Abstract
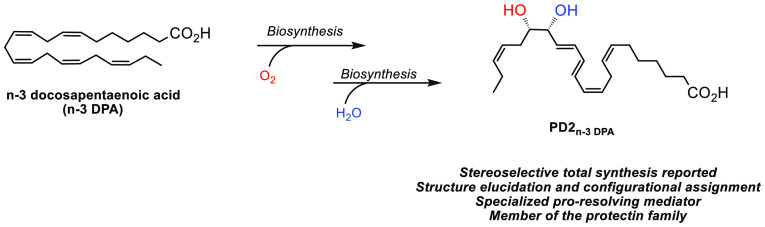
The resolution of inflammation is governed by the active biosynthesis of specialized pro-resolving mediators using ω-6 and ω-3 polyunsaturated fatty acids as substrates. These mediators act as resolution agonists and display several interesting bioactivities. PD2n-3 DPA is an oxygenated polyunsaturated fatty acid biosynthesized from n-3 docosapentaenoic acid belonging to the specialized pro-resolving lipid mediator family named protectins. The protectins exhibit anti-inflammatory properties and pro-resolving bioactivities. These endogenously produced compounds are of interest as leads in resolution pharmacology and drug development. Herein, together with its NMR, MS, and UV data, a stereoselective total synthesis of PD2n-3 DPA is presented.
Endogenous mechanisms that control resolution programs during acute inflammation are essential in maintaining health.1 If uncontrolled, chronic inflammation may result in the development of several human diseases.1,2 Individual families of specialized pro-resolving mediators (SPMs) are the lipoxins, the resolvins, the maresins, and the protectins. SPMs are oxygenated polyunsaturated fatty acids (PUFAs) that stimulate the resolution of inflammation and fight infections.2 The ω-3 PUFAs eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and n-3 docosapentaenoic acid (n-3 DPA) are, in the presence of cyclooxygenase-2 and various lipoxygenases, converted into SPMs.3 SPMs exhibit anti-inflammatory and pro-resolving bioactions in the low nanomolar range due to their agonist effects on G-protein coupled receptors (GPCRs).4 Such resolution processes have attracted interest in drug development research programs, given that approximately 30% of all approved drugs act on this receptor family.5 Because SPMs are endogenously biosynthesized in minute amounts, stereoselective total synthesis becomes necessary for configurational assignment and extensive biological evaluations facilitating drug development efforts.3
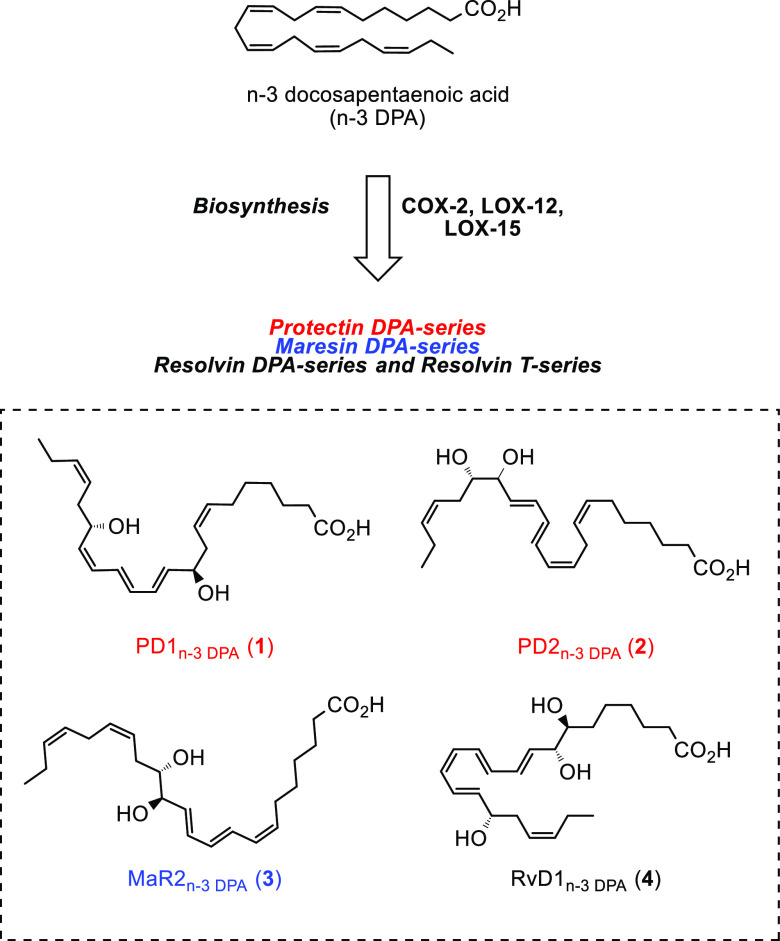
In 2013 and 2015, new SPMs biosynthesized from n-3 DPA were reported.6 N-3 DPA is a biochemical intermediate in the formation of DHA from EPA.7 Some examples of n-3 DPA derived SPMs are shown in Figure 1.6a
Figure 1.
Examples of SPMs derived from n-3 DPA.
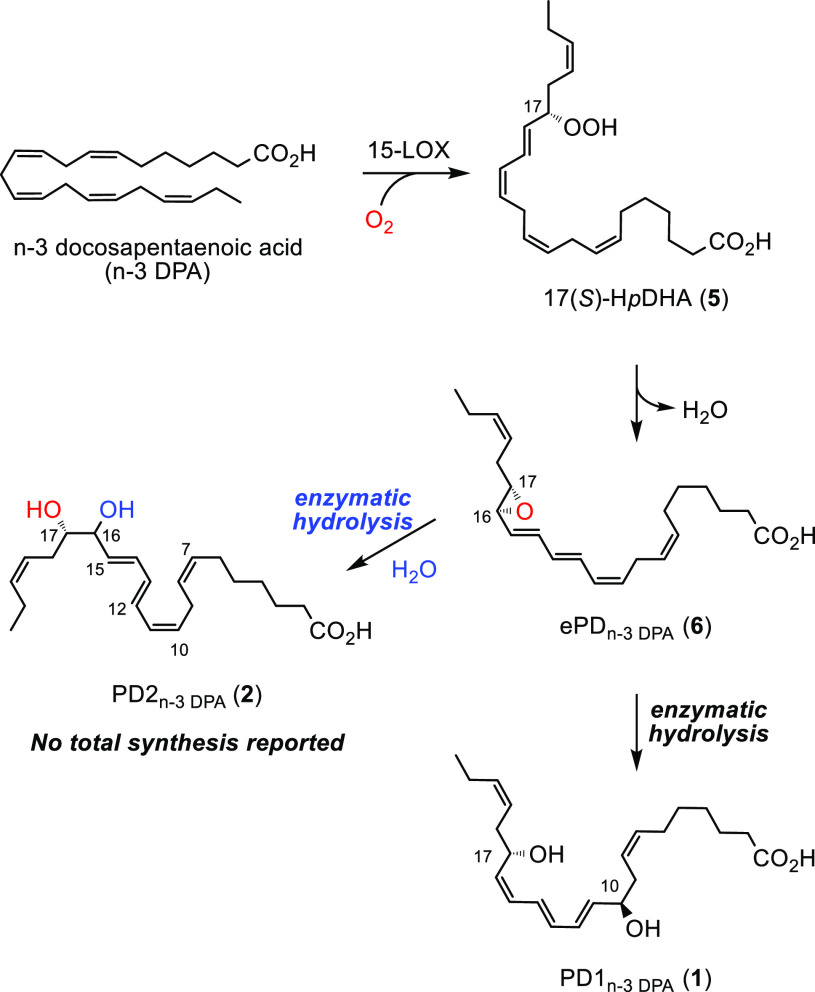
Stereoselective total synthesis of 1 confirmed its structure as shown in Figure 1.8,9 In collaboration with the Dalli group, we elucidated the biosynthetic pathway of PD1n-3 DPA (1) and PD2n-3 DPA (2) as depicted in Scheme 1.10 Oxygenation of n-3 DPA by 15-LOX gives the hydroperoxide 5, which is transformed into the epoxide ePDn-3 DPA (6). Hydrolysis of 6 in the presence of an unknown enzyme provides 1 and 2.10
Scheme 1. Biosynthetic Pathway of the n-3 DPA-Derived SPMs PD1n-3 DPA (1) and PD2n-3 DPA (2).
The SPM 1 displayed potent anti-inflammatory and pro-resolving bioactions together with stimulation of human macrophage phagocytosis and efferocytosis.8 In the resolution of inflammation these bioactions are distinct features.2−4 As of today, no total synthesis of PD2n-3 DPA (2) has been reported. Against this, and the interesting bioactions of the n-3 DPA derived SPMs,11 the first total synthesis of 2 is presented.
Results and Discussion
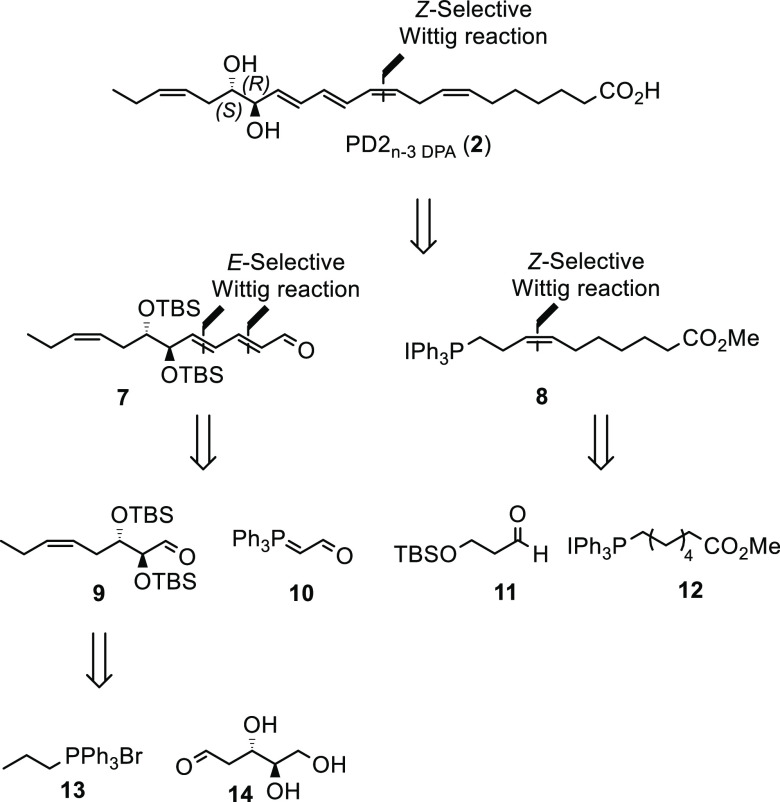
Based on the biosynthesis presented in Scheme 1, we anticipate the diol moiety in PD2n-3 DPA (2) to be anti and 16R,17S configured. Hence, our synthesis of 2 was planned as outlined in Scheme 2, leading back to the triene-aldehyde 7 and the known Wittig salt 8; the latter has been prepared from 11 and 12.12 Compound 9 may be prepared from the Wittig reagent 13 and 2-deoxy-d-ribose (14), both commercially available.
Scheme 2. Retrosynthetic Analysis of PD2n-3 DPA (2).
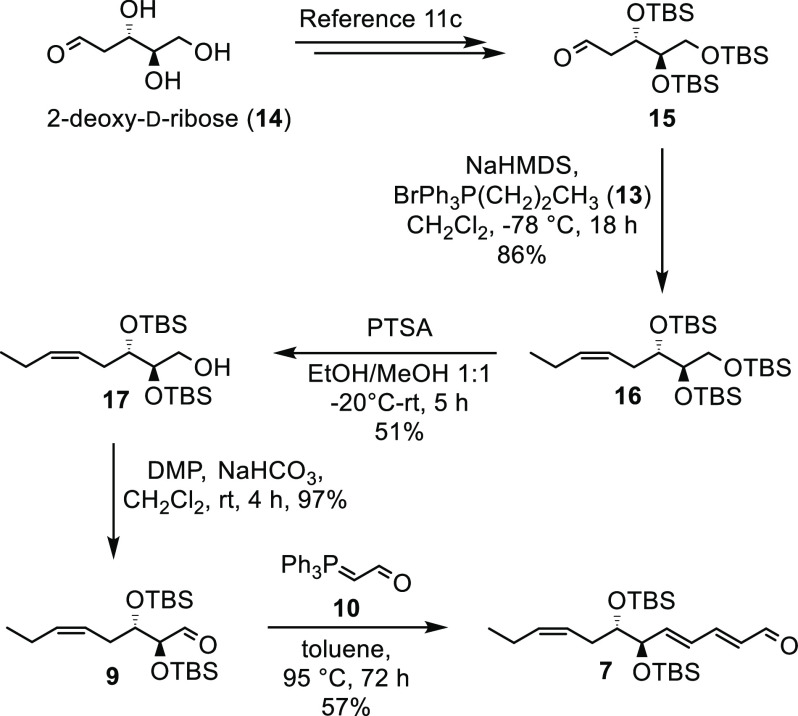
Aldehyde 15 was prepared from 2-deoxy-d-ribose (14) over three steps as previously reported11c (Scheme 3). Aldehyde 15 was reacted with the ylide of commercially available triphenyl(propyl)phosphonium bromide (13) in a Z-selective Wittig reaction. The ylide of 13 was formed after reaction with NaHMDS in CH2Cl2 at −78 °C. After column chromatography, 16 was obtained as one stereoisomer in 86% yield (Scheme 3). Next, we used our monodeprotection protocol11c in order to obtain alcohol 17, which was oxidized to the aldehyde 9. Reacting 9 with an excess of 10 afforded the desired aldehyde 7 in 28% isolated yield over the three steps. The desired product was readily separated from substantial quantities of the mono Wittig product also produced, by column chromatography. Of notice, ylide 10 had to be added in several portions over the course of the reaction due to the instability of 10 at elevated temperatures.
Scheme 3. Synthesis of Aldehyde 7.
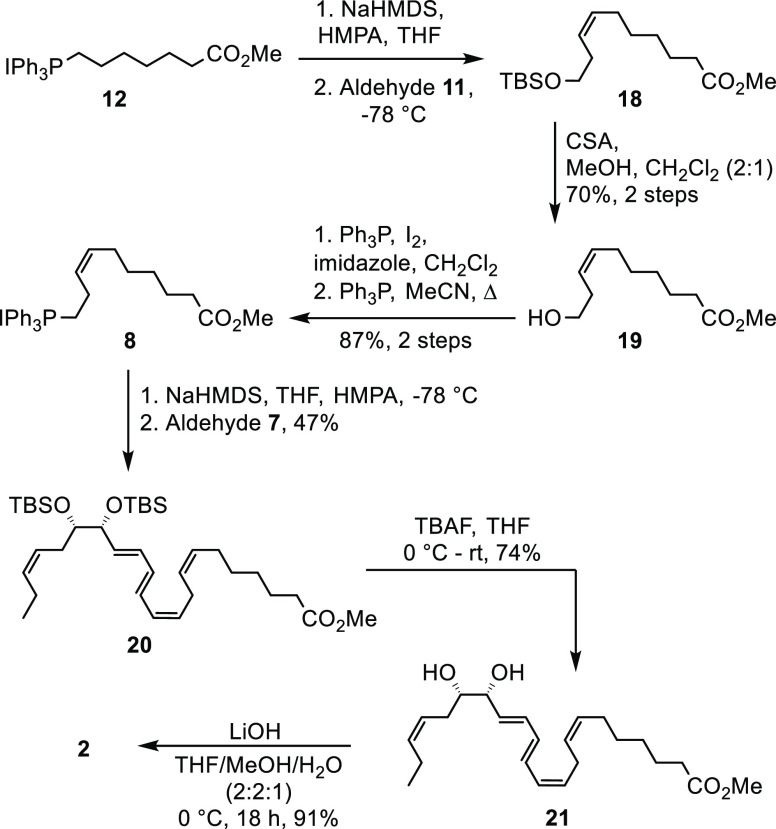
Next the ylide of known Wittig-salt 12(8) was generated (NaHMDS, THF, HMPA, −78 °C) and reacted with commercially available aldehyde 11. This yielded compound 18, which was converted (camphorsulfonic acid (CSA), MeOH, CH2Cl2) into alcohol 19. Alcohol 19 was isolated in 70% yield from 11. The Wittig-salt 8 was obtained in 87% yield from 19 over two steps (I2, PPh3, imidazole; PPh3). Again, NaHMDS in THF and HMPA at −78 °C was used in a Z-selective Wittig reaction, this time with 8 and aldehyde 7, affording 20 in 47% yield (Scheme 4). Deprotection of the two TBS-ethers was achieved with TBAF, yielding the methyl ester 21 in 74% yield and with >96% chemical purity (HPLC, Supporting Information); see Scheme 4. Basic hydrolysis gave PD2n-3 DPA (2) in 91% yield.
Scheme 4. Synthesis of PD2n-3 DPA (2).
The assignment of the Z- or E-configuration for each of the double bonds was then performed using two-dimensional NMR spectroscopy. From these experiments, connectivity between the adjacent olefinic protons (H-7/H-8, H-10/H-15, and H-19/H-20) was observed (Table 1, Scheme 1). Combining these analyses with the data from the HMBC spectra allowed the assignment of all the olefinic hydrogens. The signals at 5.83 ppm (dd, 1H, J = 15.0, 7.1 Hz) and 6.26 ppm (dd, 1H, J = 14,6, 10.7 Hz) are diagnostic for E-double bonds, while the signal observed at 6.03 ppm (apparent t, 1H, J = 11.1 Hz) correlates with a Z-double bond.
Table 1. Compilation of 1H and 13C NMR Data of PD2n-3 DPA (2).
| position | δC,amult. | δH,b mult. (J in Hz) | HMBCc | COSY |
|---|---|---|---|---|
| 1 | 177.8, C | |||
| 2 | 35.1, CH2 | 2.28, t (7.5) | 1, 3, 4 | 3 |
| 3 | 26.1, CH2 | 1.62, quint (7.3) | 1, 2, 4, 5 | 2, 4 |
| 4 | 29.9, CH2 | 1.39, m | 2, 3, 5, 6 | 3, 5 |
| 5 | 30.4, CH2 | 1.39, m | 3, 4, 6 | 4, 6 |
| 6 | 28.0, CH2 | 2.11, m | 4, 5, 7, 8 | 5, 7 |
| 7 | 131.5, CH | 5.41, m | 6, 8, 9 | 6, 8 |
| 8 | 128.5, CH | 5.38, m | 6, 7, 9, 10 | 7, 9 |
| 9 | 27.1, CH2 | 2.96, t (7.3) | 7, 8, 10, 11 | 8, 10 |
| 10 | 131.3, CH | 5.40, m | 9, 11 | 9, 11 |
| 11 | 129.6, CH | 6.03, apparent t (11.1) | 9, 10, 13 | 10, 12 |
| 12 | 129.1, CH | 6.57, dd (14.3, 11.2) | 13, 14 | 11, 13 |
| 13 | 133.7, CH | 6.26, dd (14.6, 10.7) | 11, 12, 15 | 12, 14 |
| 14 | 133.6, CH | 6.36, ddd (15.1, 10.7, 1.1) | 12, 15, 16 | 13, 15 |
| 15 | 133.8, CH | 5.83, dd (15.0, 7.1) | 13, 14, 16, 17 | 14, 16 |
| 16 | 76.3, CH | 4.01, ddd (7.0, 4.9, 1.1) | 14, 15, 17, 18 | 15, 17 |
| 17 | 76.0, CH | 3.55, dt (8.1, 4.9) | 15, 16, 18, 19 | 16, 18 |
| 18 | 31.8, CH2 | 2.33 + 2.16, m | 16, 17, 19, 20 | 17, 19 |
| 19 | 126.3, CH | 5.45, m | 17, 18, 20, 21 | 18, 20 |
| 20 | 134.4, CH | 5.46, m | 18, 19, 21, 22 | 19, 21 |
| 21 | 21.7, CH2 | 2.08, m | 19, 20, 22 | 20, 22 |
| 22 | 14.6, CH3 | 0.97, t (7.5) | 20, 21 | 21 |
Measured at 100 MHz.
Measured at 400 MHz.
HMBC correlations are from proton(s) stated to the indicated carbon(s). The ppm values listed above for δH were assigned using the center of the COSY- and HSQC-peak intensities.
The COSY spectrum was used for assigning vicinal signals for each of the two isolated Z-olefins and the Z,E,E-moiety. Moreover, two signals from the hydrogen atoms attached to the carbinol carbon atoms were observed as expected with signals at 4.01 ppm (ddd, 1H, J = 7.0, 4.9, 1.1 Hz) and 3.55 ppm (dt, 1H, J = 8.1, 4.9 Hz). MaR2n-3 DPA (3)11a and RvD1n-3 DPA (4)11c displayed similar chemical shift values and coupling pattern. Of note, these SPMs were matched against authentic materials using LC/MS-MS analysis.
The HSQC spectrum was used for assigning the signals from the methylene carbons at 27.1 and 31.8 ppm, next to the C-19/C-20 and the C-7/C-8 Z-double bonds, respectively. The data from the 1H and 13C NMR spectra, in combination with the COSY and the HMBC spectra, allowed the structural assignment of the rest of the molecule (Table 1).
Moreover, the ultraviolet absorbance profile of 2 gave absorbance characteristics of a triene chromophore that is allylic to an auxochrome with λmax (MeOH) = 272 nm with shoulders at 262 and 283 nm.
The use of 2-deoxy-d-ribose (14) as a chiral pool starting material enabled the introduction of the 16R,17S-configured diol moiety in PD2n-3 DPA (2). This sugar was used by Rodriguez and Spur in their synthesis of PD2, the 4,5-Z double-bond congener of 2.13 The 17S configuration is biosynthetically formed by a stereoselective oxygenation of n-3 DPA by the 15-LOX enzyme (Scheme 2).6a,10 The COX-2 enzyme produces an R-configured hydroperoxide intermediate with DHA,14 n-3 DPA,6b,12 or 3-oxa n-3 DPA as substrate.15 It has been reported that for anti-1,2-diol-containing protectins and resolvins, also biosynthesized via epoxide intermediates, an SN2-type opening by water takes place at the activated allylic carbon atom of the epoxide.16 This process occurs both for epoxides formed via the 15-LOX or the COX-2 pathways.3,15,16 In our biosynthetic studies of PD1n-3 DPA (1), using the epoxide ePDn-3 DPA (6),10 we also identified PD2n-3 DPA (2), rendering support for an SN2-type reaction involving epoxide 6. In 14, as well as all intermediates derived thereof, the configurations of the stereogenic centers are preserved. The same observation was reported for the synthesis of PD2.13 Moreover, the specific rotation value for synthetic 2 ([α]D25 +22 (c 0.14, MeOH) indicates 2 is dextrorotary. The same sign has been reported for the specific rotation of the constitutional isomer MaR2n-3 DPA (3).11a In addition, the anti configuration is also observed when l-glutathione is the nucleophile in the biosynthesis of conjugated protectins17 and leuoktrienes.18 These arguments, together with the spectroscopic data presented, render support for the structural assignment of PD2n-3 DPA (2) to be (7Z,10Z,12E,14E,19Z)-16R,17S-dihydroxydocosa-7,10,12,14,19-pentaenoic acid.
Conclusions
To conclude, the first total synthesis of the oxygenated PUFA product PD2n-3 DPA (2) is reported, enabling its exact structural assignment. The key synthetic reactions were E- and Z-selective Wittig reactions, avoiding the use of challenging Z-selective reduction protocols of internal alkynes.19 Multimilligrams of material are now available for biological testing.
Experimental Section
General Experimental Procedures
Optical rotations were measured using a 0.7 mL cell with a 1.0 dm path length on an Anton Paar MCP 100 polarimeter. The UV/vis spectra from 190 to 900 nm were recorded using an Agilent Technologies Cary 8485 UV–vis spectrophotometer using quartz cuvettes. NMR spectra were recorded on a Bruker AVII400 or a Bruker AVIII HD 400 spectrometer at 400 MHz or a Bruker AVII600 spectrometer at 600 MHz for 1H NMR and at 100 or 150 MHz for 13C NMR. Spectra are referenced relative to the central residual protium solvent resonance in 1H NMR (CDCl3 δH 7.26, DMSO-d6 δH 2.50 and methanol-d4 δH 3.31) and the central carbon solvent resonance in 13C NMR (CDCl3 δC 77.00, DMSO-d6 δC 39.43 and methanol-d4 δC 49.00). Mass spectra were recorded at 70 eV on a Waters Prospec Q or Micromass QTOF 2W spectrometer using ESI as the method of ionization. High-resolution mass spectra were recorded at 70 eV on a Waters Prospec Q or Micromass QTOF 2W spectrometer using ESI as the method of ionization. Thin layer chromatography was performed on silica gel 60 F254 aluminum-backed plates fabricated by Merck. Flash column chromatography was performed on silica gel 60 (40–63 μm) produced by Merck. HPLC analyses were performed using a C18 stationary phase (Eclipse XDB-C18, 4.6 × 250 mm, particle size 5 μm, from Agilent Technologies), applying the conditions stated. Separation of diastereomers of 21 were conducted using a Biotage Select purification system (Biotage Sfär C18) applying the conditions stated. Diastereomeric ratios reported have not been validated by calibration; see Wernerova and Hudlicky for discussions and guidelines.20 Unless stated otherwise, all commercially available reagents and solvents were used in the form they were supplied without any further purification. All reactions were performed under an argon atmosphere, unless otherwise stated. The stated yields are based on isolated material. Liquid chromatography (LC)-grade solvents were purchased from Fisher Scientific.
(2R,3S,5Z)-1,2,3-Tris((tert-butyldimethylsilyl)oxy)oct-5-en (16)
NaHMDS (1.0 M, 6.60 mL, 6.60 mmol, 1.05 equiv) was added to a solution of propyltriphenylphosphonium bromide (13, 2.54 g, 6.60 mmol, 1.05 equiv) in CH2Cl2 (200 mL) at −78 °C and stirred for 30 min. Aldehyde 15 (3.00 g, 6.29 mmol, 1.00 equiv), obtained from 14 as earlier reported,11a,11c was then added to this solution at −78 °C. The solution was allowed to slowly warm to room temperature (rt) in the dry ice/acetone bath over 20 h before it was quenched with phosphate buffer (50 mL, pH = 7.2). The phases were separated. The aqueous phase was extracted with CH2Cl2 (2 × 50 mL), and the combined organic layers were dried (Na2SO4), before being concentrated in vacuo. Alkene 16 (2.72 mg, 5.41 mmol, 86%) was obtained after purification by column chromatography (hexane/EtOAc, 99:1) as a colorless oil: Rf (hexane/EtOAc, 99:1) = 0.42; [α]D25 −5.3 (c 0.2, CHCl3); 1H NMR (300 MHz, CDCl3) δ 5.46–5.36 (m, 2H), 3.83–3.74 (m, 1H), 3.69 (td, J = 5.9, 2.2 Hz, 1H), 3.62 (dd, J = 9.9, 6.2 Hz, 1H), 3.47 (dd, J = 9.9, 5.8 Hz, 1H), 2.35–2.14 (m, 2H), 2.12–1.97 (m, 2H), 0.96 (t, J = 7.5 Hz, 3H), 0.90 (s, 9H), 0.89 (s, 9H), 0.88 (s, 9H), 0.08 (s, 3H), 0.06 (s, 3H), 0.06 (s, 3H), 0.05 (s, 6H), 0.04 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 133.1, 126.3, 77.3, 74.7, 65.0, 30.8, 26.2 (6C), 26.1 (3C), 20.9, 18.5, 18.4, 18.3, 14.3, −4.1, −4.3, −4.4, −4.4, −5.2, −5.3; HRESIMS m/z 525.3587 [M + Na]+ (calcd for C26H58O3Si3Na, 525.3586).
(2R,3S,5Z)-2,3-Bis((tert-butyldimethylsilyl)oxy)oct-5-en-1-ol (17)
p-Toluenesulfonic acid (PTSA) (945 mg, 4.97 mmol, 1.00 equiv) was added to a solution of alkene 16 (2.50 g, 4.97 mmol, 1.00 equiv) in EtOH/MeOH (58 mL, 1:1), and the solution was stirred for 5 h at −20 °C. The reaction was quenched by addition of a saturated aqueous solution of NaHCO3 (60 mL) and diluted with EtOAc (40 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 40 mL). The combined organic layers were washed with brine (40 mL), dried (MgSO4), and filtered, and the solvent was removed in vacuo. Alcohol 17 (985 mg, 2.53 mmol, 51%, 89% based on recovered starting material) was obtained after purification by column chromatography (hexane/EtOAc, 95:5) as a colorless oil, and unreacted starting material was reisolated. Rf (hexane/EtOAc, 95:5) = 0.28; [α]D25 +2.9 (c 0.1, MeOH); 1H NMR (400 MHz, CDCl3) δ 5.51–5.34 (m, 2H), 3.80–3.69 (m, 2H), 3.67–3.56 (m, 2H), 2.37–2.21 (m, 2H), 2.09–1.96 (m, 3H), 0.96 (t, J = 7.5 Hz, 3H), 0.91 (s, 9H), 0.89 (s, 9H), 0.10 (s, 3H), 0.09 (s, 6H), 0.07 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 133.8, 124.7, 75.4, 74.8, 63.9, 32.1, 26.1 (3C), 26.0 (3C), 21.0, 18.3, 18.3, 14.2, −4.3, −4.3, −4.4, −4.5; HRESIMS m/z 411.2721 [M + Na]+ (calcd for C20H44O3Si2Na, 411.2721).
(2S,3S,Z)-2,3-Bis((tert-butyldimethylsilyl)oxy)oct-5-enal (9)
To a solution of alcohol 17 (750 mg, 1.93 mmol, 1.00 equiv) in CH2Cl2 (57 mL) were added NaHCO3 (924 mg, 11.0 mmol, 5.70 equiv) and DMP (1.47 g, 3.47 mmol, 1.80 equiv) at rt. The reaction mixture was stirred overnight and quenched by addition of a saturated aqueous solution of Na2S2O3 (12 mL). The layers were separated, and the aqueous layer was extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with brine, dried (Na2SO4), and filtered, and the solvent was removed in vacuo. Aldehyde 9 (722 mg, 1.87 mmol, 97%) was obtained after purification by column chromatography (hexane/EtOAc, 95:5) as a colorless oil: Rf (hexane/EtOAc, 95:5) = 0.48; [α]D25 +3.0 (c 0.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 9.56 (dd, J = 1.6, 0.7 Hz, 1H), 5.55–5.41 (m, 1H), 5.32–5.17 (m, 1H), 3.97–3.86 (m, 2H), 2.48–2.36 (m, 1H), 2.27–2.15 (m, 1H), 2.13–1.99 (m, 2H), 0.96 (t, J = 7.5 Hz, 3H), 0.92 (s, 9H), 0.88 (s, 9H), 0.08 (s, 6H), 0.07 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 204.0, 135.1, 123.9, 81.1, 76.3, 31.7, 26.0 (3C), 26.0 (3C), 20.9, 18.4, 18.3, 14.2, −4.5, −4.5, −4.6, −4.6; HRESIMS m/z 409.2564 [M + Na]+ (calcd for C20H42O3Si2Na, 409.2565).
(2E,4E,6R,7S,9Z)-6,7-Bis((tert-butyldimethylsilyl)oxy)dodeca-2,4,9-trienal (7)
To a solution of aldehyde 9 (372 mg, 0.962 mmol, 1.00 equiv) in toluene (20 mL) was added (triphenylphosphoranylidene)acetaldehyde (10, 293 mg, 0.962 mmol, 1.00 equiv). The reaction mixture was warmed to 95 °C and stirred for 72 h. Another equivalent of (triphenylphosphoranylidene)acetaldehyde (10, 293 mg, 0.962 mmol, 1.00 equiv) was added after 6, 24, 48, and 60 h. After cooling to rt, the solvent was evaporated, and the residue was dissolved in hexane/EtOAc (8:2, 20 mL) and filtrated. The residue was washed with another portion of hexane/EtOAc (8:2, 20 mL). The filtrate was concentrated in vacuo. Aldehyde 7 (240 mg, 0.547 mmol, 57%) was obtained after purification by column chromatography (hexane/EtOAc, 95:5) as an orange oil: Rf (hexane/EtOAc, 95:5) = 0.20; [α]D25 +15 (c 0.8, MeOH); 1H NMR (400 MHz, CDCl3) δ 9.58 (d, J = 7.9 Hz, 1H), 7.11 (dd, J = 15.3, 10.8 Hz, 1H), 6.42 (dd, J = 15.3, 10.8 Hz, 1H), 6.27 (dd, J = 15.3, 6.4 Hz, 1H), 6.13 (dd, J = 15.3, 7.9 Hz, 1H), 5.49–5.39 (m, 1H), 5.39–5.31 (m, 1H), 4.20–4.08 (m, 1H), 3.75–3.63 (m, 1H), 2.33–2.14 (m, 2H), 2.07–1.98 (m, 2H), 0.96 (t, J = 7.5 Hz, 3H), 0.90 (s, 9H), 0.86 (s, 9H), 0.06 (s, 3H), 0.04 (s, 3H), 0.03 (s, 3H), 0.01 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 194.0, 151.7, 145.9, 133.8, 131.6, 129.0, 124.8, 76.7, 76.1, 32.0, 26.1, 26.0, 21.0, 18.4, 18.3, 14.3, −4.1, −4.2, −4.3, −4.5; HRESIMS m/z 461.2877 [M + Na]+ (calcd for C24H46O3Si2Na, 461.2878).
Methyl (7Z,10Z,12E,14E,16R,17S,19Z)-16,17-Bis((tert-butyldimethylsilyl)oxy)docosa-7,10,12,14,19-pentaenoate (20)
To the Wittig-salt 8(12) (153 mg, 0.267 mmol, 1.20 equiv) in THF (20 mL) was added HMPA (4.0 mL) before NaHMDS (0.43 mL, 0.6 M in toluene, 1.20 equiv) was slowly added at −78 °C, and then the mixture was stirred for 30 min at 0 °C. Aldehyde 7 (100 mg, 0.228 mmol, 1.00 equiv) was added at −78 °C. The solution was allowed to slowly warm to rt in a dry ice/acetone bath over 18 h before it was quenched with phosphate buffer (10 mL, pH = 7.2). Et2O (15 mL) was added, and the phases were separated. The aqueous phase was extracted with Et2O (2 × 15 mL), and the combined organic layers were dried (Na2SO4), before being concentrated in vacuo. The title compound 20 (65 mg, 0.11 mmol, 47%) was obtained after purification by column chromatography (hexane/EtOAc, 95:5) as an orange oil: Rf (hexane/EtOAc, 95:5) = 0.22; [α]D25 +13 (c 0.5, MeOH); 1H NMR (400 MHz, CDCl3) δ 6.50–6.41 (m, 1H), 6.27–6.10 (m, 2H), 6.03 (apparent t, J = 10.9, 1.7 Hz, 1H), 5.68 (dd, J = 14.5, 7.4 Hz, 1H), 5.50–5.28 (m, 5H), 4.02 (dd, J = 7.4, 4.1 Hz, 1H), 3.66 (s, 3H), 3.62 (m, 1H), 2.93 (t, J = 6.9 Hz, 2H), 2.31 (t, J = 7.5 Hz, 2H), 2.27–2.16 (m, 2H), 2.12–1.97 (m, 4H), 1.63 (p, J = 7.4 Hz, 2H), 1.49–1.20 (m, 4H), 0.95 (t, J = 7.5 Hz, 3H), 0.87 (18H), 0.49–0.94 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 174.3, 134.6, 133.3, 132.8, 131.8, 130.5, 130.5, 128.8, 127.6, 127.5, 125.5, 77.1, 76.9, 51.6, 34.2, 31.8, 29.4, 28.9, 27.2, 26.3, 26.1, 26.1, 25.0, 20.9, 18.4, 18.3, 14.3, −3.9, −4.0, −4.3, −4.5; HRESIMS m/z 627.4234 [M + Na]+ (calcd for C35H64O4Si2Na, 627.4235).
Methyl (7Z,10Z,12E,14E,16R,17S,19Z)-16,17-Dihydroxydocosa-7,10,12,14,19-pentaenoate (21)
A solution of TBS-protected diol 20 (32.5 mg, 53.7 μmol, 1.00 equiv) in THF (1.5 mL) was cooled to 0 °C, and TBAF (1.0 M in THF, 0.161 mL, 0.161 mmol, 3.00 equiv) was added. The reaction was stirred for 16 h before it was quenched with phosphate buffer (pH = 7.0, 0.5 mL). Brine (1.0 mL) and EtOAc (1.0 mL) were added, and the phases were separated. The water phase was extracted with EtOAc (2 × 1.0 mL). The combined organic phases were dried (Na2SO4) and concentrated in vacuo. The crude product was purified by column chromatography (SiO2, hexane/EtOAc, 60:40) to afford the methyl ester of PD2n-3 DPA (21) as a mixture of C-10/C-11-isomers (Z/E, 17:3). The Z-C-10/C-11 isomer 21 was obtained (15 mg, 39.8 μmol, 74%) as a pale yellow oil using a Biotage Select purification system (Biotage Sfär C18, H2O/MeOH, 60:40, to H2O/MeOH, 20:80, over 32.8 column volumes, 6.0 mL/min). The purity (>96%) was determined by HPLC analysis (Eclipse XDB-C18, MeOH/H2O, 77:23, 1.0 mL/min); Rf (hexane/EtOAc, 60:40) = 0.14; [α]D25 +8.1 (c 0.5, MeOH); UV (MeOH) λmax (log ε) 263 (4.52), 273 (4.59), 284 (4.53) nm; 1H NMR (400 MHz, CD3OD) δ 6.56 (dd, J = 14.3, 11.3 Hz, 1H), 6.36 (ddd, J = 15.0, 10.8, 1.1 Hz, 1H), 6.25 (dd, J = 14.6, 10.6 Hz, 1H), 6.03 (apparent t, J = 11.1 Hz, 1H), 5.82 (dd, J = 15.0, 7.1 Hz, 1H), 5.55–5.25 (m, 5H), 4.01 (ddd, J = 7.0, 4.9, 1.1 Hz, 1H), 3.65 (S, 3H), 3.54 (dt, J = 8.1, 4.9 Hz, 1H), 2.95 (t, J = 7.5, 2H), 2.48–2.25 (m, 3H), 2.22–1.97 (m, 5H), 1.61 (quint, J = 7.5 Hz, 2H), 1.47–1.23 (m, 4H), 0.97 (t, J = 7.5 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 176.0, 134.4, 133.8, 133.7, 133.6, 131.4, 131.3, 129.7, 129.1, 128.6, 126.3, 76.3, 76.0, 52.0, 34.8, 31.8, 30.3, 29.8, 28.0, 27.1, 25.9, 21.7, 14.6; HRESIMS m/z 399.2505 [M + Na]+ (calcd for C23H36O4Na, 399.2506).
(7Z,10Z,12E,14E,19Z)-16R,17S-Dihydroxydocosa-7,10,12,14,19-pentaenoic Acid (PD2n-3 DPA, 2)
Solid LiOH (6.7 mg, 0.28 mmol, 35 equiv) was added at 0 °C to a solution of the methyl ester 21 (3.0 mg, 8.0 μmol, 1.0 equiv) in THF/MeOH/H2O (2:2:1, 1.1 mL). The mixture was stirred at 0 °C for 20 h. The solution was acidified with aqueous saturated NaH2PO4 (1.5 mL) before EtOAc (1.5 mL) was added. The layers were separated, and the water phase was extracted with EtOAc (2 × 2.0 mL). The combined organic layer was dried (Na2SO4) before being concentrated in vacuo. PD2n-3 DPA (2) (2.6 mg, 7.2 μmol, 91%) was obtained after purification by column chromatography (4% MeOH in CH2Cl2) as a colorless oil: Rf (hexane/EtOAc, 40:60) = 0.28; [α]D25 +22 (c 0.14, MeOH); UV (MeOH) λmax (log ε) 262 (4.52), 272 (4.59), 283 (4.53) nm; 1H NMR (400 MHz, CD3OD) δ 6.57 (dd, J = 14.3, 11.2 Hz, 1H), 6.36 (ddd, J = 15.1, 10.7, 1.1 Hz, 1H), 6.26 (dd, J = 14.6, 10.7 Hz, 1H), 6.03 (apparent t, J = 11.1 Hz, 1H), 5.83 (dd, J = 15.0, 7.1 Hz, 1H), 5.50–5.32 (m, 5H), 4.01 (ddd, J = 7.0, 4.9, 1.1 Hz, 1H), 3.55 (dt, J = 8.1, 4.9 Hz, 1H), 2.96 (t, J = 7.3 Hz, 2H), 2.39–2.26 (m, 3H), 2.21–2.03 (m, 5H), 1.62 (p, J = 7.3 Hz, 2H), 1.46–1.29 (m, 4H), 0.97 (t, J = 7.5 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 177.8, 134.4, 133.8, 133.7, 133.6, 131.5, 131.3, 129.6, 129.1, 128.5, 126.3, 76.3, 76.0, 35.1, 31.8, 30.4, 29.9, 28.0, 27.1, 26.1, 21.7, 14.6; HRESIMS m/z 385.2349 [M + Na]+ (calcd for C22H34O4Na, 385.2349).
Acknowledgments
The Norwegian Research Council is gratefully acknowledged for funding to T.V.H. (FRIPRO-FRINATEK 230470).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jnatprod.0c00385.
1H and 13C NMR, UV/vis, and HPLC data of PD2n-3 DPA (2), methyl ester 21, and all synthetic intermediates (PDF)
Author Contributions
‡ J. E. Tungen and K. G. Primdahl contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Serhan C. N.; Levy B. D. J. Clin. Invest. 2018, 128, 2657–2669. 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Serhan C. N. Nature 2014, 510, 92–101. 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dalli J. Mol. Aspects Med. 2017, 58, 12–20. 10.1016/j.mam.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Serhan C. N.; Petasis N. A. Chem. Rev. 2011, 111, 5922–5943. 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dalli J.; Serhan C. N. Br. J. Pharmacol. 2019, 8, 1024–1037. 10.1111/bph.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Serhan C. N.; Chiang N. Br. J. Pharmacol. 2008, 153, S200. 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton J. N.; Gilroy D. W. Nat. Rev. Drug Discovery 2016, 15, 551–567. 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- a Dalli J.; Colas R. A.; Serhan C. N. Sci. Rep. 2013, 3, 1940. 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dalli J.; Chiang N.; Serhan C. N. Nat. Med. 2015, 21, 1071–1075. 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kaur G.; Cameron-Smith D.; Garg M.; Sinclair A. J. Prog. Lipid Res. 2011, 50, 28–34. 10.1016/j.plipres.2010.07.004. [DOI] [PubMed] [Google Scholar]; b Jakobsen M. G.; Vik A.; Hansen T. V. Tetrahedron Lett. 2012, 53, 5837–5839. 10.1016/j.tetlet.2012.08.009. [DOI] [Google Scholar]
- Aursnes M.; Tungen J. E.; Vik A.; Colas R.; Cheng C.-Y. C.; Dalli J.; Serhan C. N.; Hansen T. V. J. Nat. Prod. 2014, 77, 910–916. 10.1021/np4009865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Vik A.; Dalli J.; Hansen T. V. Bioorg. Med. Chem. Lett. 2017, 27, 2259–2066. 10.1016/j.bmcl.2017.03.079. [DOI] [PubMed] [Google Scholar]; b Hansen T. V.; Dalli J.; Serhan C. N. Prostaglandins Other Lipid Mediators 2017, 133, 103–110. 10.1016/j.prostaglandins.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Primdahl K. G.; Tungen J. E.; De Souza P. R. S.; Colas R. A.; Dalli J.; Hansen T. V.; Vik A. Org. Biomol. Chem. 2017, 15, 8606–8613. 10.1039/C7OB02113E. [DOI] [PubMed] [Google Scholar]; b Pistorius K.; Souza P. R.; De Matteis R.; Austin-Williams S.; Primdahl K. G.; Vik A.; Mazzacuva F.; Colas R. A.; Marques R. M.; Hansen T. V.; Dalli J. Cell Chem. Biol. 2018, 25, 749–760. 10.1016/j.chembiol.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sønderskov J.; Tungen J. E.; Palmas F.; Dalli J.; Serhan C. N.; Stenstrøm Y.; Hansen T. V. Tetrahedron Lett. 2020, 61, 151510. 10.1016/j.tetlet.2019.151510. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tungen J. E.; Aursnes M.; Dalli J.; Arnardottir H.; Serhan C. N.; Hansen T. V. Chem. - Eur. J. 2014, 20, 14575–14578. 10.1002/chem.201404721. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tungen J. E.; Gerstmann L.; Vik A.; De Mateis R.; Colas R. A.; Dalli J.; Chiang N.; Serhan C. N.; Kalesse M.; Hansen T. V. Chem. - Eur. J. 2019, 25, 1476–1480. 10.1002/chem.201806029. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Nesman J. I.; Tungen J. E.; Primdahl K. G.; Palmas F.; Dalli J.; Hansen T. V. Molecules 2019, 24, 3228. 10.3390/molecules24183228. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Hansen T. V.; Vik A.; Serhan C. N. Front. Pharmacol. 2019, 9, 1582–1598. 10.3389/fphar.2018.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Gobbetti T.; Dalli J.; Colas R. A.; Canova D. F.; Aursnes M.; Bonnet D.; Alric L.; Vergnolle N.; Deraison C.; Hansen T. V.; Serhan C. N.; Perretti M. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 3963–3968. 10.1073/pnas.1617290114. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Frigerio F.; Pasqualini G.; Craparotta I.; Marchini S.; Van Vliet E. A.; Foerch P.; Vandenplas C.; Leclercq K.; Aronica E.; Porcu L.; Pistorius K.; Colas R. A.; Hansen T. V.; Perretti M.; Kaminski R. M.; Dalli J.; Vezzani A. Brain 2018, 141, 3130–3143. 10.1093/brain/awy247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primdahl K. G.; Aursnes M.; Walker M. E.; Colas R. A.; Serhan C. N.; Dalli J.; Hansen T. V.; Vik A. J. Nat. Prod. 2016, 79, 2693–2702. 10.1021/acs.jnatprod.6b00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A. R.; Spur B. W. Tetrahedron Lett. 2018, 59, 1143–1146. 10.1016/j.tetlet.2018.02.029. [DOI] [Google Scholar]
- a Serhan C. N.; Hong S.; Gronert K.; Colgan S. P.; Devchand P. R.; Mirick G.; Moussignac R. L. J. Exp. Med. 2002, 196, 1025–1037. 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Serhan C. N.; Fredman G.; Yang R.; Karamnov S.; Belayev L. S.; Bazan N. G.; Zhu M.; Winkler J. W.; Petasis N. A. Chem. Biol. 2011, 18, 976–987. 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sun Y. P.; Oh S. F.; Uddin J.; Yang R.; Gotlinger K.; Campbell E.; Colgan S. P.; Petasis N. A.; Serhan C. N. J. Biol. Chem. 2007, 282, 9323–9334. 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Pangopoulos M. K.; Nolsøe J. M. N.; Antonsen S. G.; Colas R. A.; Dalli J.; Aursnes M.; Stenstrøm Y.; Hansen T. V. Bioorg. Chem. 2020, 96, 103653. 10.1016/j.bioorg.2020.103653. [DOI] [PubMed] [Google Scholar]
- a Dalli J.; Zhu M.; Vlasenko N. A.; Deng B.; Haeggström J. Z.; Petasis N. A.; Serhan C. N. FASEB J. 2013, 27, 2573–2583. 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tungen J. E.; Aursnes M.; Vlasakov I.; Colas R. A.; Dalli J.; Serhan C. N.; Hansen T. V. J. Nat. Prod. 2015, 78, 2924–2931. 10.1021/acs.jnatprod.5b00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon S.; Dalli J.; Sanger J. M.; Winkler J. W.; Aursnes M.; Tungen J. E.; Hansen T. V.; Serhan C. N. Am. J. Pathol. 2016, 186, 962–971. 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggström J. Z.; Funk C. D. Chem. Rev. 2011, 111, 5866–5898. and references cited therein. 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- Oger C.; Balas L.; Durand T.; Galano J.-M. Chem. Rev. 2013, 113, 1313–1350. 10.1021/cr3001753. [DOI] [PubMed] [Google Scholar]
- Wernerova M.; Hudlicky T. Synlett 2010, 18, 2701–2707. 10.1055/s-0030-1259018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.