Abstract
Background
Almost half of all patients with non-small-cell lung cancer (nsclc) present with stage iv disease. The objective of the present study was to characterize treatment patterns and survival outcomes in patients with advanced nsclc.
Methods
We conducted a longitudinal population-level study in patients diagnosed with stage iv nsclc in Ontario between 1 April 2010 and 31 March 2015, with follow-up to 31 March 2017 for overall survival and treatment sequence. Patients were stratified as nonsquamous or squamous histology. A sub-analysis was conducted for patients with nonsquamous histology who received targeted therapies, on the assumption that their tumours were EGFR mutation–positive (EGFRm+). Treatment patterns were determined, and survival was calculated from date of diagnosis to death or censoring.
Results
Of 24,729 nsclc cases identified, stage iv disease was diagnosed in 49.2%, histology was nonsquamous in 10,103, and EGFRm+ was assumed in 508. Median patient age ranged from 69 to 72 years for the three cohorts. For patients with nonsquamous histology, palliative radiotherapy was the most frequently used first-line treatment (44.4%), followed by no treatment (26.7%) and chemotherapy (14.9%). In the EGFRm+ cohort, 75.6% received gefitinib as first- or second-line therapy, and almost half (47.4%) the 473 patients with squamous histology treated with first-line chemotherapy received cisplatin or carboplatin with gemcitabine. Median overall survival in the nonsquamous and squamous cohorts was 4.9 and 4.6 months respectively; it was 17.6 months for patients who were EGFRm+.
Conclusions
Survival of patients with stage iv nsclc remains poor, with the exception of patients who are EGFRm+. Only 14.9% of patients received first-line chemotherapy; the mainstay of treatment was palliative radiotherapy.
Keywords: Lung cancer, stage iv disease, real-world evidence
BACKGROUND
Lung cancer is the most commonly diagnosed cancer in Canada. In 2020, approximately 29,800 new cases will be diagnosed, with an estimated 21,200 deaths1. Of the individuals diagnosed, 80%–85% have non-small-cell lung cancer (nsclc), and approximately 40% are diagnosed with stage iv disease2. Survival for patients with stage iv disease is poor, with 5-year survival rates ranging from 2% to 13%3.
Guidelines for the treatment of advanced nsclc continue to recommend either single-agent or doublet platinum-based chemotherapy4–6, although the identification of driver mutations and the introduction of molecularly targeted therapies are changing the treatment paradigm7. In addition, immunotherapy has emerged as an effective treatment strategy alone or in combination with chemotherapy. Consequently, treatment options for this patient population have increased markedly.
To select patients who would most likely benefit from targeted therapies, guidelines from both the U.S. National Comprehensive Cancer Network and the American Society of Clinical Oncology recommend testing for biomarkers such as EGFR, ALK, BRAF, ROS1, and PD-L1 in all patients with advanced nonsquamous nsclc6,8. First-generation epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis) such as gefitinib and erlotinib provide short-term benefits9, but better therapies are needed because tumour resistance typically emerges after a few months on-therapy. Second-generation tkis have been associated with modest improvements in progression-free or overall survival (os) in patients positive for an EGFR mutation (EGFRm+). Osimertinib, a third-generation egfr tki, has been associated with significantly longer progression-free survival than is seen with either gefitinib or erlotinib (18.9 months vs. 10.2 months)10.
Checkpoint inhibitors are a new class of immunotherapy agents that provide an effective clinical response in some patients with lung cancer, either through the PD-1 or PD-L1 protein11,12. As a result, nivolumab and atezolizumab are approved in Canada for patients with high expression of PD-1 and PD-L113–15.
The objective of the present retrospective longitudinal cohort study was to understand treatment patterns in patients with stage iv nsclc in Ontario at the population level and the associated stage-specific survival.
METHODS
Study Design
This retrospective longitudinal cohort study considered patients with stage iv nsclc diagnosed in Ontario between 1 April 2010 and 31 March 2015, with follow-up to March 2017. The code C34 in the International Classification of Diseases for Oncology, 3rd edition (icd), was used to identify cases with a diagnosis of nsclc in the Ontario Cancer Registry; histologic subtypes were determined from relevant morphology codes16. Patients with a second primary cancer 3 years before or after the nsclc diagnosis, patients who died less than 2 weeks after their nsclc diagnosis, and those who did not have a valid ohip (Ontario Health Insurance Plan) number or ices key number were excluded. Patients were then stratified by disease stage (those with an unknown stage were excluded). Patients with stage iv disease were further stratified into nonsquamous and squamous histologies (icd codes 80703–80783). A subgroup analysis explored endpoints in the EGFRm+ group (identified by receipt of a targeted therapy such as gefitinib, afatinib, or erlotinib), regardless of stage at diagnosis. No other agents were funded for EGFRm+ cases by the Ontario public health system, and therefore EGFRm+ cases in which other agents were used were excluded from the analysis. Treatment patterns were reported up to the end of third-line treatment.
Data Sources
Ontario is the largest Canadian province, with a population of 14 million, and it provides publicly funded health care services through the ohip, with patient-level data accessible through the ices Data and Analytic Services. For our study, a cohort of patients with stage iv nsclc was created, and each individual patient in the cohort was linked to applicable health administrative datasets to obtain data relating to the trajectory of their care through the health system over time. Linkages were made to 12 provincial or national datasets: Activity Level Reporting (alr), the Continuing Care Reporting System, the Home Care Database, the Hospital Discharge Abstract Database, the National Ambulatory Care Reporting System, the National Rehabilitation Reporting System, the New Drug Funding Program, the Ontario Cancer Registry, the Ontario Drug Benefit (odb) Program, the ohip Schedule of Benefits, the Ontario Mental Health Reporting System, and the Registered Persons Database. Systemic treatment data for oral treatments were obtained from alr, the New Drug Funding Program, and the odb formulary. Intravenous systemic chemotherapies, which are newer and expensive, are funded by Ontario Health (Cancer Care Ontario) [oh(cco)] through the New Drug Funding Program, provided that patients meet eligibility criteria. Data related to older systemic therapies, particularly older intravenous chemotherapy agents and all radiotherapy are recorded in alr. The odb formulary includes all oral anticancer drugs and a wide range of supportive care drugs (for example, analgesics and antiemetics) prescribed to patients 65 years of age and older. Information about cancer clinic visits was collected through a combination of the alr and the National Ambulatory Care Reporting System datasets. The ohip database includes data about physician visits and medical or diagnostic procedures. Inpatient rehabilitation admissions for respiratory and exercise rehabilitation are reported in the National Rehabilitation Reporting System. The Registered Persons Database contains demographic information for all individuals registered with ohip (for example, date of birth, date of death).
All outcomes are reported at an aggregate level. Comorbidity data were collected using two methods: score on the Charlson comorbidity index, which includes 17 conditions tracked using International Statistical Classification of Diseases and Related Health Problems diagnostic codes during inpatient hospitalizations for the 5 years before the nsclc diagnosis17, and the Johns Hopkins (Baltimore, MD, U.S.A.) Aggregated Diagnosis Groups (adgs), which include 32 conditions derived from diagnostic codes recorded during outpatient health system encounters 2 years before the nsclc diagnosis18. The adgs were also assigned to a simplified morbidity category called “predicted resource utilization bands.” A mean score of 0 indicates no comorbidities; however, the higher the mean score, the more likely the patient is to incur high resource utilization or to die.
Ethics
This study obtained approval from the Research Ethics Board at Sunnybrook Health Sciences Centre.
Statistical Methods
Descriptive statistics are used to summarize patient characteristics and treatment patterns. Single-agent and doublet or combination chemotherapy regimens are both reported as “chemotherapy.” Radiotherapy was assumed to be palliative. Chemoradiotherapy was recategorized as chemotherapy. Patients who received targeted therapies such as afatinib, erlotinib, or gefitinib were categorized as EGFRm+, being that those agents were the only targeted agents funded in the Ontario public system at the time of analysis. Patients were categorized as having had no treatment if no treatment was reported or if the patient died before receiving any treatment. The time from the stop date of first- or second-line treatment to the start of second- or third-line treatment was set at 6 weeks or more based on consensus for the analysis plan. The Kaplan–Meier method was used to calculate os from the date of diagnosis to the date of death or to the last date of follow-up, and results were stratified by the type of first-line treatment used for stage iv nonsquamous or squamous disease. The log-rank test identified differences in os between the treatment or tumour histology type groups.
RESULTS
Cohorts and Baseline Characteristics
Based on the relevant icd diagnostic codes, 24,729 individuals were diagnosed with nsclc. Of that group, 49.2% (n = 12,159) had stage iv disease, with 83.1% (n = 10,103) having nonsquamous histology, 16.9% (n = 2056) having squamous histology, and 508 being classified as EGFRm+. As Table I shows, median age was comparable for all groups and ranged from 69 to 72 years. A greater proportion of men had squamous histology (65.1%) than nonsquamous histology (50.7%). In line with the published literature, the EGFRm+ group contained a higher proportion of women (61.2%). Mean score on the Charlson comorbidity index ranged from 0.6 to 1.2 in the groups. Although that score was lowest in the EGFRm+ population, the adg score was comparable for all groups, ranging from 7.5 to 8.1. About 95% of each cohort showed a moderate to very high level of health care resource utilization in the 2 years before the nsclc diagnosis, suggesting that they were “sicker” patients. Of all patients with stage IV disease and of those with nonsquamous histology, 44.3% and 43.6% respectively were categorized into the two lowest income quintiles; fewer patients in the EGFRm+ group (38.2%) were so categorized. Notably, patients with squamous histology had the highest health care resource utilization and were categorized into the lowest income quintiles.
TABLE I.
Baseline characteristics for patients with stage IV non-small-cell lung cancer
| Variable | Patient cohort | |||
|---|---|---|---|---|
|
| ||||
| Overall | Nonsquamous | Squamous | EGFR mutation–positive | |
| Patients (n) | 12,159 | 10,103 | 2,056 | 508 |
| Age (years) | ||||
|
| ||||
| Median | 69 | 69 | 72 | 70 |
| IQR | 62–77 | 61–77 | 64–79 | 63–77 |
|
| ||||
| Sex [n (%)] | ||||
| Women | 5,694 (46.8) | 4,976 (49.3) | 718 (34.9) | 308 (61.2) |
| Men | 6,465 (53.2) | 5,127 (50.7) | 1,338 (65.1) | 195 (38.8) |
|
| ||||
| Mean score on the CCI | 1.0±1.5 | 0.9±1.5 | 1.2±1.7 | 0.6±1.2 |
|
| ||||
| Mean ADGsa (n) | 7.6±3.7 | 7.5±3.6 | 7.8±3.7 | 8.1±3.4 |
|
| ||||
| Predicted RUB [n (%)] | ||||
| Non users | 216 (1.8) | 182 (1.8) | 34 (1.7) | 1–5b |
| Healthy users | 153 (1.3) | 131 (1.3) | 22 (1.1) | 1–5b |
|
| ||||
| Resource utilization [n (%)] | ||||
| Low | 487 (4.0) | 425 (4.2) | 62 (3.0) | 13 (2.6) |
| Moderate | 4,984 (41.0) | 4,215 (41.7) | 769 (37.4) | 210 (41.7) |
| High | 3,556 (29.2) | 2,931 (29.0) | 625 (30.4) | 174 (34.6) |
| Very high | 2,763 (22.7) | 2,219 (22.0) | 544 (26.5) | 98 (19.5) |
|
| ||||
| Census-based income quintilec [n (%)] | ||||
| 1 (lowest) | 2,713 (22.4) | 2,207 (22.0) | 506 (24.8) | 98 (19.6) |
| 2 | 2,650 (21.9) | 2,175 (21.6) | 475 (23.3) | 93 (18.6) |
| 3 | 2,381 (19.7) | 1,985 (19.7) | 396 (19.4) | 103 (20.6) |
| 4 | 2,295 (19.0) | 1,938 (19.3) | 357 (17.5) | 104 (20.8) |
| 5 (highest) | 2,057 (17.0) | 1,749 (17.4) | 308 (15.1) | 101 (20.2) |
Johns Hopkins, Baltimore, MD, U.S.A.
Value less than 5 suppressed because of data use agreement.
Excludes patients with unknown income quintile values.
IQR = 25%–75% interquartile range; CCI = Charlson comorbidity index; ADG = Aggregated Diagnosis Groups; RUB = resource utilization band.
Treatment Patterns
Nonsquamous Histology
Table II shows that almost 15% of patients with stage iv nonsquamous nsclc received first-line chemotherapy, a percentage that increased slightly to 19% in the second and third lines. In the first line, the greatest percentage of patients received either no treatment (26.7%) or palliative radiotherapy (44.4%). Of the 1506 patients with nonsquamous histology who were treated with first-line chemotherapy, 1074 (71.3%) received platinum-based doublet chemotherapy: cisplatin or carboplatin with either gemcitabine (51.3%), pemetrexed (20.3%), or paclitaxel (15.1%). Of the 1434 patients with nonsquamous histology treated with second-line chemotherapy, pemetrexed was the most frequently prescribed treatment (44.4%). Of the 663 patients with nonsquamous histology treated with third-line chemotherapy, 56.6% received pemetrexed. Of patients with nonsquamous histology, 3.0% received an egfr tki in the first line, and proportionately more received such an agent in the second and third lines (4.5% and 6.1% respectively).
TABLE II.
Treatment distribution by line of therapy, stratified by histology
| Treatment | Histology group [n (%)] | ||||
|---|---|---|---|---|---|
|
| |||||
| Nonsquamous | Squamous | ||||
|
|
|
||||
| First line (n=10,103) | Second line (n=7,238) | Third line (n=3,467) | First line (n=2,056) | Second line (n=1,469) | |
| Chemoradiation | 1,110 (11.0) | 469 (6.5) | 199 (5.7) | 234 (11.4) | 59 (4.0 |
|
| |||||
| Chemotherapy | 1,506 (14.9) | 1,434 (19.8) | 663 (19.1) | 239 (11.6) | 209 (14.2) |
|
| |||||
| Radiotherapy | 4,483 (44.4) | 1,168 (16.1) | 532 (15.3) | 1,029 (50.0) | 245 (16.7) |
|
| |||||
| Targeted therapy | 305 (3.0) | 323 (4.5) | 210 (6.1) | 554 (26.9)a | 15 (1.0) |
|
|
|
||||
| No treatment | 2,699 (26.7) | 3,844 (53.1) | 1,863 (53.7) | 941 (64.1) | |
Results merged. One treatment type with a value <6 required suppression because of the data use agreement.
EGFRm+ Population
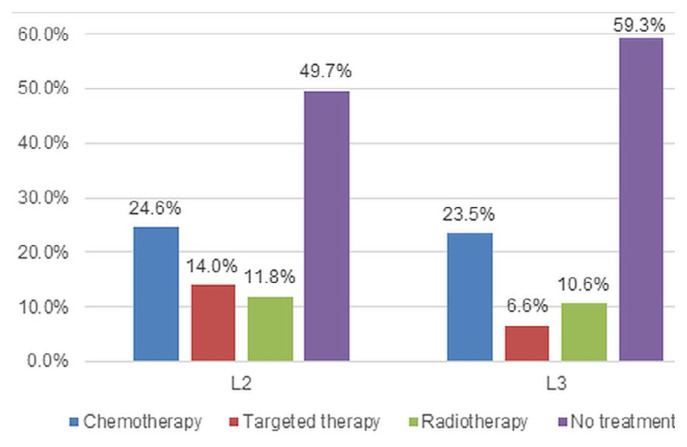
Although 305 patients with stage iv nonsquamous disease received first-line targeted therapy, the subgroup analysis of patients classified EGFRm+ was expanded to include all patients with nonsquamous histology, regardless of stage at diagnosis, who received targeted therapy (n = 508). Gefitinib was the most frequently used agent; the distribution of first-line gefitinib, erlotinib, and afatinib in the expanded cohort was 75.6%, 19.7%, and 4.7% respectively. Figure 1 presents the various types of treatments received in the second (n = 501) and third lines (n = 302). Of the 249 patients who did not receive any second-line treatment (49.7%), most died before receiving treatment (n = 197). For those who did receive additional therapy, chemotherapy was the treatment most frequently received in both the second line (24.6%) and third line (23.5%).
FIGURE 1.
Treatment distribution in the second line (2L) and third line (3L) for patients with a positive EGFR mutation status.
Squamous Population
Only 239 of the 2056 patients with squamous histology (11.6%) were treated with first-line chemotherapy (Table II), and of those 239, 120 received cisplatin or carboplatin with gemcitabine. In the 209 patients treated with second-line chemotherapy, docetaxel was the most frequently used treatment (30.0%). Third-line treatment information was not analyzed.
Survival
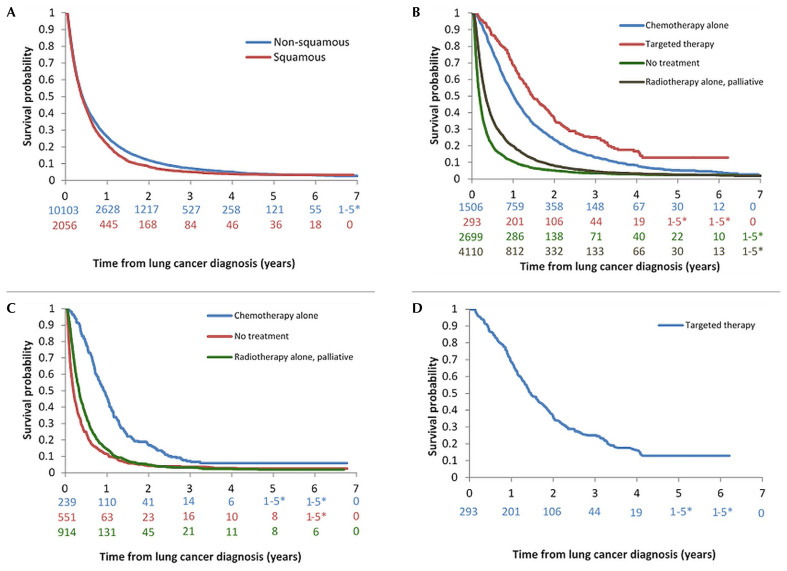
In the overall cohort of 12,159 patients with stage iv disease, 11,509 deaths occurred, and 650 patients were censored. The median os (mos) for all patients with stage iv disease was 4.8 months [95% confidence interval (ci): 4.7 months to 5.0 months]. For patients with nonsquamous and squamous histology [Figure 2(A)], the mos was 4.9 months (95% ci: 4.7 months to 5.0 months) and 4.6 months (95% ci: 4.4 months to 5.1 months) respectively. At 5 years, 3.7% of patients with stage iv disease overall, 3.7% of those with nonsquamous histology, and 3.5% of those with squamous histology were still alive.
FIGURE 2.
Overall survival curves. (A) In stage IV nonsquamous and squamous disease. (B) In nonsquamous disease stratified by patient’s first-line treatment. (C) In squamous disease stratified by patient’s first-line treatment. (D) In patients with a positive EGFR mutation status.
In the cohort of 8608 patients with nonsquamous histology, 8172 deaths occurred, and 436 patients were censored [Figure 2(B)]. Those who received an egfr tki had the best median survival at 17.6 months (95% ci: 15.8 months to 20.2 months), followed by those treated with chemotherapy (12.1 months; 95% ci: 11.4 months to 12.8 months) and radiotherapy (3.9 months; 95% ci: 3.8 months to 4.1 months). Patients who received no active treatment had the poorest survival, at a median of 2.2 months (95% ci: 2.1 months to 2.3 months). Of the patients with nonsquamous histology, 12.9% of those who received targeted therapy, 5.2% of those who received chemotherapy, 2.7% of those who received radiotherapy, and 2.4% of those who received no treatment were still alive at 5 years.
In the cohort of 1704 patients with squamous histology, 1648 deaths occurred, and 56 patients were censored [Figure 2(C)]. Those who received chemotherapy had the best survival, at a median of 11.1 months (95% ci: 9.6 months to 12.4 months), followed by those treated with radiotherapy (3.9 months; 95% ci: 3.6 months to 4.1 months). Patients who received no active treatment had the poorest survival, at a median of 2.3 months (95% ci: 2.0 months to 2.6 months). Of the patients with squamous histology, 5.9% of those who received chemotherapy, 2.0% of those who received radiotherapy, and 2.6% of those who received no treatment were still alive at 5 years.
In the cohort of patients classified EGFRm+ who were stage iv at diagnosis and who received first-line targeted therapy, os from initial treatment was 17.6 months [Figure 2(D)]. In patients who survived and received second-line treatment, mos at initiation of second-line treatment was 5.3 months (95% ci: 4.2 months to 6.8 months).
DISCUSSION
Our retrospective study provides a population-based real-world perspective about how patients with stage iv lung cancer were managed in Ontario. The study period provided data concerning some of the more recent practice changes affecting lung cancer and also sufficient time to assess the survival associated with those treatments. Importantly, the study timeframe pre-dates the introduction of immunotherapy and the newer targeted treatments that are transforming nsclc management. Payers in publicly funded health care systems increasingly require information about the clinical benefit, cost-effectiveness, and potential budget impacts of new cancer treatments. By understanding real-world treatment patterns, estimating the economic burden of the new immuno-oncology or targeted agents for advanced nsclc will be easier.
One of the key observations from our study is that only a small proportion of patients received any active treatment, and only about one quarter received systemic therapy. The fact that most patients with stage iv nsclc received no active treatment has been observed by others19–25. The percentage varies by country, with the rate of no treatment ranging from as high as 50% in Ireland19 and 43% in Scotland20 to as low as 20% in the United States21. Our rate of no treatment (no systemic or palliative radiotherapy) was 26.7% for nonsquamous and 26.9% for squamous nsclc. The reasons for those low rates of active treatment are not fully explained by the administrative data and might be hypothesized to reflect the older median age of our study cohort compared with clinical trial cohorts. Others have noted that elderly patients are less likely to be considered for systemic therapy for nsclc26. Patients in our cohort had several comorbidities, judging from their high (29.2%) or very high (22.7%) resource utilization and their high median adg score of 7.6 ± 3.7, reflecting high use of outpatient health care services in the 2 years preceding their diagnosis. Many patients also resided in lower-income areas, which might have resulted in barriers to care access even in a publicly funded health care system.
Clinical practice guidelines for advanced (stage iv) nsclc from oh(cco) recommend platinum-based doublet chemotherapy as the standard of care for newly diagnosed patients who do not have an actionable mutation4. For those with a mutation that can be targeted (such as EGFR, ALK, or ROS1), a tki is the standard of care4. Our study found that only 28.9% (n = 2921) of patients with nonsquamous nsclc received any type of first-line systemic therapy. Most of the systemic therapy was chemotherapy, only 3.0% of which was attributable to egfr-targeted therapies, reflecting the funding status of those agents at the time of treatment. When chemotherapy was used, it was a platinum-based doublet in 71.3% of cases. For the 19.8% of patients with nonsquamous histology who received second-line chemotherapy (n = 1438), pemetrexed was used as a single agent in 44.4% of cases. In an earlier study, Sacher and colleagues22 also observed a low rate of chemotherapy use in 8113 Ontario patients with metastatic nsclc during 2005–2009. Only 24% of their cohort received first-line chemotherapy, with 89% of that group receiving platinum-based doublet chemotherapy, 41% of whom received second-line pemetrexed. Their results, coupled with findings in our study, suggest that, during the period from 2005 to 2015, the initial treatment approach to metastatic nsclc did not change significantly.
The stigma associated with a diagnosis of lung cancer might lead patients to be passive about accepting treatment, and their physicians might have a negative bias toward the treatment of lung cancer. Vinod and colleagues in Australia24 reported that differences between “guideline recommended no treatment” and “multidisciplinary meeting no treatment” were largely attributable to patient comorbidities and clinical factors, but that differences between “multidisciplinary meeting no treatment” and “actual no treatment” were attributable to patient preference and declining performance status. In Ontario, rurality and distance to health care institutions that provide systemic chemotherapy administration could be a factor contributing to low first-line treatment rates.
A few limitations of this study can be noted. The administrative databases do not collect certain data elements that might clarify the reasons for no active treatment, such as performance status, weight loss, and clinical disease characteristics (such as tumour size and location) that might also have prognostic significance. Another limitation is the possibility that the use of targeted therapies might be underreported, given that data for the use of oral therapy has been underreported in the alr database, and the odb reimburses patients for the use of such agents only if they are 65 years of age or older, or receiving social assistance. Younger patients have to pay for oral therapies through private insurance or out of pocket, and so the relevant utilization information is not available in provincial administrative databases.
In our study, os in the stage iv nonsquamous and squamous populations remained poor (the mos being 4.9 and 4.6 months respectively), and only 3.7% survived 5 years. Sacher and colleagues22 stratified their metastatic nsclc cohort by whether they received first-line chemotherapy. For those treated with first-line chemotherapy in our study (n = 1944), the mos was 8.2 months compared with 12.1 months. Both values fall within the 8- to 12-month range that Leighl reported in a 2012 overview of first-line chemotherapy results27. Nonetheless, in our study, patients who received first-line targeted therapies experienced the longest survival.
CONCLUSIONS
Our study underscores the need for new and effective therapies, with limited toxicities, that can be prescribed to most patients. Immunotherapies and agents that target various actionable mutations are showing promising results, with less toxicity than chemotherapy and longer progression-free survival and os. In estimating the proportion of patients who could benefit from the new therapies, it will be useful to know the proportion of patients who would qualify for active treatment. In our study period, that proportion was remarkably low. Less-toxic therapies will be more acceptable to patients, and improved treatment outcomes will overcome therapeutic nihilism and encourage more physicians to refer patients for treatment. Periodic updates of practice patterns will be necessary to observe the rate of uptake of new therapies and to provide data about real-world clinical effectiveness and economic impact.
ACKNOWLEDGMENTS
This study made use of de-identified data from the ices Data Repository, which is managed by ices with support from its funders and partners: Canada’s Strategy for Patient-Oriented Research (spor), the Ontario spor Support Unit, the Canadian Institutes of Health Research, and the Government of Ontario. The opinions, results, and conclusions reported are those of the authors. No endorsement by ices or any of its funders or partners is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (cihi). However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not necessarily those of cihi. Parts of this material are based on data and information provided by oh(cco). The opinions, results, views, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of oh(cco). No endorsement by oh(cco) is intended or should be inferred.
The authors thank Shazia Hassan for assistance in manuscript preparation.
This work was previously presented at the 19th World Conference on Lung Cancer of the International Association for the Study of Lung Cancer; Toronto, ON; 23–26 September 2018; and at the Canadian Association for Population Therapeutics/Association Canadienne pour la Thérapeutiques des Populations 2018 Annual Meeting; Toronto, ON; 22–23 October 2018.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: SJS received an unrestricted grant from AstraZeneca Canada Inc. to conduct this study. MH and RNW are employees of AstraZeneca Canada Inc. WKE has received fees as an advisory board member from AbbVie, Astellas, Bristol–Myers Squibb, Eisai, Gilead, Lilly, Takeda, and Merck, and consulting fees from AstraZeneca, Boehringer Ingelheim, Celgene, Janssen, Lilly, Roche, Servier, and Sanofi Genzyme.
REFERENCES
- 1.Canadian Cancer Society. Lung cancer statistics [Web page] Toronto, ON: Canadian Cancer Society; 2020. [Available at: https://www.cancer.ca/en/cancer-information/cancer-type/lung/statistics/; cited 28 July 2020] [Google Scholar]
- 2.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute (nci) Non–Small Cell Lung Cancer Treatment (PDQ) – Health professional version: Stage Information for NSCLC [Web resource] Bethesda, MD: NCI; 2020. [Available at: https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq#_470; cited 28 July 2020] [Google Scholar]
- 3.Canadian Cancer Society. Survival statistics for non–small cell lung cancer [Web page] Toronto, ON: Canadian Cancer Society; n.d.. [Available at: https://www.cancer.ca/en/cancer-information/cancer-type/lung/prognosis-and-survival/non-small-cell-lung-cancer-survival-statistics/; cited 1 November 2018. [Google Scholar]
- 4.Ellis PM, Vella ET, Ung YC on behalf of the Lung Cancer Disease Site Group. Systemic Treatment for Patients with Advanced Non–Small Cell Lung Cancer. Toronto, ON: Ontario Health (Cancer Care Ontario); 2016. [Available online at: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/31811; cited 1 November 2018. [Google Scholar]
- 5.Alberta Health Services (ahs) Non–Small Cell Lung Cancer, Stage IV. Clinical practice guideline ver. 6. Edmonton, AB: AHS; 2013. [Available online at: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-lu004-nsclc-stage4.pdf; cited 1 November 2018. [Google Scholar]
- 6.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Non–Small Cell Lung Cancer. Ver. 6.2018. Fort Washington, PA: NCCN; 2018. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (free registration required); cited 1 November 2018] [Google Scholar]
- 7.Li T, Kung HJ, Mack PC, Gandra DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31:1039–49. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering first-line egfr tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29:2121–7. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 9.Kanellakis NI, Jacinto T, Psallidas I. Targeted therapies for lung cancer: how did the game begin? Breathe (Sheff) 2016;12:177–9. doi: 10.1183/20734735.006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang T, Su C, Ren S, et al. on behalf of the ame Lung Cancer Collaborative Group. A consensus on the role of osimertinib in non–small cell lung cancer from the ame Lung Cancer Collaborative Group. J Thorac Dis. 2018;10:3909–21. doi: 10.21037/jtd.2018.07.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone DP, Gandara DR, Antonia SJ, Zielinski C, Paz-Ares L. Non-small-cell lung cancer: role of the immune system and potential for immunotherapy. J Thorac Oncol. 2015;10:974–84. doi: 10.1097/JTO.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanwal B, Biswas S, Seminara RS, Jeet C. Immunotherapy in advanced non–small cell lung cancer patients: ushering chemotherapy through the checkpoint inhibitors? Cureus. 2018;10:e3254. doi: 10.7759/cureus.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.pan-Canadian Oncology Drug Review (pcodr) Nivolumab (Opdivo) for Metastatic Non–Small Cell Lung Cancer. Ottawa, ON: pCODR; 2016. [Available online at: https://www.cadth.ca/sites/default/files/pcodr/pcodr-provfund_nivolimab_opdivo_nsclc.pdf; cited 1 November 2018. [Google Scholar]
- 14.pan-Canadian Oncology Drug Review (pcodr) Pembrolizumab (Keytruda) for Advanced Non–Small Cell Lung Carcinoma (First Line) Ottawa, ON: pCODR; 2017. [Available online at: https://www.cadth.ca/sites/default/files/pcodr/pcodr_profund_pembrolizumab_keytruda_nsclc_1stln.pdf; cited 1 November 2018. [Google Scholar]
- 15.pan-Canadian Oncology Drug Review (pcodr) Atezolizumab (Tecentriq) for Non–Small Cell Lung Cancer. Ottawa, ON: pCODR; 2018. [Available online at: https://www.cadth.ca/sites/default/files/pcodr/pcodr_profund_atezolizumab_tecentriq_nsclc.pdf; cited 1 November 2018. [Google Scholar]
- 16.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 17.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (adgs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49:932–9. doi: 10.1097/MLR.0b013e318215d5e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmud SM, Reilly M, Comber H. Patterns of initial management of lung cancer in the Republic of Ireland: a population-based observational study. Lung Cancer. 2003;41:57–64. doi: 10.1016/S0169-5002(03)00148-X. [DOI] [PubMed] [Google Scholar]
- 20.Gregor A, Thomson CS, Brewster DH, et al. Management and survival of patients with lung cancer in Scotland diagnosed in 1995: results of the national population-based study. Thorax. 2001;56:212–17. doi: 10.1136/thorax.56.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chute CG, Greenberg ER, Barron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56:2107–11. doi: 10.1002/1097-0142(19851015)56:8<2107::AID-CNCR2820560837>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Sacher AG, Le LW, Lau A, Earle CC, Leighl NB. Real-world chemotherapy treatment patterns in metastatic non–small cell lung cancer: are patients undertreated? Cancer. 2015;121:2562–9. doi: 10.1002/cncr.29386. [DOI] [PubMed] [Google Scholar]
- 23.Vinod SK, O’Connell DL, Simonella L, et al. Gaps in optimal care for lung cancer. J Thorac Oncol. 2008;3:871–9. doi: 10.1097/JTO.0b013e31818020c3. [DOI] [PubMed] [Google Scholar]
- 24.Vinod SK, Sidhom MA, Gabriel GS, Lee MT, Delaney GP. Why do some lung cancer patients receive no anticancer treatment? J Thorac Oncol. 2010;5:1025–32. doi: 10.1097/JTO.0b013e3181da85e4. [DOI] [PubMed] [Google Scholar]
- 25.Carrato A, Vergnenegre M, Thomas M, McBride K, Medina J, Cruciani G. Clinical management patterns and treatment outcomes in patients with non–small cell lung cancer (nsclc) across Europe: epiclin–Lung study. Curr Med Res Opin. 2014;30:447–61. doi: 10.1185/03007995.2013.860372. [DOI] [PubMed] [Google Scholar]
- 26.Dawe DE, Pond GR, Ellis PM. Assessment of referral and chemotherapy treatment patterns for elderly patients with non-small-cell lung cancer. Clin Lung Cancer. 2016;17:563–72. doi: 10.1016/j.cllc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol. 2012;19(suppl 1):S52–8. doi: 10.3747/co.19.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]