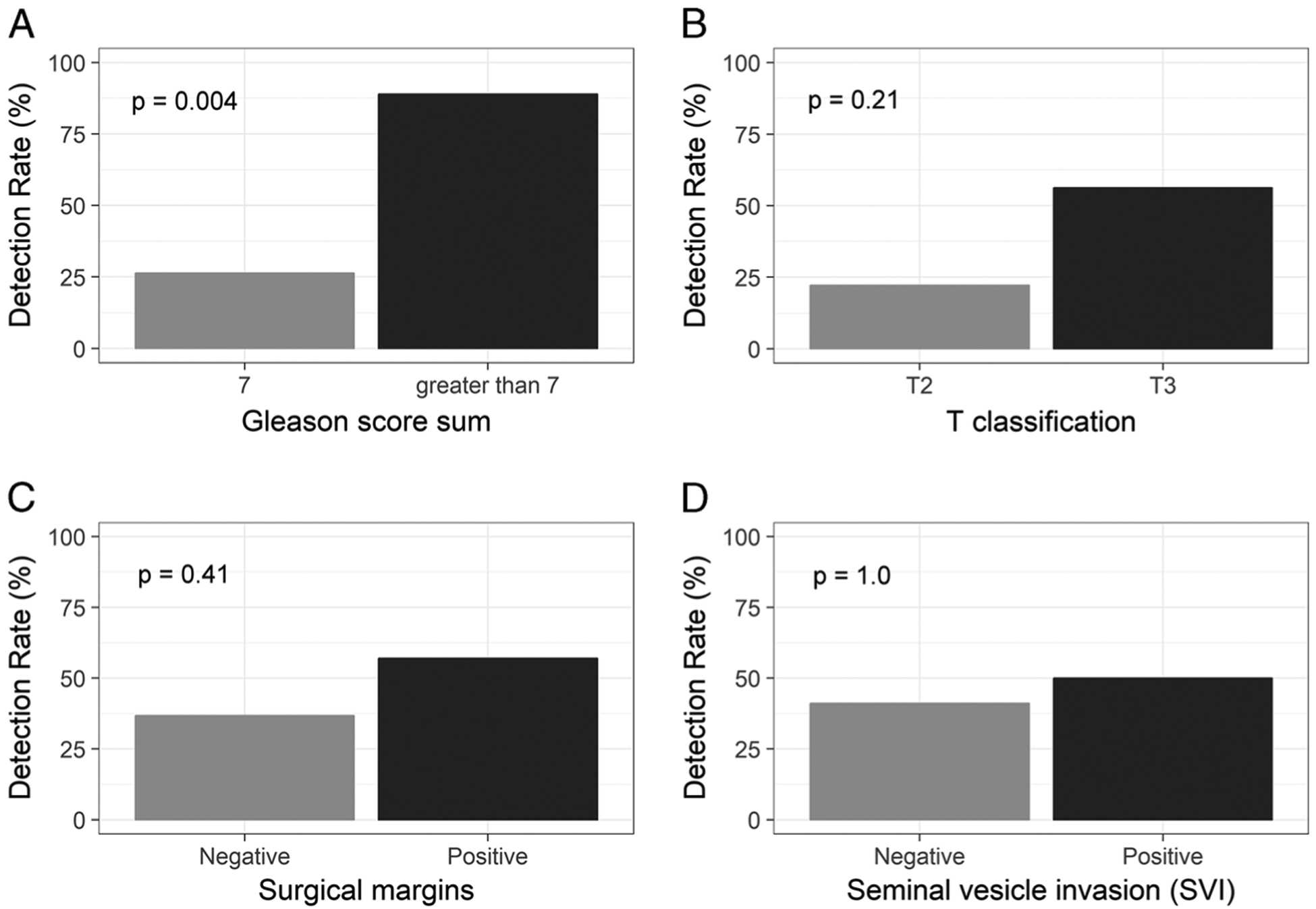
Comparisons of detection rate in relation to Gleason score sum (A), T classification (B), surgical margins (C), and seminal vesicle invasion (D) are presented in bar charts.
PSA and PSA Doubling Time
Mean PSA and PSA doubling time are reported in Table 1, and comparisons between patients with positive and negative scans are depicted in box-and-whisker plots in Figure 3. PSA and PSA doubling time were not significantly different between patients with positive and negative scans (P = 0.29 and 0.70, respectively).
FIGURE 3.
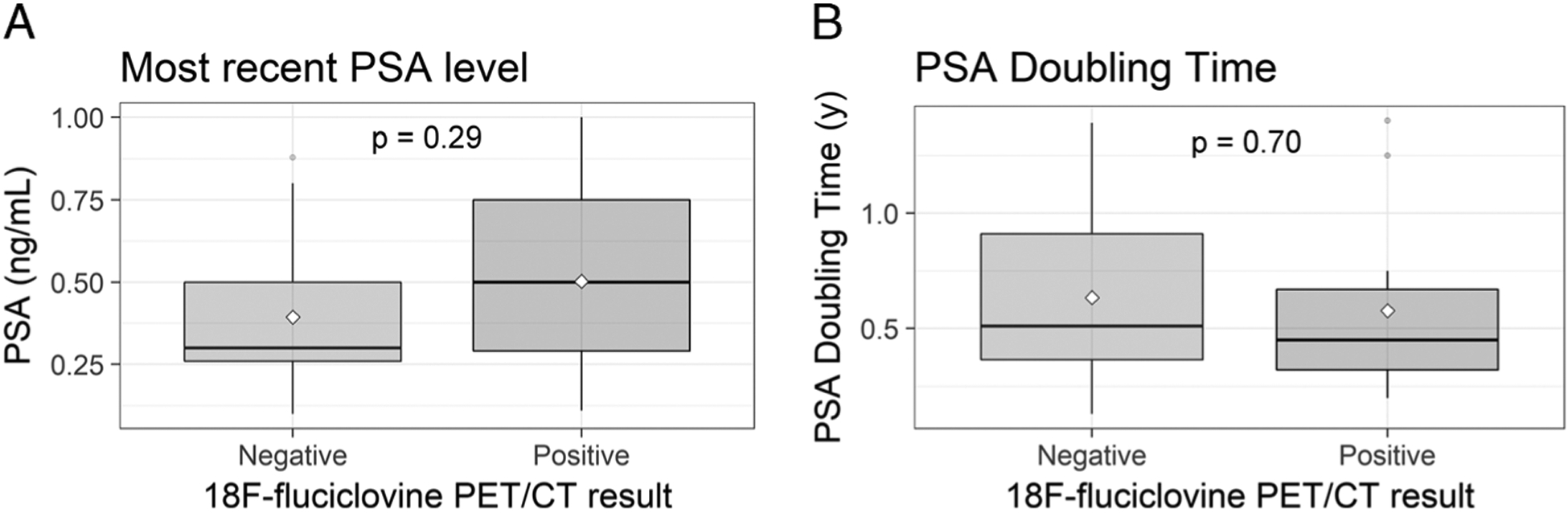
Comparisons of PSA (A) and PSA doubling time (B) in patients with negative and positive 18F-fluciclovine PET/CT scans are presented in box-and-whisker plots.
Follow-up
Follow-up imaging and/or pathology was available for 4 of the 13 patients with positive scans and was not available for the 15 patients with negative scan results. In the 4 patients with positive findings for whom follow-up was available (tissue pathology results in 2 patients and follow-up 18F-fluciclovine PET/CT imaging in 2 patients), there was 1 false-positive and 3 true-positives. In one true-positive case, 18F-fluciclovine PET/CT showed local recurrence in a patient who originally underwent nonsurgical treatment with external beam radiation therapy and ADT, and biopsy confirmed the presence of malignancy. In the 2 other true-positive cases, 18F-fluciclovine PET/CT showed a single mildly avid pelvic lymph node, and follow-up 18F-fluciclovine scan showed increase in size and radiotracer avidity, consistent with metastasis (Fig. 4). In the one false-positive case, 18F-fluciclovine PET/CT showed multiple mildly avid pelvic and retroperitoneal lymph nodes, and the patient underwent salvage pelvic and retroperitoneal lymph node dissection with no evidence of malignancy at pathology.
FIGURE 4.
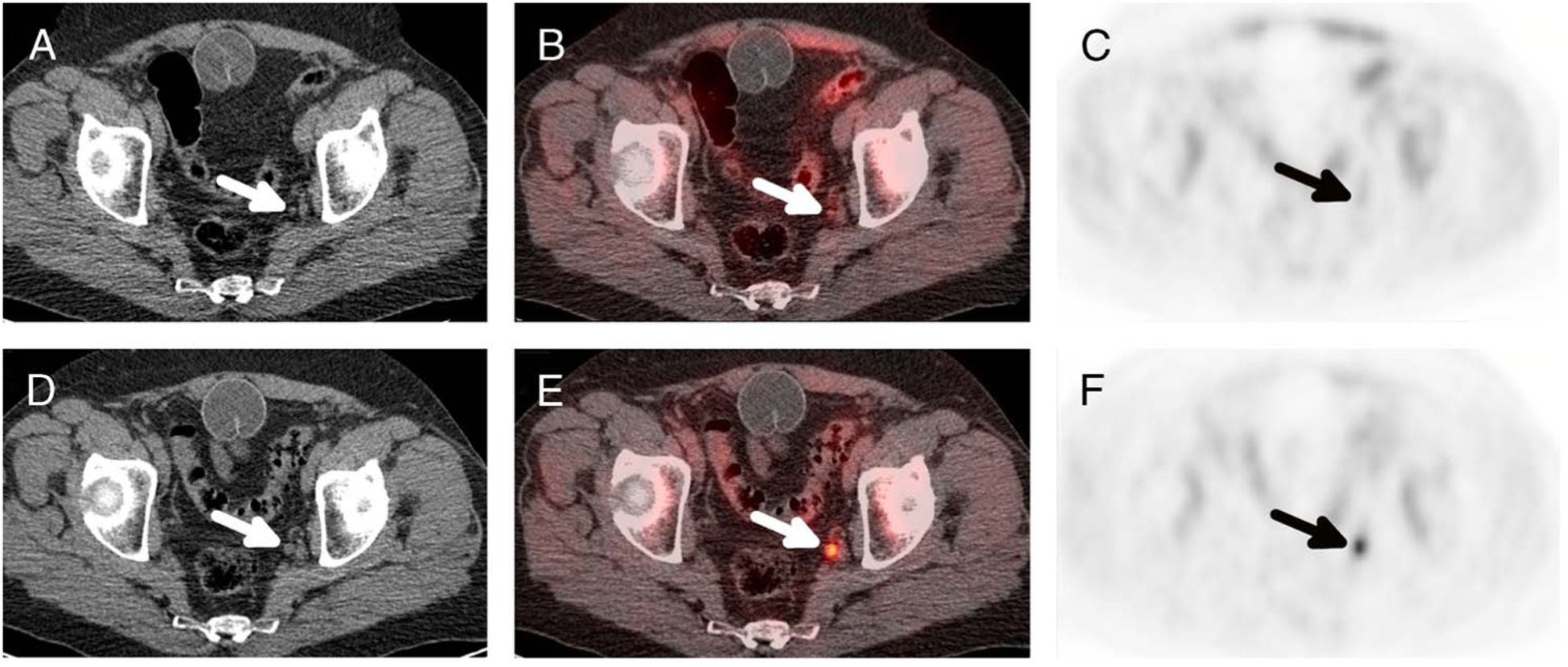
True-positive case of nodal recurrence in a 77-year-old man with history of prostate carcinoma, Gleason 4 + 4, T3aN0, originally treated with RP who presented with BCR, PSA from 0.16 to 0.59 ng/mL in a 6-month period. Initial axial CT (A), PET/CT fusion (B), and PET (C) images show a 5-mm left internal iliac lymph node (arrows) that exhibits 18F-fluciclovine activity above blood pool, SUVmax of 2.2 compared with blood pool SUVmean of 1.3. Follow-up scan (D–F) performed 6 months later shows interval increase in size, now 8 mm, and activity, now SUVmax of 6.7. PSA also increased to 3.02 ng/mL.
DISCUSSION
Increasing use of local salvage therapy in the context of BCR of prostate cancer has led to increasing importance of accurate imaging identification of disease recurrence.14 With the recent development and Food and Drug Administration approval of 18F-fluciclovine PET/CT, sites of prostate cancer recurrence can be identified at lower PSA levels than with standard-of-care imaging modalities, but reported detection rates are still relatively low in patients with PSA level less than 1 ng/mL. In the present study, we report on our early experience using 18F-fluciclovine PET/CT to detect recurrent prostate carcinoma in patients with low but rising PSA level less than or equal to 1 ng/mL.
18F-Fluciclovine PET/CT scans demonstrated at least one site suspicious for disease recurrence in 46.4% of patients, a detection rate that is similar to previously reported rates ranging from 21.0% to 41.4%. Prevalence of nodal and local recurrence far exceeded distant metastasis, which was identified in only 1 patient, a finding consistent with previous studies and with the observation that identifiable distant metastasis is seldom present in early BCR with PSA level of 1 ng/mL or less.8,9,15
Previous studies have reported that 18F-fluciclovine PET/CT detection rate in patients with PSA level less than 1 ng/mL is correlated with high PSA doubling time and some have recommended the use of rapid PSA kinetics to select patients for expedited imaging.6,16 In our study, PSA level and PSA doubling time were not significantly different between patients with positive and negative scans. Pathologic T classification, positive surgical margins, and SVI, all known predictors of poor clinical outcomes, also demonstrated no significant association with positive scan findings.17–19 The only clinical characteristic associated with a high detection rate was Gleason score greater than 7 with a detection rate of 88.8%. Our results suggest that Gleason score may be of equal or greater importance than PSA kinetics in selecting patients with PSA level of 1 ng/mL or less who are likely to have positive 18F-fluciclovine PET/CT imaging results.
We acknowledge several limitations in our investigation. First, our sample size was relatively small. This is a limitation of all existing studies of 18F-fluciclovine PET/CT in patients with PSA level 1 ng/mL or less, but hopefully as imaging and treatment of this patient population become more common, additional robust data will become available. Second, pathologic and imaging follow-up were only available for 4 of 13 patients with positive results. This is also a limitation of prior literature and is a problem that is likely to continue given that only a minority of recurrence sites are ever confirmed by tissue sampling and many are treated presumptively with radiation therapy or systemic ADT. Given that treatment is often presumptive based on imaging findings, the detection rate can be interpreted as the likelihood that any potentially useful information will be discovered. Detection rate, even without pathologic confirmation, is therefore an important measure of the clinical utility of 18F-fluciclovine PET/CT in the management of prostate cancer recurrence.
CONCLUSIONS
18F-Fluciclovine PET/CT detection of recurrent prostate cancer is possible in patients with low but rising PSA levels less than or equal to 1 ng/mL, and detection rate is higher in those with Gleason score greater than 7. Gleason score is therefore of potential use in selecting patients most likely to benefit from early 18F-fluciclovine PET/CT imaging.
Conflicts of interest and sources of funding:
Hossein Jadvar was supported by the National Institutes of Health grant numbers R21-EB017568 and P30-CA014089. The rest of the authors declare no potential conflicts of interest.
REFERENCES
- 1.Bach-Gansmo T, Nanni C, Nieh PT, et al. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce JY, Lang JM, McNeel DG, et al. Current controversies in the management of biochemical failure in prostate cancer. Clin Adv Hematol Oncol. 2012;10:716–722. [PubMed] [Google Scholar]
- 3.Cary KC, Paciorek A, Fuldeore MJ, et al. Temporal trends and predictors of salvage cancer treatment after failure following radical prostatectomy or radiation therapy: an analysis from the CaPSURE registry. Cancer. 2014;120: 507–512. [DOI] [PubMed] [Google Scholar]
- 4.Punnen S, Cooperberg MR, D’Amico AV, et al. Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2013;64:905–915. [DOI] [PubMed] [Google Scholar]
- 5.Schuster DM, Nanni C, Fanti S, et al. Anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med. 2014;55:1986–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savir-Baruch B, Zanoni L, Schuster DM. Imaging of prostate cancer using fluciclovine. PET Clin. 2017;12:145–157. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. [DOI] [PubMed] [Google Scholar]
- 8.Nanni C, Schiavina R, Brunocilla E, et al. 18F-fluciclovine PET/CT for the detection of prostate cancer relapse: a comparison to 11C-choline PET/CT. Clin Nucl Med. 2015;40:e386–e391. [DOI] [PubMed] [Google Scholar]
- 9.Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3-[18F] FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging. 2016;43:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MP, Kostakoglu L, Pryma D, et al. Reader training for the restaging of biochemically recurrent prostate cancer using 18F-fluciclovine PET/CT. J Nucl Med. 2017;58:1596–1602. [DOI] [PubMed] [Google Scholar]
- 11.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013: Available at: http://www.R-project.org/. [Google Scholar]
- 12.Time Doubling. Available at: http://www.doubling-time.com/compute-PSA-doubling-time.php. [Google Scholar]
- 13.Vickers AJ, Brewster SF. PSA velocity and doubling time in diagnosis and prognosis of prostate cancer. Br J Med Surg Urol. 2012;5:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballas LK, de Castro Abreu AL, Quinn DI. What medical, urologic, and radiation oncologists want from molecular imaging of prostate cancer. J Nucl Med. 2016;57:6S–12S. [DOI] [PubMed] [Google Scholar]
- 15.Jadvar H, Desai B, Ji L, et al. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kairemo K, Rasulova N, Partanen K, et al. Preliminary clinical experience of trans-1-Amino-3-(18)F-fluorocyclobutanecarboxylic Acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. Biomed Res Int. 2014; 2014:305182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humphrey PA. Seminal vesicle invasion by adenocarcinoma of the prostate. J Urol. 2015;194:1757–1758. [DOI] [PubMed] [Google Scholar]
- 18.Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59: 893–899. [DOI] [PubMed] [Google Scholar]
- 19.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. [DOI] [PubMed] [Google Scholar]


