Abstract
Combined immune checkpoint blockade with nivolumab and ipilimumab is standard therapy for the treatment of patients with previously untreated advanced renal cell carcinoma who are at intermediate or poor risk. However, data about the safety and efficacy of combined immune checkpoint blockade with nivolumab and ipilimumab in patients on hemodialysis are limited. Renal function has no known clinically important effects on the pharmacokinetics and clearance of nivolumab and ipilimumab. Further, most immune-related adverse events in patients on hemodialysis are thought to be manageable with the same treatments applied in patients with normal renal function.
We present a case of advanced clear-cell renal cell carcinoma in a patient on hemodialysis who received combined immune checkpoint blockade with nivolumab and ipilimumab and who showed no evident signs of immune-related adverse events. Here, we confirm the safety and efficacy of combined immune checkpoint blockade with nivolumab and ipilimumab in a patient on hemodialysis.
Keywords: Hemodialysis, ipilimumab, renal cell carcinoma, nivolumab
INTRODUCTION
Immune checkpoint blockade offers significant benefit in the treatment of cancer1. It enhances the antitumour immune response by reducing endogenous immune downregulators such as PD-1 and ctla-42. Nivolumab is a PD-1 immune checkpoint inhibitor antibody whose monotherapy shows favourable antitumour efficacy in several types of tumours, including advanced renal cell carcinoma (rcc)3. Ipilimumab is an anti–ctla-4 antibody whose monotherapy shows favourable antitumour efficacy in unresectable or metastatic melanoma4. On the basis of a clinical trial5, combined immune checkpoint blockade with nivolumab and ipilimumab has become standard therapy for the treatment of patients with previously untreated advanced rcc who are at intermediate or poor risk as stratified by the International Metastatic RCC Database Consortium risk score. However, data concerning the safety and efficacy of immune checkpoint blockade monotherapy or combined therapy in patients on hemodialysis are limited because such patients were excluded from the clinical trials5,6. Although several case studies have suggested that patients on hemodialysis can be treated safely and efficaciously with nivolumab or ipilimumab monotherapy7,8, no reports have been published describing the safety and efficacy of combined therapy in patients on hemodialysis. We present the case of a patient on hemodialysis whose advanced clear-cell rcc was treated with combined immune checkpoint blockade using nivolumab and ipilimumab.
CASE PRESENTATION
A 77-year-old man was diagnosed in 2008 with chronic renal failure derived from gouty nephropathy and was receiving hemodialysis 3 times weekly. A right renal tumour was detected by screening abdominal ultrasound in July 2015. The patient had no significant symptoms, and his Karnofsky performance status was 100%. His past history included appendicitis, gastric ulcer, and colonic polyp, but no history of autoimmune disease, interstitial pneumonitis, or organ transplantation.
Contrast-enhanced computed tomography (ct) showed a hypervascular mass 26 mm in diameter in the right kidney and no signs of metastasis. The patient was diagnosed as having a right renal tumour (cT1aN0M0), and we performed right radical nephrectomy using a retroperitoneal approach. Pathology findings showed clear-cell rcc (grade 1, pT1), and the patient was subsequently followed with no additional therapy.
In December 2017, the patient’s serum prostate-specific antigen rose to 17.3 ng/mL, and he was diagnosed with prostate carcinoma (Gleason 3+4 = 7, cT1cN0M0). He was started on androgen deprivation therapy (leuprorelin) in January 2018, and in October 2019, his serum prostate-specific antigen had decreased to 0.24 ng/mL. At that time, he was considered to have stable disease.
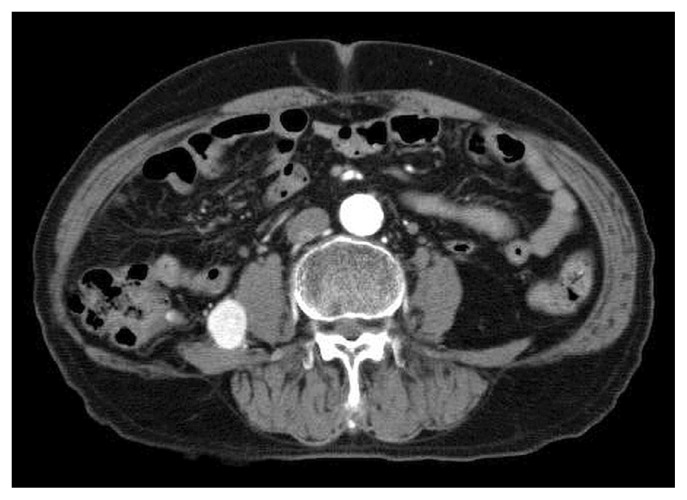
Contrast-enhanced ct in January 2019 revealed a hypervascular mass 22 mm in diameter in the retroperitoneum, and no obvious tumours otherwise (Figure 1). We performed ct-guided biopsy of the retroperitoneal mass in February 2019 and diagnosed metastatic clear-cell rcc.
FIGURE 1.
A retroperitoneal mass observed on computed tomography was concordant with metastatic clear-cell renal cell carcinoma.
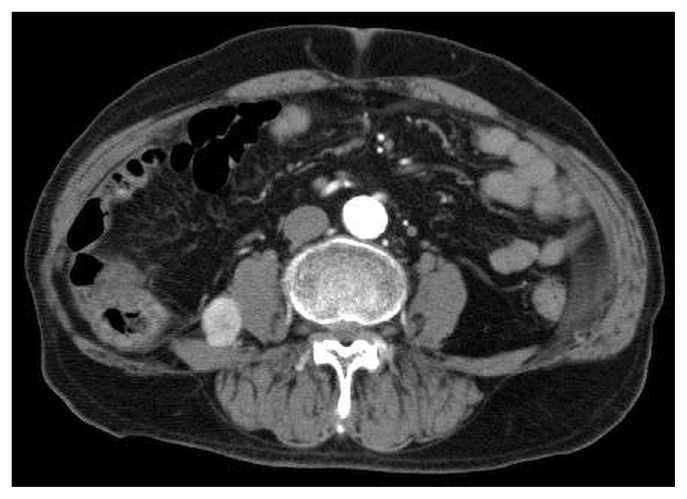
After a discussion of treatment options and the limited data concerning immune checkpoint inhibitors or molecularly targeted drugs in patients on hemodialysis, the patient decided to accept combined immune checkpoint blockade with nivolumab and ipilimumab. His risk score was 1 (hemoglobin 11.7 g/dL, below the normal limit), and he was classified as being at intermediate risk. Blood chemistry showed chronic renal failure (blood urea nitrogen 30 mg/dL, serum creatinine 5.55 mg/dL), but no electrolyte abnormalities. His liver and coagulation function and endocrine system were almost all within normal limits. Abdominal ct in March 2019 showed no remarkable change in the retroperitoneal mass, and no additional tumours (Figure 2).
FIGURE 2.
The retroperitoneal mass on computed tomography before initiation of combined immune checkpoint blockade.
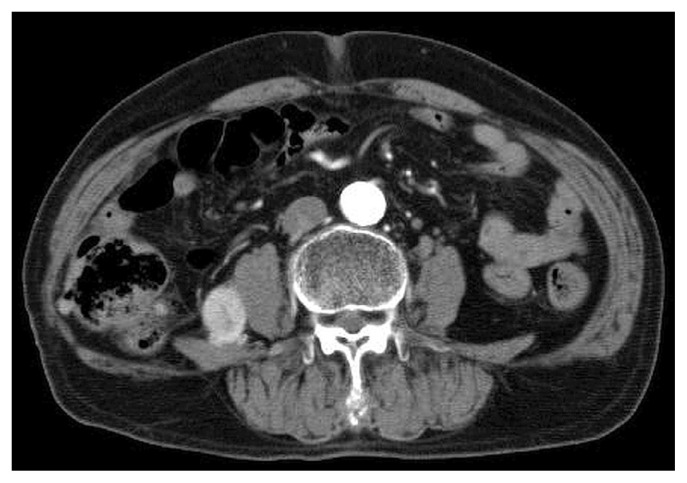
The patient was started on combined immune checkpoint blockade with nivolumab and ipilimumab in April 2019. He received nivolumab 240 mg plus ipilimumab 1 mg/kg intravenously every 3 weeks for 4 doses, followed by nivolumab 240 mg every 2 weeks. Imaging by ct in December 2019 showed stable disease in terms of the size of the retroperitoneal metastasis, and no appearance of new disease (Figure 3).
FIGURE 3.
At 8 months after the first cycle of combined immune checkpoint blockade, the retroperitoneal mass was considered, based on computed tomography imaging, to be stable disease.
The patient continued to receive hemodialysis 3 times weekly, with a stable serum creatinine level and no electrolyte abnormalities. He had no clinical symptoms, and blood chemistry tests showed normal C-reactive protein, liver function, and no significant changes in coagulation and endocrine function. He had no evident signs of immune-related adverse events (iraes), with stable disease at 8 months after initiation of the combined immune checkpoint blockade with ipilimumab and nivolumab.
DISCUSSION
Nivolumab is a PD-1–blocking monoclonal antibody and an immunoglobulin G4κ with a weight of 146 kDa9. Nivolumab attaches to PD-1 receptors, preventing interactions with PD-L1 and PD-L2, and increasing T cell proliferation and cytokine production10. Ipilimumab is a ctla-4 blocking monoclonal antibody and immunoglobulin G1κ with a weight of 148 kDa11. Ipilimumab attaches to ctla-4 and prevents interaction with its ligands (CD80 and CD86). It also increases T cell activation and proliferation, and decreases regulatory T cell function12. In addition, ipilimumab expands the cancer-specific CD8 T cell repertoire13. Combined immune checkpoint blockade with nivolumab and ipilimumab enhances T cell function more than the effect of either nivolumab or ipilimumab alone and, as a result, improves antitumour responses in advanced rcc5.
Information about the efficacy and safety of immunotherapies such as nivolumab and ipilimumab in patients on hemodialysis is limited because such patients were excluded from clinical trials of those drugs5,6. Renal function has no effect on the pharmacokinetics of monoclonal antibodies such as nivolumab and ipilimumab14,15 and no significant effect on the clearance of the drugs, because monoclonal antibodies are metabolized to peptides and amino acids just as endogenous immunoglobulin G is9,11,14. As a result, nivolumab and ipilimumab do not require dose adjustment for patients with renal dysfunction9,11.
One concern is a potential decrease in potency because of ultrafiltration, but given the high molecular weight of monoclonal antibodies, including nivolumab and ipilimumab, hemodialysis does not clear the antibody form8,16. Because the metabolism of nivolumab and ipilimumab is similar regardless of hemodialysis, we think that it is possible to apply the usual immune checkpoint blockade in patients on hemodialysis.
Immune checkpoint blockade often causes iraes by enhancing the activity of the immune system, which results in inflammatory side effects17. Recently, one study reported that C-reactive protein can be a predictive marker for the onset of iraes after infectious disease has been excluded. However, the exact pathophysiology of iraes is unknown, and there are no standard early markers for the onset of iraes18. However, most iraes are manageable by starting a glucocorticoid or an additional immunosuppressive agent such as infliximab19.
Although the overall safety of immune checkpoint blockade for patients on hemodialysis is unknown, use of nivolumab or ipilimumab monotherapy appears to be safe and efficacious, and iraes in such patients are manageable with the same treatment applied in patients with normal renal function7,8. Unlike previously reported monotherapies, combined immune checkpoint blockade with nivolumab and ipilimumab might enhance the risk of iraes. Clinical trials showed that 46% of patients experience grade 3 and 4 iraes with combined immune checkpoint blockade using nivolumab and ipilimumab, but only 19% experience iraes while receiving nivolumab monotherapy3,5. Although combined immune checkpoint blockade with nivolumab and ipilimumab clearly imparts a higher risk for iraes, clinicians can maximize safety and efficacy by detecting clinical signs as soon as possible. In the present case, we cautiously monitored clinical symptoms, performed blood chemistry tests and chest radiography every 2 weeks, and monitored parameters of endocrine function such as adrenocorticotropic hormone, cortisol, and thyroid function every month.
CONCLUSIONS
To our best knowledge, this case of a patient on hemodialysis who had advanced clear-cell rcc is the first to report treatment with combined immune checkpoint blockade using nivolumab and ipilimumab. Combined immune checkpoint blockade using nivolumab and ipilimumab was safe and efficacious in this patient on dialysis. We trust that clinicians will find this study useful in discussing the risks and benefits of combined immune checkpoint blockade using nivolumab and ipilimumab in patients with advanced malignancies who are undergoing hemodialysis.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–85. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 2.Sweis RF, Luke JJ. Mechanistic and pharmacologic insights on immune checkpoint inhibitors. Pharmacol Res. 2017;120:1–9. doi: 10.1016/j.phrs.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheun H, Kim M, Lee H, Oh KH, Keam B. Safety and efficacy of immune checkpoint inhibitors for end-stage renal disease patients undergoing dialysis: a retrospective case series and literature review. Invest New Drugs. 2019;37:579–83. doi: 10.1007/s10637-018-0673-y. [DOI] [PubMed] [Google Scholar]
- 7.Vitale MG, Baldessari C, Milella M, et al. Immunotherapy in dialysis-dependent cancer patients: our experience in patients with metastatic renal cell carcinoma and a review of the literature. Clin Genitourin Cancer. 2019;17:e903–8. doi: 10.1016/j.clgc.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Cavalcante L, Amin A, Lutzky J. Ipilimumab was safe and effective in two patients with metastatic melanoma and end-stage renal disease. Cancer Manag Res. 2015;7:47–50. doi: 10.2147/CMAR.S73389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bristol–Myers Squibb. Opdivo (Nivolumab) Injection, for Intravenous Use [highlights of prescribing information] Princeton NJ: Bristol–Myers Squibb; 2019. [Available online at: http://packageinserts.bms.com/pi/pi_opdivo.pdf; cited 19 December 2019. [Google Scholar]
- 10.Brahmer JR, Drake CG, Wollner I, et al. Phase i study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bristol–Myers Squibb Canada. Product Monograph: Yervoy (Ipilimumab for Injection) Saint-Laurent, QC: Bristol–Myers Squibb Canada; 2019. [Available online at: https://packageinserts.bms.com/pi/pi_yervoy.pdf; cited 19 December 2019. [Google Scholar]
- 12.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti–cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kvistborg P, Philips D, Kelderman S, et al. Anti–ctla-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]
- 14.Keizer RJ, Huitema AD, Schellens JHM, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Sheng J, Srivastava S, Sanghavi K, et al. Clinical pharmacology considerations for the development of immune checkpoint inhibitors. J Clin Pharmacol. 2017;57(suppl 10):S26–42. doi: 10.1002/jcph.990. [DOI] [PubMed] [Google Scholar]
- 16.Carlo MI, Feldman DR. Response to nivolumab in a patient with metastatic clear cell renal cell carcinoma and end-stage renal disease on dialysis. Eur Urol. 2016;70:1082–3. doi: 10.1016/j.eururo.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 18.Abolhassani AR, Schuler G, Kirchberger MC, Heinzerling L. C-Reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol. 2019;145:2625–31. doi: 10.1007/s00432-019-03002-1. [DOI] [PubMed] [Google Scholar]
- 19.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–53. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]